Enhancing Stereocomplexation Ability of Polylactide by Coalescing from Its Inclusion Complex with Urea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the PLA/Urea Complex and Coalesced PLA
2.3. Characterizations
3. Results
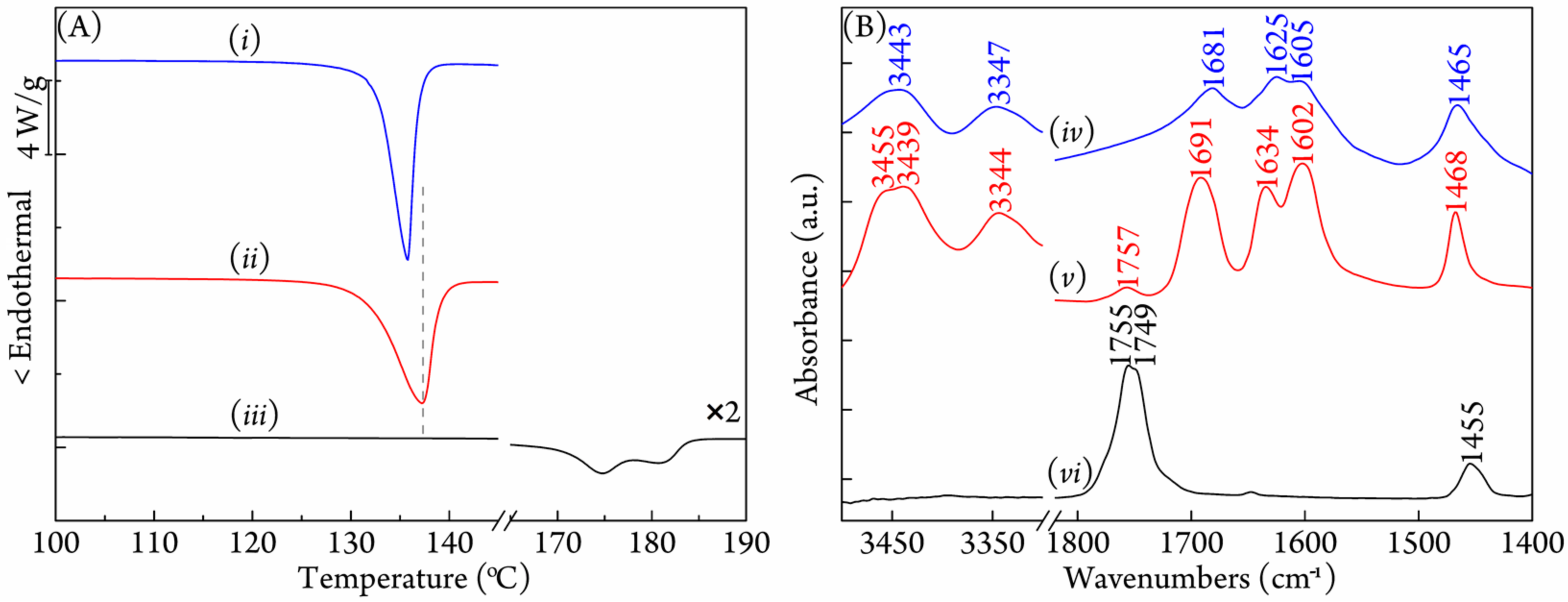
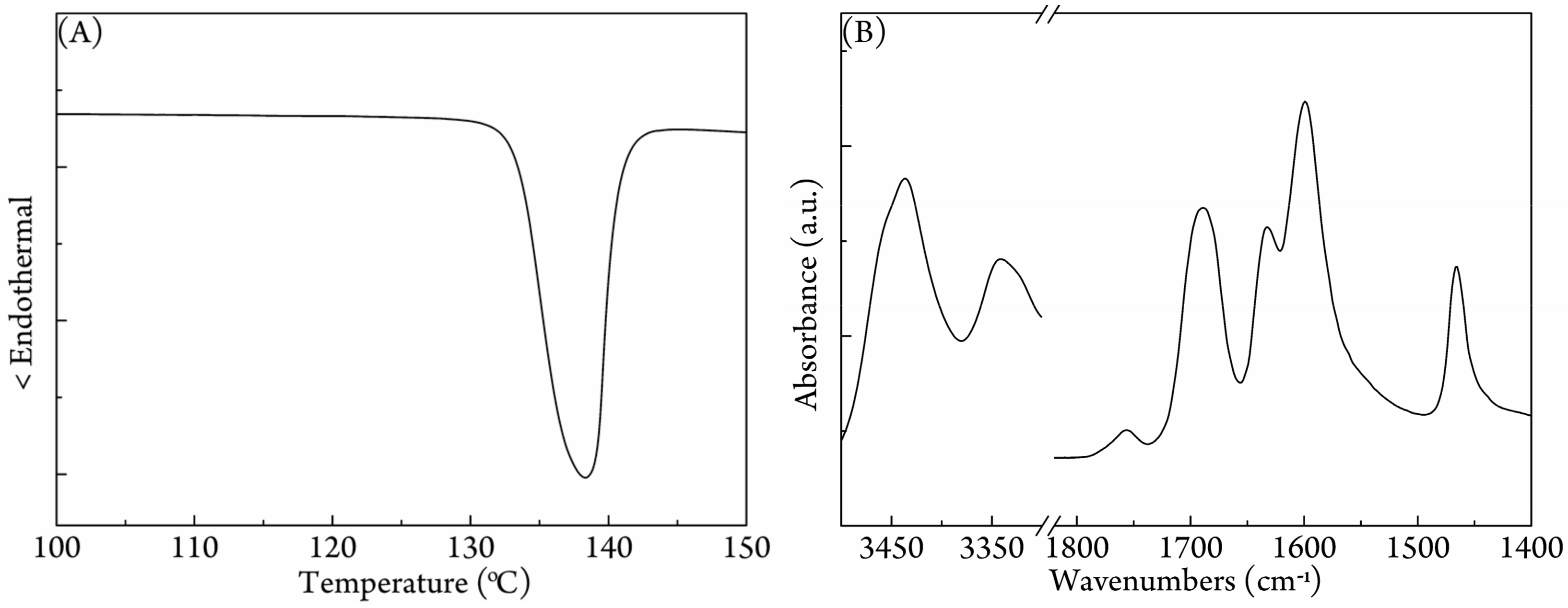
3.1. Characterization of the PLA/Urea Complex
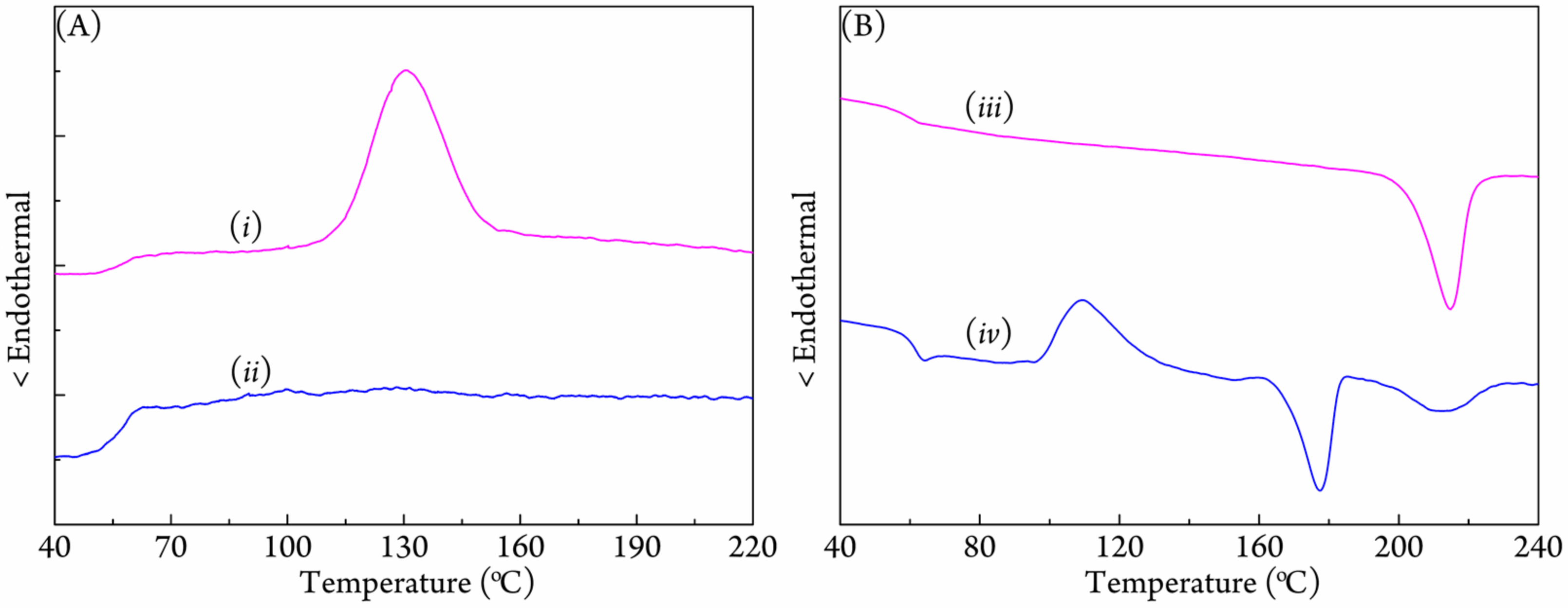
3.2. Crystallization Behavior of Coalesced PLLA/PDLA Blend
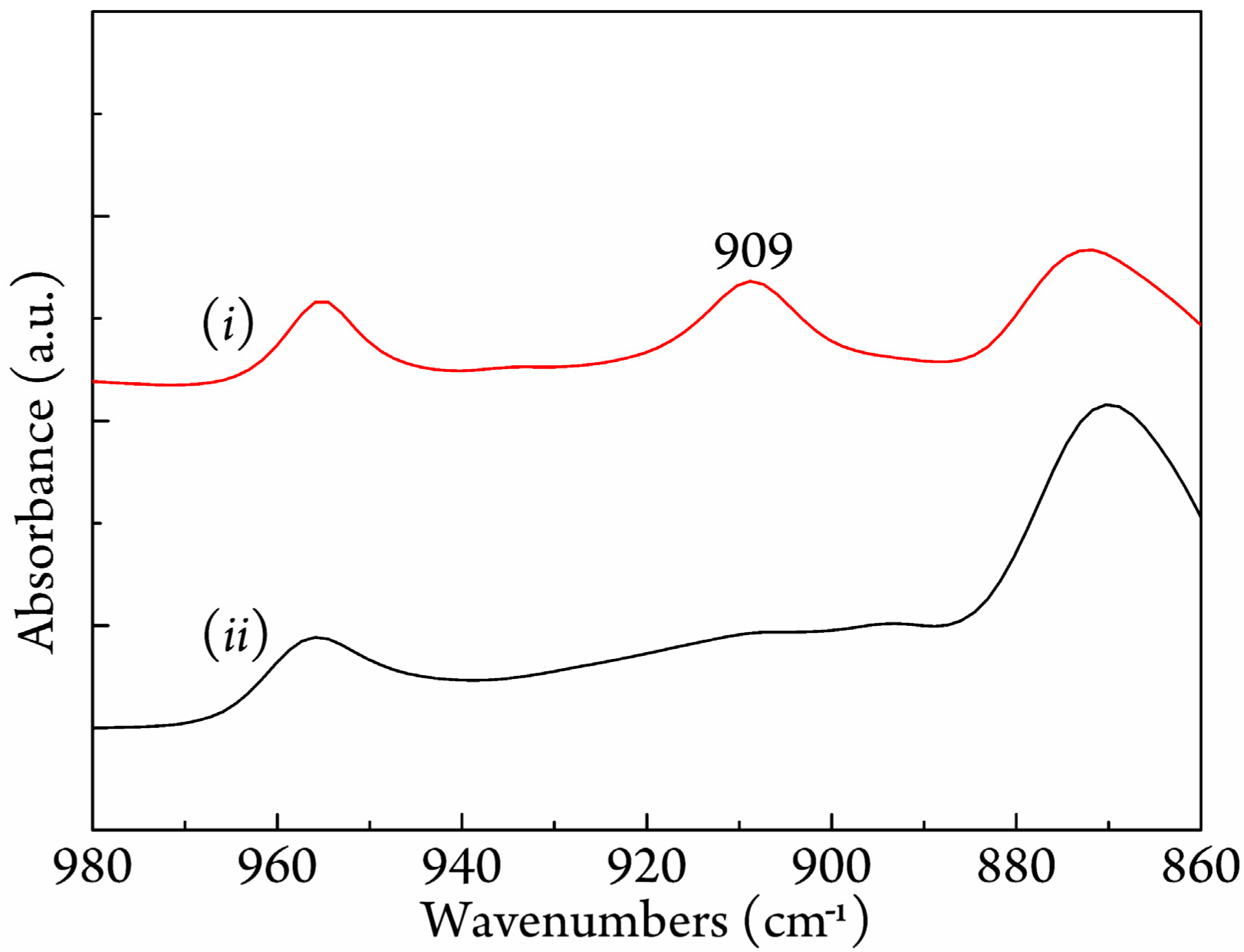
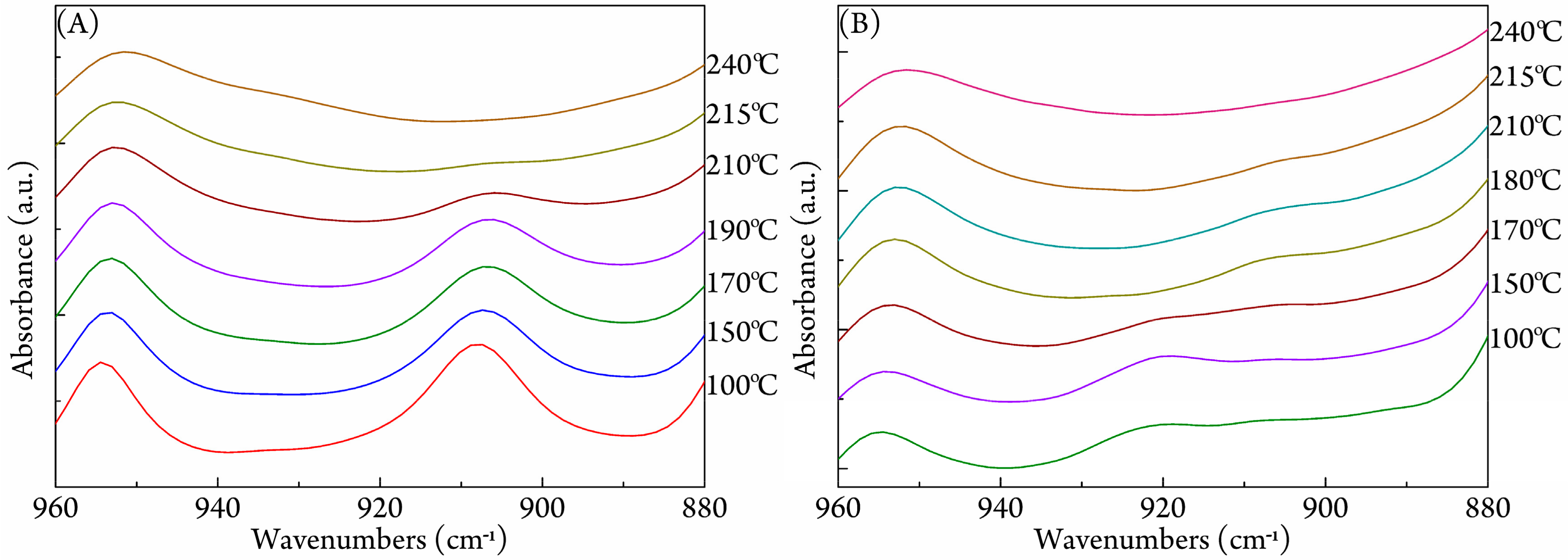
3.3. FTIR Study on Stereocomplexation Ability
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, G.Q.; Patel, M.K. Plastics Derived from Biological Sources: Present and Future: A Technical and Environmental Review. Chem. Rev. 2012, 112, 2082–2099. [Google Scholar] [CrossRef] [PubMed]
- Cicero, J.A.; Dorgan, J.R.; Garrett, J.; Runt, J.; Lin, J.S. Effects of molecular architecture on two-step, melt-spun poly(lactic acid) fibers. J. Appl. Polym. Sci. 2002, 86, 2839–2846. [Google Scholar] [CrossRef]
- Drieskens, M.; Peeters, R.; Mullens, J.; Lemstra, P.J.; Hristova-Bogaerds, D.G. Structure versus properties relationship of poly(lactic acid). I. Effect of crystallinity on barrier properties. J. Polym. Sci. Part B Polym. Phys. 2009, 47, 2247–2258. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.N.; Huang, Z.G.; Weng, Y.X. Heat resistance, crystallization behavior, and mechanical properties of polylactide/nucleating agent composites. Mater. Des. 2015, 66, 7–15. [Google Scholar] [CrossRef]
- Courgneau, C.; Domenek, S.; Lebossé, R.; Guinault, A.; Avérous, L.; Ducruet, V. Effect of crystallization on barrier properties of formulated polylactide. Polym. Int. 2012, 61, 180–189. [Google Scholar] [CrossRef] [Green Version]
- Loiola, L.M.D.; Más, B.A.; Duek, E.A.R.; Felisberti, M.I. Amphiphilic multiblock copolymers of PLLA, PEO and PPO blocks: Synthesis, properties and cell affinity. Eur. Polym. J. 2015, 68, 618–629. [Google Scholar] [CrossRef]
- Lu, J.; Qiu, Z.; Yang, W. Fully biodegradable blends of poly(l-lactide) and poly(ethylene succinate): Miscibility, crystallization, and mechanical properties. Polymer 2007, 48, 4196–4204. [Google Scholar] [CrossRef]
- Pan, P.; Shan, G.; Bao, Y. Enhanced nucleation and crystallization of poly(l-lactic acid) by immiscible blending with poly(vinylidene fluoride). Ind. Eng. Chem. Res. 2014, 53, 3148–3156. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, Z. Effect of poly(vinyl alcohol) as an efficient crystallization-assisting agent on the enhanced crystallization rate of biodegradable poly(l-lactide). RSC Adv. 2015, 5, 49216–49223. [Google Scholar] [CrossRef]
- Ye, H.M.; Hou, K.; Zhou, Q. Improve the thermal and mechanical properties of poly(l-lactide) by forming nanocomposites with pristine vermiculite. Chin. J. Polym. Sci. 2016, 34, 1–12. [Google Scholar] [CrossRef]
- Bai, H.W.; Huang, C.M.; Xiu, H.; Zhang, Q.; Fu, Q. Enhancing mechanical performance of polylactide by tailoring crystal morphology and lamellae orientation with the aid of nucleating agent. Polymer 2014, 55, 6924–6934. [Google Scholar] [CrossRef]
- Barrau, S.; Vanmansart, C.; Moreau, M.; Addad, A.; Stoclet, G.; Lefebvre, J.M.; Seguela, R. Crystallization behavior of carbon nanotube-polylactide nanocomposites. Macromolecules 2011, 44, 6496–6502. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, X.; Zhang, R.; He, H.F.; Jin, T.; Zhang, J. Unique morphology in polylactide/graphene oxide nanocomposites. J. Macromol. Sci. Phys. 2015, 54, 45–57. [Google Scholar] [CrossRef]
- Ikada, Y.; Jamshidi, K.; Tsuji, H.; Hyon, S.H. Stereocomplex formation between enantiomeric poly(lactides). Macromolecules 1987, 20, 904–906. [Google Scholar] [CrossRef]
- Tsuji, H. Poly(lactide) stereocomplexes: Formation, structure, properties, degradation, and applications. Macromol. Biosci. 2005, 5, 569–597. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.C.; Hillmyer, M.A. Polylactide stereocomplex crystallites as nucleating agents for isotactic polylactide. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 300–313. [Google Scholar] [CrossRef]
- Tsuji, H.; Tezuka, Y. Stereocomplex formation between enantiomeric poly(lactic acid)s. 12. Spherulite growth of low-molecular-weight poly(lactic acid)s from the melt. Biomacromolecules 2004, 5, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Takai, H.; Saha, S.K. Isothermal and non-isothermal crystallization behavior of poly(l-lactic acid): Effects of stereocomplex as nucleating agent. Polymer 2006, 47, 3826–3837. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Stereocomplex formation between enantiomeric poly(lactic acid)s. XI. Mechanical properties and morphology of solution-cast films. Polymer 1999, 40, 6699–6708. [Google Scholar] [CrossRef]
- Ma, P.M.; Shen, T.F.; Xu, P.W.; Dong, W.F.; Lemstra, P.J.; Chen, M.Q. Superior performance of fully biobased poly(lactide) via stereocomplexation-induced phase separation: Structure versus property. ACS Sustain. Chem. Eng. 2015, 3, 1470–1478. [Google Scholar] [CrossRef]
- Tsuji, H.; Fukui, I. Enhanced thermal stability of poly(lactide)s in the melt by enantiomeric polymer blending. Polymer 2003, 44, 2891–2896. [Google Scholar] [CrossRef]
- Tsuji, H.; Hyon, S.H.; Ikada, Y. Stereocomplex formation between enantiomeric poly(lactic acid)s. 3. Calorimetric studies on blend films cast from dilute solution. Macromolecules 1991, 24, 5651–5656. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Stereocomplex formation between enantiomeric poly(lactic acids). 9. Stereocomplexation from the melt. Macromolecules 1993, 26, 6918–6926. [Google Scholar] [CrossRef]
- Pan, P.J.; Han, L.L.; Bao, J.N.; Xie, Q.; Shan, G.R.; Bao, Y.Z. Competitive stereocomplexation, homocrystallization, and polymorphic crystalline transition in poly(l-lactic acid)/poly(d-lactic acid) racemic blends: Molecular weight effects. J. Phys. Chem. B 2015, 119, 6462–6470. [Google Scholar] [CrossRef] [PubMed]
- Han, L.L.; Pan, P.J.; Shan, G.R.; Bao, Y.Z. Stereocomplex crystallization of high-molecular-weight poly(l-lactic acid)/poly(d-lactic acid) racemic blends promoted by a selective nucleator. Polymer 2015, 63, 144–153. [Google Scholar] [CrossRef]
- Tsuji, H.; Tashiro, K.; Bouapao, L.; Hanesaka, M. Synchronous and separate homo-crystallization of enantiomeric poly(l-lactic acid)/poly(d-lactic acid) blends. Polymer 2012, 53, 747–754. [Google Scholar] [CrossRef]
- Han, L.L.; Shan, G.R.; Bao, Y.Z.; Pan, P.J. Exclusive stereocomplex crystallization of linear and multiarm star-shaped high-molecular-weight stereo diblock poly(lactic acid)s. J. Phys. Chem. B 2015, 119, 14270–14279. [Google Scholar] [CrossRef] [PubMed]
- Han, L.L.; Yu, C.T.; Zhou, J.; Shan, G.R.; Bao, Y.Z.; Yun, X.Y.; Dong, T.; Pan, P.J. Enantiomeric blends of high-molecular-weight poly(lactic acid)/poly(ethylene glycol) triblock copolymers: Enhanced stereocomplexation and thermomechanical properties. Polymer 2016, 103, 376–386. [Google Scholar] [CrossRef]
- Fukushima, K.; Kimura, Y. An efficient solid-state polycondensation method for synthesizing stereocomplexed poly(lactic acid)s with high molecular weight. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3714–3722. [Google Scholar] [CrossRef]
- Li, S.H.; Woo, E.M. Kinetic Analysis on Effect of Poly(4-vinyl phenol) on Complex-Forming Blends of Poly(l-lactide) and Poly (d-lactide). Polym. J. Tokyo Jpn. 2009, 41, 374–382. [Google Scholar] [CrossRef]
- Pan, P.J.; Bao, J.N.; Han, L.L.; Xie, Q.; Shan, G.R.; Bao, Y.Z. Stereocomplexation of high-molecular-weight enantiomeric poly(lactic acid)s enhanced by miscible polymer blending with hydrogen bond interactions. Polymer 2016, 98, 80–87. [Google Scholar] [CrossRef]
- Bao, R.Y.; Yang, W.; Jiang, W.R.; Liu, Z.Y.; Xie, B.H.; Yang, M.B.; Fu, Q. Stereocomplex formation of high-molecular-weight polylactide: A low temperature approach. Polymer 2012, 53, 5449–5454. [Google Scholar] [CrossRef]
- Lu, J.; Mirau, P.A.; Tonelli, A.E. Chain conformations and dynamics of crystalline polymers as observed in their inclusion compounds by solid-state NMR. Prog. Polym. Sci. 2002, 27, 357–401. [Google Scholar] [CrossRef]
- Gurarslan, A.; Joijode, A.S.; Tonelli, A.E. Polymers Coalesced from Their Cyclodextrin Inclusion Complexes: What Can They Tell Us about the Morphology of Melt-Crystallized Polymers? J. Polym. Sci. Part B Polym. Phys. 2012, 50, 813–823. [Google Scholar] [CrossRef]
- Wei, M.; Shuai, X.; Tonelli, A.E. Melting and crystallization behaviors of biodegradable polymers enzymatically coalesced from their cyclodextrin inclusion complexes. Biomacromolecules 2003, 4, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Williamson, B.R.; Krishnaswamy, R.; Tonelli, A.E. Physical properties of poly(ε-caprolactone) coalesced from its α-cyclodextrin inclusion compound. Polymer 2011, 52, 4517–4527. [Google Scholar] [CrossRef]
- Gurarslan, A.; Shen, J.; Tonelli, A.E. Behavior of poly(ε-caprolactone)s (PCLs) coalesced from their stoichiometric urea inclusion compounds and their use as nucleants for crystallizing PCL melts: Dependence on PCL molecular weights. Macromolecules 2012, 45, 2835–2840. [Google Scholar] [CrossRef]
- Wei, M.; Davis, W.; Urban, B.; Song, Y.; Porbeni, F.E.; Wang, X.; White, J.L.; Balik, C.M.; Rusa, C.C.; Fox, J.; Tonelli, A.E. Manipulation of Nylon-6 Crystal Structures with Its α-Cyclodextrin Inclusion Complex. Macromolecules 2002, 35, 8039–8044. [Google Scholar] [CrossRef]
- Gurarslan, A.; Caydamli, Y.; Shen, J.L.; Tse, S.; Yetukuri, M.; Tonelli, A.E. Coalesced poly(ε-caprolactone) fibers are stronger. Biomacromolecules 2015, 16, 890–893. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Shin, I.D.; Urban, B.; Tonelli, A.E. Partial miscibility in a nylon-6/nylon-66 blend coalesced from their common α-cyclodextrin inclusion complex. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 1369–1378. [Google Scholar] [CrossRef]
- Ye, H.M.; Chen, X.T.; Liu, P.; Wu, S.Y.; Jiang, Z.Y.; Xiong, B.J.; Xu, J. Preparation of Poly(butylene succinate) Crystals with Exceptionally High Melting Point and Crystallinity from Its Inclusion Complex. Macromolecules 2017, 50, 5425–5433. [Google Scholar] [CrossRef]
- Howe, C.; Vasanthan, N.; MacClamrock, C.; Sanker, S.; Skin, I.D.; Simonsen, I.K.; Tonelli, A.E. Inclusion compound formed between poly(l-lactic acid) and urea. Macromolecules 1994, 27, 7433–7436. [Google Scholar] [CrossRef]
- Howe, C.; Sankar, S.; Tonelli, A.E. 13C n.m.r observation of poly (l-lactide) in the narrow channels of its inclusion compound with urea. Polymer 1993, 34, 2674–2676. [Google Scholar] [CrossRef]
- Eaton, P.; Vasanthan, N.; Shin, I.D.; Tonelli, A.E. Formation and Characterization of Polypropylene-Urea Inclusion Compounds. Macromolecules 1996, 29, 2531–2536. [Google Scholar] [CrossRef]
- Ravindran, P.; Vasanthan, N. Formation of Poly(3-hydroxybutyrate) (PHB) Inclusion Compound with Urea and Unusual Crystallization Behavior of Coalesced PHB. Macromolecules 2015, 48, 3080–3087. [Google Scholar] [CrossRef]
- Okihara, T.; Tsuji, M.; Kawaguchi, A.; Katayama, K.I.; Tsuji, H.; Hyon, S.H.; Ikada, Y. Crystal structure of stereocomplex of poly(l-lactide) and poly(d-lactide). J. Macromol. Sci. Part B Phys. 1991, 30, 119–140. [Google Scholar] [CrossRef]
- Kister, G.; Cassanas, G.; Vert, M.; Pauvert, B.; Térol, A. Vibrational analysis of poly(l-lactic acid). J. Raman Spectrosc. 1995, 26, 307–311. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Chen, X.-T.; Ye, H.-M. Enhancing Stereocomplexation Ability of Polylactide by Coalescing from Its Inclusion Complex with Urea. Polymers 2017, 9, 592. https://doi.org/10.3390/polym9110592
Liu P, Chen X-T, Ye H-M. Enhancing Stereocomplexation Ability of Polylactide by Coalescing from Its Inclusion Complex with Urea. Polymers. 2017; 9(11):592. https://doi.org/10.3390/polym9110592
Chicago/Turabian StyleLiu, Ping, Xiao-Tong Chen, and Hai-Mu Ye. 2017. "Enhancing Stereocomplexation Ability of Polylactide by Coalescing from Its Inclusion Complex with Urea" Polymers 9, no. 11: 592. https://doi.org/10.3390/polym9110592




