Reducing Water Sensitivity of Chitosan Biocomposite Films Using Gliadin Particles Made by In Situ Method
Abstract
:1. Introduction
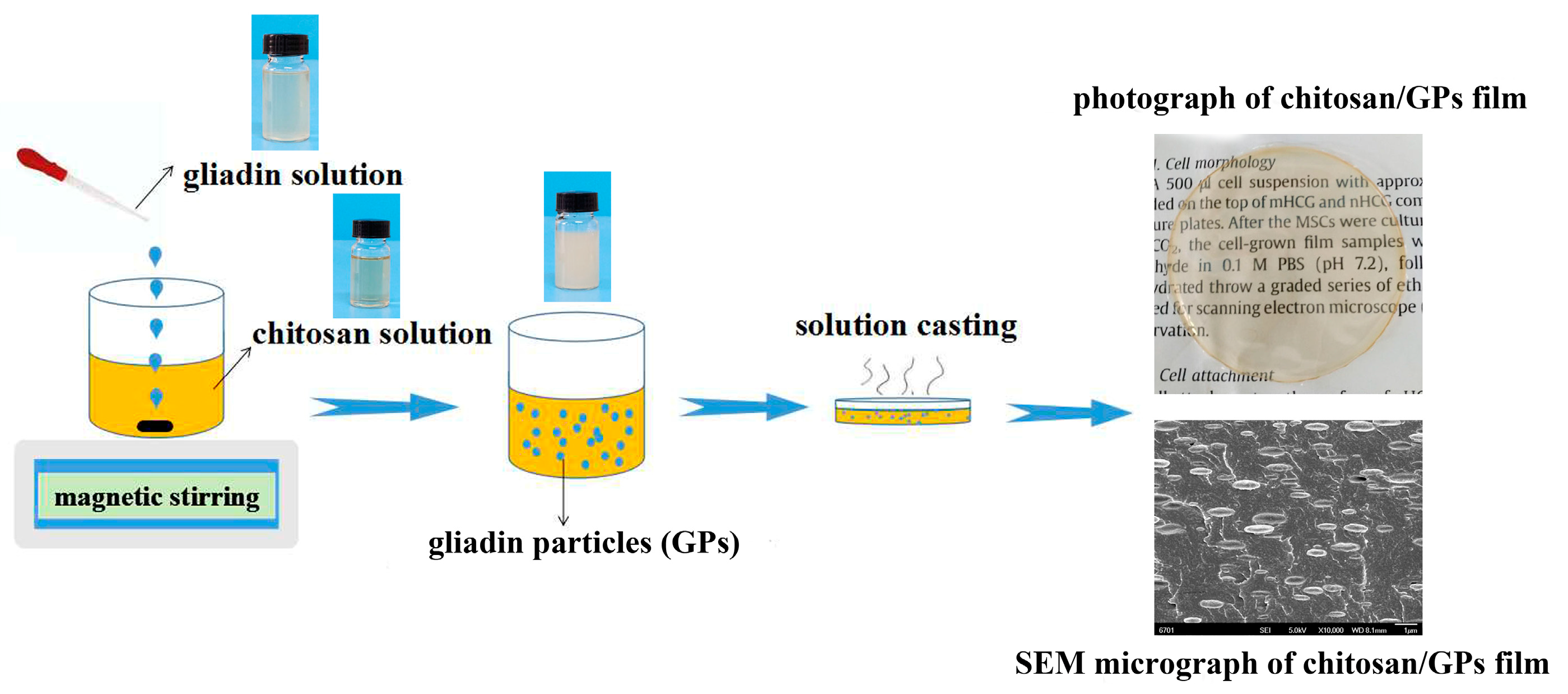
2. Materials and Methods
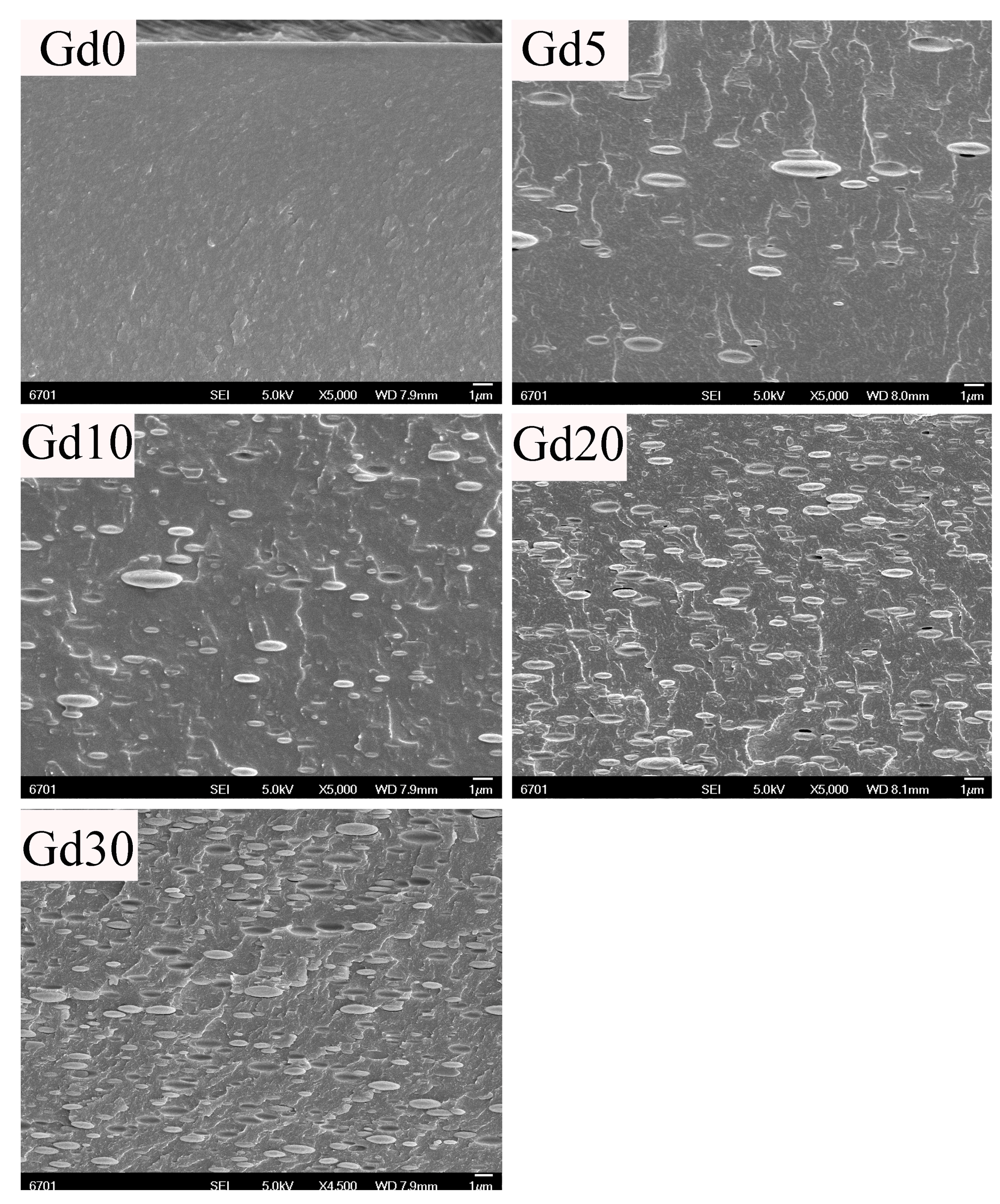
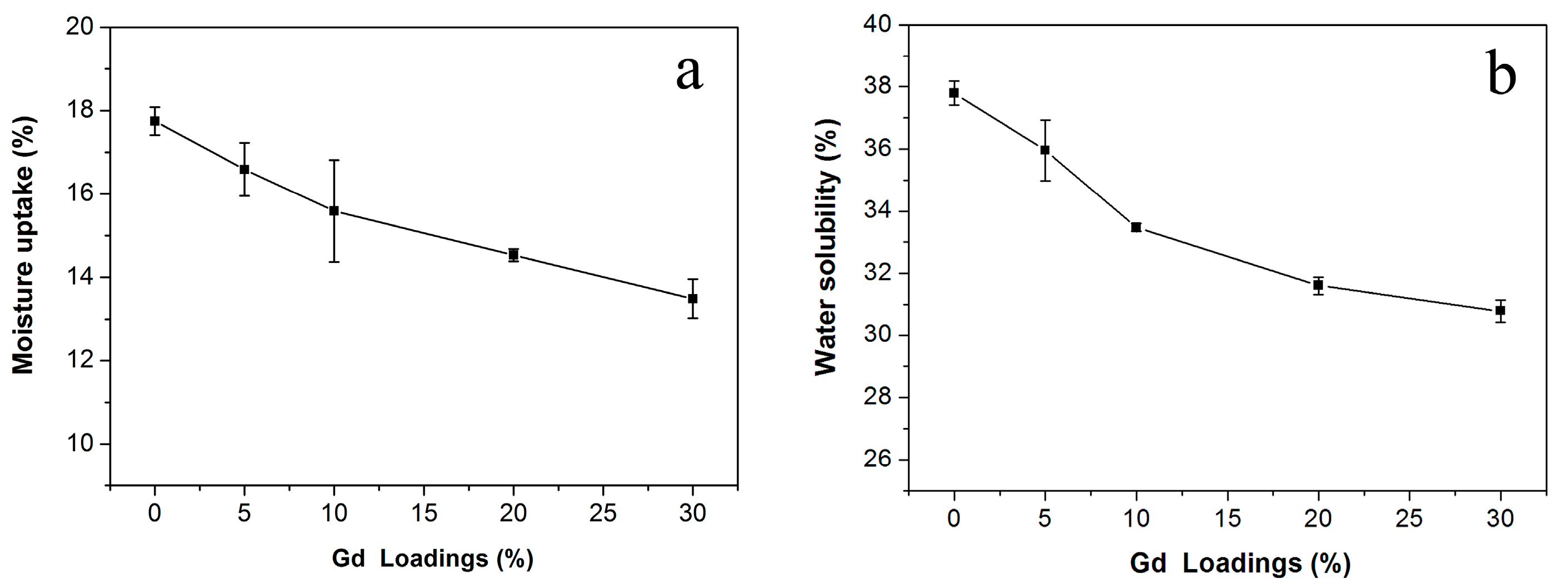
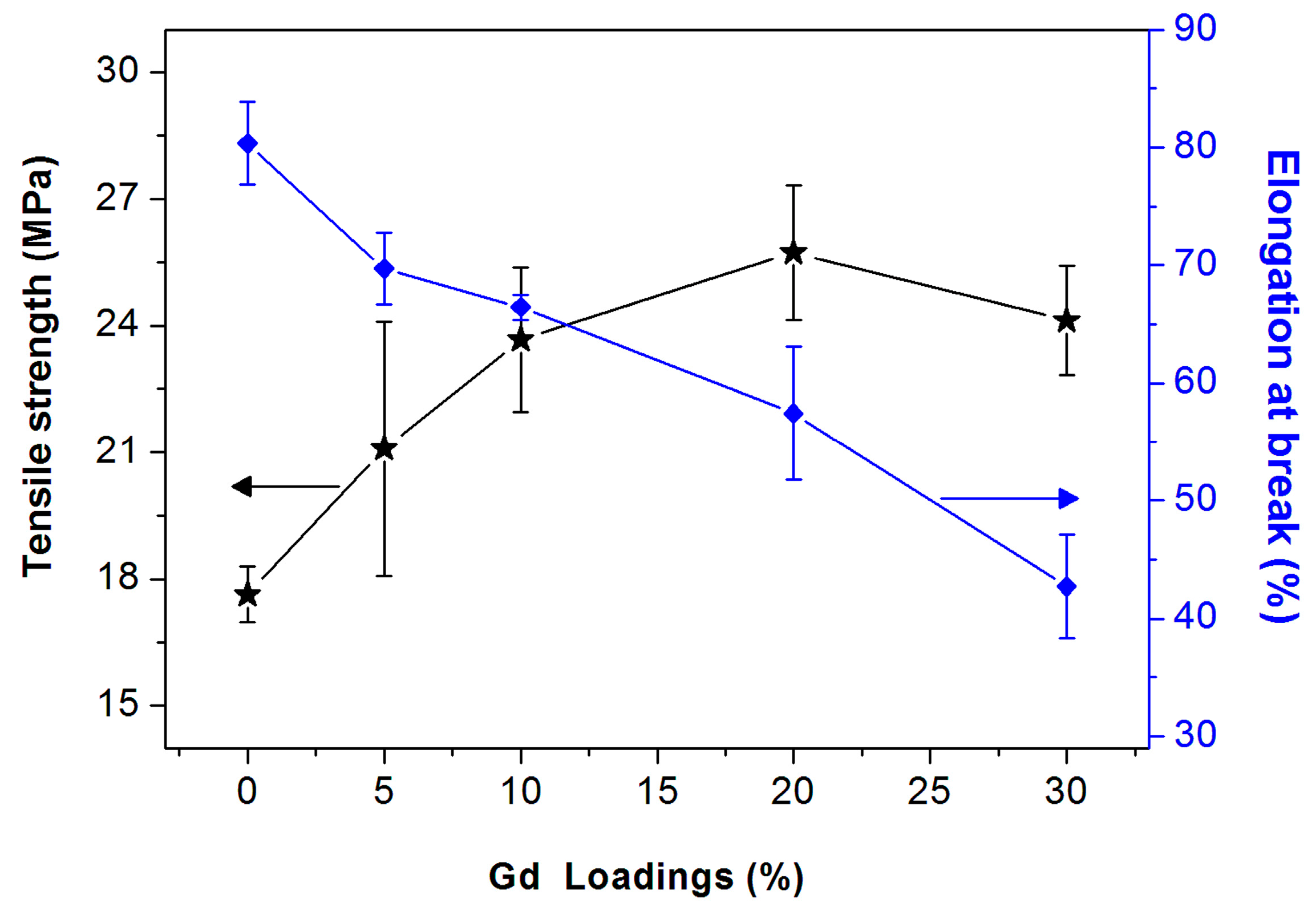
3. Results
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Romero-Bastida, C.A.; Bello-Perez, L.A.; Velazquez, G.; Alvarez-Ramirez, J. Effect of the addition order and amylose content on mechanical, barrier and structural properties of films made with starch and montmorillonite. Carbohydr. Polym. 2015, 127, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jia, Z.; Zhou, X.; Zhou, D.; Chen, M.; Xie, D.; Luo, Y.; Jia, D. Preparation of a biodegradable poly(vinyl alcohol)-starch composite film and its application in pesticide controlled release. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Melo Da Costa, M.P.; de Mello Ferreira, I.L.; de Macedo Cruz, M.T. New polyelectrolyte complex from pectin/chitosan and montmorillonite clay. Carbohydr. Polym. 2016, 146, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Flores-Hernandez, C.G.; Colin-Cruz, A.; Velasco-Santos, C.; Castano, V.M.; Rivera-Armenta, J.L.; Almendarez-Camarillo, A.; Garcia-Casillas, P.E.; Martinez-Hernandez, A.L. All Green Composites from Fully Renewable Biopolymers: Chitosan-Starch Reinforced with Keratin from Feathers. Polymers 2014, 6, 686–705. [Google Scholar] [CrossRef]
- Mueller, K.; Bugnicourt, E.; Latorre, M.; Jorda, M.; Echegoyen Sanz, Y.; Lagaron, J.M.; Miesbauer, O.; Bianchin, A.; Hankin, S.; Boelz, U.; et al. Review on the Processing and Properties of Polymer Nanocomposites and Nanocoatings and Their Applications in the Packaging, Automotive and Solar Energy Fields. Nanomaterials 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Abas, Z.; Kim, H.S.; Kim, J. Recent Progress on Cellulose-Based Electro-Active Paper, Its Hybrid Nanocomposites and Applications. Sensors 2016, 16. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wang, W.; Wang, A. Ethanol-NaOH solidification method to intensify chitosan/poly(vinyl alcohol)/attapulgite composite film. RSC Adv. 2015, 5, 17775–17781. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Chand, N.; Ahuja, S.; Roy, M.K. Curcumin/cellulose micro crystals/chitosan films: Water absorption behavior and in vitro cytotoxicity. Int. J. Biol. Macromol. 2015, 75, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wang, W.; Kang, Y.; Wang, A. A chitosan/poly(vinyl alcohol) nanocomposite film reinforced with natural halloysite nanotubes. Polym. Compos. 2012, 33, 1693–1699. [Google Scholar] [CrossRef]
- Vu, K.H.B.; Park, D.; Lee, Y. Chitosan Combined with ZnO, TiO2 and Ag Nanoparticles for Antimicrobial Wound Healing Applications: A Mini Review of the Research Trends. Polymers 2017, 9. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Zhou, Z.; Lai, H.; Xu, P.; Liao, L.; Wei, J. Biodegradable Polymer Membranes Applied in Guided Bone/TissueRegeneration: A Review. Polymers 2016, 8, 115. [Google Scholar] [CrossRef]
- Thakhiew, W.; Devahastin, S.; Soponronnarit, S. Physical and mechanical properties of chitosan film as affected by drying methods and addition of antimicrobial agent. J. Food Eng. 2013, 119, 140–149. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, Y.; Zhang, Y.; Yu, C.; Cao, S. Preparation and structural analysis of chitosan films with and without sorbitol. Food Hydrocoll. 2013, 33, 186–191. [Google Scholar] [CrossRef]
- Huang, D.; Wang, W.; Xu, J.; Wang, A. Mechanical and water resistance properties of chitosan/poly(vinyl alcohol) films reinforced with attapulgite dispersed by high-pressure homogenization. Chem. Eng. J. 2012, 210, 166–172. [Google Scholar] [CrossRef]
- Ma, J.; Liu, C.; Li, R.; Wang, J. Properties and structural characterization of oxide starch/chitosan/graphene oxide biodegradable nanocomposites. J. Appl. Polym. Sci. 2012, 123, 2933–2944. [Google Scholar] [CrossRef]
- Darder, M.; Lopez-Blanco, M.; Aranda, P.; Aznar, A.J.; Bravo, J.; Ruiz-Hitzky, E. Microfibrous chitosan-sepiolite nanocomposites. Chem. Mater. 2006, 18, 1602–1610. [Google Scholar] [CrossRef]
- Mallakpour, S.; Khadem, E. Chitosan reinforced with modified CaCO3 nanoparticles to enhance thermal, hydrophobicity properties and removal of cu(II) and cd(II) ions. J. Polym. Res. 2017, 24. [Google Scholar] [CrossRef]
- Li, K.; Jin, S.; Liu, X.; Chen, H.; He, J.; Li, J. Preparation and Characterization of Chitosan/Soy Protein Isolate Nanocomposite Film Reinforced by Cu Nanoclusters. Polymers 2017, 9. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Kehoe, T.; Latorre, M.; Serrano, C.; Philippe, S.; Schmid, M. Recent Prospects in the Inline Monitoring of Nanocomposites and Nanocoatings by Optical Technologies. Nanomaterials 2016, 6, 150. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Vermerris, W. Antimicrobial Nanomaterials Derived from Natural Products—A Review. Materials 2016, 9, 255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiao, G.; Wang, Y.; Zhao, Y.; Su, H.; Tan, T. Preparation of chitosan-TiO2 composite film with efficient antimicrobial activities under visible light for food packaging applications. Carbohydr. Polym 2017, 169, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Badawi, A.; Ahmed, E.M.; Mostafa, N.Y.; Abdel-Wahab, F.; Alomairy, S.E. Enhancement of the optical and mechanical properties of chitosan using Fe2O3 nanoparticles. J. Mater. Sci. Mater. Electron. 2017, 28, 10877–10884. [Google Scholar] [CrossRef]
- Atif, R.; Shyha, I.; Inam, F. Mechanical, Thermal, and Electrical Properties of Graphene-Epoxy Nanocomposites-A Review. Polymers 2016, 8, 281. [Google Scholar] [CrossRef]
- Goncalves, C.; Goncalves, I.C.; Magalhaes, F.D.; Pinto, A.M. Poly(lactic acid) Composites Containing Carbon-Based Nanomaterials: A Review. Polymers 2017, 9, 269. [Google Scholar] [CrossRef]
- Hu, Y.; Yin, S.; Zhu, J.; Qi, J.; Guo, J.; Wu, L.; Tang, C.; Yang, X. Fabrication and characterization of novel Pickering emulsions and Pickering high internal emulsions stabilized by gliadin colloidal particles. Food Hydrocoll. 2015, 44, 86–93. [Google Scholar] [CrossRef]
- Tarhini, M.; Greige-Gerges, H.; Elaissari, A. Protein-based nanoparticles: From preparation to encapsulation of active molecules. Int. J. Pharm. 2017, 522, 172–197. [Google Scholar] [CrossRef] [PubMed]
- Joye, I.J.; Nelis, V.A.; McClements, D.J. Gliadin-based nanoparticles: Fabrication and stability of food-grade colloidal delivery systems. Food Hydrocoll. 2015, 44, 86–93. [Google Scholar] [CrossRef]
- Feng, J.; Wu, S.; Wang, H.; Liu, S. Gliadin nanoparticles stabilized by a combination of thermally denatured ovalbumin with gemini dodecyl O-glucoside: The modulating effect of cosurfactant. Colloids Surf. A 2017, 516, 94–105. [Google Scholar] [CrossRef]
- Alcantara, A.C.S.; Darder, M.; Aranda, P.; Ruiz-Hitzky, E. Polysaccharide-fibrous clay bionanocomposites. Appl. Clay Sci. 2014, 96, 2–8. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic-inorganic nanocomposites-A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Zhang, Y.; Niu, Y.; Luo, Y.; Ge, M.; Yang, T.; Yu, L.L.; Wang, Q. Fabrication, characterization and antimicrobial activities of thymol-loaded zein nanoparticles stabilized by sodium caseinate-chitosan hydrochloride double layers. Food Chem. 2014, 142, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Cervera, M.F.; Karjalainen, M.; Airaksinen, S.; Rantanen, J.; Krogars, K.; Heinamaki, J.; Colarte, A.I.; Yliruusi, J. Physical stability and moisture sorption of aqueous chitosan-amylose starch films plasticized with polyols. Eur. J. Pharm. Biopharm. 2004, 58, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Oymaci, P.; Altinkaya, S.A. Improvement of barrier and mechanical properties of whey protein isolate based food packaging films by incorporation of zein nanoparticles as a novel bionanocomposite. Food Hydrocoll. 2016, 54, 1–9. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, H. Preparation and properties of zein-rutin composite nanoparticle/corn starch films. Carbohydr. Polym. 2017, 169, 385–392. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, D.; Ma, Z.; Zhang, Z.; Quan, Q. Reducing Water Sensitivity of Chitosan Biocomposite Films Using Gliadin Particles Made by In Situ Method. Polymers 2017, 9, 583. https://doi.org/10.3390/polym9110583
Huang D, Ma Z, Zhang Z, Quan Q. Reducing Water Sensitivity of Chitosan Biocomposite Films Using Gliadin Particles Made by In Situ Method. Polymers. 2017; 9(11):583. https://doi.org/10.3390/polym9110583
Chicago/Turabian StyleHuang, Dajian, Zonghong Ma, Zhuo Zhang, and Qiling Quan. 2017. "Reducing Water Sensitivity of Chitosan Biocomposite Films Using Gliadin Particles Made by In Situ Method" Polymers 9, no. 11: 583. https://doi.org/10.3390/polym9110583



