Preparation, Characterization and Application of UV-Curable Flexible Hyperbranched Polyurethane Acrylate
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
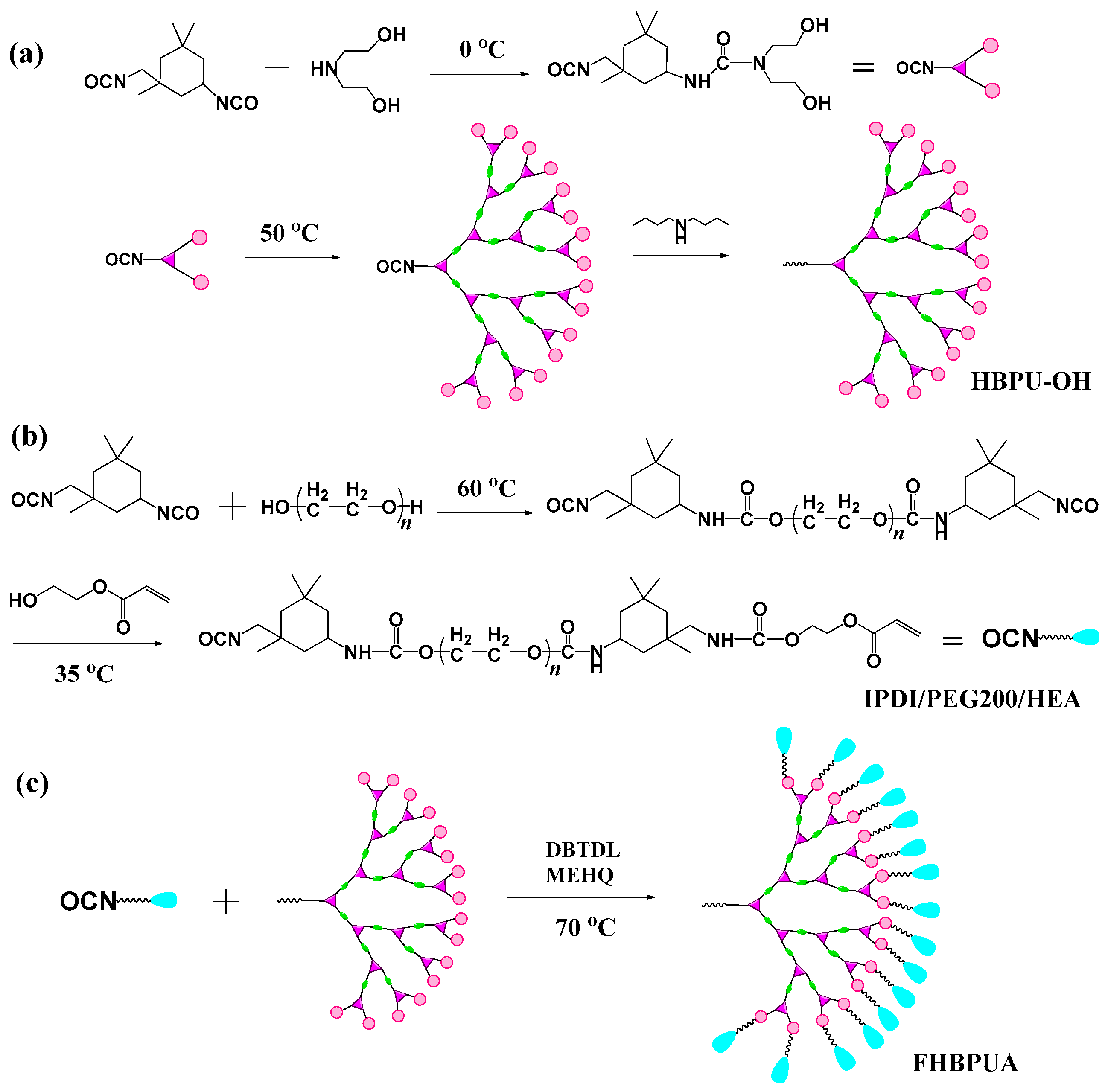
2.2. Preparation of Hydroxyl Terminated Hyperbranched Polyurethane (HBPU–OH)
2.3. Preparation of Semiadduct Urethane Monoacrylate (IPDI/PEG200/HEA)
2.4. Preparation of Flexible Hyperbranched Polyurethane Acrylate (FHBPUA)
2.5. Preparation of UV-Cured Films
2.6. Characterization
3. Result and Discussion
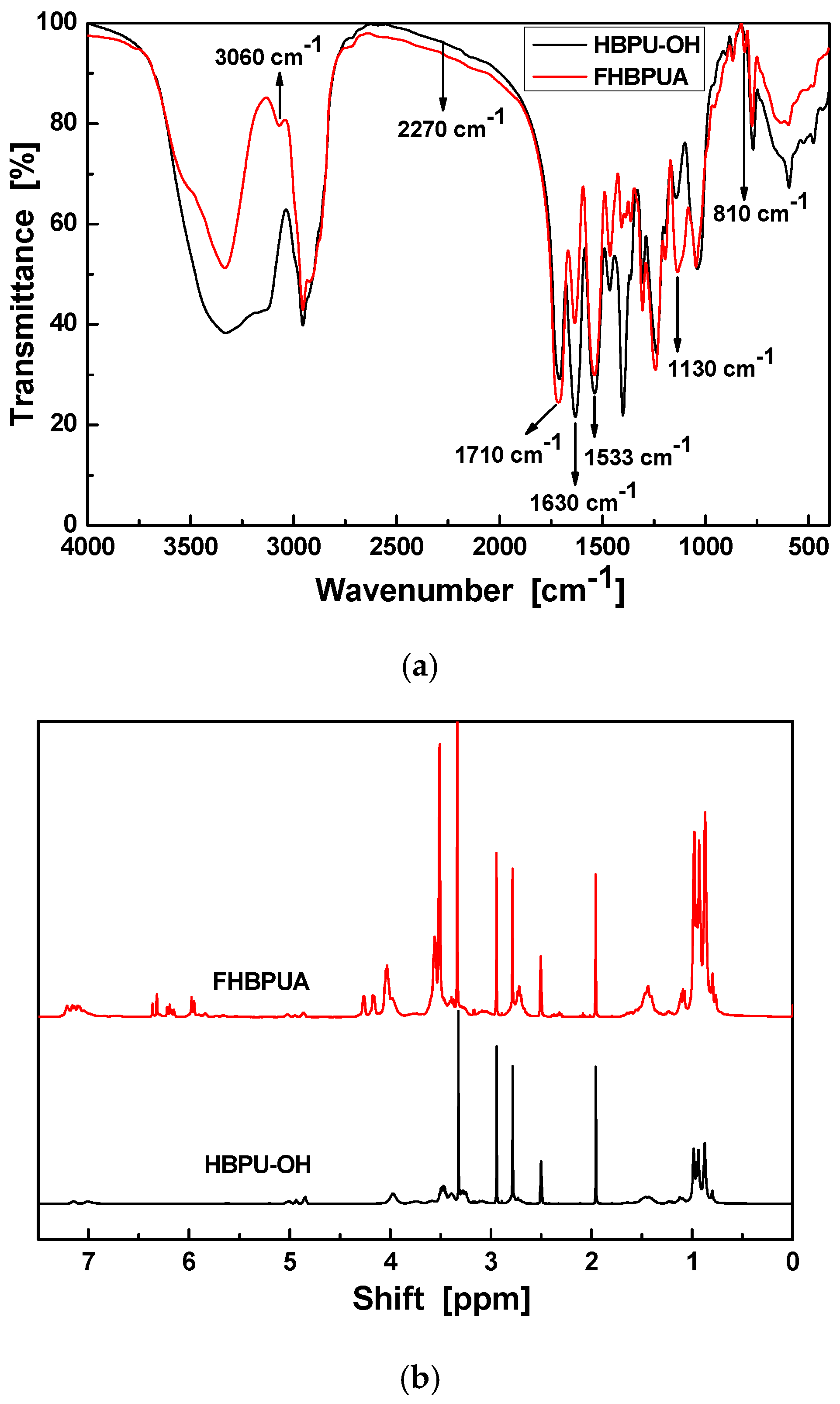
3.1. Characterization of FHBPUA
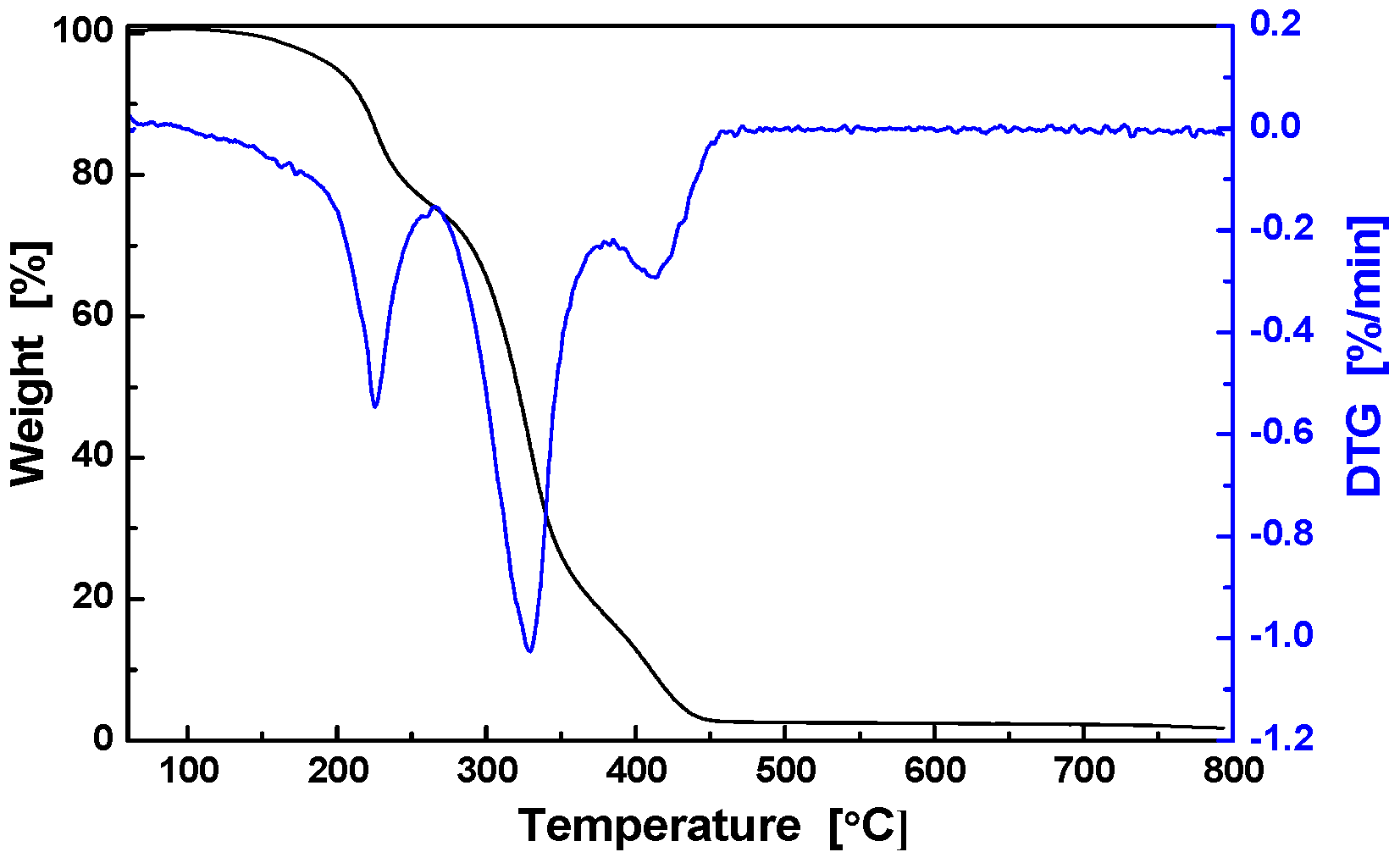
3.2. Thermal Properties
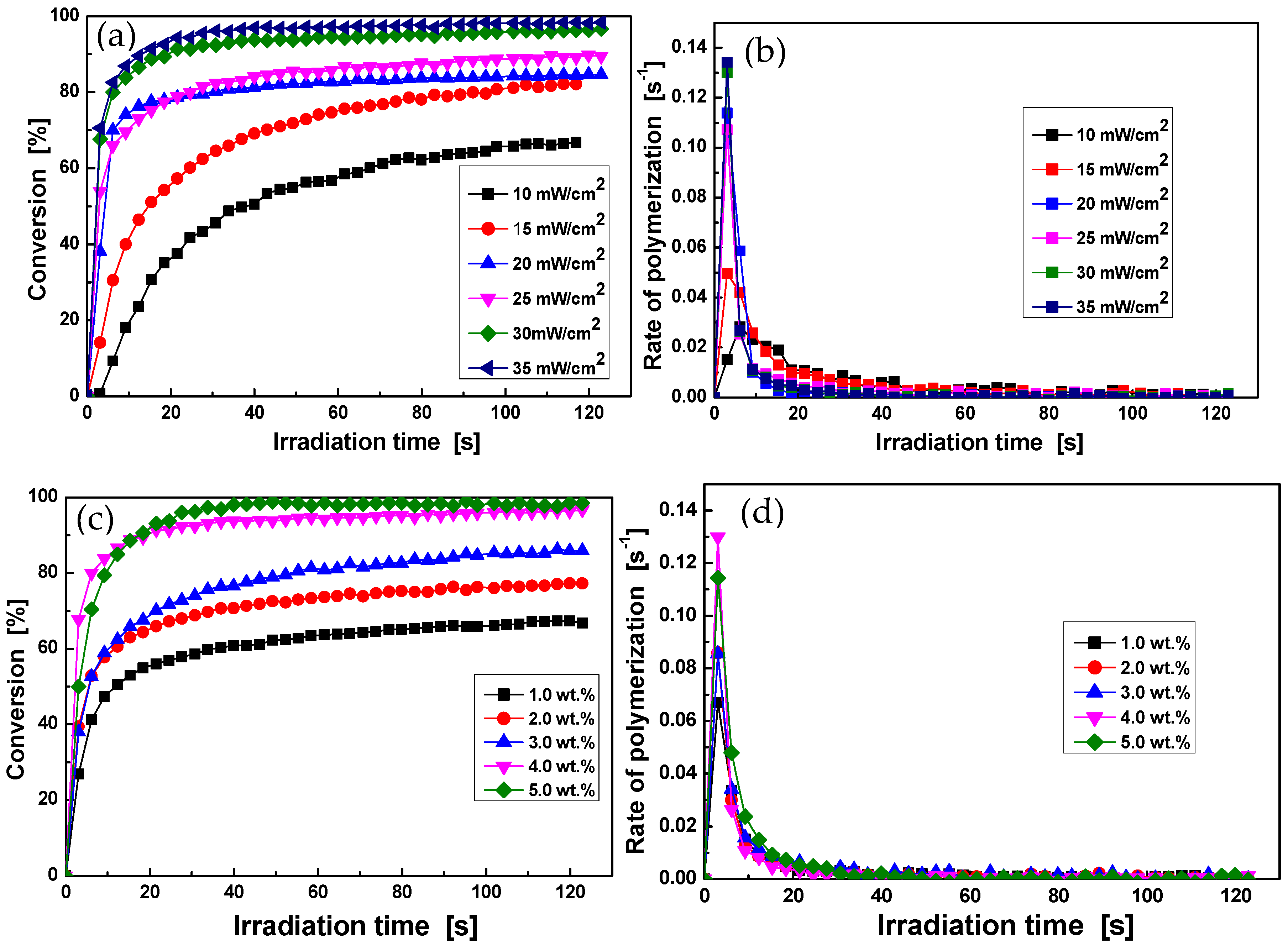
3.3. Photopolymerization Kinetics
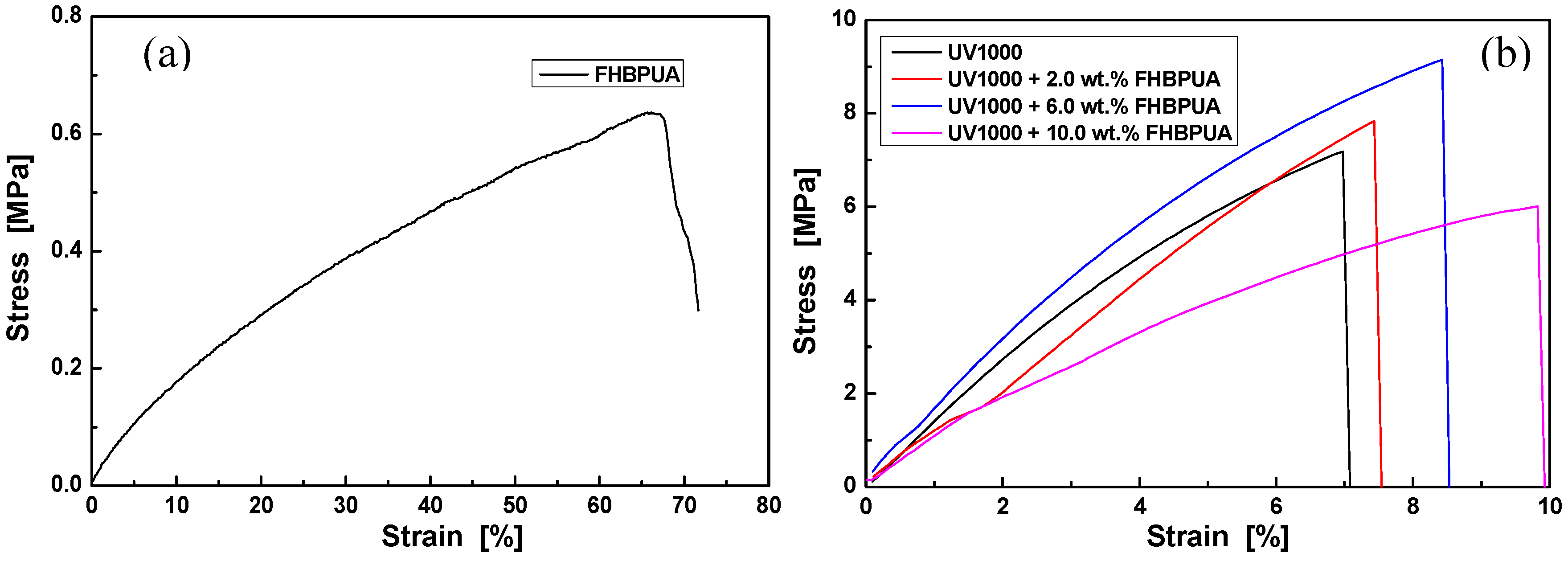
3.4. Properties of UV-Cured Films
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sun, F.; Luo, X.; Kang, L.; Peng, X.; Lu, C. Synthesis of hyperbranched polymers and their applications in analytical chemistry. Polym. Chem. 2015, 6, 1214–1225. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, D.; Zhu, X.; Yan, D.; Chen, R. Synthesis and therapeutic applications of biocompatible or biodegradable hyperbranched polymers. Polym. Chem. 2015, 6, 2794–2812. [Google Scholar] [CrossRef]
- Wang, D.; Jin, Y.; Zhu, X.; Yan, D. Synthesis and applications of stimuli-responsive hyperbranched Polymers. Prog. Polym. Sci. 2017, 64, 114–153. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, S.; Weng, Z.; Gao, C. Hyperbranched polymers: Advances from synthesis to applications. Chem. Soc. Rev. 2015, 44, 4091–4130. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhou, Y.; Yan, D. Hyperbranched polymer vesicles: From self-assembly, characterization, mechanisms, and properties to applications. Chem. Soc. Rev. 2015, 44, 3874–3889. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Tang, R.; Li, Q.; Li, Z. Functional hyperbranched polymers with advanced optical, electrical and magnetic properties. Chem. Soc. Rev. 2015, 44, 3997–4022. [Google Scholar] [CrossRef] [PubMed]
- Kurniasih, I.N.; Keilitz, J.; Haag, R. Dendritic nanocarriers based on hyperbranched polymers. Chem. Soc. Rev. 2015, 44, 4145–4164. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhao, T.; Zhu, X.; Yan, D.; Wang, W. Bioapplications of hyperbranched polymers. Chem. Soc. Rev. 2015, 44, 4023–4071. [Google Scholar] [CrossRef] [PubMed]
- Sangermano, M.; Razza, N.; Crivello, J.V. Cationic UV-curing: Technology and applications. Macromol. Mater. Eng. 2014, 299, 775–793. [Google Scholar] [CrossRef]
- Fertier, L.; Koleilat, H.; Stemmelen, M.; Giani, O.; Duhamel, C.J. The use of renewable feedstock in UV-curable materials—A new age for polymers and green chemistry. Prog. Polym. Sci. 2013, 38, 932–962. [Google Scholar] [CrossRef]
- Lin, J.; Zeng, X.; Hou, Y.; Shi, H.; Lin, J. Synthesis and characterization of UV-curable hyperbranched urethane acrylate. Polym. Plast. Technol. 2008, 47, 237–241. [Google Scholar] [CrossRef]
- Mishra, R.S.; Mishra, A.K.; Raju, K.V.S.N. Synthesis and property study of UV-curable hyperbranched polyurethane acrylate/ZnO hybrid coatings. Eur. Polym. J. 2009, 45, 960–966. [Google Scholar] [CrossRef]
- Şabani, S.; Önen, A.H.; Güngör, A. Preparation of hyperbranched polyester polyol-based urethane acrylates and applications on UV-curable wood coatings. J. Coat. Technol. Res. 2012, 9, 703–716. [Google Scholar] [CrossRef]
- Xu, G.; Shi, W. Synthesis and characterization of hyperbranched polyurethane acrylates used as UV curable oligomers for coatings. Prog. Org. Coat. 2005, 52, 110–117. [Google Scholar] [CrossRef]
- Xu, G.; Zhao, Y.; Shi, W. Properties and morphologies of UV-cured epoxy acrylate blend films containing hyperbranched polyurethane acrylate/hyperbranched polyester. J. Polym. Sci. Part B Polym. Phys. 2010, 43, 3159–3170. [Google Scholar] [CrossRef]
- Džunuzović, E.; Tasić, S.; Božić, B.; Jeremić, K.; Dunjić, B. Photoreactive hyperbranched urethane acrylates modified with a branched saturated fatty acid. React. Funct. Polym. 2006, 66, 1097–1105. [Google Scholar] [CrossRef]
- Dzunuzovic, E.; Tasic, S.; Bozic, B.; Babic, D.; Dunjic, B. UV-curable hyperbranched urethane acrylate oligomers containing soybean fatty acids. Prog. Org. Coat. 2005, 52, 136–143. [Google Scholar] [CrossRef]
- Džunuzović, E.S.; Tasić, S.V.; Božić, B.R.; Džunuzović, J.V.; Dunjić, B.M. Mechanical and thermal properties of UV cured mixtures of linear and hyperbranched urethane acrylates. Prog. Org. Coat. 2012, 74, 158–164. [Google Scholar] [CrossRef]
- Bao, F.; Shi, W. Synthesis and properties of hyperbranched polyurethane acrylate used for UV curing coatings. Prog. Org. Coat. 2012, 68, 334–339. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, C.; Wang, H.; Hu, M.; Li, H.; Liu, X. UV-curable coating crosslinked by a novel hyperbranched polyurethane acrylate with excellent mechanical properties and hardness. R. Soc. Chem. Adv. 2016, 6, 107942–107950. [Google Scholar] [CrossRef]
- Han, W.; Lin, B.; Zhou, Y.; Song, J. Synthesis and properties of UV-curable hyperbranched polyurethane acrylate oligomers containing photoinitiator. Polym. Bull. 2012, 68, 729–743. [Google Scholar] [CrossRef]
- Han, W.; Lin, B.; Yang, H.; Zhang, X. Synthesis and properties of UV-curable hyperbranched polyurethane acrylate oligomers containing carboxyl groups. Polym. Bull. 2012, 68, 1009–1022. [Google Scholar] [CrossRef]
- Yin, W.; Zeng, X.; Li, H.; Hou, Y.; Gao, Q. Synthesis, photopolymerization kinetics and thermal properties of UV-curable waterborne hyperbranched polyurethane acrylate dispersions. J. Coat. Technol. Res. 2011, 8, 577–584. [Google Scholar] [CrossRef]
- Asif, A.; Huang, C.; Shi, W. Photopolymerization of waterborne polyurethane acrylate dispersions based on hyperbranched aliphatic polyester and properties of the cured films. Colloid Polym. Sci. 2005, 283, 721–730. [Google Scholar] [CrossRef]
- Asif, A.; Huang, C.; Shi, W. Structure-property study of waterborne, polyurethane acrylate dispersions based on hyperbranched aliphatic polyester for UV-curable coatings. Colloid Polym. Sci. 2004, 283, 200–208. [Google Scholar] [CrossRef]
- Asif, A.; Hu, L.; Shi, W. Synthesis, rheological and thermal properties of waterborne hyperbranched polyurethane acrylate dispersions for UV curable coatings. Colloid Polym. Sci. 2009, 287, 1041–1049. [Google Scholar] [CrossRef]
- Hu, H.; Yuan, Y.; Shi, W. Preparation of waterborne hyperbranched polyurethane acrylate/LDH nanocomposite. Prog. Org. Coat. 2012, 75, 474–479. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, S.; Qian, J. Synthesis and properties of a novel UV-curable waterborne hyperbranched polyurethane. J. Coat. Technol. Res. 2014, 11, 319–328. [Google Scholar] [CrossRef]
- And, M.T.; Hedrick, J.L. Hyperbranched Poly(ε-caprolactone) Derived from Intrinsically Branched AB2 Macromonomers. Macromolecules 1998, 31, 4390–4395. [Google Scholar]
- Kwak, S.Y.; Ahn, D.U.; Choi, J.; Song, H.J.; Lee, S.H. Amelioration of mechanical brittleness in hyperbranched polymer. 1. Macroscopic evaluation by dynamic viscoelastic relaxation. Polymer 2004, 45, 6889–6896. [Google Scholar] [CrossRef]
- Trollsås, M.; Atthoff, B.; Claesson, H.; Hedrick, J.L. Hyperbranched Poly(ε-caprolactone)s. Macromolecules 1998, 31, 3439–3445. [Google Scholar] [CrossRef]
- Trollsås, M.; Hedrick, J.L.; Mecerreyes, D.; Dubois, P.; Jérôme, R.; Ihre, H.; Hult, A. Versatile and controlled synthesis of star and branched macromolecules by dentritic initiation. Macromolecules 1997, 30, 8508–8511. [Google Scholar] [CrossRef]
- Schmaljohann, D.; Häußler, L.; Pötschke, P.; Voit, B.I.; Loontjens, T.J.A. Modification with alkyl chains and the influence on thermal and mechanical properties of aromatic hyperbranched polyesters. Macromol. Chem. Phys. 2000, 201, 303–316. [Google Scholar] [CrossRef]
- Hong, X.; Chen, Q.; Zhang, Y.; Liu, G. Synthesis and Characterization of a Hyperbranched Polyol with Long Flexible Chains and Its Application in Cationic UV Curing. J. Appl. Polym. Sci. 2015, 77, 1353–1356. [Google Scholar] [CrossRef]
- Hawker, C.J.; Chu, F. Hyperbranched poly(ether ketones): Manipulation of structure and physical properties. Macromolecules 1996, 29, 4370–4380. [Google Scholar] [CrossRef]
- Kakimoto, M.; Grunzinger, S.J.; Hayakawa, T. Hyperbranched poly(ether sulfone)s: Preparation and application to ion-exchange membranes. Polym. J. 2010, 42, 697–705. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Stukenbrock, T. New polymer syntheses. XC. A-B-A triblock copolymers with hyperbranched polyester A-blocks. J. Polym. Sci. Part A Polym. Chem. 1998, 36, 31–38. [Google Scholar] [CrossRef]
- Yin, S.; Sun, N.; Feng, C.Y.; Wu, Z.M.; Xu, Z.H.; Jiang, S.H. Synthesis, characterization and application of multi-generations hyperbranched polyurethane based on isophorone diisocyanate. Adv. Mater. Res. 2012, 554, 126–129. [Google Scholar] [CrossRef]
- Gao, Q.; Li, H.; Zeng, X. Preparation and characterization of UV-curable hyperbranched polyurethane acrylate. J. Coat. Technol. Res. 2011, 8, 61–66. [Google Scholar] [CrossRef]
- Sun, F.; Liao, B.; Zhang, L.; Du, H.; Huang, Y. Synthesis and Characterization of Alkali-Soluble Hyperbranched Photosensitive Polysiloxane Urethane Acrylate. Ind. Eng. Chem. Res. 2015, 120, 3604–3612. [Google Scholar] [CrossRef]
- Ahmed, A.; Sarkar, P.; Ahmad, I.; Das, N.; Bhowmick, A.K. Influence of the Nature of Acrylates on the Reactivity, Structure, and Properties of Polyurethane Acrylates. Ind. Eng. Chem. Res. 2015, 54, 47–54. [Google Scholar] [CrossRef]
- Abdelrehim, M.; Komber, H.; Langenwalter, J.; Voit, B.; Bruchmann, B. Synthesis and characterization of hyperbranched poly(urea-urethane)s based on AA* and B2B* monomers. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 3062–3081. [Google Scholar] [CrossRef]
- Zou, Z.P.; Liu, X.B.; Wu, Y.P.; Tang, B.; Chen, M.; Zhao, X.L. Hyperbranched polyurethane as a highly efficient toughener in epoxy thermosets with reaction-induced microphase separation. RSC. Adv. 2016, 6, 18060–18070. [Google Scholar] [CrossRef]
- Hawker, C.J.; Lee, R.; Fréchet, J.M.J. One-step synthesis of hyperbranched dendritic polyesters. J. Am. Chem. Soc. 2002, 113, 4583–4588. [Google Scholar] [CrossRef]
- Tasic, S.; Bozic, B.; Dunjic, B. Synthesis of new hyperbranched urethane-acrylates and their evaluation in UV-curable coatings. Prog. Org. Coat. 2004, 51, 320–327. [Google Scholar] [CrossRef]
- Mishra, A.K.; Narayan, R.; Raju, K.V.S.N.; Aminabhavi, T.M. Hyperbranched polyurethane (HBPU)-urea and HBPU-imide coatings: Effect of chain extender and NCO/OH ratio on their properties. Prog. Org. Coat. 2004, 74, 134–141. [Google Scholar] [CrossRef]
- Lecamp, L.; Youssef, B.; Bunel, C.; Lebaudy, P. Photoinitiated polymerization of a dimethacrylate oligomer: 1. Influence of photoinitiator concentration, temperature and light intensity. Polymer 1997, 38, 6089–6096. [Google Scholar] [CrossRef]
- Sun, F.; Shi, J.; Du, H.; Nie, J. Synthesis and characterization of hyperbranched photosensitive polysiloxane urethane acrylate. Prog. Org. Coat. 2009, 66, 412–419. [Google Scholar] [CrossRef]
- Zhang, Y.; Asif, A.; Shi, W. Highly branched polyurethane acrylates and their waterborne UV curing coating. Prog. Org. Coat. 2011, 71, 295–301. [Google Scholar] [CrossRef]
- Chuang, F.S.; Tsen, W.C.; Shu, Y.C. The effect of different siloxane chain-extenders on the thermal degradation and stability of segmented polyurethanes. Polym. Degrad. Stab. 2004, 84, 69–77. [Google Scholar] [CrossRef]
- Sang, S.L.; Koo, J.H.; Lee, S.S.; Chai, S.G.; Lim, J.C. Gloss reduction in low temperature curable hybrid powder coatings. Prog. Org. Coat. 2003, 46, 266–272. [Google Scholar]


| Samples | FHBPUA | Curing Time/s | Pencil Hardness | Flexibility/mm | Adhesive Force/Grade | Glossiness/Gu |
|---|---|---|---|---|---|---|
| FHBPUA | 100% | 3 | HB | 1 | 3 | 92.1 |
| UV1000 | 0% | 6 | 3H | 10 | 5 | 98.3 |
| 2% | 3 | 3H | 10 | 5 | 97.7 | |
| 4% | 3 | 3H | 5 | 4 | 97.6 | |
| 6% | 3 | 2H | 3 | 4 | 97.8 | |
| 8% | 3 | 2H | 2 | 4 | 97.1 | |
| 10% | 3 | H | 1 | 3 | 97.4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, H.; Wang, X.; Lin, G.; Xi, L.; Yang, Y.; Lei, D.; Dong, H.; Su, J.; Cui, Y.; Liu, X. Preparation, Characterization and Application of UV-Curable Flexible Hyperbranched Polyurethane Acrylate. Polymers 2017, 9, 552. https://doi.org/10.3390/polym9110552
Xiang H, Wang X, Lin G, Xi L, Yang Y, Lei D, Dong H, Su J, Cui Y, Liu X. Preparation, Characterization and Application of UV-Curable Flexible Hyperbranched Polyurethane Acrylate. Polymers. 2017; 9(11):552. https://doi.org/10.3390/polym9110552
Chicago/Turabian StyleXiang, Hongping, Xiaowei Wang, Guanghong Lin, Lu Xi, Yan Yang, Dehua Lei, Haihui Dong, Jiahui Su, Yanyan Cui, and Xiaoxuan Liu. 2017. "Preparation, Characterization and Application of UV-Curable Flexible Hyperbranched Polyurethane Acrylate" Polymers 9, no. 11: 552. https://doi.org/10.3390/polym9110552




