Selective Adsorption of Ag+ on a New Cyanuric-Thiosemicarbazide Chelating Resin with High Capacity from Acid Solutions
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
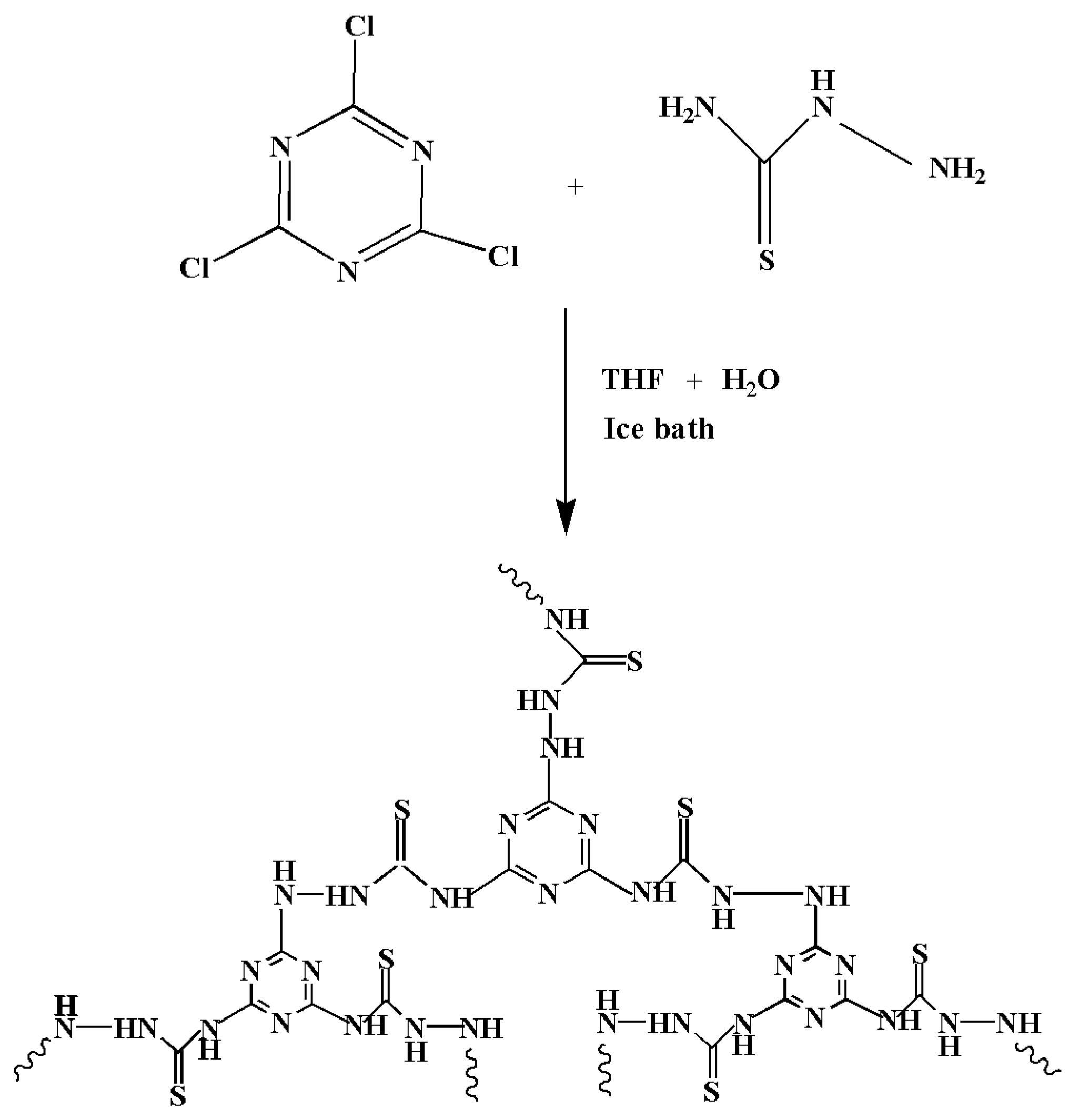
2.2.1. Synthesis of Chelating Resin
2.2.2. Adsorption Experiments
2.3. Analysis
3. Results and Discussion
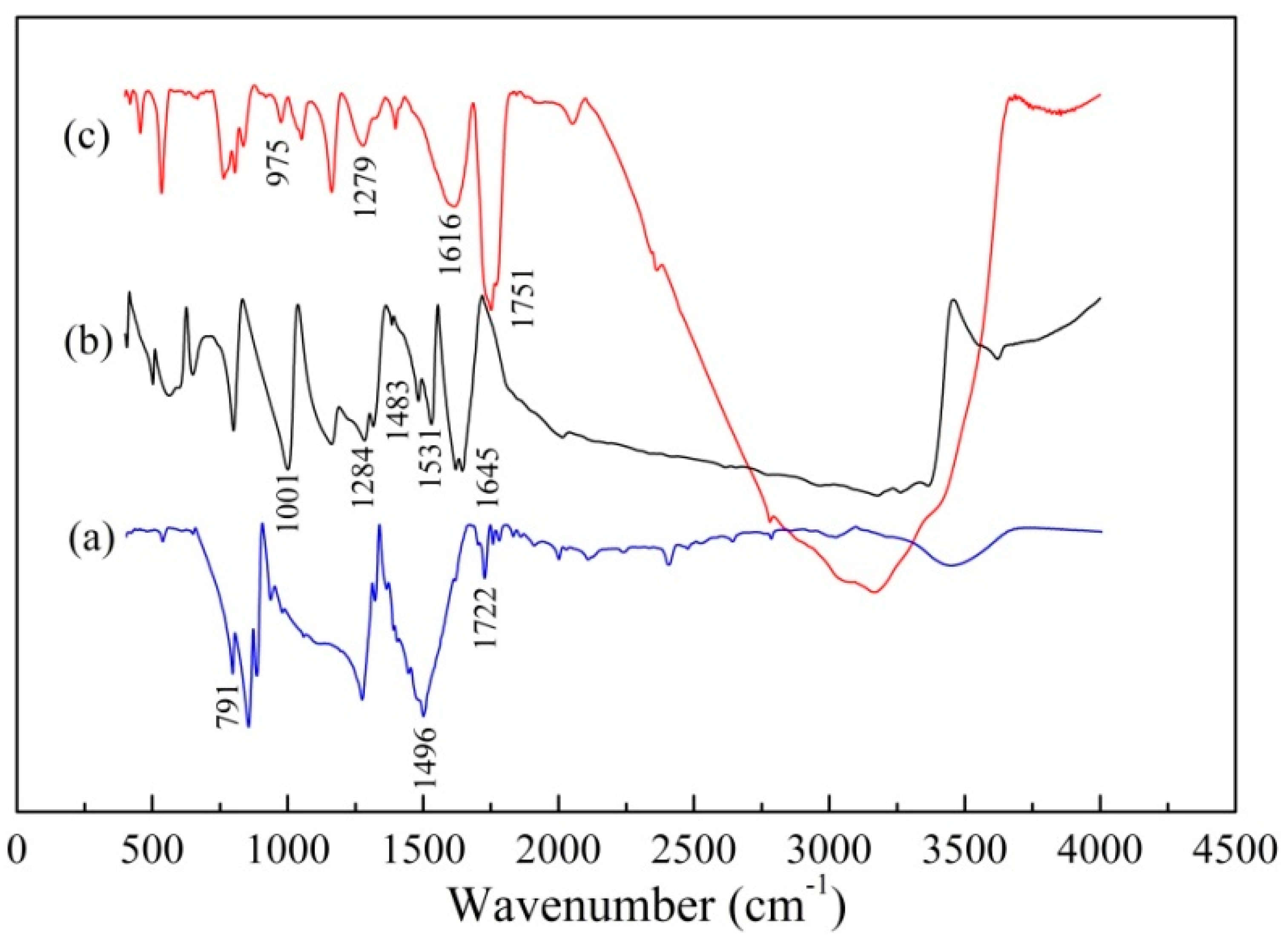
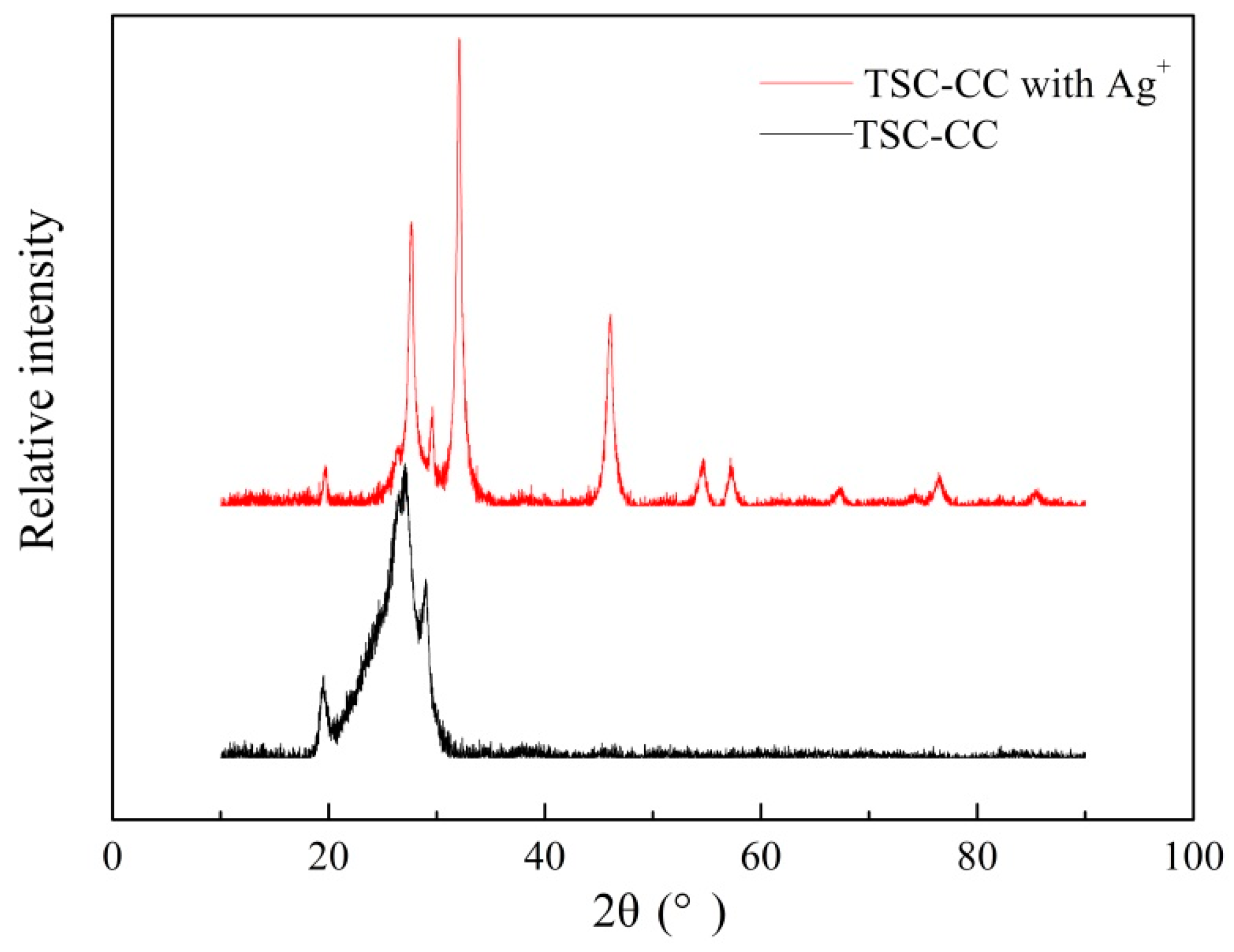
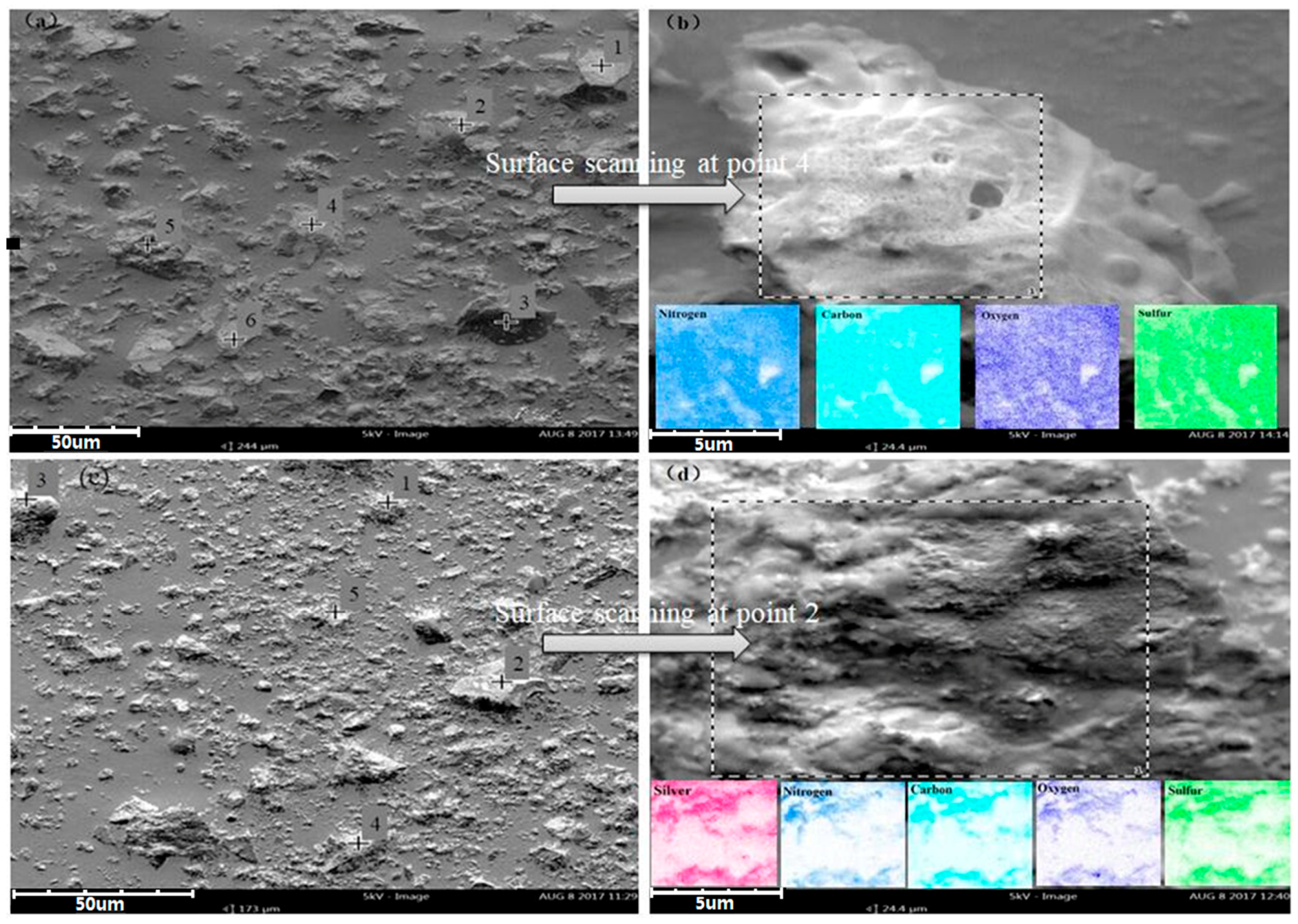
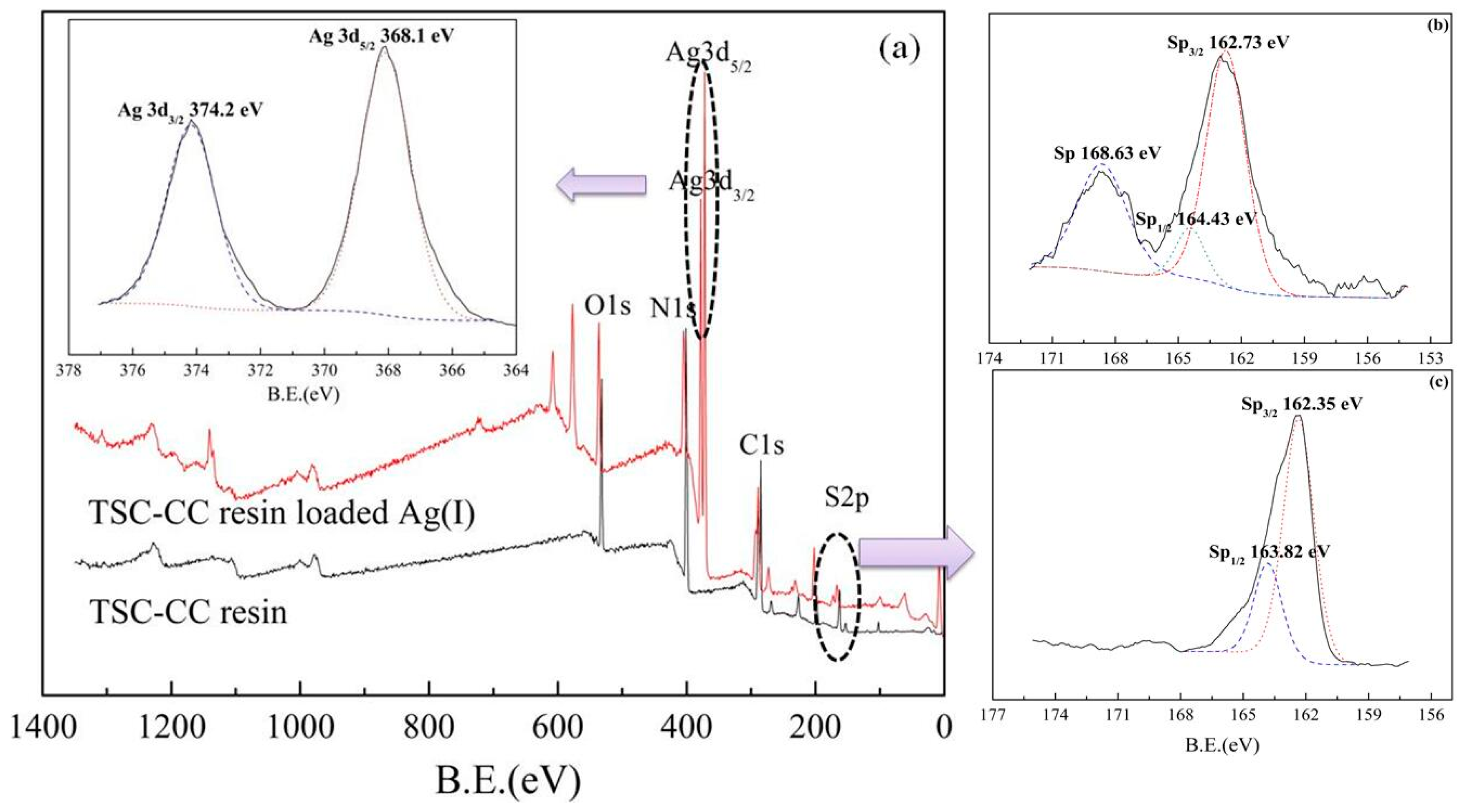
3.1. Characterization of Cyanuric-Thiosemicarbazide Chelating Resin
3.2. Effect of Initial Acidity on Adsorption
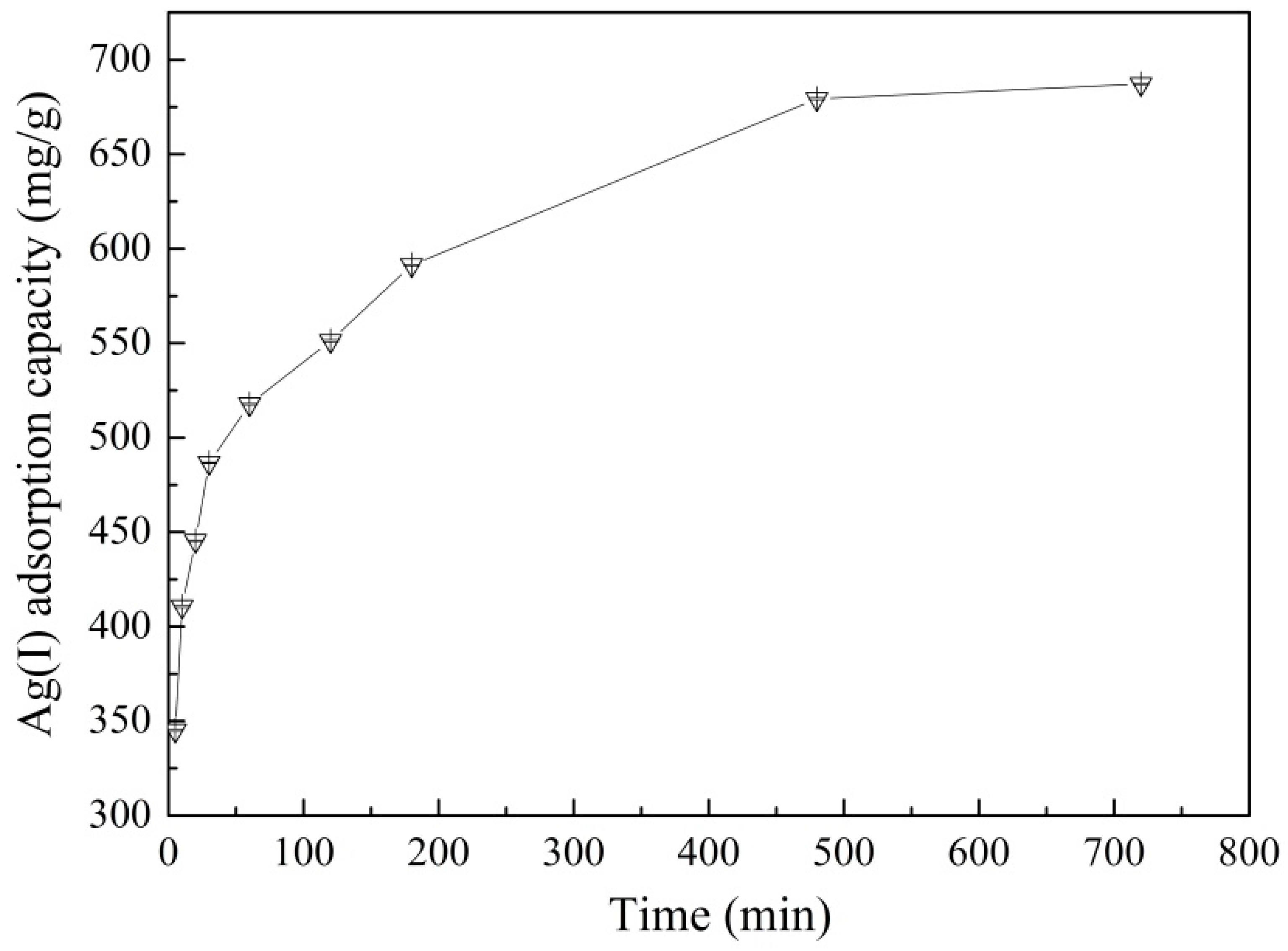
3.3. Effect of Contact Time on Adsorption and Adsorption Kinetics
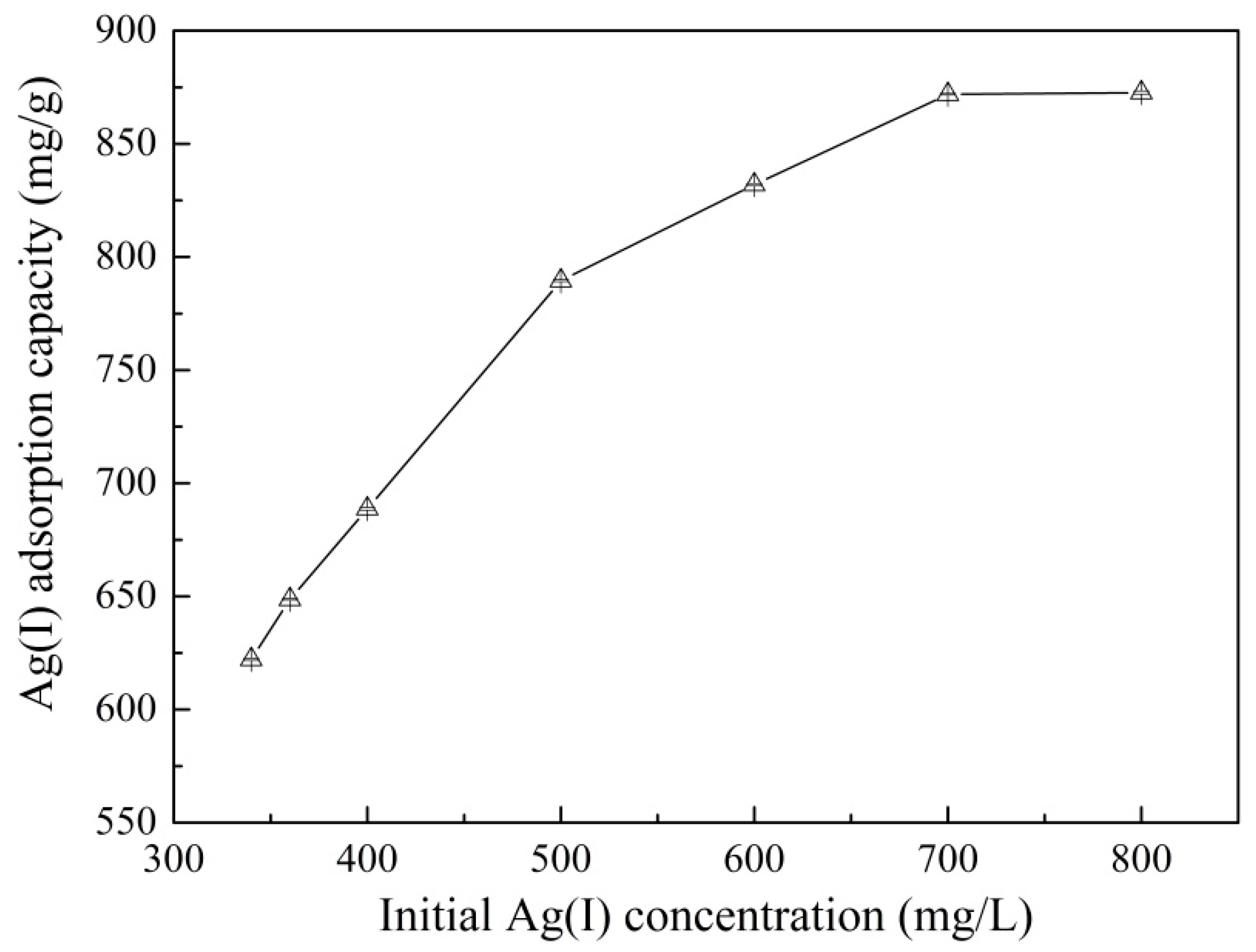
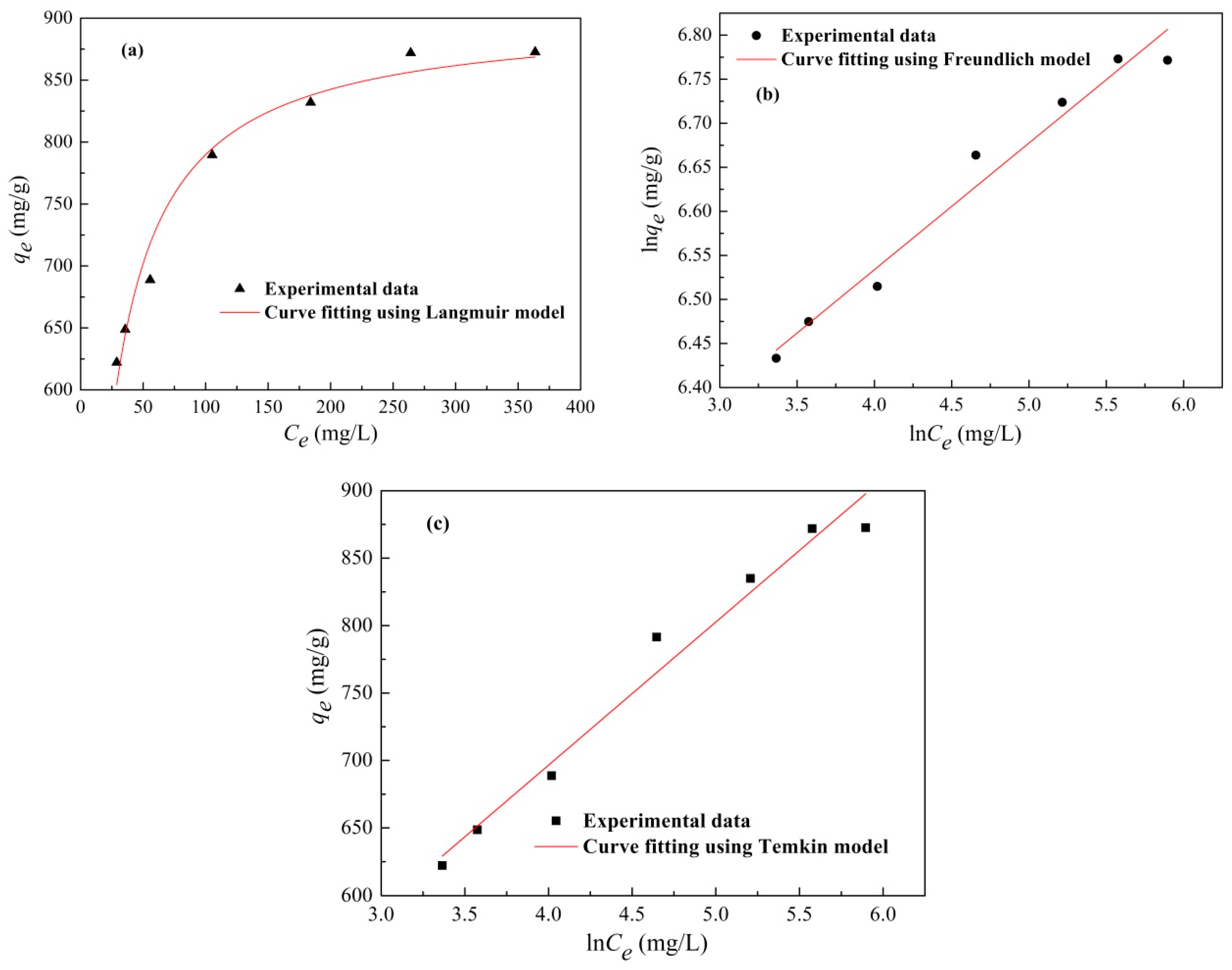
3.4. Effect of Initial Ag+ Concentration on Adsorption and Adsorption Isotherms
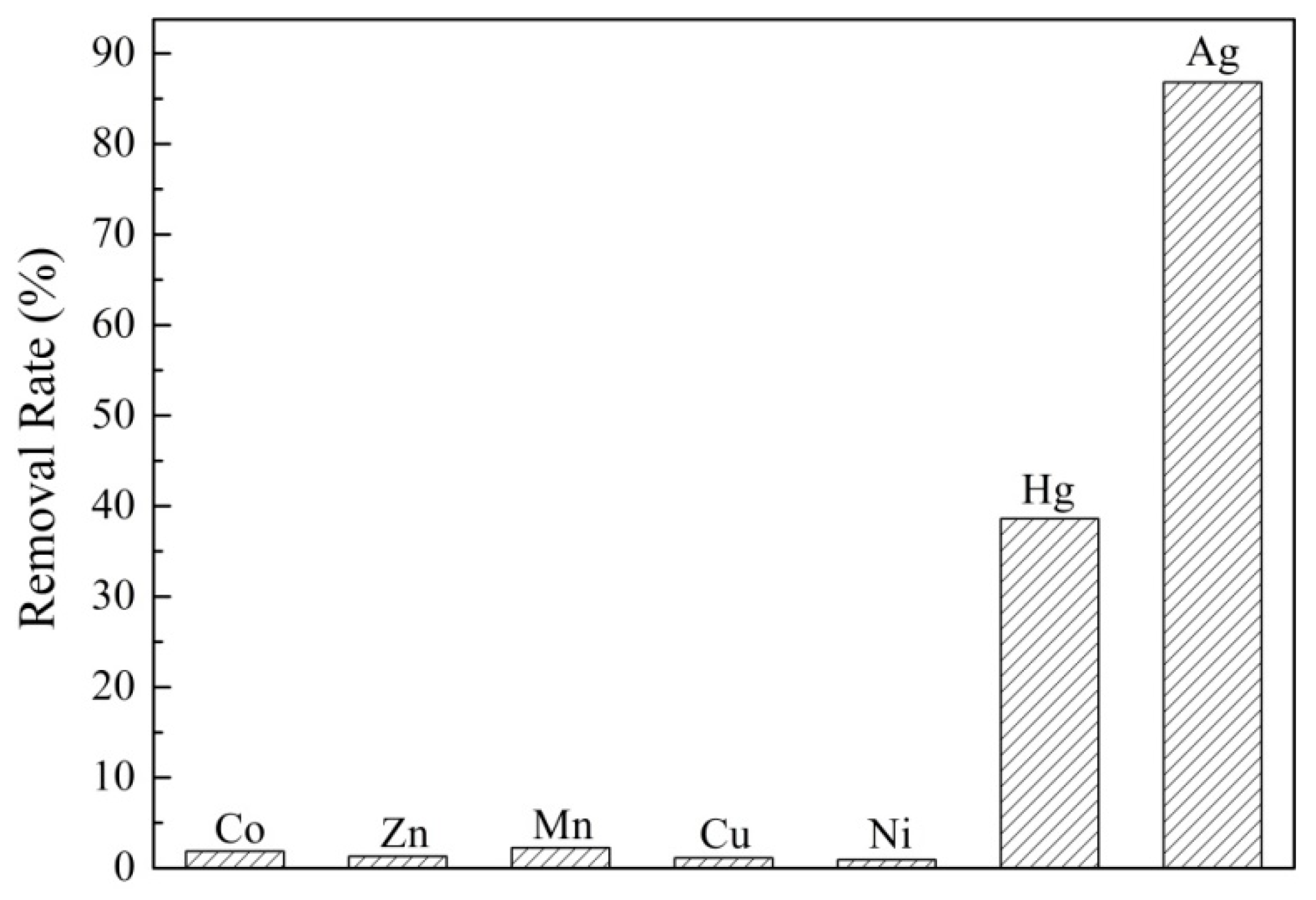
3.5. Selective Adsorption
3.6. Desorption and Recyclability of TSC-CC Resin
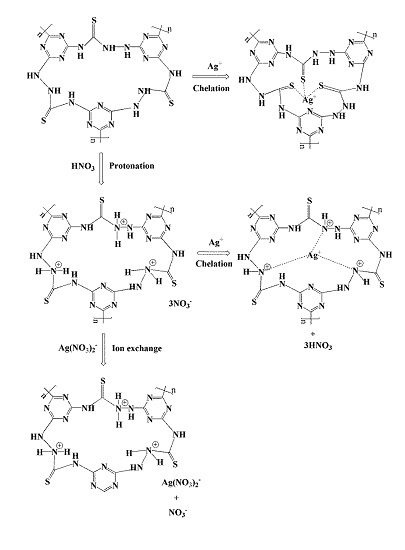
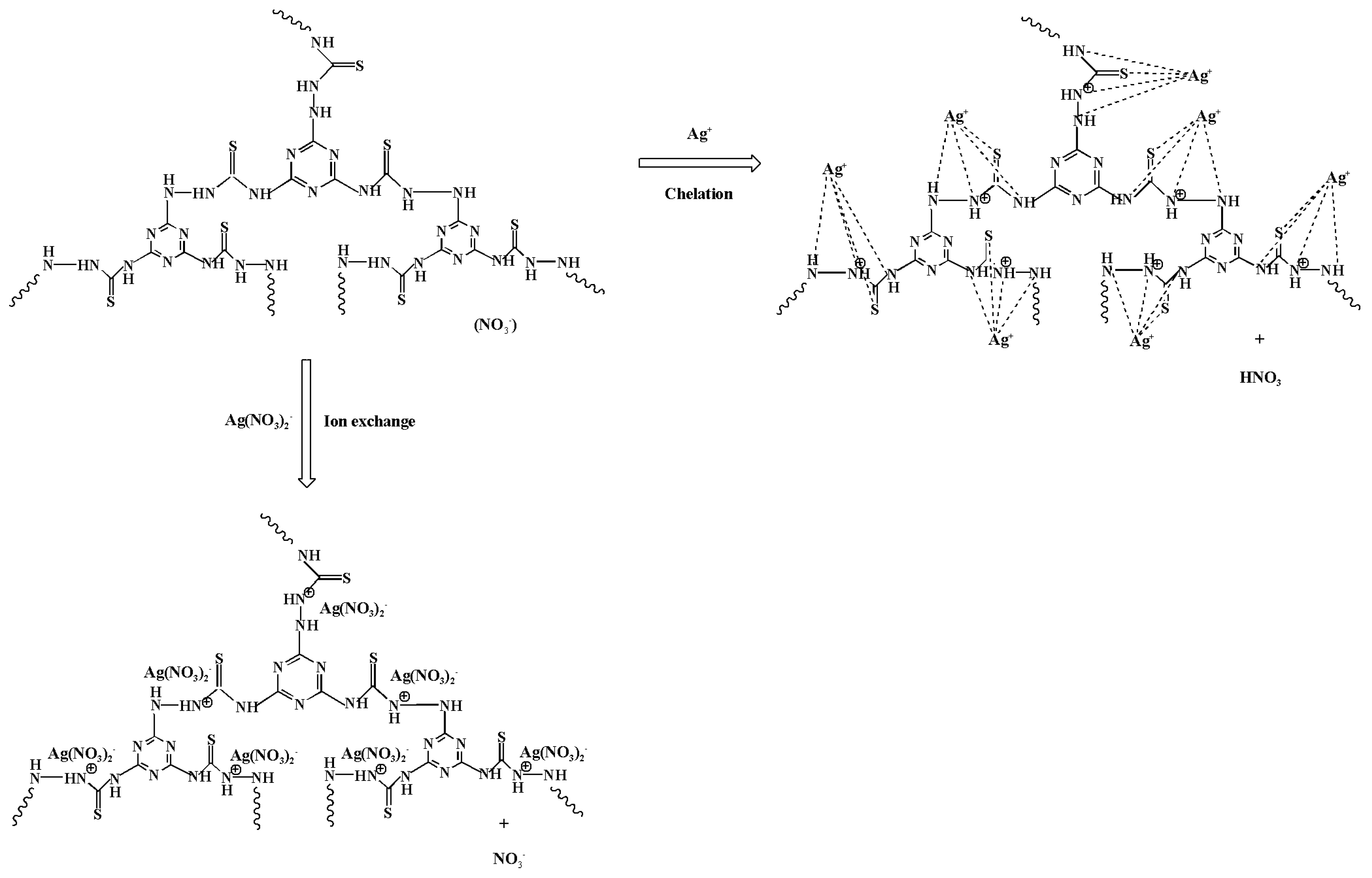
3.7. Adsorption Mechanism
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, Y.J.; Choi, J.H. Selective removal of nitrate ion using a novel composite carbon electrode in capacitive deionization. Water Res. 2012, 46, 6033–6039. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Li, W.; Jing, X.; Wang, Y.; Xing, Z.; Shan, W.; Lou, Z. Selective recovery of Ag(I) coordination anion from simulate nickel electrolyte using corn stalk based adsorbent modified by ammonia-thiosemicarbazide. J. Hazard. Mater. 2016, 301, 277–285. [Google Scholar]
- Petrova, Y.S.; Pestov, A.V.; Usoltseva, M.K.; Neudachina, L.K. Selective adsorption of silver(I) ions over copper(II) ions on a sulfoethyl derivative of chitosan. J. Hazard. Mater. 2015, 299, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, C.P.; Pirestani, D. Interactions of silver with wastewater constituents. Water Res. 2003, 37, 4444–4452. [Google Scholar] [CrossRef]
- Jeon, C. Adsorption of silver ions from industrial wastewater using waste coffee grounds. Korean J. Chem. Eng. 2017, 34, 384–391. [Google Scholar] [CrossRef]
- Liu, C.; Bai, R.; San Ly, Q. Selective removal of copper and lead ions by diethylenetriamine-functionalized adsorbent: Behaviors and mechanisms. Water Res. 2008, 42, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, G.; Wang, S.; Peng, J.; Cui, W. Sulfoethyl functionalized silica nanoparticle as an adsorbent to selectively adsorb silver ions from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2017, 71, 330–337. [Google Scholar] [CrossRef]
- Song, X.; Gunawan, P.; Jiang, R.; Leong, S.S.; Wang, K.; Xu, R. Surface activated carbon nanospheres for fast adsorption of silver ions from aqueous solutions. J. Hazard. Mater. 2011, 194, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, H.; Chen, X.; Yang, M.; Qi, Y. Selective adsorption of silver ions from aqueous solution using polystyrene-supported trimercaptotriazine resin. J. Environ. Sci. 2012, 24, 2166–2172. [Google Scholar] [CrossRef]
- Atia, A.A.; Donia, A.M.; Yousif, A.M. Comparative study of the recovery of silver(I) from aqueous solutions with different chelating resins derived from glycidyl methacrylate. J. Appl. Polym. Sci. 2005, 97, 806–812. [Google Scholar] [CrossRef]
- Yirikoglu, H.; Gülfen, M. Separation and Recovery of Silver(I) Ions from Base Metal Ions by Melamine-formaldehyde-thiourea (MFT) Chelating Resin. Sep. Sci. Technol. 2008, 43, 376–388. [Google Scholar] [CrossRef]
- Xue, C.; Zhu, H.; Zhang, T.; Cao, D.; Hu, Z. Synthesis and properties of novel alkylbetaine zwitterionic gemini surfactants derived from cyanuric chloride. Colloids Surf. A 2011, 375, 141–146. [Google Scholar] [CrossRef]
- Zhang, L.; Ni, C.; Zhu, C.; Jiang, X.; Liu, Y.; Huang, B. Preparation and adsorption properties of chelating resins from thiosemicarbazide and formaldehyde. J. Appl. Polym. Sci. 2009, 112, 2455–2461. [Google Scholar] [CrossRef]
- Celik, Z.; Gülfen, M.; Aydin, A.O. Synthesis of a novel dithiooxamide-formaldehyde resin and its application to the adsorption and separation of silver ions. J. Hazard. Mater. 2010, 174, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Juang, R.S.; Shao, H.J. Effect of pH on competitive adsorption of Cu(II), Ni(II), and Zn(II) from water onto chitosan beads. Adsorption 2002, 8, 71–78. [Google Scholar] [CrossRef]
- Fu, L.; Zhang, L.; Wang, S.; Peng, J.; Zhang, G. Selective adsorption of Ag+ by silica nanoparticles modified with 3-Amino-5-mercapto-1,2,4-triazole from aqueous solutions. J. Mol. Liq. 2017, 241, 292–300. [Google Scholar] [CrossRef]
- Gülfen, M.; Kirci, S.; Aydin, A.O. Separation and Recovery of Silver(I) Ions from Base Metal Ions by Thiourea- or Urea-Formaldehyde Chelating Resin. Sep. Sci. Technol. 2009, 44, 1869–1883. [Google Scholar]
- Birinci, E.; Gülfen, M.; Aydın, A.O. Separation and recovery of palladium(II) from base metal ions by melamine–formaldehyde–thiourea (MFT) chelating resin. Hydrometallurgy 2009, 95, 15–21. [Google Scholar] [CrossRef]
- Febrianto, J.; Kosasih, A.N.; Sunarso, J.; Ju, Y.H.; Indraswati, N.; Ismadji, S. Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: A summary of recent studies. J. Hazard. Mater. 2009, 162, 616–645. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Sun, W.Y.; Guo, N.; Ding, S.L.; Su, S.J. Comparison of Co(2+) adsorption by chitosan and its triethylene-tetramine derivative: Performance and mechanism. Carbohydr. Polym. 2016, 151, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Liu, X.; Lin, C.; Zhang, X.; Ma, J.; Tan, H.; Ye, W. Degradation of sulfamethoxazole by microwave-activated persulfate: Kinetics, mechanism and acute toxicity. Chem. Eng. J. 2014, 249, 6–14. [Google Scholar] [CrossRef]
- Hou, H.; Yu, D.; Hu, G. Preparation and Properties of Ion-Imprinted Hollow Particles for the Selective Adsorption of Silver Ions. Langmuir 2015, 31, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Kuang, S.P.; Wang, Z.Z.; Liu, J.; Wu, Z.C. Preparation of triethylene-tetramine grafted magnetic chitosan for adsorption of Pb(II) ion from aqueous solutions. J. Hazard. Mater. 2013, 260, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Elwakeel, K.Z.; El-Sayed, G.O.; Darweesh, R.S. Fast and selective removal of silver(I) from aqueous media by modified chitosan resins. Int. J. Miner. Process. 2013, 120, 26–34. [Google Scholar] [CrossRef]
- Donia, A.M.; Atia, A.A.; El-Boraey, H.A.; Mabrouk, D.H. Adsorption of Ag(I) on glycidyl methacrylate/N,N′-methylene bis-acrylamide chelating resins with embedded iron oxide. Sep. Purif. Technol. 2006, 48, 281–287. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, L.; Xia, H.; Peng, J.; Shu, J. Adsorption behavior of methylene blue onto waste-derived adsorbent and exhaust gases recycling. RSC Adv. 2017, 7, 27331–27341. [Google Scholar] [CrossRef]
- Tang, X.H.; Zhang, X.M.; Guo, C.C.; Zhou, A.L. Adsorption of Pb2+ on Chitosan Cross-Linked with Triethylene-Tetramine. Chem. Eng. Technol. 2010, 30, 955–961. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, L.; Xia, H.; Peng, J.; Shu, J.; Li, C. Ultrasound and microwave-assisted preparation of Fe-activated carbon as an effective low-cost adsorbent for dyes wastewater treatment. RSC Adv. 2016, 6, 78936–78946. [Google Scholar] [CrossRef]
- Donia, A.M.; Atia, A.A.; Elwakeel, K.Z. Selective separation of mercury(II) using magnetic chitosan resin modified with Schiff’s base derived from thiourea and glutaraldehyde. J. Hazard. Mater. 2008, 151, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Monier, M.; Kenawy, I.M.; Hashem, M.A. Synthesis and characterization of selective thiourea modified Hg(II) ion-imprinted cellulosic cotton fibers. Carbohydr. Polym. 2014, 106, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Y.; Helleur, R. Selective adsorption of Ag+ by ion-imprinted O-carboxymethyl chitosan beads grafted with thiourea-glutaraldehyde. Chem. Eng. J. 2015, 264, 56–65. [Google Scholar] [CrossRef]

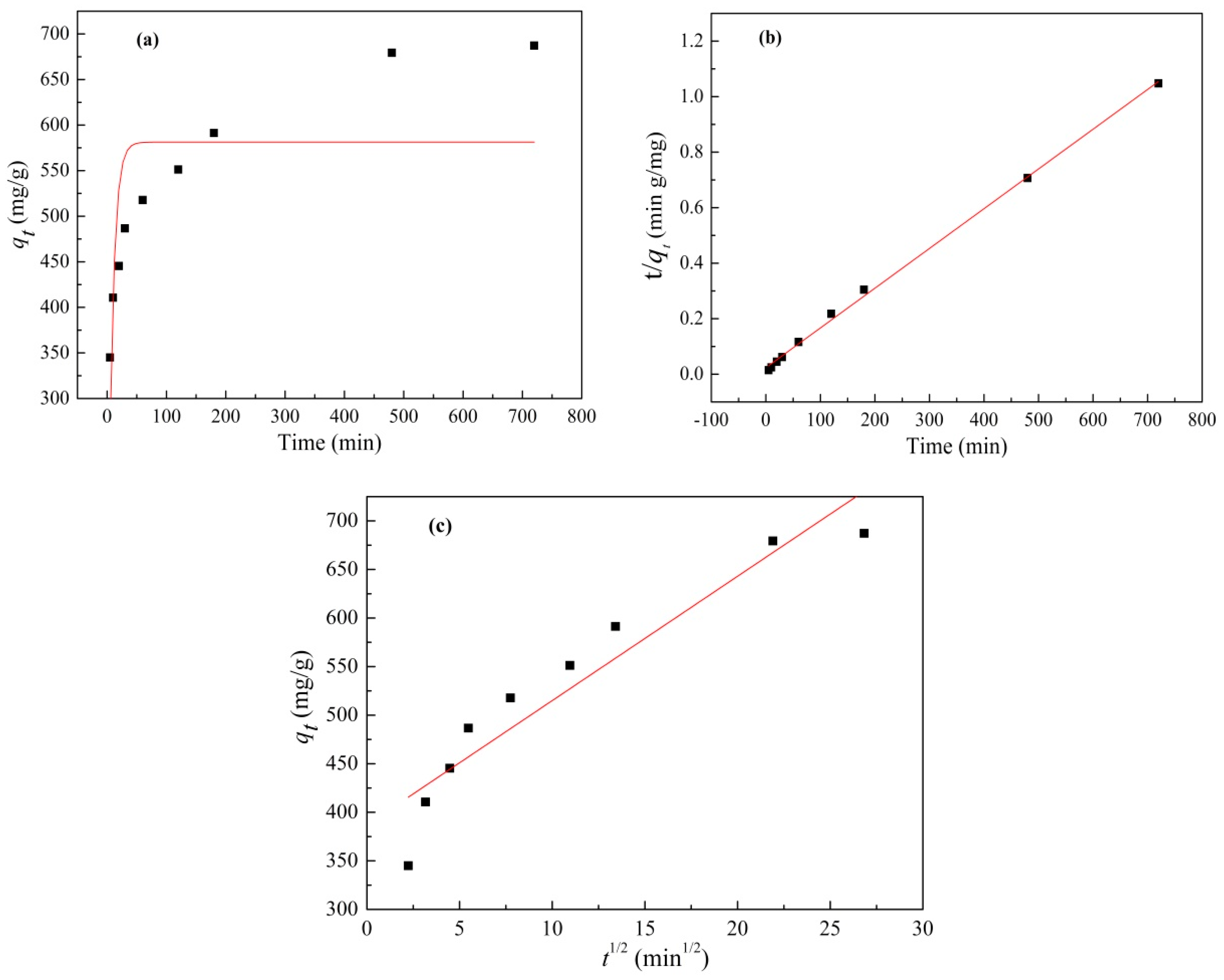


| Kinetic Models | (mg/g) Calculated | Rate Constant | R2 |
|---|---|---|---|
| Pseudo-first-order | 581.18 | 0.1229 (min−1) | 0.5181 |
| Pseudo-second-order | 699.3 | 0.000087 (g/mg min) | 0.9982 |
| Intraparticle diffusion | 12.799 (mg/g min0.5) | 0.8863 |
| Adsorbent | Adsorption Capacity (mg/g) | References |
|---|---|---|
| Chitosan resin | 122.04 | [24] |
| SE-SNPs | 21.9 | [7] |
| Chelating resin | 313.2 | [25] |
| Amino/thiol-bearing resin | 308.88 | [10] |
| Ag-IISHPs | 80.5 | [22] |
| TSC-CC resin | 872.63 | This study |
| Isotherm Model | Parameter | Value |
|---|---|---|
| Langmuir | (mg/g) | 905.237 |
| (L/g) | 0.0695 | |
| R2 | 0.9742 | |
| Freundlich | (mg·g−1 (L·mg−1)1/n) | 387.021 |
| 1/n | 0.1438 | |
| R2 | 0.9672 | |
| Temkin | (L/g) | 12.982 |
| (J/mol) | 106.121 | |
| R2 | 0.971 |
| Number | Ultimate Analysis (%) | |||
|---|---|---|---|---|
| N | C | O | S | |
| 1 | 60.84 | 27.63 | 9.06 | 2.47 |
| 2 | 60.58 | 27.38 | 9.09 | 2.95 |
| 3 | 45.16 | 35.73 | 15.04 | 4.08 |
| 4 | 59.91 | 27.17 | 7.97 | 4.88 |
| 5 | 46.60 | 36.12 | 11.32 | 5.96 |
| 6 | 54.68 | 30.50 | 7.16 | 7.66 |
| Surface scanning | 58.92 | 27.48 | 9.23 | 4.38 |
| Number | Ultimate Analysis (%) | ||||
|---|---|---|---|---|---|
| Ag | N | C | O | S | |
| 1 | 46.53 | 15.22 | 28.42 | 9.83 | |
| 2 | 29.30 | 28.53 | 27.46 | 11.7 | 2.93 |
| 3 | 49.36 | 13.80 | 25.85 | 8.28 | 2.70 |
| 4 | 41.40 | 16.14 | 22.62 | 16.25 | 3.52 |
| 5 | 44.04 | 17.49 | 28.19 | 8.39 | 1.89 |
| Surface scanning | 46.18 | 12.47 | 24.88 | 11.84 | 4.64 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, G.; Wang, S.; Zhang, L.; Hu, T.; Peng, J.; Cheng, S.; Fu, L. Selective Adsorption of Ag+ on a New Cyanuric-Thiosemicarbazide Chelating Resin with High Capacity from Acid Solutions. Polymers 2017, 9, 568. https://doi.org/10.3390/polym9110568
Lin G, Wang S, Zhang L, Hu T, Peng J, Cheng S, Fu L. Selective Adsorption of Ag+ on a New Cyanuric-Thiosemicarbazide Chelating Resin with High Capacity from Acid Solutions. Polymers. 2017; 9(11):568. https://doi.org/10.3390/polym9110568
Chicago/Turabian StyleLin, Guo, Shixing Wang, Libo Zhang, Tu Hu, Jinhui Peng, Song Cheng, and Likang Fu. 2017. "Selective Adsorption of Ag+ on a New Cyanuric-Thiosemicarbazide Chelating Resin with High Capacity from Acid Solutions" Polymers 9, no. 11: 568. https://doi.org/10.3390/polym9110568