Surface Modification of Cardiovascular Stent Material 316L SS with Estradiol-Loaded Poly (trimethylene carbonate) Film for Better Biocompatibility
Abstract
:1. Introduction
2. Materials and Methods
2.1 Reagents and Materials
2.2 Preparation of PTMC-E5 Film
2.3 Surface Characterization of PTMC-E5 Film
2.4 Platelet Adhesion Test of PTMC-E5 Film
2.5 Smooth Muscle Cells Culture
2.6 Endothelial Cells Culture
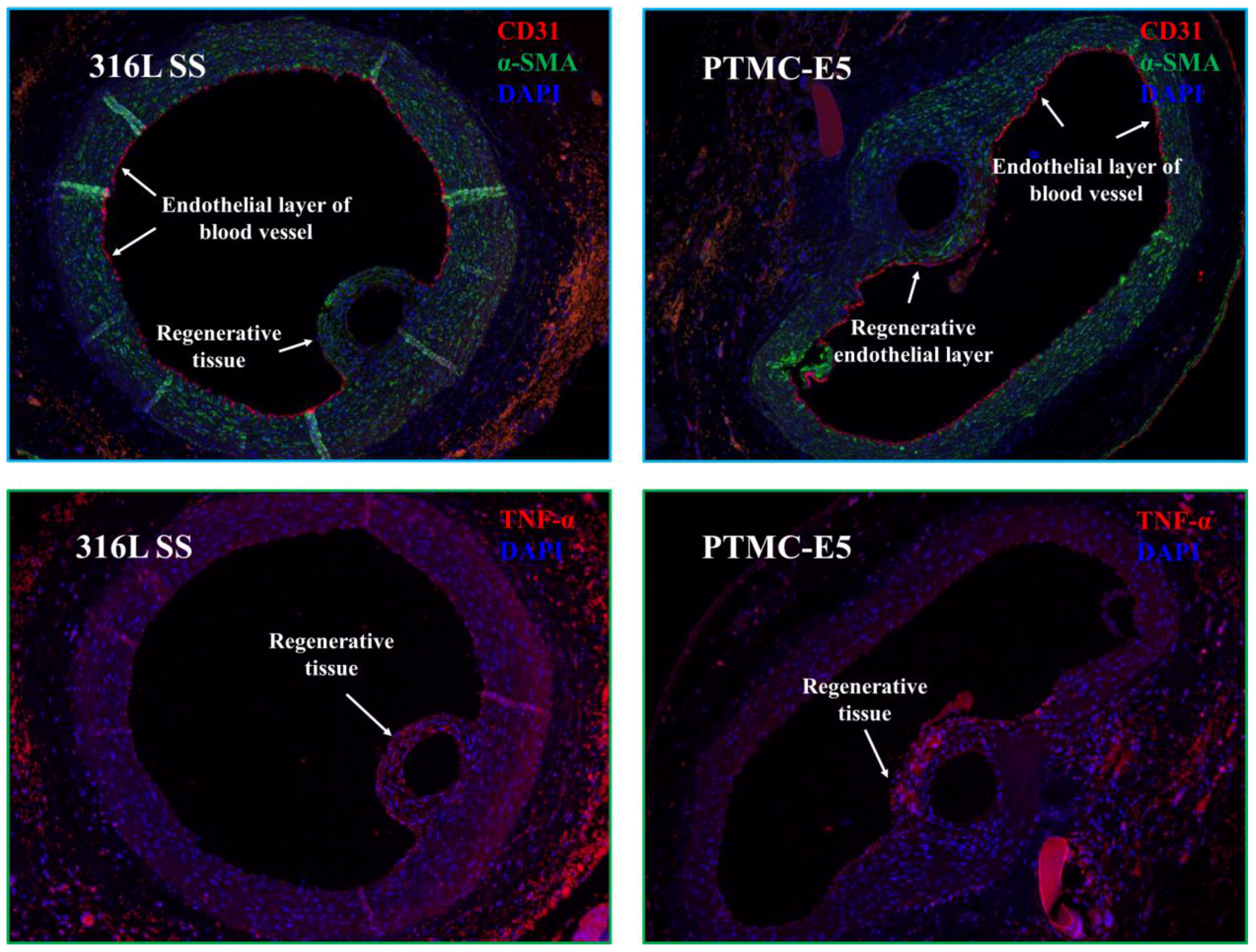
2.7 In Vivo Tissue-Response Test of PTMC-E5 Film
2.8 Statistical Analysis
3. Results and Discussion
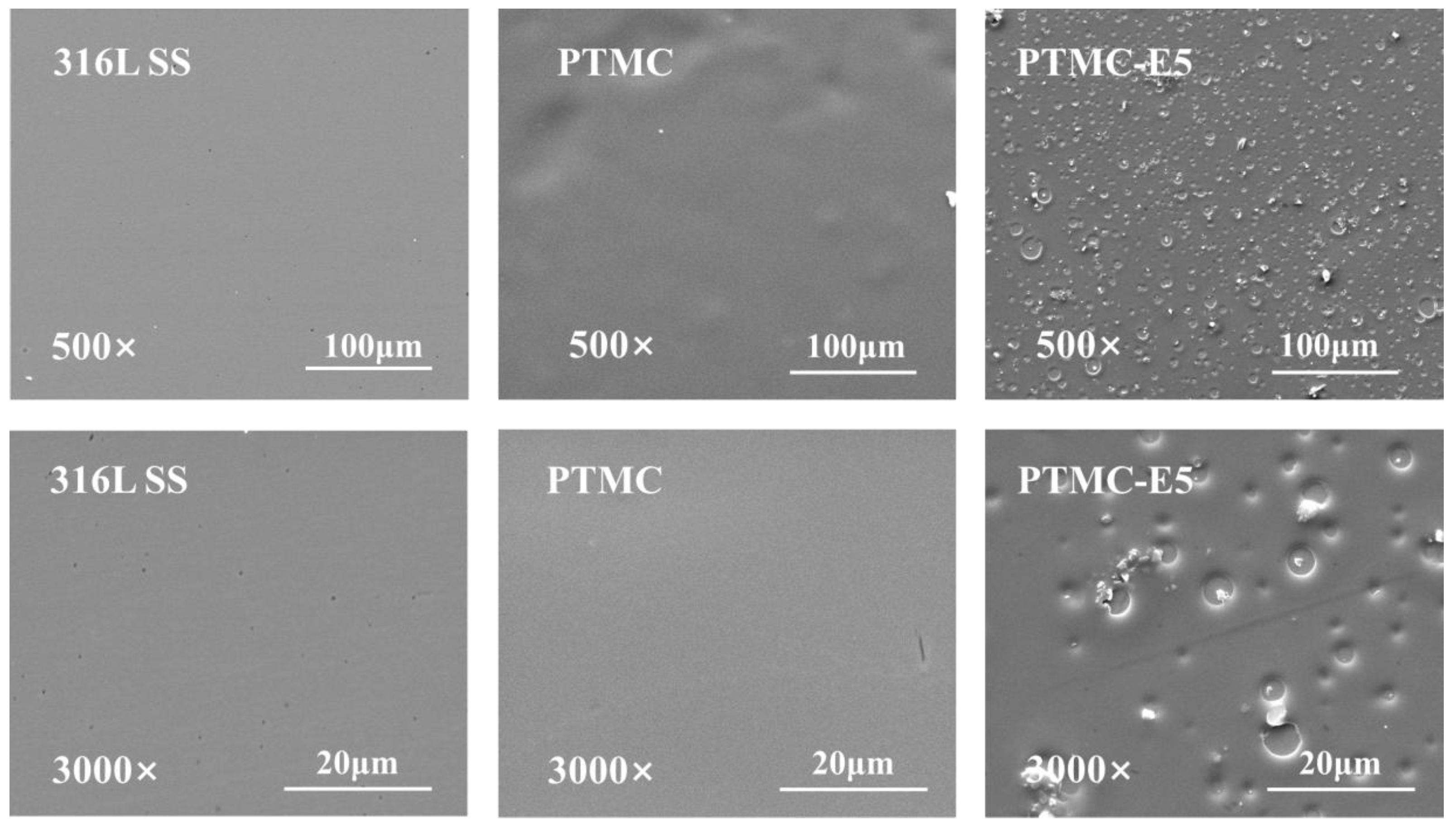
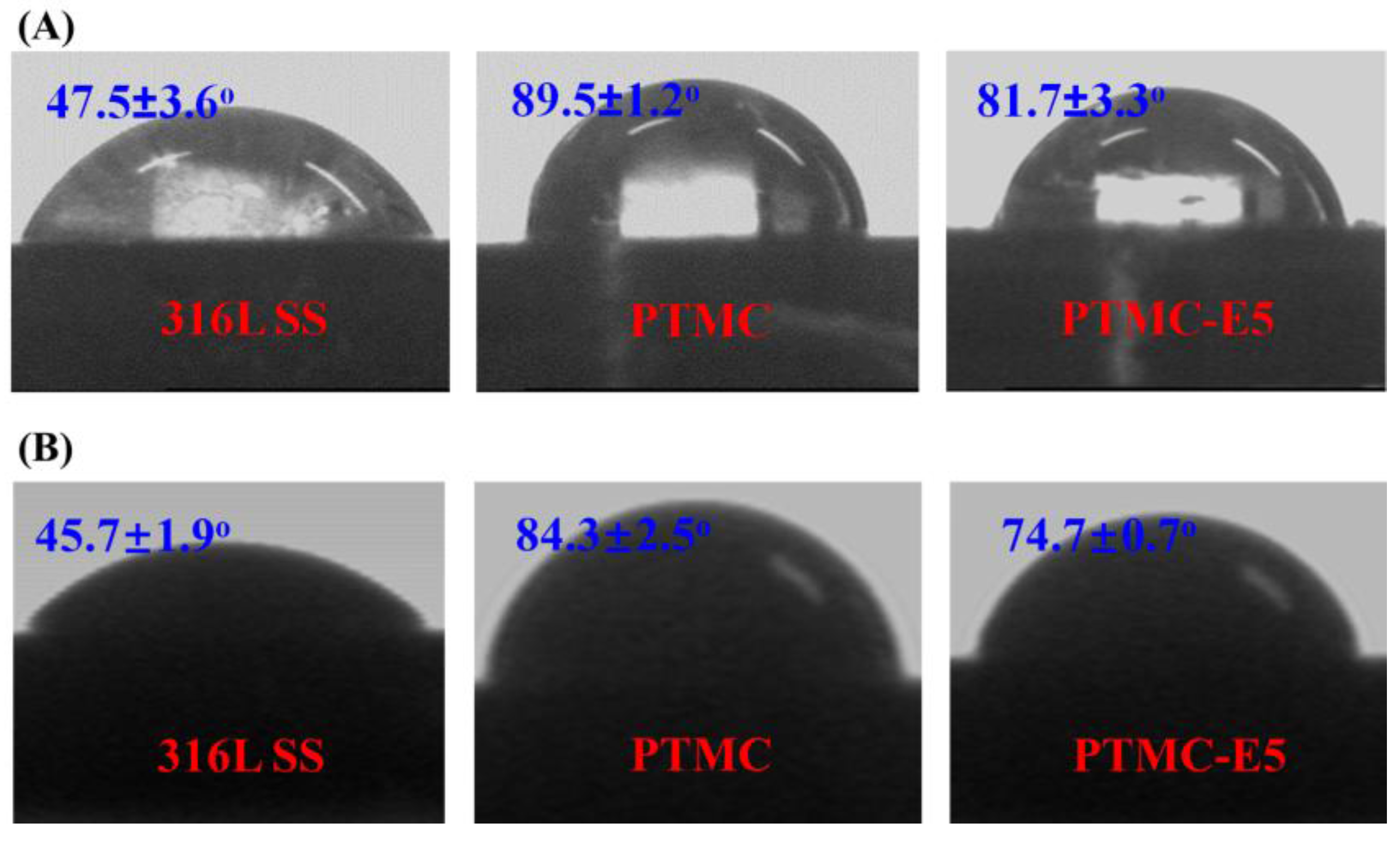
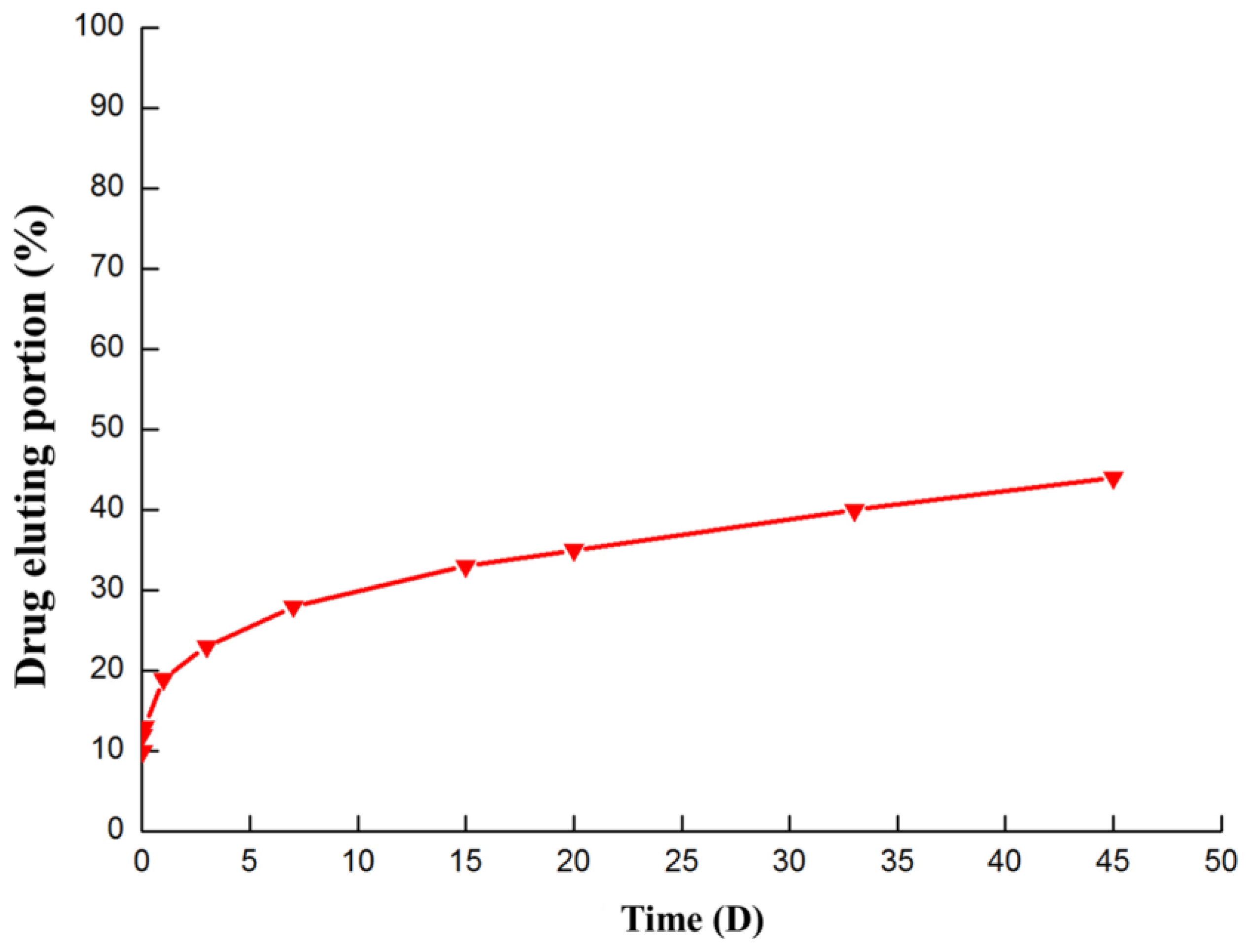
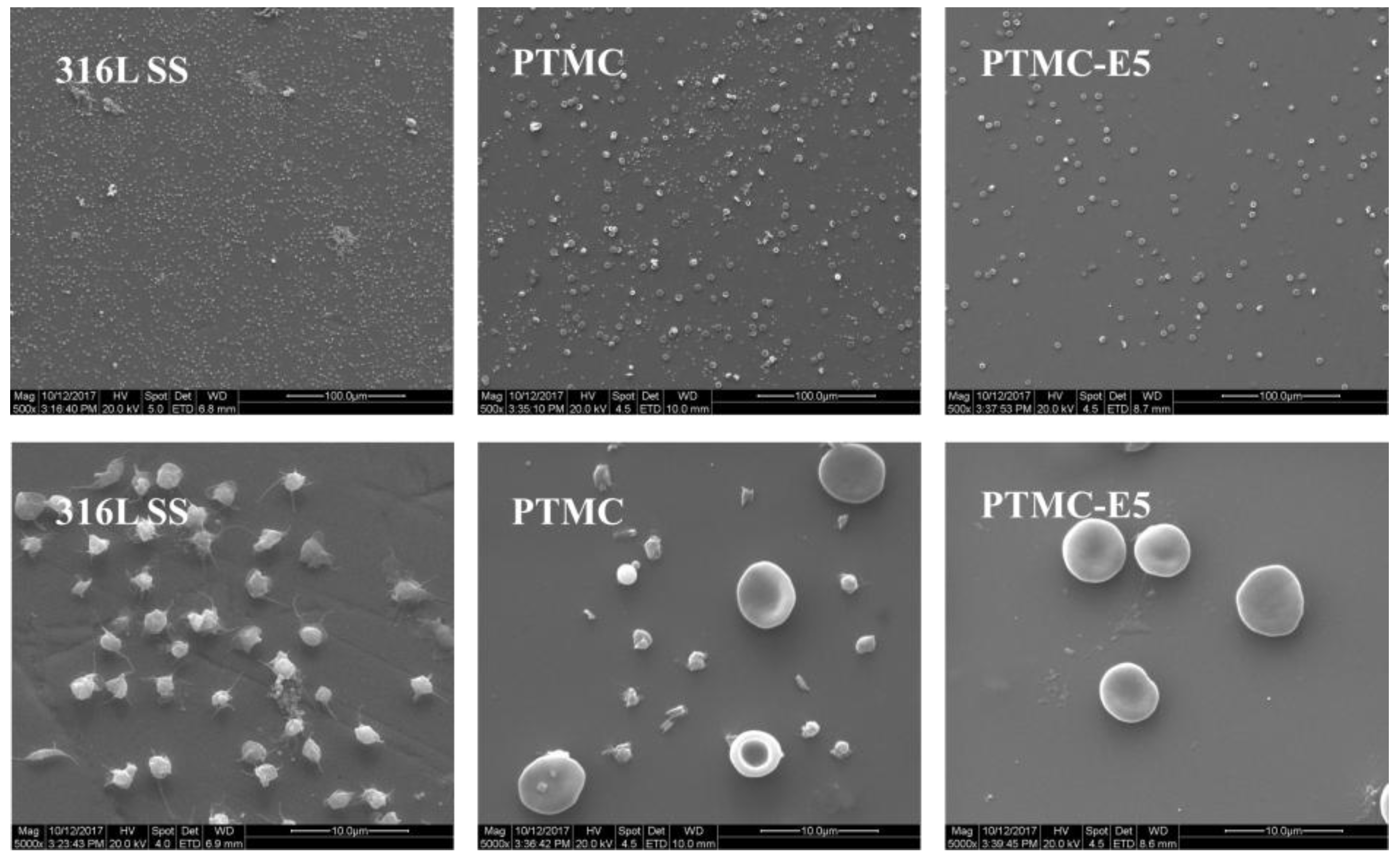
3.1. Surface Characterization
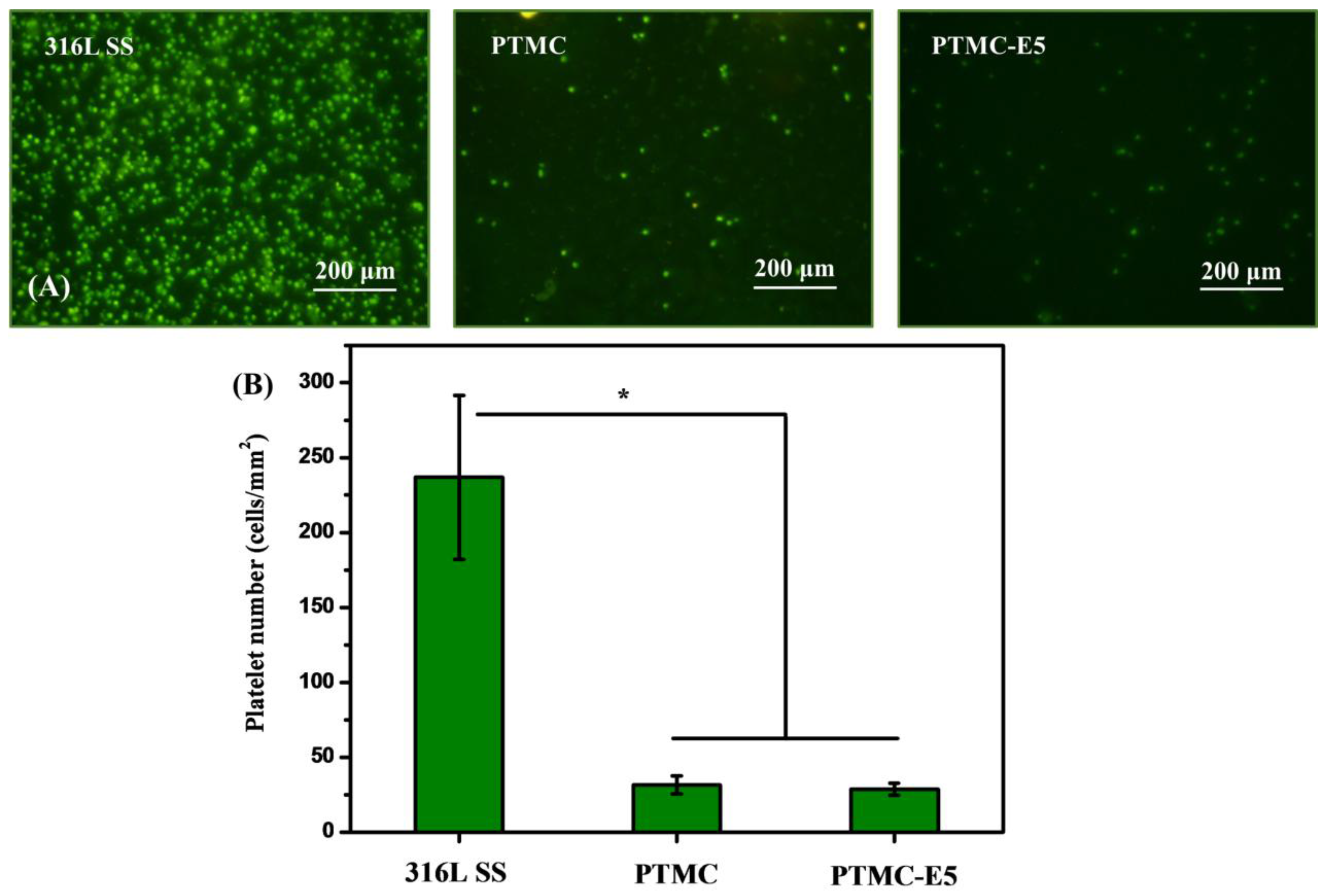
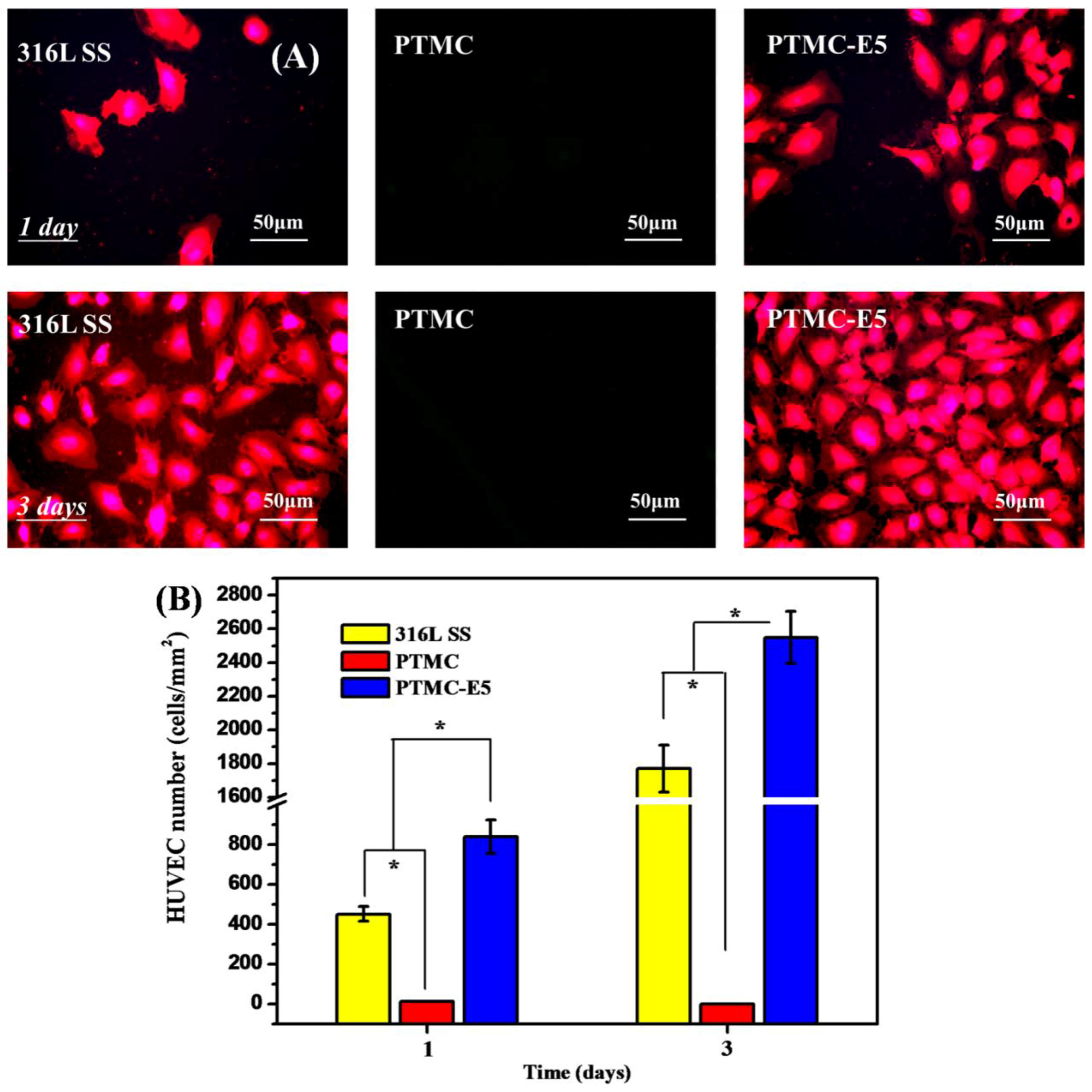
3.2. Biocompatibility of PTMC-E5 Film
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. Global Status Report on Noncommunicable Diseases 2014; World Health Organization: Geneva, Switzerland, 2014; Volume 1, p. 9. [Google Scholar]
- Fuster, V. Top 10 cardiovascular therapies and interventions for the next decade. Nat. Rev. Cardiol. 2014, 11, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.; Pendyala, L.K.; Ormiston, J.A.; Waksman, R. Review: Stent fracture in the drug-eluting stent era. Cardiovasc. Revasc. Med. 2016, 17, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, H.; Ruparelia, N.; Latib, A.; Miyazaki, T.; Sato, K.; Mangieri, A.; Contri, R.; Stella, S.; Figini, F.; Chieffo, A.; et al. Drug-Coated Balloons Versus Second-Generation Drug-Eluting Stents for the Management of Recurrent Multimetal-Layered In-Stent Restenosis. JACC Cardiovasc. Interv. 2015, 8, 1586–1594. [Google Scholar] [CrossRef] [PubMed]
- Rittger, H.; Waliszewski, M.; Brachmann, J.; Hohenforst-Schmidt, W.; Ohlow, M.; Brugger, A.; Thiele, H.; Birkemeyer, R.; Kurowski, V.; Schlundt, C.; et al. Long-Term Outcomes after Treatment with a Paclitaxel-Coated Balloon Versus Balloon Angioplasty: Insights from the PEPCAD-DES Study (Treatment of Drug-eluting Stent [DES] in-Stent Restenosis with SeQuent Please Paclitaxel-Coated Percutaneous Transluminal Coronary Angioplasty [PTCA] Catheter). JACC Cardiovasc. Interv. 2015, 8, 1695–1700. [Google Scholar] [PubMed]
- Li, J.A.; Zhang, K.; Yang, P.; Liao, Y.Z.; Wu, L.L.; Chen, J.L.; Zhao, A.S.; Li, G.C.; Huang, N. Research of smooth muscle cells response to fluid flow shear stress by hyaluronic acid micro-pattern on a titanium surface. Exp. Cell Res. 2013, 319, 2663–2672. [Google Scholar] [CrossRef] [PubMed]
- Li, J.A.; Zhang, K.; Wu, F.; He, Z.K.; Yang, P.; Huang, N. Constructing bio-functional layers of hyaluronan and type IV collagen on titanium surface for improving endothelialization. J. Mater. Sci. 2015, 50, 3226–3236. [Google Scholar] [CrossRef]
- Chen, J.L.; Li, Q.L.; Li, J.; Maitz, F.M. The effect of anti-CD34 antibody orientation control on endothelial progenitor cell capturing cardiovascular devices. J. Bioact. Compat. Pol. 2016, 31, 583–599. [Google Scholar] [CrossRef]
- Ozaki, H.; Narita, T.; Koga, T.; Indei, T. Theoretical Analysis of Critical Flowable Physical Gel Cross-Linked by Metal Ions and Polyacrylamide-Derivative Associating Polymers Containing Imidazole Groups. Polymers 2017, 9, 256. [Google Scholar] [CrossRef]
- Wu, F.; Li, J.A.; Zhang, K.; He, Z.K.; Yang, P.; Zou, D.; Huang, N. Multi-Functional Coating Based on Hyaluronic Acid and Dopamine Conjugate for Potential Application on Surface Modification of Cardiovascular Implanted Devices. ACS Appl. Mater. Interfaces 2016, 8, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Bai, Y.X.; Wang, X.F.; Li, Q.; Guan, F.X.; Li, J.A. Surface modification of esophageal stent materials by a polyethylenimine layer aiming at anti-cancer function. J. Mater. Sci. Mater. Med. 2017, 28, 125. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.W.; Yu, J.; Cao, J.; Gao, F.; Zhu, Y.Q.; Cheng, Y.S.; Cui, W.G. Fabrication of a Delaying Biodegradable Magnesium Alloy-Based Esophageal Stent via Coating Elastic Polymer. Materials 2016, 9, 384. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.Q.; Li, J.X.; Li, M.; Gu, Z.W. The in Vitro and in Vivo Degradation of Cross-Linked Poly(trimethylene carbonate)-Based Networks. Polymers 2016, 8, 151. [Google Scholar] [CrossRef]
- Sayyar, S.; Bjorninen, M.; Haimi, S.; Miettinen, S.; Gilmore, K.; Grijpma, D.; Wallace, G. UV Cross-Linkable Graphene/Poly(trimethylene Carbonate) Composites for 3D Printing of Electrically Conductive Scaffolds. ACS Appl. Mater. Interfaces 2016, 8, 31916–31925. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Thouas, G.A.; Chen, Q.Z. Biodegradable soft elastomers: Synthesis/properties of materials and fabrication of scaffolds. RSC Adv. 2012, 2, 8229–8242. [Google Scholar] [CrossRef]
- He, Y.H.; Wang, J.; Yan, W.; Huang, N. Gallic acid and gallic acid-loaded coating involved in selective regulation of platelet, endothelial and smooth muscle cell fate. RSC Adv. 2014, 4, 212–221. [Google Scholar] [CrossRef]
- York-Duran, M.J.; Godoy-Gallardo, M.; Labay, C.; Urquhart, A.J.; Andresen, T.L.; Hosta-Rigau, L. Recent advances in compartmentalized synthetic architectures as drug carriers, cell mimics and artificial organelles. Colloids Surf. B 2017, 152, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.X.; Wu, L.G.; Wang, J.; Huang, N. Investigation on biological properties of tacrolimus-loaded poly(1,3-trimethylene carbonate) in vitro. Appl. Surf. Sci. 2010, 256, 5000–5005. [Google Scholar] [CrossRef]
- Shetkar, S.S.; Parakh, N.; Singh, B.; Mishra, N.K.; Ray, R.; Karthikeyan, G.; Yadav, R.; Goswami, K.C. Cardio-embolic stroke due to valve tissue embolization during Percutaneous Transseptal Mitral Commissurotomy (PTMC). Indian Heart J. 2014, 66, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.F.; Zhang, X.Z.; Wu, K.K.; Liu, X.Y.; Jiao, Y.P.; Zhou, C.R. Improving cytoactive of endothelial cell by introducing fibronectin to the surface of poly L-Lactic acid fiber mats via dopamine. Mater. Sci. Eng. C 2016, 69, 373–379. [Google Scholar] [CrossRef] [PubMed]
- DeHahn, K.C.; Gonzales, M.; Gonzalez, A.M.; Hopkinson, S.B.; Chandel, N.S.; Brunelle, J.K.; Jones, J.C.R. The α4 laminin subunit regulates endothelial cell survival. Exp. Cell Res. 2004, 294, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Li, J.A.; Zhang, K.; Ma, W.Y.; Wu, F.; Yang, P.; He, Z.K.; Huang, N. Investigation of enhanced hemocompatibility and tissue compatibility associated with multi-functional coating based on hyaluronic acid and type IV collagen. Regener. Biomater. 2016, 3, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, J.A.; Wang, J.; Liu, T.; Wang, X.; Chen, J.Y.; Huang, N.; Guan, F.X. Combined REDV Polypeptide and Heparin onto Titanium Surface for the Hemocompatibility and Selectively Endothelialization. J. Cell Sci. Ther. 2015, 6, 1. [Google Scholar]
- Su, H.; Xue, G.N.; Ye, C.R.; Wang, Y.; Zhao, A.S.; Huang, N.; Li, J.A. The effect of anti-CD133/fucoidan bio-coatings on hemocompatibility and EPC capture. J. Biomater. Sci. Polym. Ed. 2017, 28, 2066–2081. [Google Scholar] [CrossRef] [PubMed]
- Regal, P.; Blokland, M.H.; Fente, C.A.; Sterk, S.S.; Cepeda, A.; Ginkel, L.A. Evaluation of the Discriminative Potential of a Novel Biomarker for Estradiol Treatments in Bovine Animals. J. Agric. Food Chem. 2015, 63, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.L.; Roy, R. Cell Culture Systems for Studies of Bone and Tooth Mineralization. Chem. Rev. 2008, 108, 4716–4733. [Google Scholar] [CrossRef] [PubMed]
- Lange, A.; Paull, G.C.; Coe, T.S.; Katsu, Y.; Urushitani, H.; Iguchi, T.; Tyler, C.R. Sexual Reprogramming and Estrogenic Sensitization in Wild Fish Exposed to Ethinylestradiol. Environ. Sci. Technol. 2009, 43, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.D.; Wang, G.; He, X.J.; Wang, J.F.; Wu, R. 17β-Estradiol and progesterone decrease MDP induced NOD2 expression in bovine mammary epithelial cells. Vet. Immunol. Immunopathol. 2017, 188, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Chen, J.; Xiang, L.J.; Xu, Y.; Yang, P.; Li, J.A.; Wu, J.J.; Huang, N. Fabrication of 3D TiO2 micromesh on Silicon surface and its effects on platelet adhesion. Mater. Lett. 2014, 132, 149–152. [Google Scholar] [CrossRef]
- Li, J.A.; Li, G.C.; Zhang, K.; Liao, Y.Z.; Yang, P.; Maitz, M.F.; Huang, N. Co-culture of vascular endothelial cells and smooth muscle cells by hyaluronic acid micro-pattern on titanium surface. Appl. Surf. Sci. 2013, 273, 24–31. [Google Scholar] [CrossRef]
- Li, J.A.; Wu, F.; Zhang, K.; He, Z.K.; Zou, D.; Luo, X.; Fan, Y.H.; Yang, P.; Zhao, A.S.; Huang, N. Controlling Molecular Weight of Hyaluronic Acid Conjugated on Amine-rich Surface: Towards Better Multifunctional Biomaterials for Cardiovascular Implants. ACS Appl. Mater. Interfaces 2017, 9, 30343–30358. [Google Scholar] [CrossRef] [PubMed]
- Han, C.Z.; Li, J.A.; Zou, D.; Luo, X.; Yang, P.; Zhao, A.S.; Huang, N. Mechanical Property of TiO2 Micro/nano Surfaces based on the Investigation of Residual Stress, Tensile Force and Fluid Flow Shear Stress: For Potential Application of Cardiovascular Devices. J. Nano Res. 2017, 49, 190–201. [Google Scholar] [CrossRef]
- Xu, Y.; Li, J.A.; Yao, L.F.; Li, L.H.; Yang, P.; Huang, N. Preparation and characterization of Cu-doped TiO2 thin films and effects on platelet adhesion. Surf. Coat. Technol. 2015, 261, 436–441. [Google Scholar] [CrossRef]
- Wu, J.J.; Li, J.A.; Wu, F.; He, Z.K.; Yang, P.; Huang, N. Effect of micropatterned TiO2 nanotubes thin film on the deposition of endothelial extracellular matrix: For the purpose of enhancing surface biocompatibility. Biointerphases 2015, 10, 04A302. [Google Scholar] [CrossRef] [PubMed]
- Li, J.A.; Zhang, K.; Xu, Y.; Chen, J.; Yang, P.; Zhao, Y.C.; Zhao, A.S.; Huang, N. A novel co-culture models of human vascular endothelial cells and smooth muscle cells by hyaluronic acid micro-pattern on titanium surface. J. Biomed. Mater. Res. A 2014, 102A, 1950–1960. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Xu, Y.; Zhou, Z.; Chen, J.; Yang, P.; Yang, Y.H.; Li, J.A.; Huang, N. The effects of Cu-doped TiO2 thin films on hyperplasia, inflammation and bacteria infection. Appl. Sci. Basel 2015, 5, 1016–1032. [Google Scholar] [CrossRef]
- Li, J.A.; Zhang, K.; Yang, P.; Qin, W.; Li, G.C.; Zhao, A.S.; Huang, N. Human vascular endothelial cell morphology and functional cytokine secretion influenced by different size of HA micro-pattern on titanium substrate. Colloids Surf. B Biointerfaces 2013, 110, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Li, J.A.; Qin, W.; Zhang, K.; Wu, F.; Yang, P.; He, Z.K.; Zhao, A.S.; Huang, N. Controlling Mesenchymal Stem Cells Differentiate into Contractile Smooth Muscle Cells on a TiO2 Micro/nano Interface: Towards Benign Pericytes Environment for Endothelialization. Colloids Surf. B Biointerfaces 2016, 145, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.J.; Li, J.A.; He, Z.K.; Wu, J.J.; Yang, P.; Huang, N. Design and construction of TiO2 nanotubes in microarray using two-step anodic oxidation for application of cardiovascular implanted devices. Micro Nano Lett. 2015, 10, 287–291. [Google Scholar] [CrossRef]
- Li, J.A.; Zhang, K.; Wu, J.J.; Zhang, L.J.; Yang, P.; Tu, Q.F.; Huang, N. Tailoring of the titanium surface by preparing cardiovascular endothelial extracellular matrix layer on the hyaluronic acid micro-pattern for improving biocompatibility. Colloids Surf. B Biointerfaces 2015, 128, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.O.; Yang, L.; Deutsch, S.; Manning, K.B. Development of a platelet adhesion transport equation for a computational thrombosis model. J. Biomech. 2017, 40, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, F.; Finn, A.V.; Yazdani, S.K.; Nakano, M.; Kolodgie, F.D.; Virmani, R. The Importance of the Endothelium in Atherothrombosis and Coronary Stenting. Nat. Rev. Cardiol. 2012, 9, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Li, J.A.; Zhang, K.; Chen, H.Q.; Liu, T.; Yang, P.; Zhao, Y.C.; Huang, N. A novel coating of type IV collagen and hyaluronic acid on stent material-titanium for promoting smooth muscle cells contractile phenotype. Mater. Sci. Eng. C 2014, 38, 235–243. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, H.; Li, J.; Li, N.; Wang, K.; Li, X.; Wang, J. Surface Modification of Cardiovascular Stent Material 316L SS with Estradiol-Loaded Poly (trimethylene carbonate) Film for Better Biocompatibility. Polymers 2017, 9, 598. https://doi.org/10.3390/polym9110598
Yao H, Li J, Li N, Wang K, Li X, Wang J. Surface Modification of Cardiovascular Stent Material 316L SS with Estradiol-Loaded Poly (trimethylene carbonate) Film for Better Biocompatibility. Polymers. 2017; 9(11):598. https://doi.org/10.3390/polym9110598
Chicago/Turabian StyleYao, Hang, Jingan Li, Na Li, Kebing Wang, Xin Li, and Jin Wang. 2017. "Surface Modification of Cardiovascular Stent Material 316L SS with Estradiol-Loaded Poly (trimethylene carbonate) Film for Better Biocompatibility" Polymers 9, no. 11: 598. https://doi.org/10.3390/polym9110598




