Experimental Evidence of Large Amplitude pH Mediated Autonomous Chemomechanical Oscillation
Abstract
:1. Introduction
2. Materials and Methods
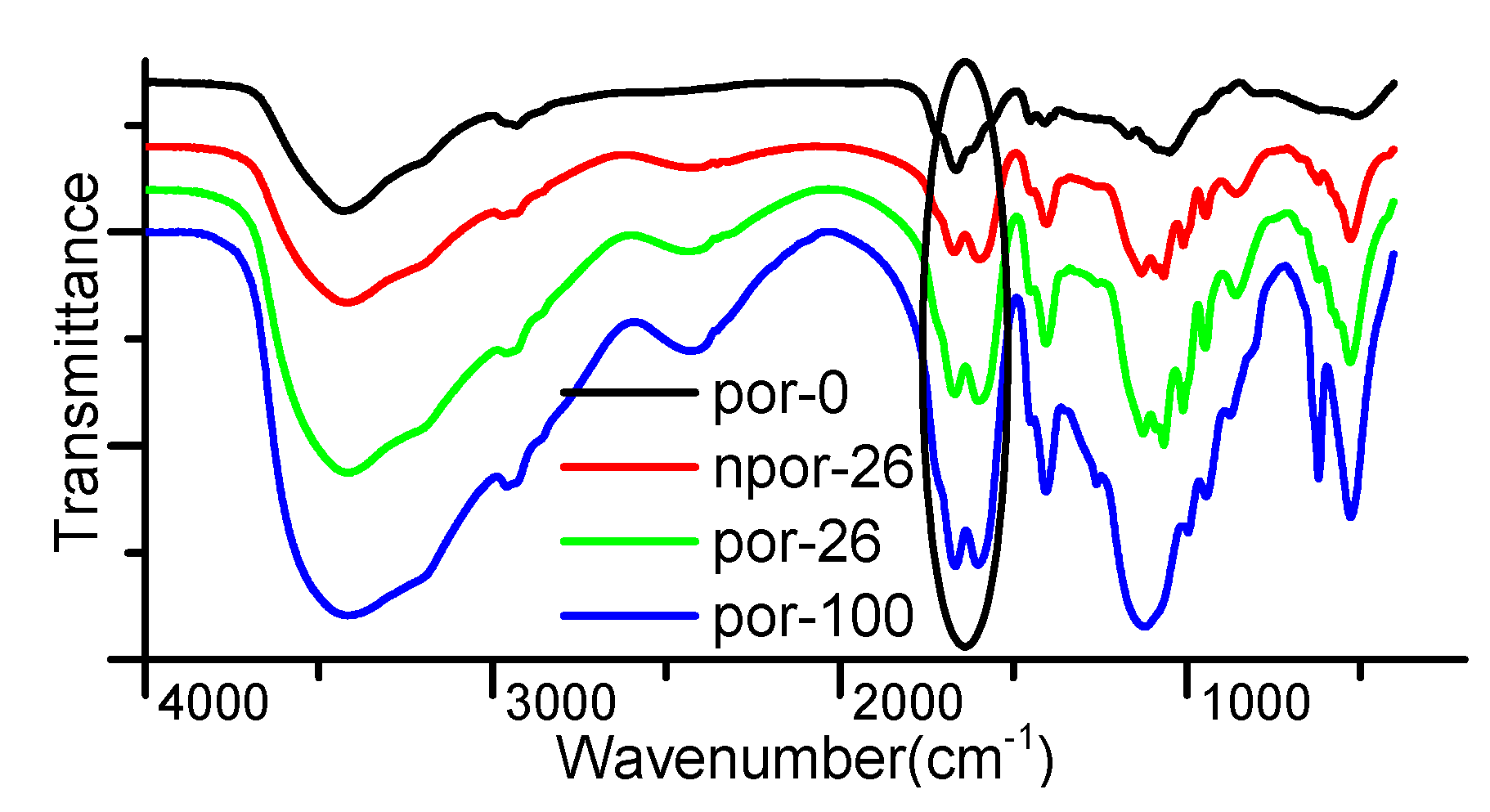
2.1. Hydrogel Synthesis
2.2. pH Oscillation Setting
2.3. Hydrogel pH-Responsiveness Test
3. Results and Discussion
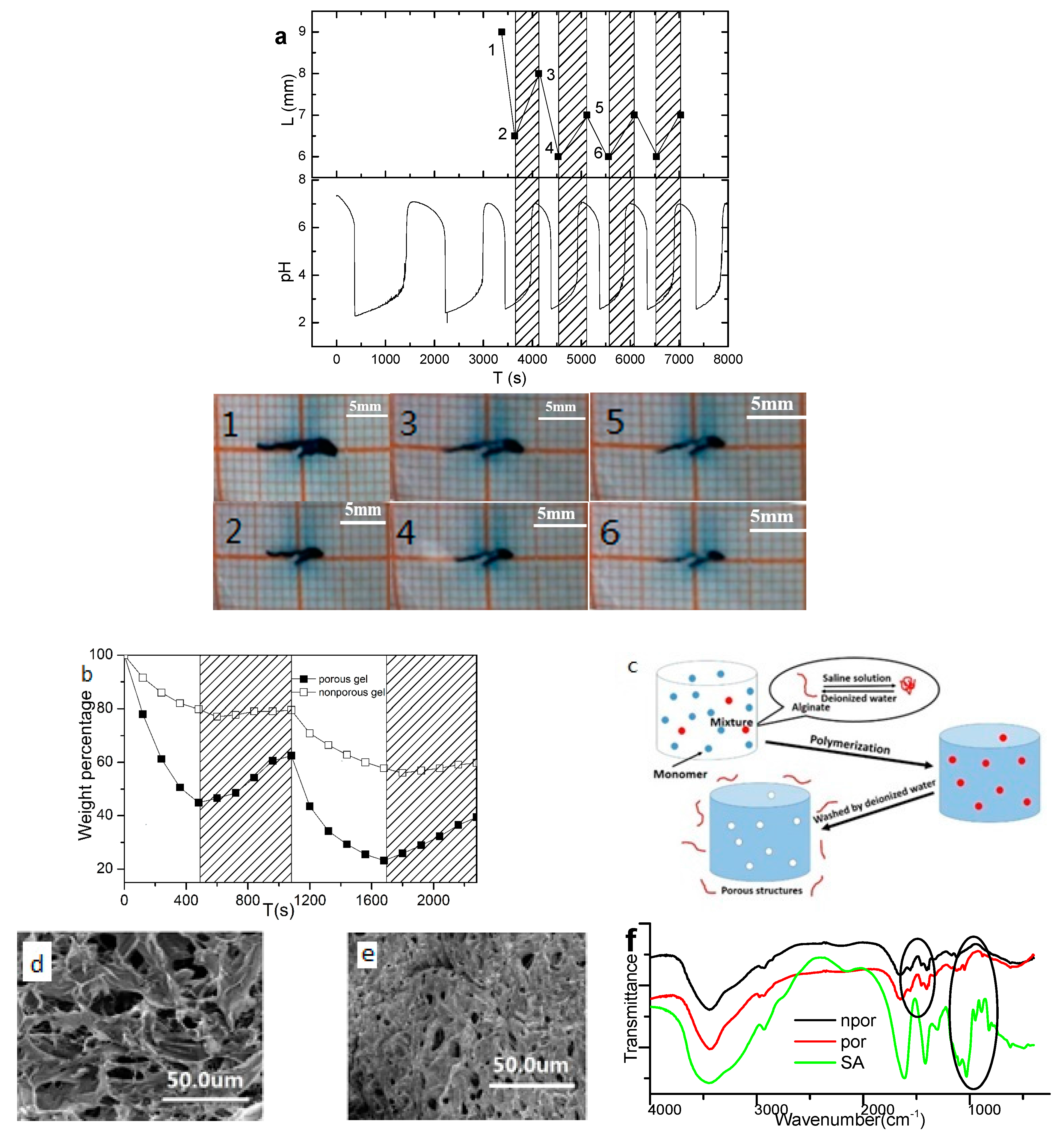
3.1. Coupled Chemomechanical Oscillations
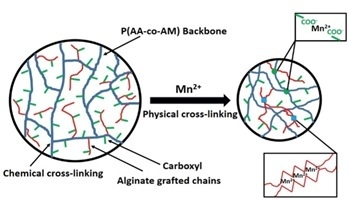
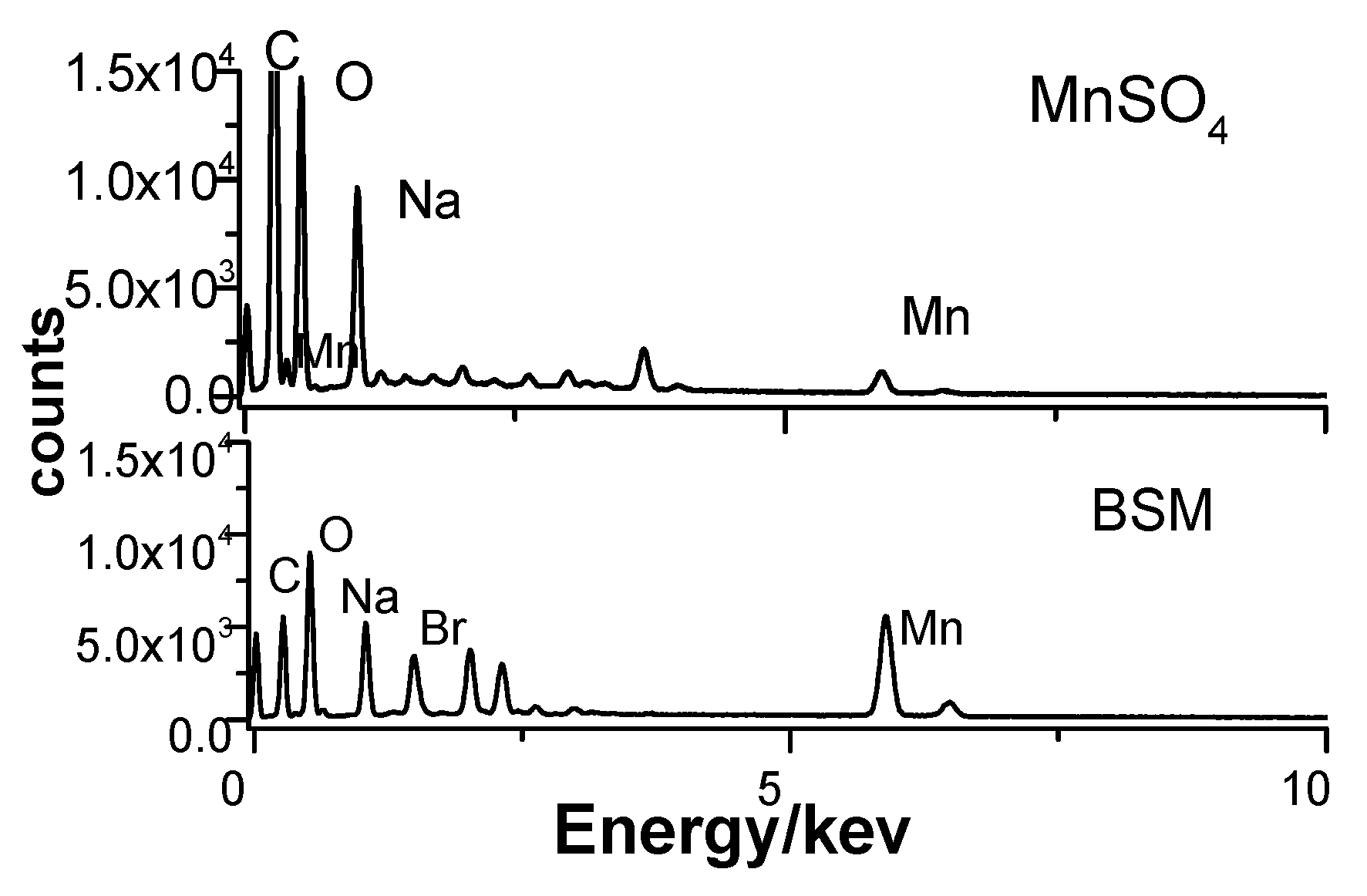
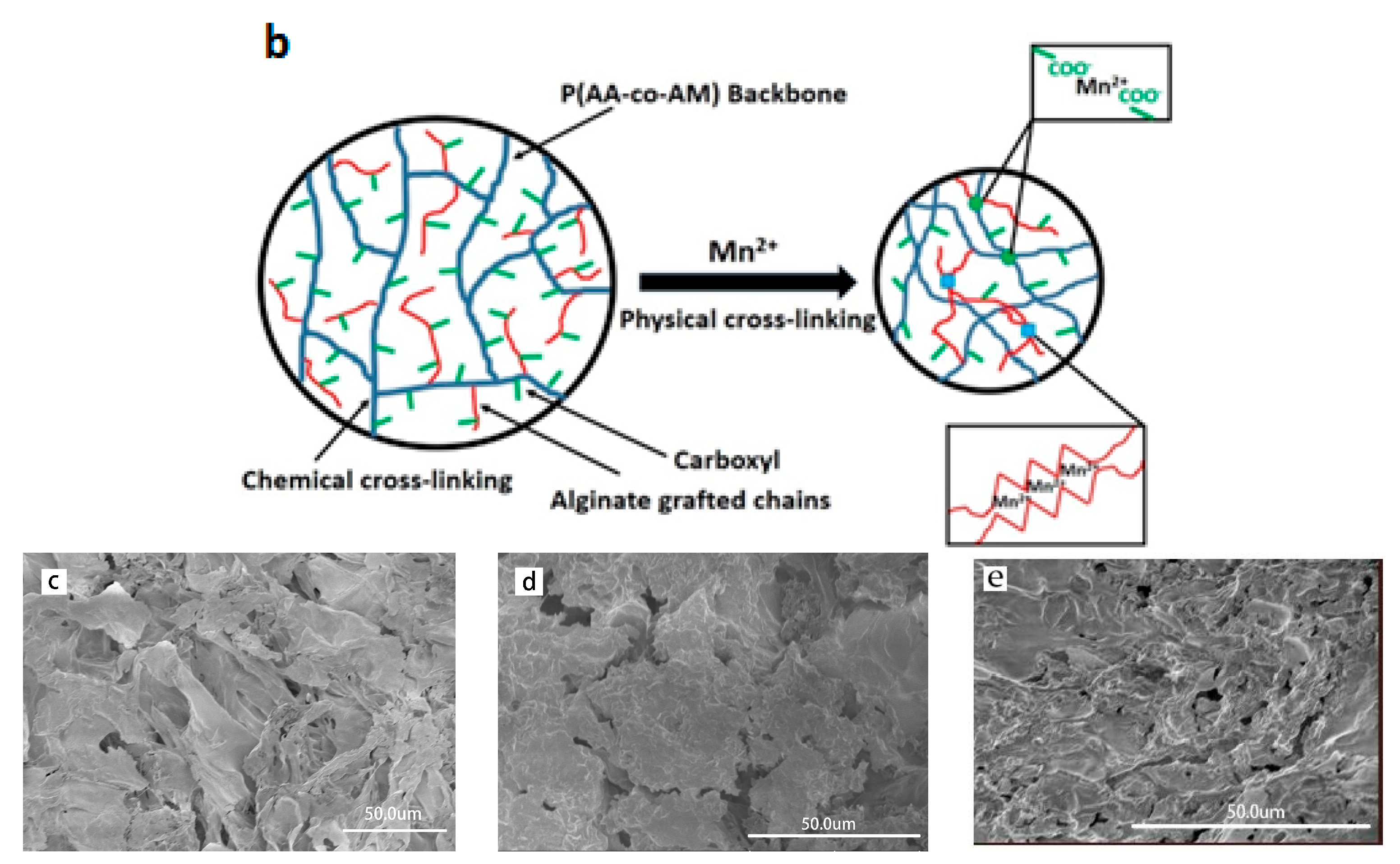
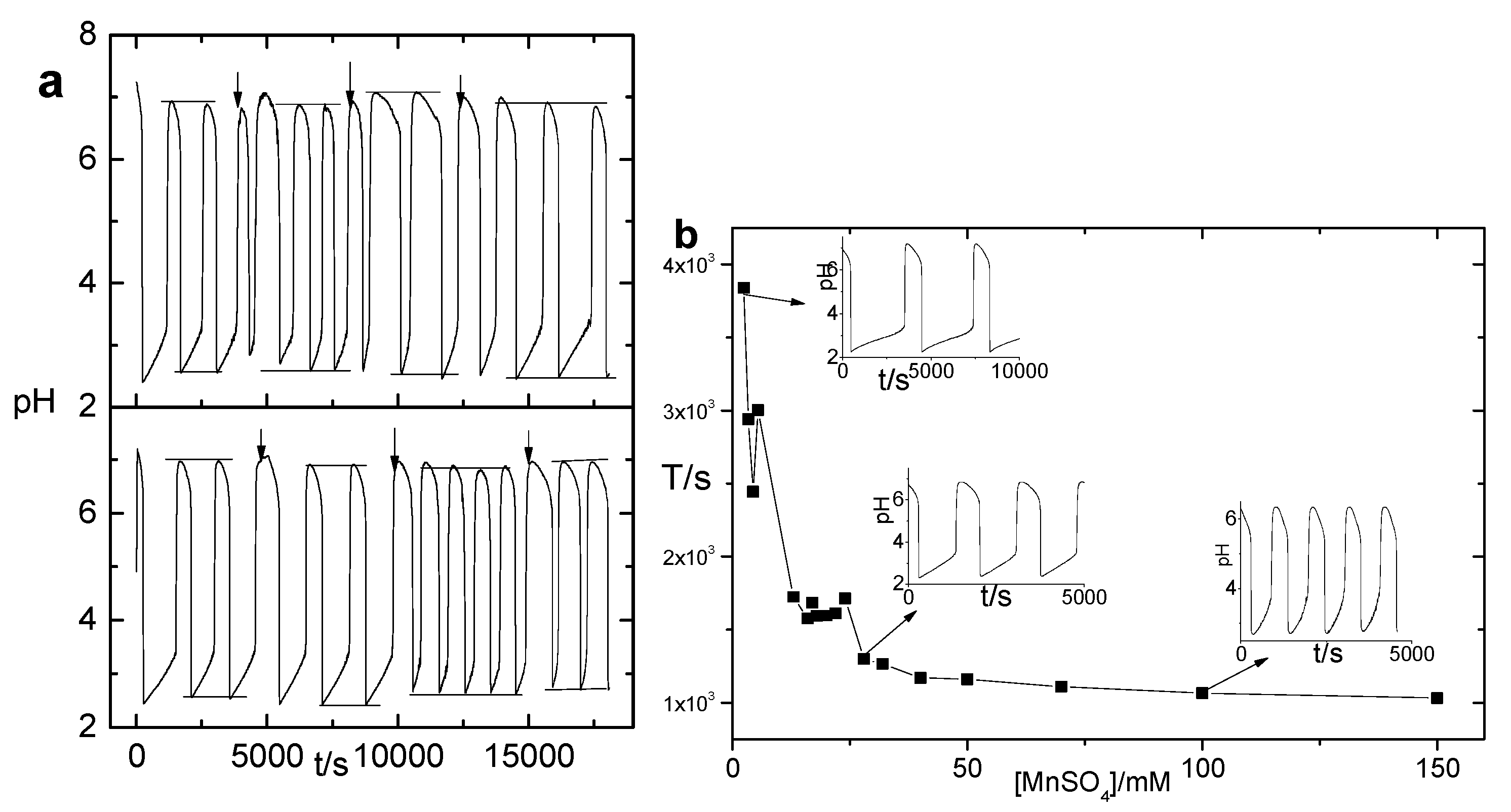
3.2. Mn2+ Coupled to the Hydrogel
3.3. Mn2+ Binding Alternated Oscillation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.A. Autonomous Rhythmic Drug Delivery Systems Based on Chemical and Biochemomechanical Oscillators; Borckmans, P., Kepper, P., Khokhlov, A.R., Métens, S., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 175–201. [Google Scholar]
- Howse, J.R.; Topham, P.; Crook, C.J.; Gleeson, A.J.; Bras, W.; Jones, R.A.; Ryan, A.J. Reciprocating power generation in a chemically driven synthetic muscle. Nano Lett. 2006, 6, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Jiang, H. Autonomous microfluidics with stimuli-responsive hydrogels. Soft Matter 2007, 3, 1223–1230. [Google Scholar] [CrossRef]
- He, X.; Aizenberg, M.; Kuksenok, O.; Zarzar, L.D.; Shastri, A.; Balazs, A.C.; Aizenberg, J. Synthetic homeostatic materials with chemo-mechano-chemical self-regulation. Nature 2012, 487, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Yoshida, R. Control of oscillating behavior for the self-oscillating polymer with pH-control site. Langmuir 2005, 21, 9773–9776. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R. Design of self-oscillating gels and application to biomimetic actuators. Sensors 2010, 10, 1810–1822. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Ueki, T. Evolution of self-oscillating polymer gels as autonomous polymer systems. NPG Asia Mater. 2014, 6, e107. [Google Scholar] [CrossRef]
- Yoshida, R.; Ichijo, H.; Hakuta, T.; Yamaguchi, T. Self-oscillating swelling and deswelling of polymer gels. Macromol. Rapid Commun. 1995, 16, 305–310. [Google Scholar] [CrossRef]
- Ryan, A.J.; Crook, C.J.; Howse, J.R.; Topham, P.; Jones, R.A.L.; Geoghegan, M.; Parnell, A.J.; Ruiz-Perez, L.; Martin, S.J.; Cadby, A.; et al. Responsive brushes and gels as components of soft nanotechnology. Faraday Discuss. 2005, 128, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Bilici, C.; Karayel, S.; Demir, T.T.; Okay, O. Self-oscillating ph-responsive cryogels as possible candidates of soft materials for generating mechanical energy. J. Appl. Polym. Sci. 2010, 118, 2981–2988. [Google Scholar] [CrossRef]
- Wang, L.P.; Ren, J.; Zhang, X.Y.; Yang, X.C.; Yang, W. Synthesis and Characterization of pH-sensitive and Self-oscillating IPN Hydrogel in a pH Oscillator. Polym. Korea 2015, 39, 359–364. [Google Scholar] [CrossRef]
- Sasaki, S.; Koga, S.; Yoshida, R.; Yamaguchi, T. Mechanical oscillation coupled with the belousov-zhabotinsky reaction in gel. Langmuir 2003, 19, 5595–5600. [Google Scholar] [CrossRef]
- Sakai, T.; Yoshida, R. Self-oscillating nanogel particles. Langmuir 2004, 20, 1036–1038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, N.; Li, N.; Sun, M.; Kim, D.; Fraden, S.; Epstein, I.R.; Xu, B. Giant volume change of active gels under continuous flow. J. Am. Chem. Soc. 2014, 136, 7341–7347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, N.; Akella, S.; Kuang, Y.; Kim, D.; Schwartz, A.; Bezpalko, M.; Foxman, B.M.; Fraden, S.; Epstein, I.R.; et al. Active cross-linkers that lead to active gels. Angew. Chem. Int. Ed. Engl. 2013, 52, 11494–11498. [Google Scholar] [CrossRef] [PubMed]
- Boissonade, J.; de Kepper, P.; Gauffre, F.; Szalai, I. Spatial bistability: A source of complex dynamics. From spatiotemporal reaction-diffusion patterns to chemomechanical structures. Chaos 2006, 16, 037110. [Google Scholar] [PubMed]
- Fuentes, M.; Kuperman, M.N.; Boissonade, J.; Dulos, E.; Gauffre, F.; de Kepper, P. Dynamical effects induced by long range activation in a nonequilibrium reaction-diffusion system. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2002, 66, 056205. [Google Scholar] [CrossRef] [PubMed]
- Boissonade, J. Simple chemomechanical process for self-generation of rhythms and forms. Phys. Rev. Lett. 2003, 90, 188302. [Google Scholar] [CrossRef] [PubMed]
- Borckmans, P. Chemomechanical Instabilities in Responsive Materials; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Boissonade, J. Self-oscillations in chemoresponsive gels: A theoretical approach. Chaos 2005, 15, 23703. [Google Scholar] [CrossRef] [PubMed]
- Dhanarajan, A.P.; Urban, J.; Siegel, A.R. Nonlinear Dynamics in Polymeric Systems; Tran-Cong-Miyata, J.P.A.Q., Ed.; American Chemical Society: Washington, DC, USA, 2003; pp. i–v. [Google Scholar]
- Dhanarajan, A.P.; Misra, G.P.; Siegel, R.A. Autonomous Chemomechanical Oscillations in a Hydrogel/Enzyme System Driven by Glucose. J. Phys. Chem. A 2002, 106, 8835–8838. [Google Scholar] [CrossRef]
- Horváth, J.; Szalai, I.; Boissonade, J.; Kepper, P.D. Oscillatory dynamics induced in a responsive gel by a non-oscillatory chemical reaction: Experimental evidence. Soft Matter 2011, 7, 8462. [Google Scholar] [CrossRef]
- Horvath, J. Sustained large-amplitude chemomechanical oscillations induced by the Landolt clock reaction. J. Phys. Chem. B 2014, 118, 8891–8900. [Google Scholar] [CrossRef] [PubMed]
- Horvath, J. Peristaltic waves in a responsive gel sustained by a halogen-free non-oscillatory chemical reaction. Polymer 2015, 79, 243–254. [Google Scholar] [CrossRef]
- Orbán, M.; Kurin-Csörgei, K.; Epstein, I.R. pH-Regulated Chemical Oscillators. Acc. Chem. Res. 2015, 48, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Fillmore, D.J. Kinetics of swelling of gels. J. Chem. Phys. 1979, 70, 1214–1218. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Yang, Y.Y.; Chung, T.S.; Ma, K.X. Preparation and characterization of fast response macroporous poly(N-isopropylacrylamide) hydrogels. Langmuir 2001, 17, 6094–6099. [Google Scholar] [CrossRef]
- Chaterji, S.; Kwon, I.K.; Park, K. Smart polymeric gels: Redefining the limits of biomedical devices. Prog. Polym. Sci. 2007, 32, 1083–1122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, R.; Shi, J.F.; Zhou, N.; Epstein, I.R.; Xu, B. Post-self-assembly cross-linking to integrate molecular nanofibers with copolymers in oscillatory hydrogels. J. Phys. Chem. B 2013, 117, 6566–6573. [Google Scholar] [CrossRef] [PubMed]
- Shibayama, M.; Tanaka, T. Volume phase-transition and related phenomena of polymer gels. Adv. Polym. Sci. 1993, 109, 1–62. [Google Scholar]
- Shinohara, S.-I.; Seki, T.; Sakai, T.; Yoshida, R.; Takeoka, Y. Chemical and optical control of peristaltic actuator based on self-oscillating porous gel. Chem. Commun. 2008, 4735–4737. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, N.; Rabai, G.; Hanazaki, I. Discovery of novel bromate-sulfite pH oscillators with Mn2+ or MnO4− as a negative-feedback species. J. Phys. Chem. A 1999, 103, 10915–10920. [Google Scholar] [CrossRef]
- Yashin, V.V.; Balazs, A.C. Modeling polymer gels exhibiting self-oscillations due to the Belousov-Zhabotinsky reaction. Macromolecules 2006, 39, 2024–2026. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.B.; Kim, S.J.; Lee, Y.M. Rapid temperature/pH response of porous alginate-g-poly(N-isopropylacrylamide) hydrogels. Polymer 2002, 43, 7549–7558. [Google Scholar] [CrossRef]
- Pouijavadi, A.; Ghasemzadeh, H.; Hosseinzadeh, H. Preparation and swelling behaviour of a novel anti-salt superabsorbent hydrogel based on κ-carrageenan and sodium alginate grafted with polyacrylamide. E-Polymers 2004, 4. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Samadi, M.; Ghasemzadeh, H. Temperature sensitive superabsorbent hydrogels from poly(N-t-butyl acrylamide-co-acrylamide) grafted on sodium alginate. Macromol. Symp. 2008, 274, 177–183. [Google Scholar] [CrossRef]
- Lee, W.-F.; Hsu, C.-H. Thermoreversible hydrogels: 3. Synthesis and swelling behavior of the (N-isopropylacrylamide-co-trimethylacrylamidopropyl ammonium iodide) copolymeric hydrogels. Polymer 1998, 39, 5393–5403. [Google Scholar] [CrossRef]
- Lee, W.F.; Yuan, W.Y. Thermoreversible hydrogels XI: Effect of salt on the swelling properties of the (N-isopropylacrylamide-co-sodium 2-acrylamido-2-methylpropyl sulfonate) copolymeric hydrogels. J. Appl. Polym. Sci. 2001, 79, 1675–1684. [Google Scholar] [CrossRef]
- Marandi, G.B.; Sharifnia, N.; Hosseinzadeh, H. Synthesis of an alginate-poly(sodium acrylate-co-acrylamide) superabsorbent hydrogel with low salt sensitivity and high pH sensitivity. J. Appl. Polym. Sci. 2006, 101, 2927–2937. [Google Scholar] [CrossRef]
- Harry, P.G.; Lionel, B.L.; Ernest, M.L. Metal-Polyelectrolyte Complexes. IV. Complexes of Polyacrylic Acid with Magnesium, Calcium, Manganese, Cobalt and Zinc. J. Phys. Chem. 1955, 55, 990–991. [Google Scholar]
- Koda, S.; Nomura, H.; Nagasawa, M. Raman-Spectroscopic Studies on the Interaction between Divalent Counterion and Polyion. Biophys. Chem. 1983, 18, 361–367. [Google Scholar] [CrossRef]
- De Gennes, P.G. Scaling Concepts in Polymer Physics; Cornell University Press: Ithaca, NY, USA, 1979. [Google Scholar]
- Tokarev, I.; Gopishetty, V.; Zhou, J.; Pita, M.; Motornov, M.; Katz, E.; Minko, S. Stimuli-Responsive Hydrogel Membranes Coupled with Biocatalytic Processes. ACS Appl. Mater. Interfaces 2009, 1, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Misra, G.P.; Siegel, R.A. Ionizable drugs and pH oscillators: Buffering effects. J. Pharm. Sci. 2002, 91, 2003–2015. [Google Scholar] [CrossRef] [PubMed]
- Kurin-Csörgei, K.; Epstein, I.R.; Orbán, M. Systematic design of chemical oscillators using complexation and precipitation equilibria. Nature 2005, 433, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Horvath, K.K.-C.V.; Epstein, I.R.; Orban, M. Oscillations in the Concentration of Fluoride Ions Induced by a pH Oscillator. J. Phys. Chem. A 2008, 112, 4271–4276. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Zhou, Y.; Ji, L.; Ding, Y.; Wang, J.; Liang, X. Experimental Evidence of Large Amplitude pH Mediated Autonomous Chemomechanical Oscillation. Polymers 2017, 9, 554. https://doi.org/10.3390/polym9110554
Yang X, Zhou Y, Ji L, Ding Y, Wang J, Liang X. Experimental Evidence of Large Amplitude pH Mediated Autonomous Chemomechanical Oscillation. Polymers. 2017; 9(11):554. https://doi.org/10.3390/polym9110554
Chicago/Turabian StyleYang, Xin, Yi Zhou, Lin Ji, Yanhui Ding, Jianquan Wang, and Xin Liang. 2017. "Experimental Evidence of Large Amplitude pH Mediated Autonomous Chemomechanical Oscillation" Polymers 9, no. 11: 554. https://doi.org/10.3390/polym9110554