1. Introduction
Bamboo fiber (BF) has become an ecologically viable substitute for wood fiber and presents great potential for various applications in the polymer composite industry. BF-reinforced polymer composites have been extensively explored because of the properties of BFs [
1,
2]. Similarly to other cellulosic fiber-reinforced polymer composites, the critical concerns regarding BF in reinforced composites is the poor interfacial adhesion between the BF and matrix, which results from the hydrophilic nature of BF that acts in opposition to the hydrophobic nature of the polymer matrix. The conflict between hydrophilic and hydrophobic components restricts the properties of the composites, leading to poor fiber partition and dispersion throughout the composites [
3,
4]. The interfacial properties are a key area of focus when studying plant fiber-reinforced polymer composites. At the interface, a high adhesive strength can transfer stress between the fiber and polymer, but a low adhesive strength will see the composite damaged (layered or broken) when it sustains an external load. Hence, it is essential to improve the matrix-fiber adhesion, which will ensure effective stress transfer in the composite, whereas the transmission efficiency depends mainly upon the interface bonding between the fiber and matrix.
There are two types of modification methods that can be used to improve interface bonding in fiber-reinforced polymer composites: the first uses a hydrophobic group to replace the hydroxy group on the fiber’s surface, thus making the BF more compatible with the hydrophobic polymer [
5,
6,
7]. The second method, which is considered to be more effective, is to reduce the number of hydroxy group on the fiber surface via forming a chemical bond with the matrix [
8,
9,
10]. Shi et al. [
11] reported that inorganic nanoparticle impregnation is an effective way to improve the interface bonding between the natural fibers and polymer matrix. The inorganic nanoparticles deposited into the cell wall and on the surface, serving as nucleation sites, have a potential to improve the crystalline formation in the polymer matrix, which may provide strong static electric attractive forces to the non-polar polymer surface; hence the interface compatibility between the fibers and the matrix may be improved. Among the inorganic nanoparticle modification methods, nano calcium carbonate (CaCO
3) modification has been developing considerable prospects because of the low cost, nano-scale, and ultra-fine solid structure of CaCO
3, as well as the high economic value of the process. Thus, in previous research, BF-reinforced composites were treated with nano CaCO
3 in situ modification, for which CaCO
3 particles were successfully deposited into the BF [
12,
13]. The modification was studied using multiple analytical methods and parameters (e.g., environmental scanning electron microscopy (ESEM), atomic force microscopy (AFM), contact angle (CA) and tensile test), and it was observed that there was significant improvement in the interface compatibility, tensile strength and modulus of elasticity (MOE) of the composites. In a later study [
14], impregnation modification (IM) was used to treat BF with nano CaCO
3, and although this saw an increase in tensile properties of single BFs and composites, it was inferior to CaCO
3 in situ modification. The optimal conditions for nano CaCO
3 IM were obtained, and with this method, the technology used for fiber modification can be simplified.
Presently, the interfacial property of BF-reinforced polymer composites is studied on the basis of the interface bonding theory. The characterization methods for the interfacial property mainly include a fiber pullout test, a micro-bond test, a single-fiber fragmentation test, a micro-indentation test, AFM, static mechanics SEM, a CA test, dynamic mechanical analysis, and so forth [
15]. In this study, one of the aims was to establish a connection between the macroscopic property and interface performance, and the surface free energy calculated by the CA and dynamic mechanical analysis was used. The surface free energy is a measurement of the energy on the material’s surface, which is also the macro embodiment of micro-mechanics at the atomic or molecular level of the material. The surface free energy not only evaluates the macroscopic property of the material, but it can also associate with the microscopic phenomena of the material [
16]. Additionally, the structure of the composites, the compatibility of each component in the composites, and the relationships between mobility of the polymer chains and performance of the composites can be reflected by dynamic mechanical analysis [
17]. Hong et al. [
18] noted that dynamic mechanical flexural properties exhibit sensitivity to the interfacial adhesion between the lignocellulose and the polymer matrix. Ibarra and Panos [
19] also reported that the interpretation of the dynamic properties and other parameters (e.g., glass transition temperature, apparent activation energy and interface performance parameter, etc.) could help to explain the existence of such interactions.
In this work, BF-reinforced high-density polyethylene (HDPE) composites were made from extrusion molding, and the BFs were treated by nano CaCO3 using blending modification (BM) and IM. The interfacial property was characterized by a CA test and dynamic mechanical analysis (DMA); moreover, the water absorption of the composites was also studied. The aim of this study was to build a relationship between the macro-performance and the micro-characteristics of the composites and to explore the interface enhancements of nano CaCO3, which may provide new theory and practice foundations for studying inorganic particle-reinforcing bamboo plastic composites for practical applications.
2. Materials and Methods
2.1. Materials
BF was obtained from Guizhou CHTH paper Co., Ltd., Guizhou, China. HDPE with a density of 0.945 g/cm3 (DGDK-3364, 0.075 g/min melt flow index at 190 °C and 2.16 kg, 22.1 MPa tensile strength) was purchased from Guangzhou Zhangmutou Plastic Co., Ltd., Guangzhou, China. The lubricant polyethylene wax (PE-wax; LPE-F4) was provided by Beijing Chemical University Keyi Fine Chemical Co., Ltd., Beijing, China. Maleic anhydride-grafted polyethylene (MAPE; 2909A#, 2.5–7 g/10 min melt-flow index at 190 °C and 2.16 kg) was obtained from Shenzhen Hualin Chemical Co., Ltd., Shenzhen, China. Nano CaCO3 with a grain diameter of 15–40 nm (NCCa40) was supplied by Beijing Boyu Gaoke New Material Technology Co., Ltd., Beijing, China. Ethylenediamine tetraacetic acid disodium salt (EDTA-2Na, analytically pure) serving as the dispersant was purchased from Beijing Huabo Stand Biological Analysis Technology Co., Ltd., Beijing, China.
2.2. Nano CaCO3 Modification Process
In this study, three types of BF/HDPE composites were produced. The first samples were made from unmodified BF, which served as a control, while the other two samples were made from BF treated with nano CaCO3 IM and BM.
IM: At 25 °C, 100 g of BF was dissolved into 2 L of deionized water for 0.5 h at 60 rpm; then 1.7 g of EDTA-2Na and 20 g of nano CaCO3 were introduced to the mixture, which was mixed for 25 min. The suspensions were washed on a 200-mesh nylon net with deionized water and were then air dried. Modified bamboo fibers (MBFs) were obtained and preserved at a constant temperature and humidity of 23 °C and 50% relative humidity (RH). BM: An equivalent content of nano CaCO3 was used to blend with BF in accordance with the loading of nano CaCO3 in the process of IM.
2.3. Composite Manufacture through Extrusion Molding Process
BF and IMBF were dried at 103 °C in an oven until the moisture content was reduced to less than 2 wt %.
BF, IMBF or BMBF with a mass fraction of 30 wt %, PE-wax with a mass fraction of 1 wt %, HDPE with a mass fraction of 65 wt % and MAPE with a mass fraction of 4 wt % were mixed together. The composites were obtained when the mixture was granulated by a twin-screw extruder (SD-30) (Shanghai Sunlight Plastics Machinery Manufacturing Co., Ltd., Shanghai, China) and crushed by a crushing machine (KCP150) (Beijing Kaichuang Tonghe Technology Development Co., Ltd., Beijing, China), obtaining granules. The granules were placed in a single-screw extruder (30 × 25) (Shanghai Sunlight Plastics Machinery Manufacturing Co., Ltd., Shanghai, China), where they were subjected to melt mixing and then water cooling to produce the final composites. BF/HDPE, BMBF/HDPE and IMBF/HDPE composites with a size of 15,000 mm × 30 mm × 4 mm (5000 mm × 30 mm × 4 mm each, length by width by thickness) were prepared by extrusion molding. In addition, the barrel temperatures used by the single-screw extruder were 160, 170, 180 and 175 °C, respectively. The process flow diagram of extrusion molding is shown in
Figure 1.
2.4. Water Absorption Tests
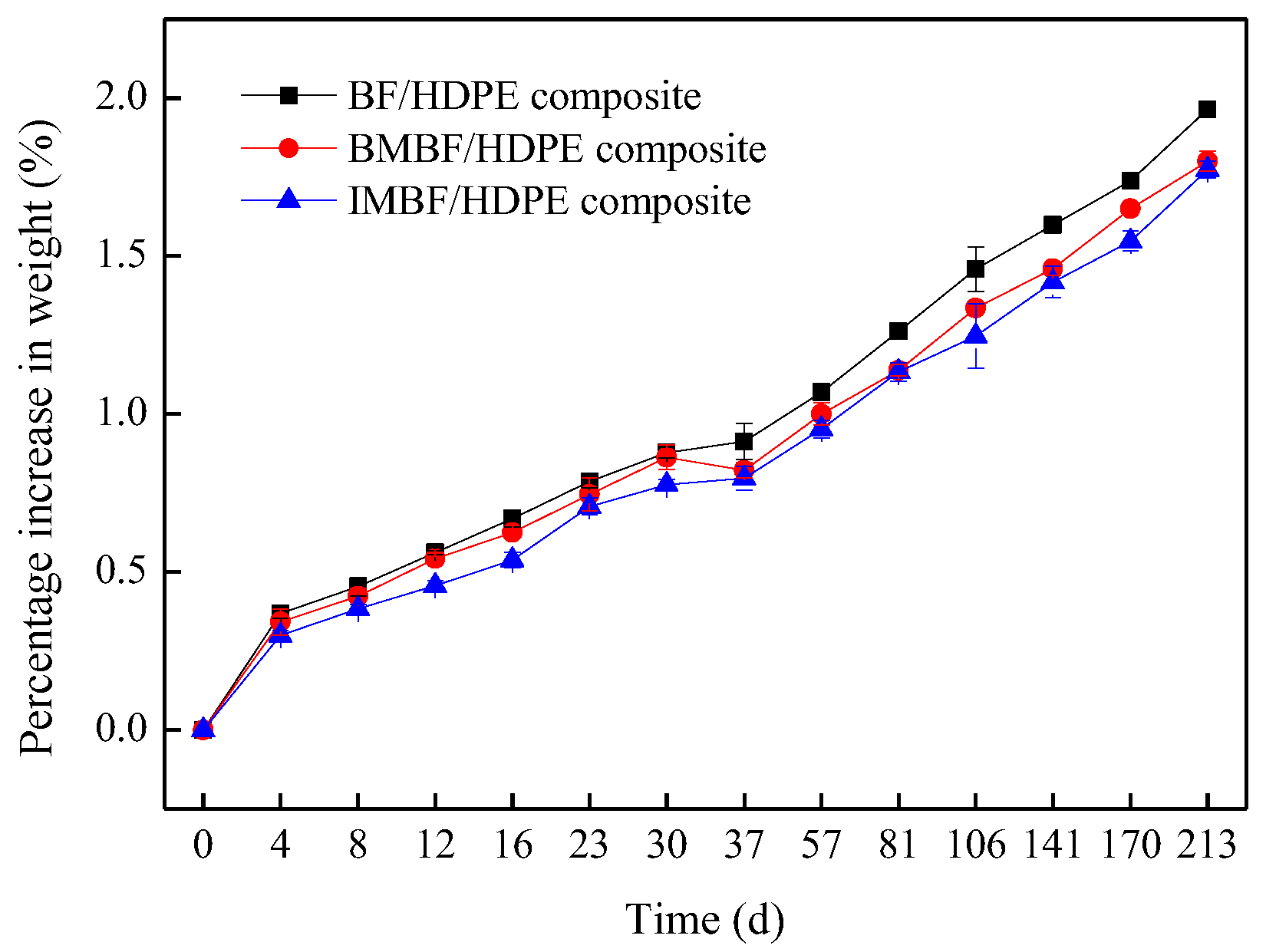
Nine composites with dimensions of 76.2 mm × 25.4 mm × 4 mm prepared from the BF-, BMBF- and IMBF-reinforced composites (three for each) were used for the weight-gain rate measurements for water absorption testing. The water absorption test was carried out in accordance with the American Society for Testing and Materials (ASTM) D570-98 standard for the determination of the weight gain rate of the composites. The percentage increase in weight during immersion was calculated to the nearest 0.01% as follows:
where
WA is the percentage increase in weight (%),
Ww is the wet weight (g), and
Wc is the conditioned weight (g).
2.5. Contact Angle Tests
The CAs of the composites were measured using an optical CA measurement instrument (Dataphsics OCA20). A charge-coupled device (CCD) with 7X objective and 25 fps was used to record the whole process of a liquid drop (deionized water, diiodomethane, formamide and glycerol) contacting with the sample. The CAs were calculated using the ellipse method and nine positions of each composite were tested in an ambient environment of 22 ± 1 °C and 50 ± 10% RH.
2.6. The Calculation of Surface Free Energy
There are many theoretical methods to calculate the solid surface energy, such as the Zisman method, the Fowkes method, the Owens-Wendt-Rabel-Kaelble (OWRK) method, the extended Fowkes method, acid-base theory formula, the theory of equilibrium state, the Schultz method, and so forth. These theoretical methods were obtained on the basis of Young’s equation. The polarity effect between the measured solid and liquid is not considered by the Fowkes method when calculating the surface energy [
16]. Thus, the OWRK method and acid-base theory formula were suitable for this study because of the hydroxy group of BFs; the surface free energy of the composites was obtained through the OWRK method and acid-base theory formula according to the measured CA values.
Table 1 shows the surface free energy parameters of the control liquid.
The OWRK method [
20,
21] divides the interfacial tension of each phase into two parts, that is, the dispersion component
γd and the polar component
γp (Equation (2)). Provided that the interaction between the polarity and dispersion force exists in the two phases of liquid and solid, the solid-liquid surface free energy
γSL can be represented as the geometric average function of
γd and
γp [
22] (Equation (3)). Furthermore, the relationship between the CA
θ formed on the solid surface and the solid surface free energy can be expressed by Young’s equation [
23] (Equation (4)):
where
γL is the surface tension of the liquid;
γLd and
γLp are the dispersion component and polar component of the liquid surface tension, respectively;
γS is the solid surface free energy; and
γSd and
γSp are the dispersion component and polar component of the solid surface free energy, respectively.
By combining Equations (3) and (4), Equation (5) is obtained:
Therefore, if the CA θ, γL, γLd and γLp of at least two liquids are known, according to Equation (5) and the graphing method, and serve as the x axis and y axis, respectively, allowing the slope and intercept of the line to be used to calculate γSp and γSd. Thus, the solid surface free energy γS can be obtained by Equation (2).
Acid-base theory [
24,
25,
26,
27] converts
γd to the Lifshitz-van der Waals component
γLW and divides
γp into the Lewis acid component
γ+ and Lewis alkali component
γ−. Then Equation (3) can be converted to Equation (6):
By uniting Equations (4) and (6), Equation (7) is then derived:
According to Equation (7), if the CA θ, γL, γLLW, γL+ and γL− of at least three liquids are known, γSLW, γS+ and γS− can be calculated; thus it was possible for γ to be obtained.
2.7. Dynamic Mechanical Property Tests
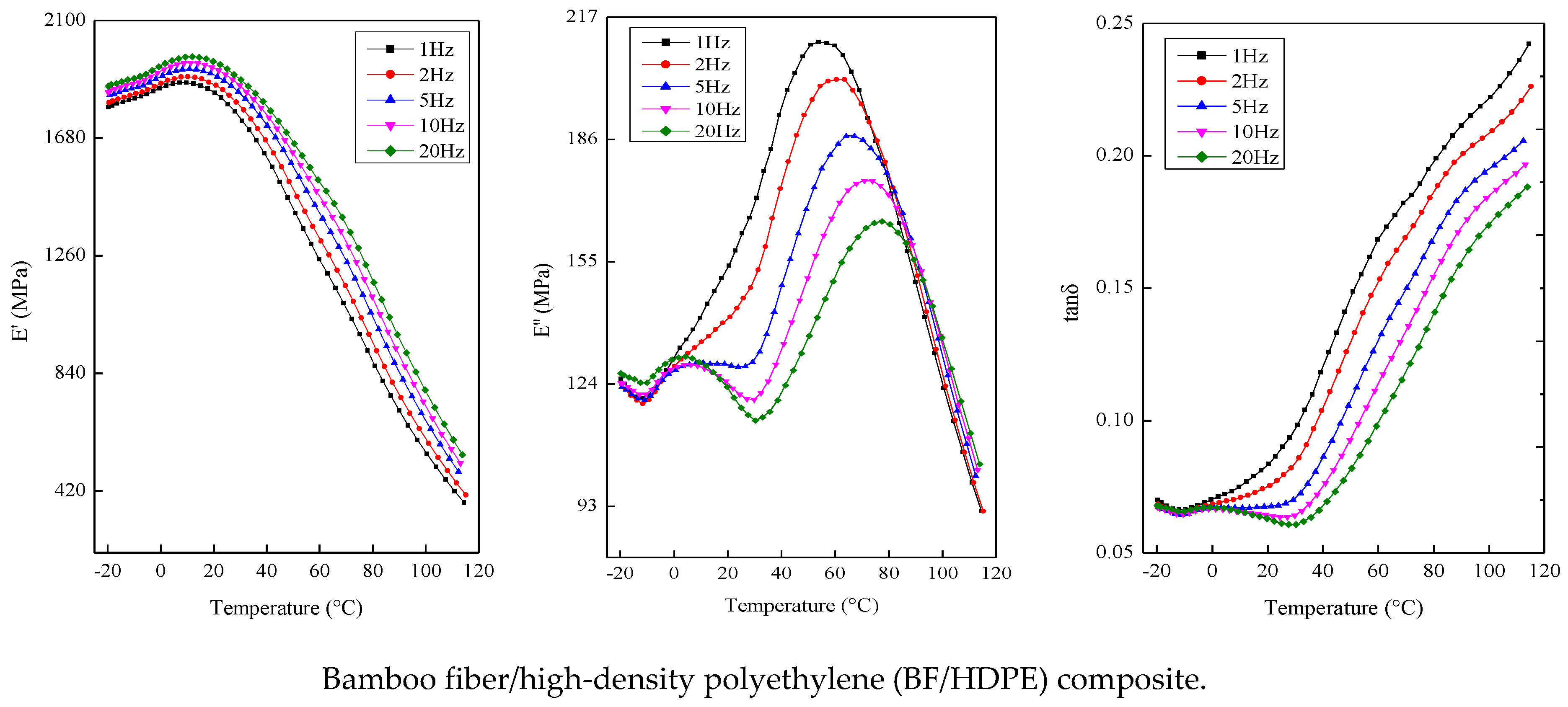
The dynamic mechanical properties of the composites were measured in the dual-cantilever mode (Q800 DMA, Thermal Analysis Instruments, New Castle, DE, USA) at frequencies of 1, 2, 5, 10 and 20 Hz. The dimensions of the sample were 60 mm × 14 mm with a thickness of 4 mm. Tests were conducted in the range of −20 to 120 °C at a heating rate of 2 °C/min with a strain amplitude of 25 μm (ASTM standard D7028-07ε1).
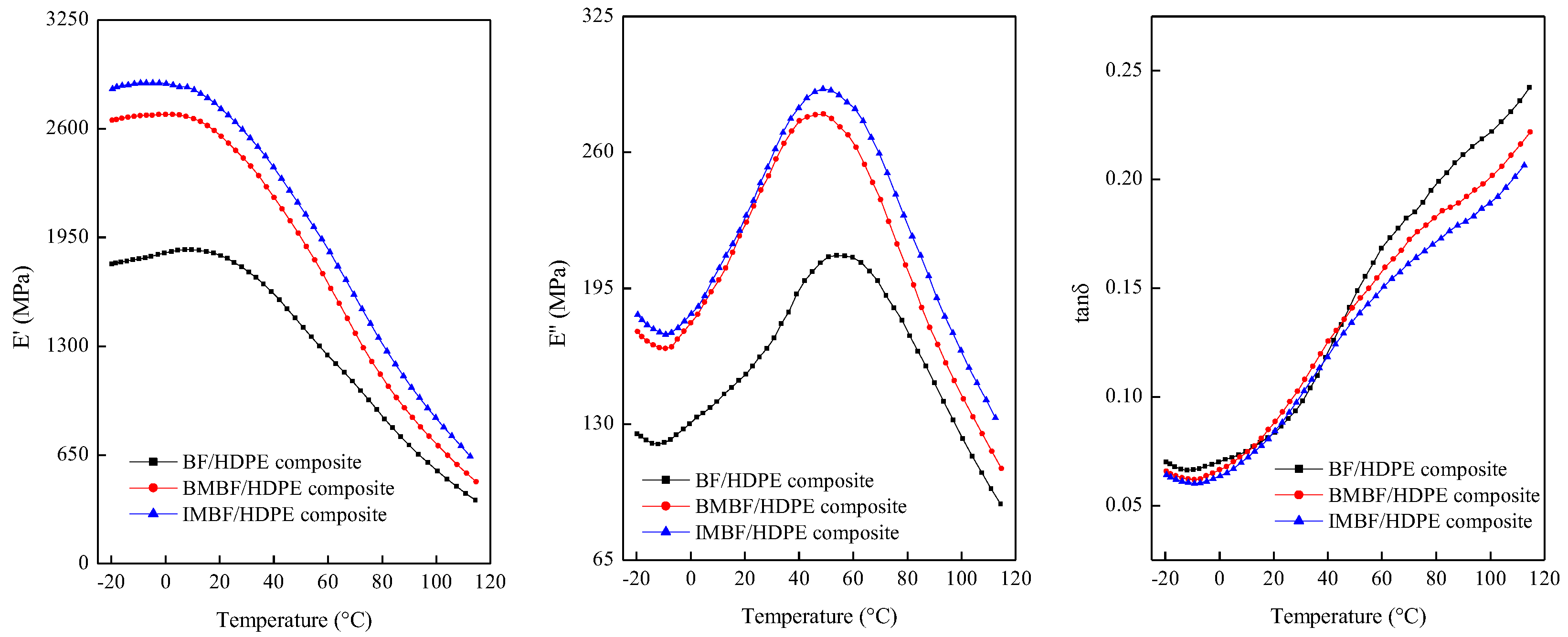
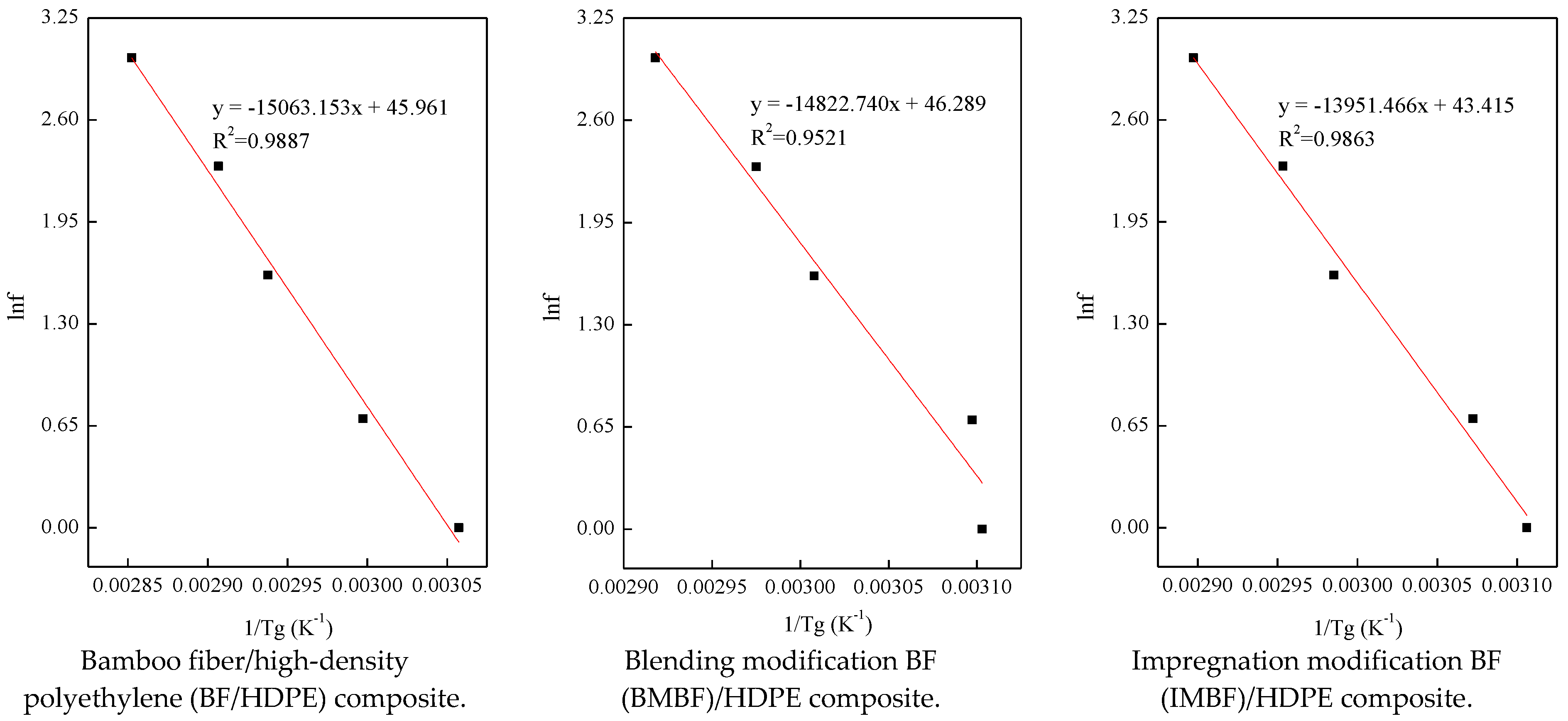
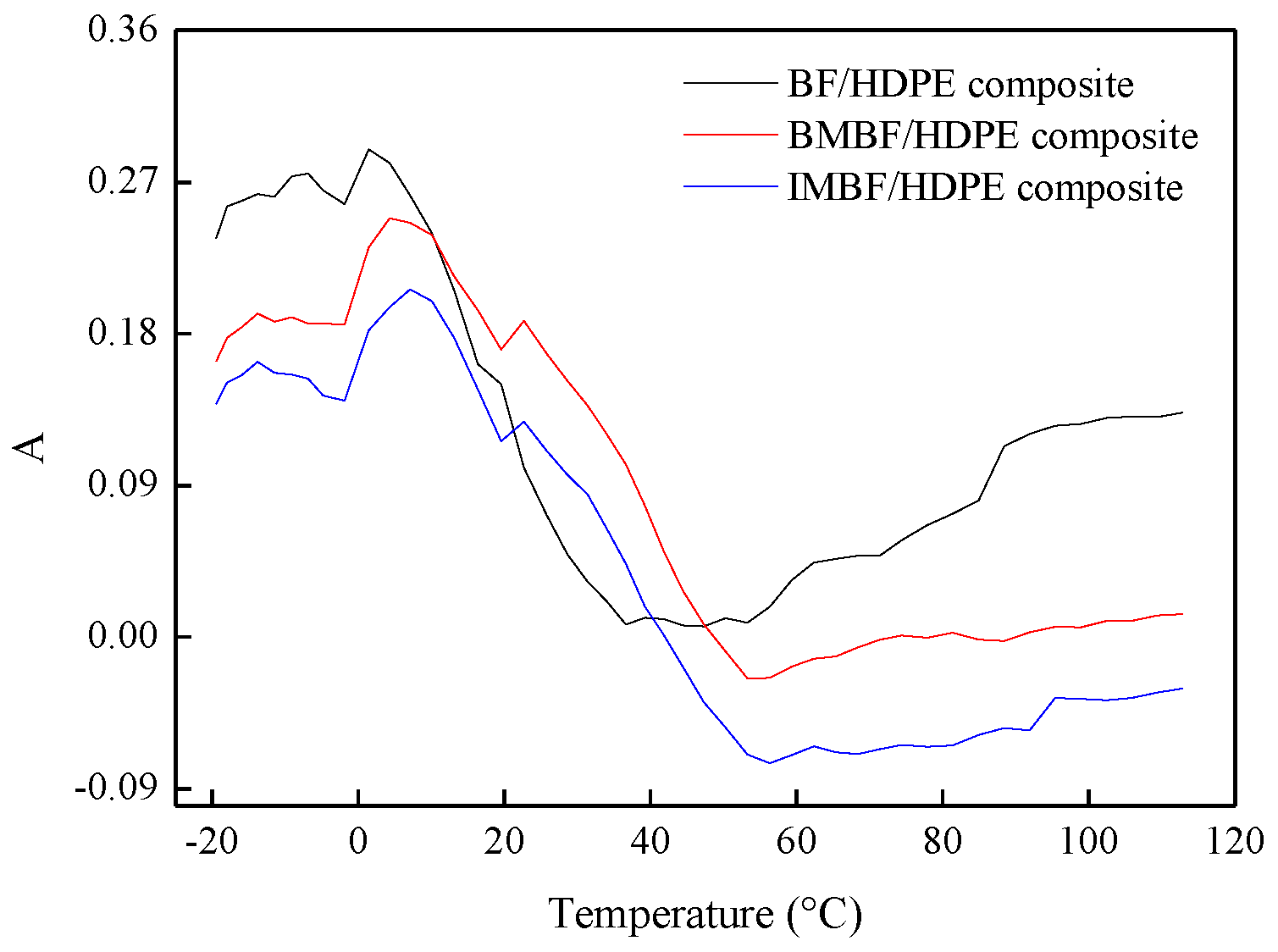
4. Conclusions
In this study, nano CaCO3 was introduced by IM and BM, and the BF/HDPE, BMBF/HDPE and IMBF/HDPE composites were prepared successfully by an extrusion molding process (the mass fraction of HDPE was 65%). Nano CaCO3 treatment reduced the percentage increase in the weight of the composites, and the reduction by IM was higher than that by BM. The acid-base model according to both the Lewis and OWRK equations was suitable for calculating the surface energy of the composites. Meanwhile, the change trend of the surface energy in the two methods was consistent, and the surface energy of the composites was between 40 and 50 mJ/m2. As deduced from the dynamic mechanical analysis (DMA) testing, the structural stability of the IMBF/HDPE composite was higher than that of the BMBF/HDPE and BF/HDPE composites under the conditions of a single frequency, while the E′max value of the BMBF/HDPE and IMBF/HDPE composites increased 1.43- and 1.53-fold, respectively, when compared to the control sample. The phase-to-phase interaction parameter B and k values of the composites treated by nano CaCO3 were higher than those of the control sample; in particular, the effect of IM was remarkable. When compared to the control sample, the Ea value of the BMBF/HDPE and IMBF/HDPE composites decreased by 1.60% and 7.39%, respectively, which indicated that the interactions between the IMBF and HDPE at the interface were strong. It was observed that nano CaCO3 decreased the A value of composites in the temperature range of −20 to 120 °C, which confirmed this finding. Thus, the CaCO3 produced by IM and BM has an effect on the interfacial properties of BF/HDPE composites manufactured by the extrusion molding process, and the effect of IM was more apparent than that of BM.