Hygral Behavior of Superabsorbent Polymers with Various Particle Sizes and Cross-Linking Densities
Abstract
:1. Introduction
2. Materials and Methods
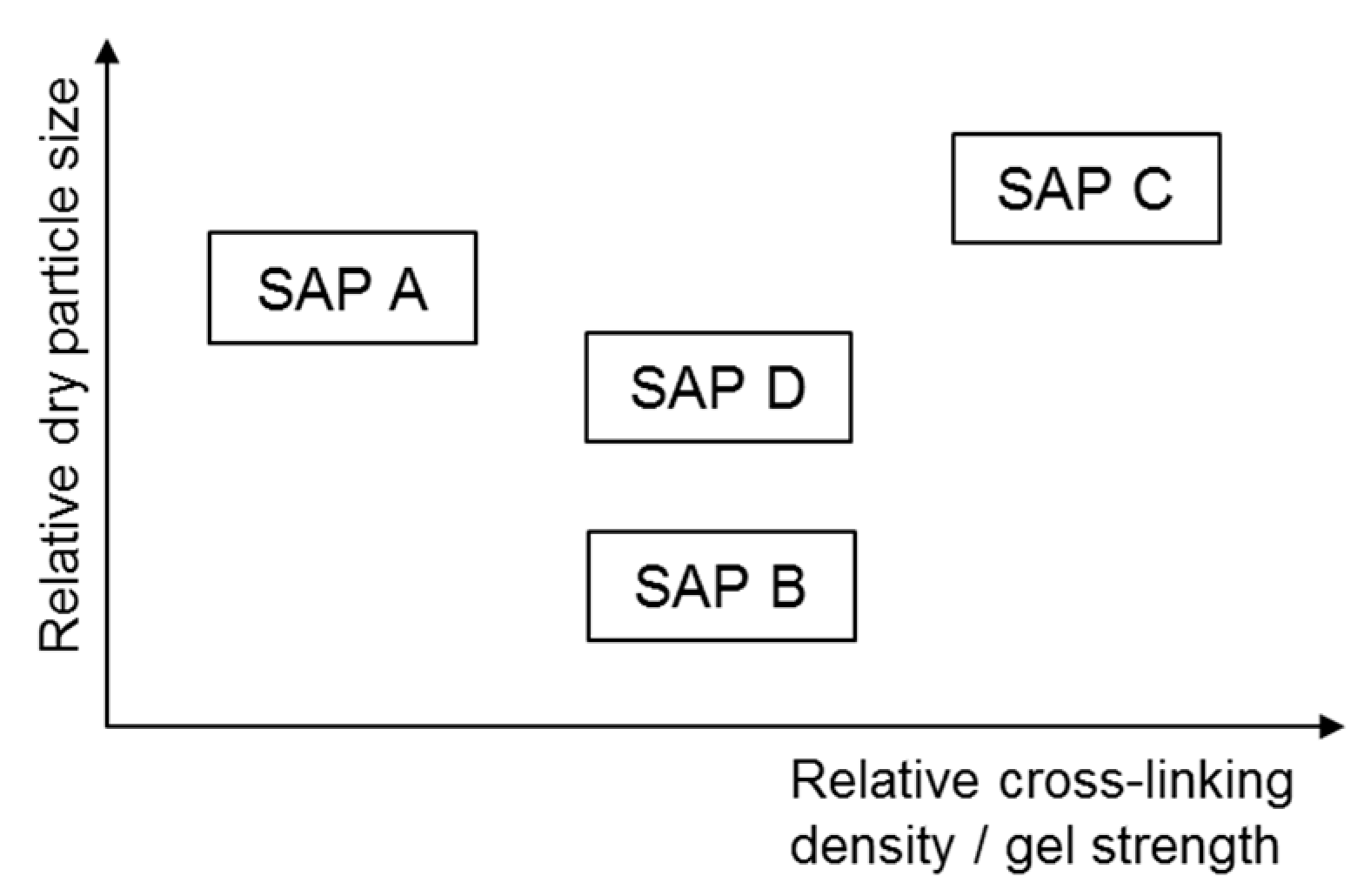
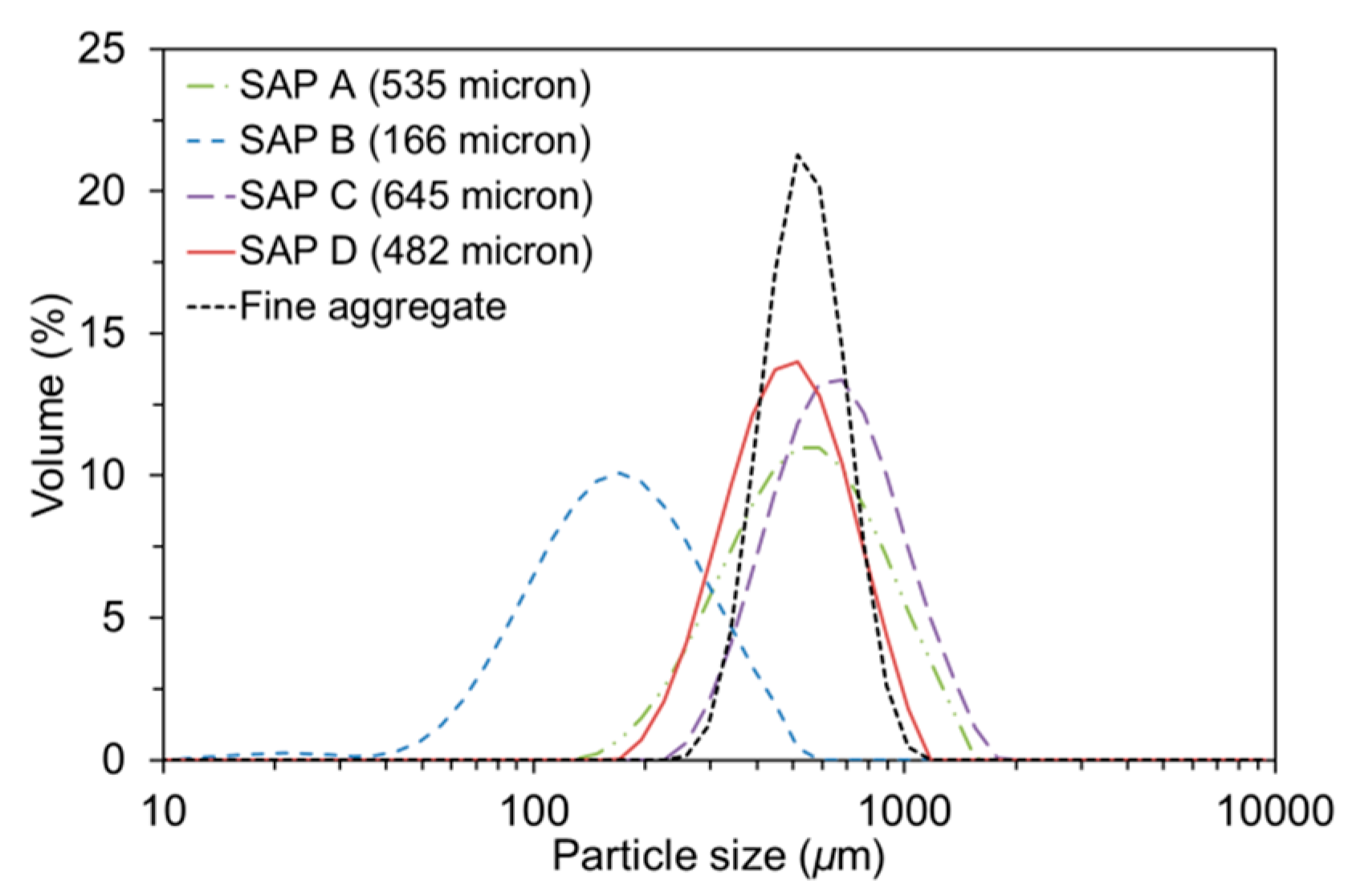
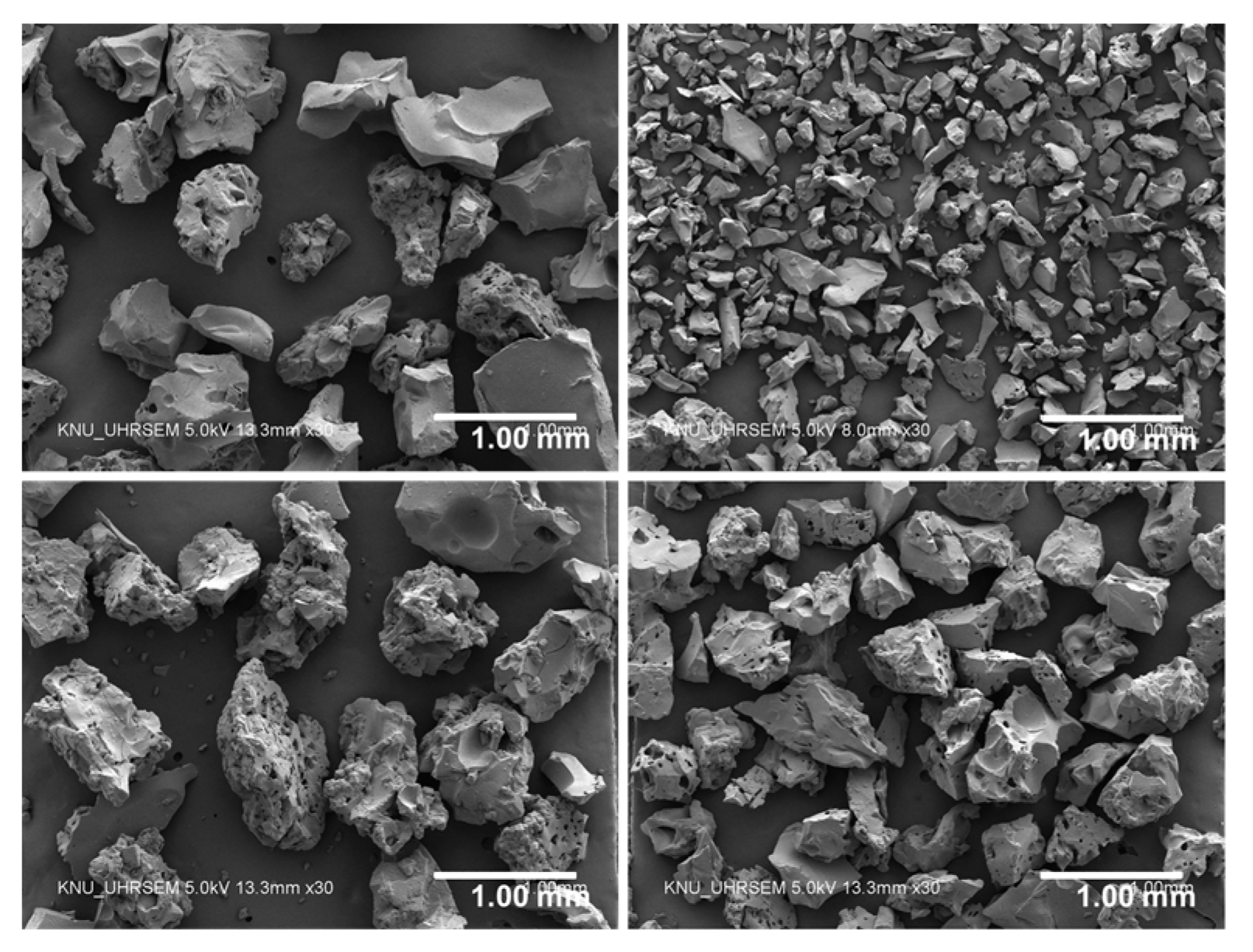
2.1. Materials
2.2. Methods
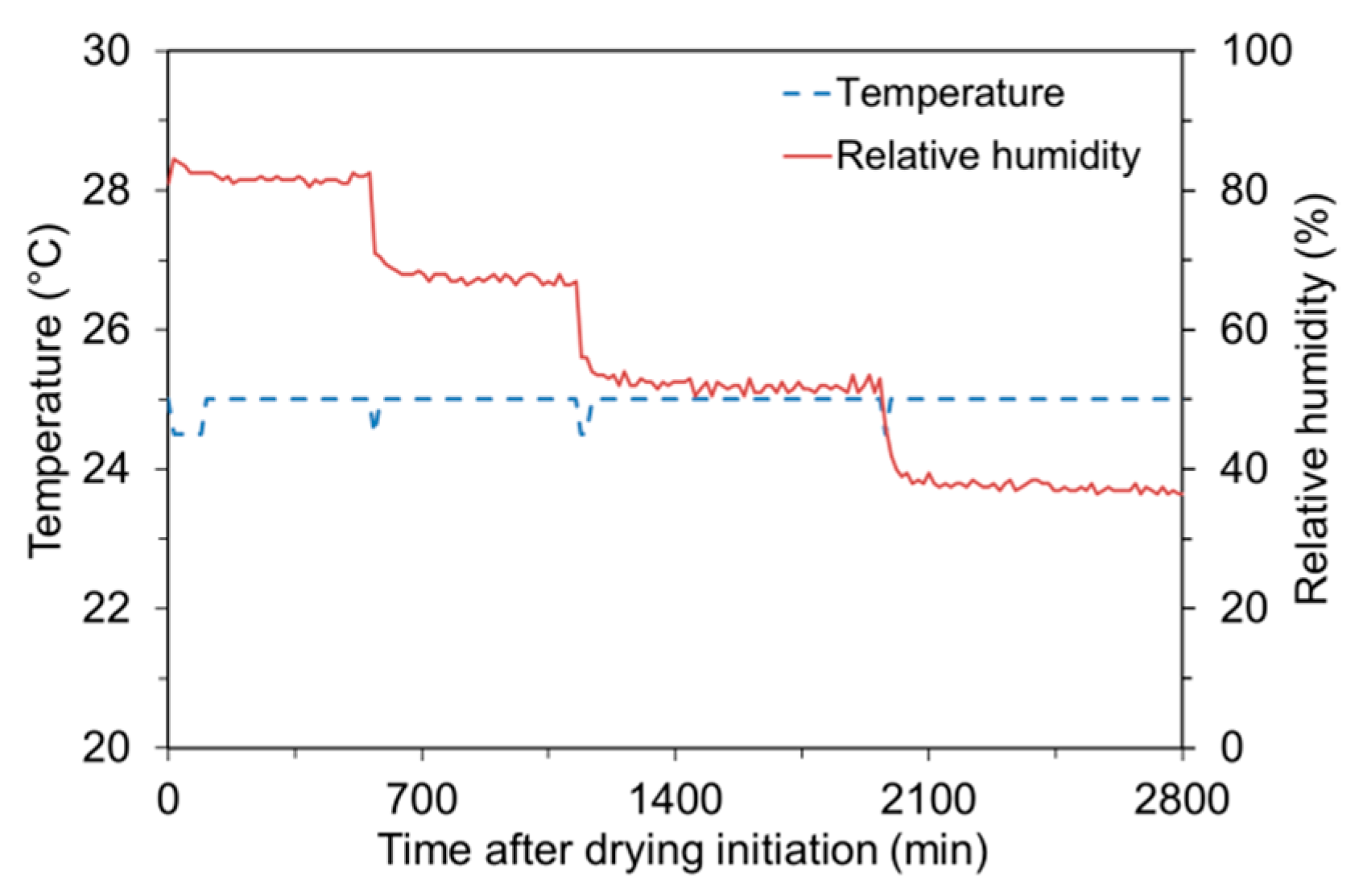
2.2.1. Absorption Test
2.2.2. Desorption Test
2.2.3. Sorption Isotherm Test
2.2.4. Absorption Test under Mixing Conditions
- Dry mix all the dry ingredients (cement, sand, and dry SAP) for 1 min with 140 rpm.
- Add water for 30 s with a mixing intensity of 140 rpm.
- Mix for 1 min with a mixing intensity of 285 rpm.
- Scrap mortar from the walls and manual mix for 1 min.
- Final mix for 1 min with a mixing intensity of 285 rpm.
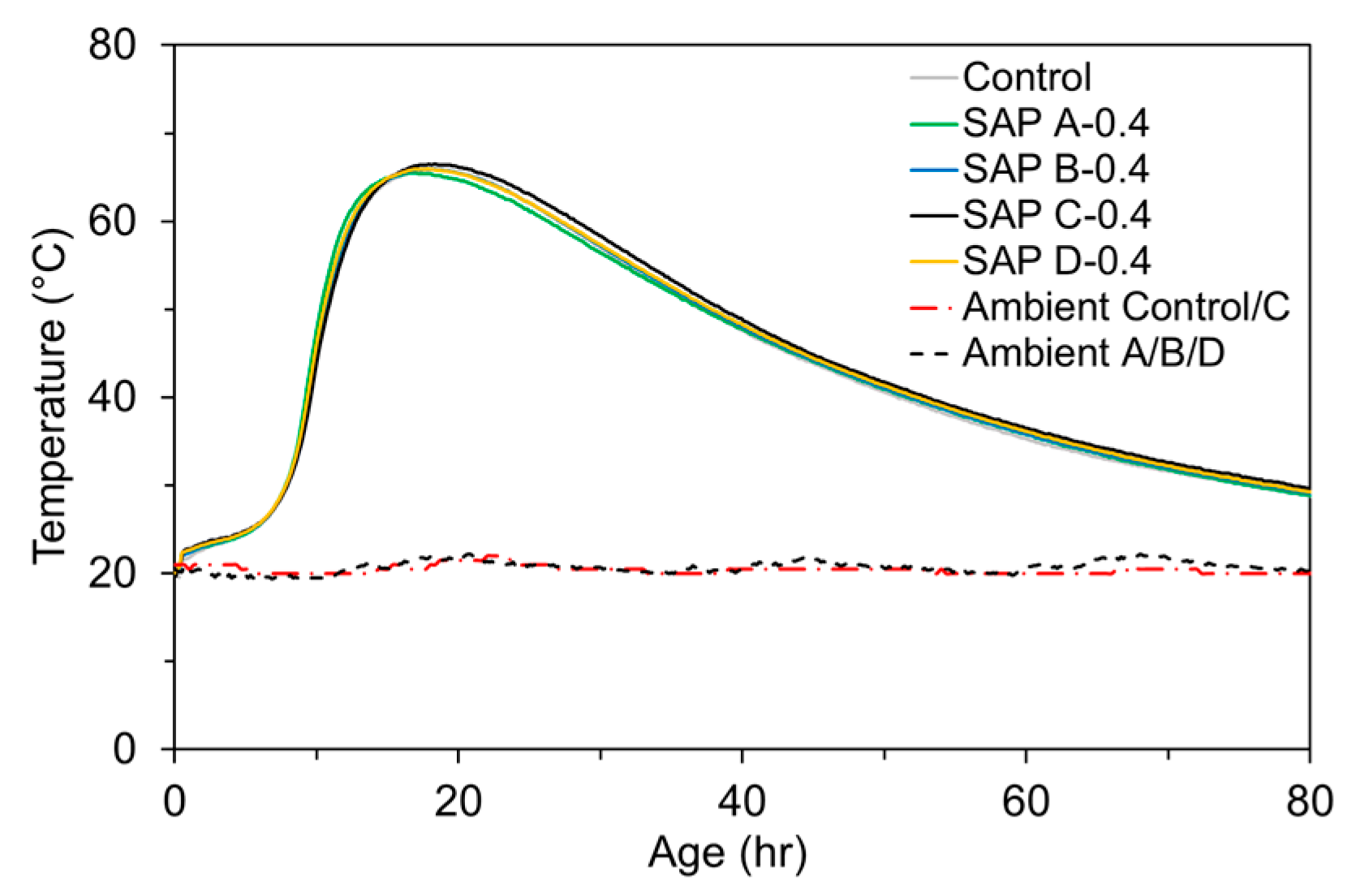
2.2.5. Semi-Adiabatic Temperature Rise Test
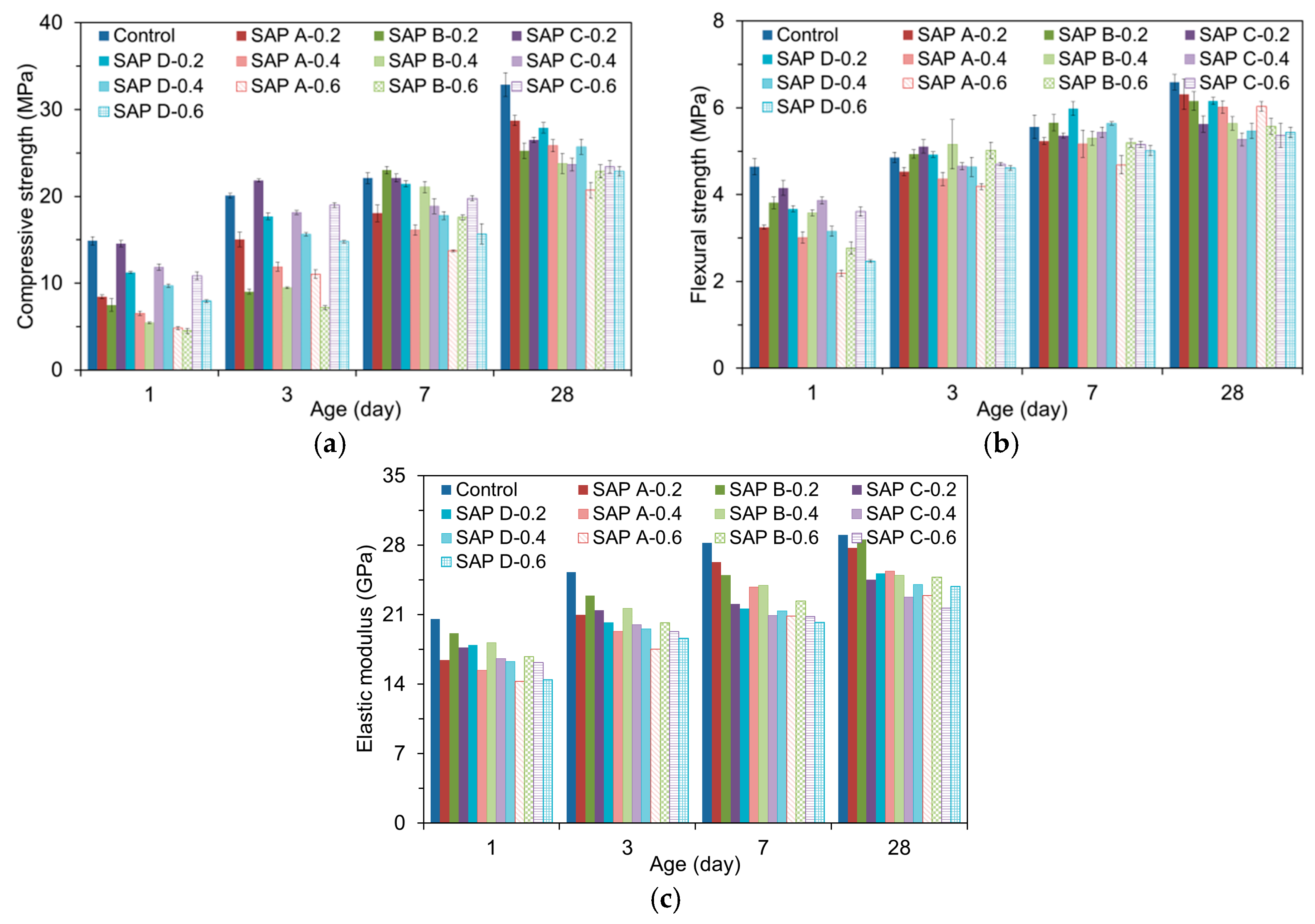
2.2.6. Flexural, Compressive Strength, and Elastic Modulus Tests
3. Results and Discussion
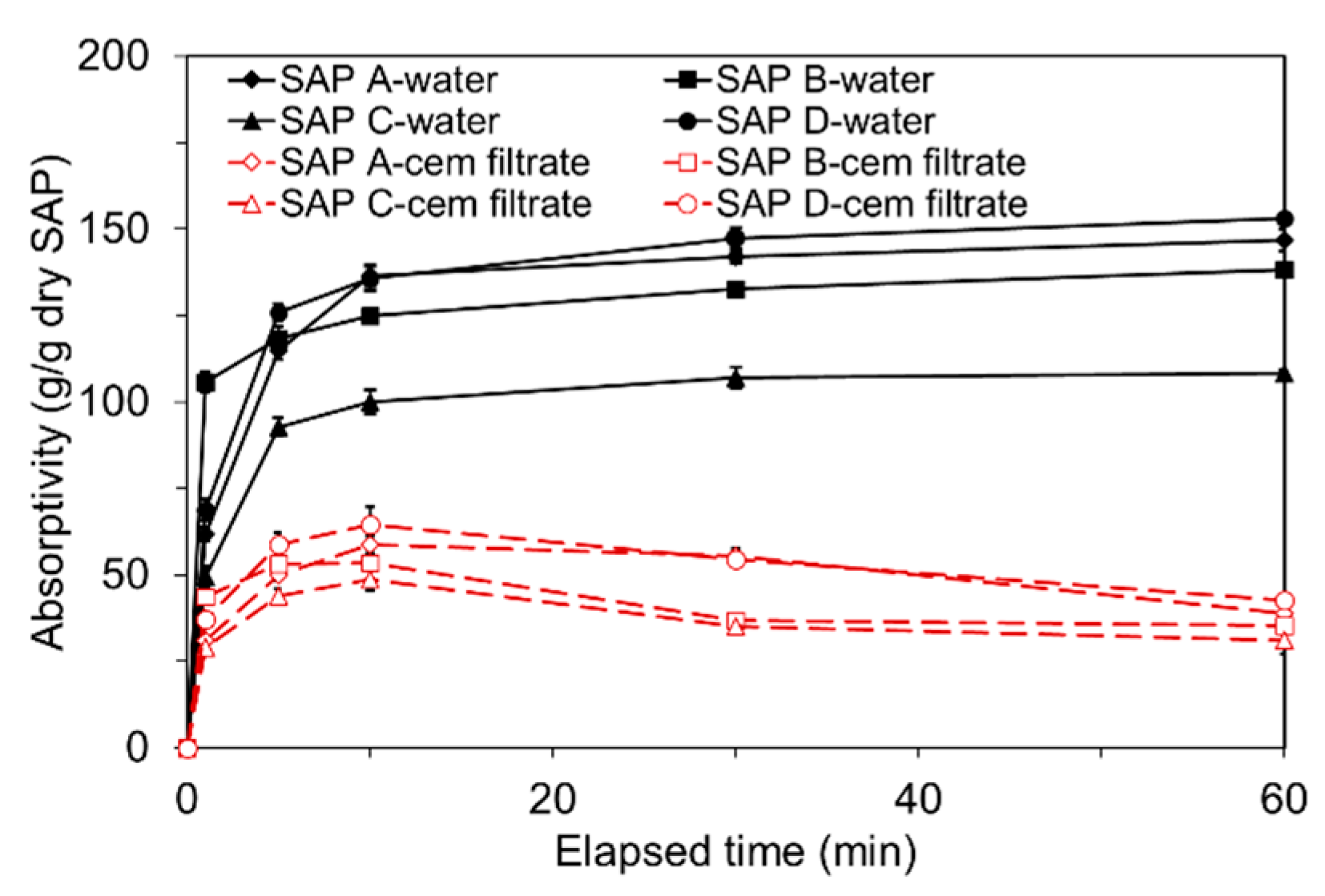
3.1. Absorption Kinetics
3.2. Desorption Kinetics
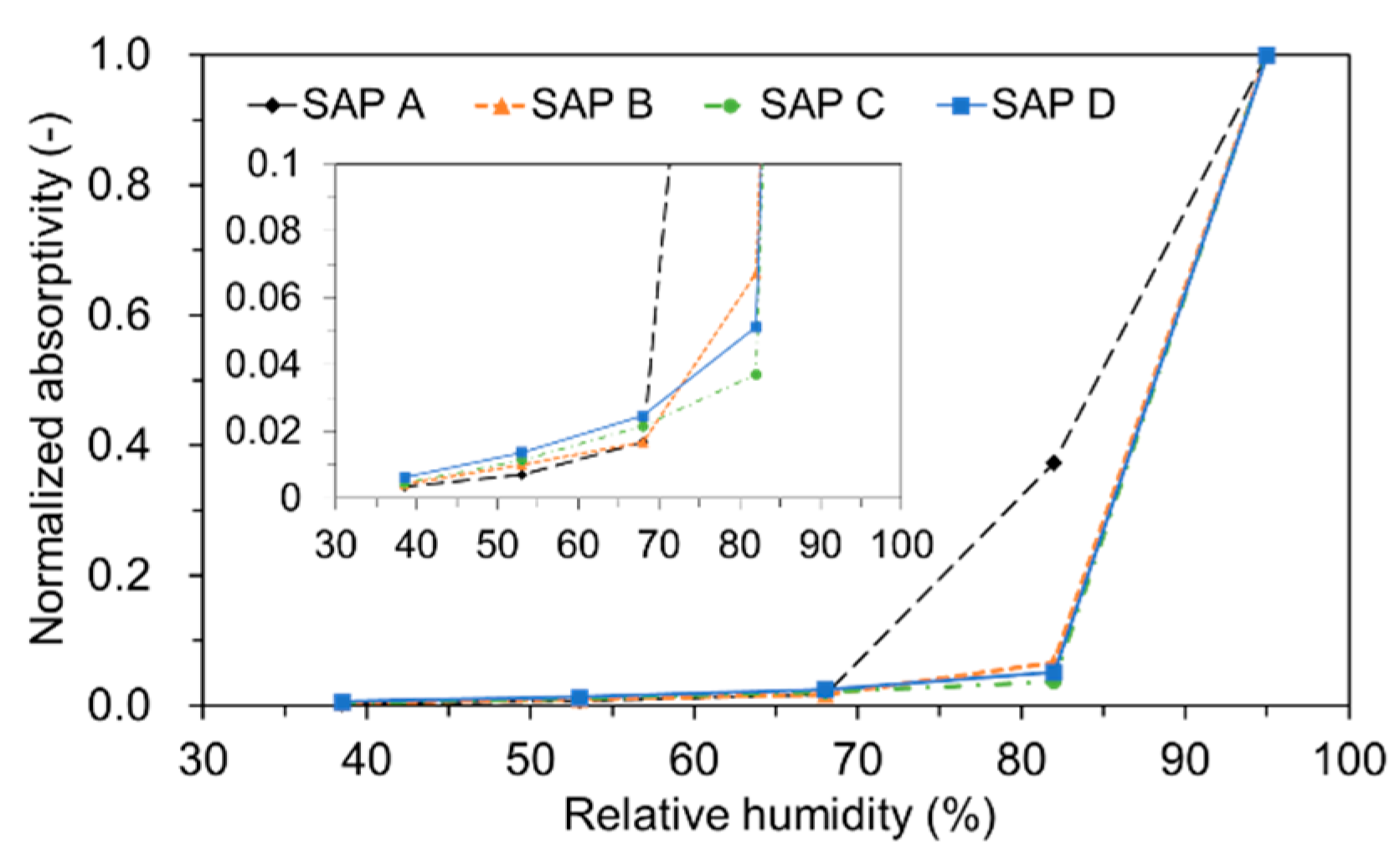
3.3. Sorption Isotherm
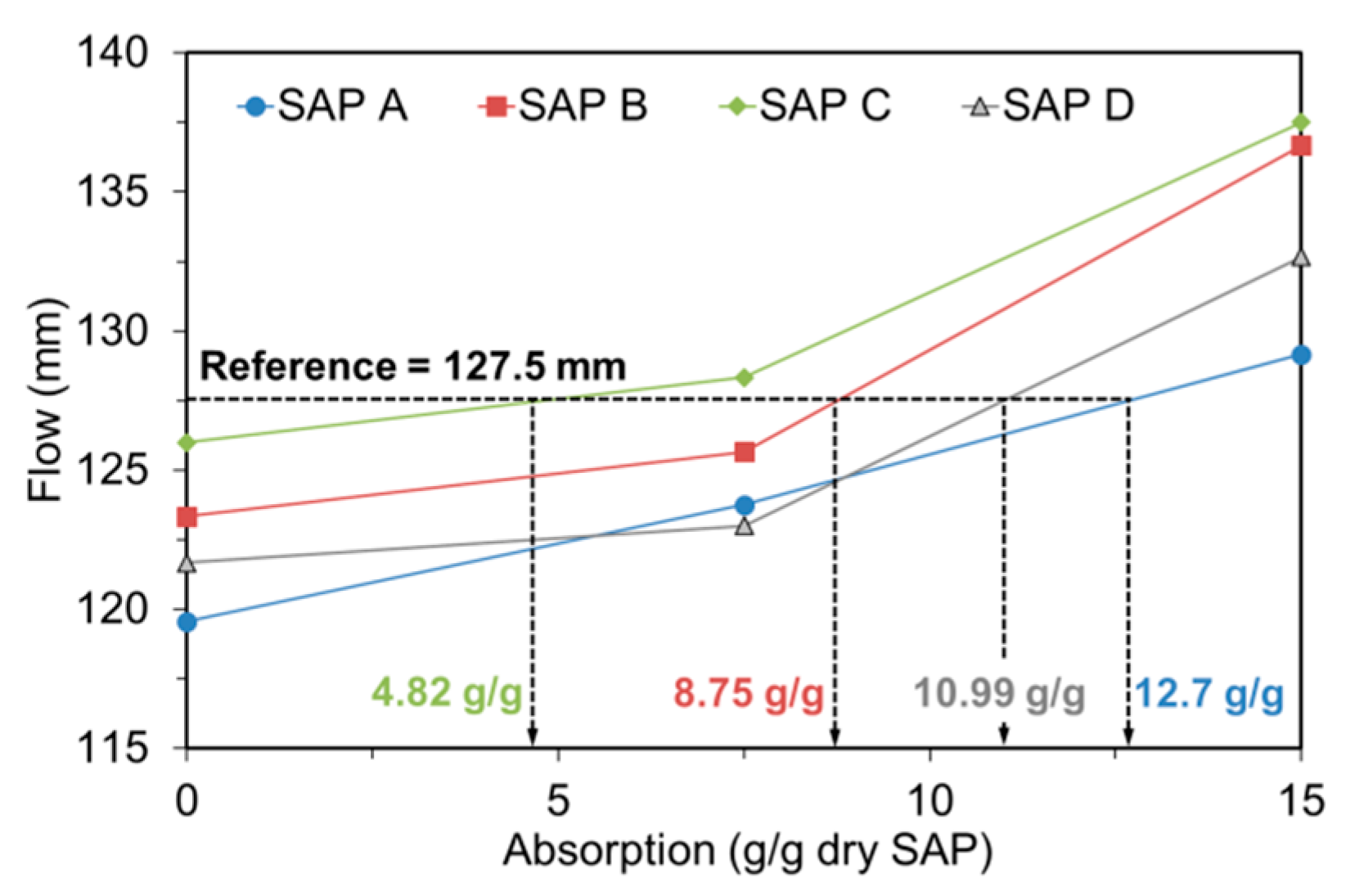
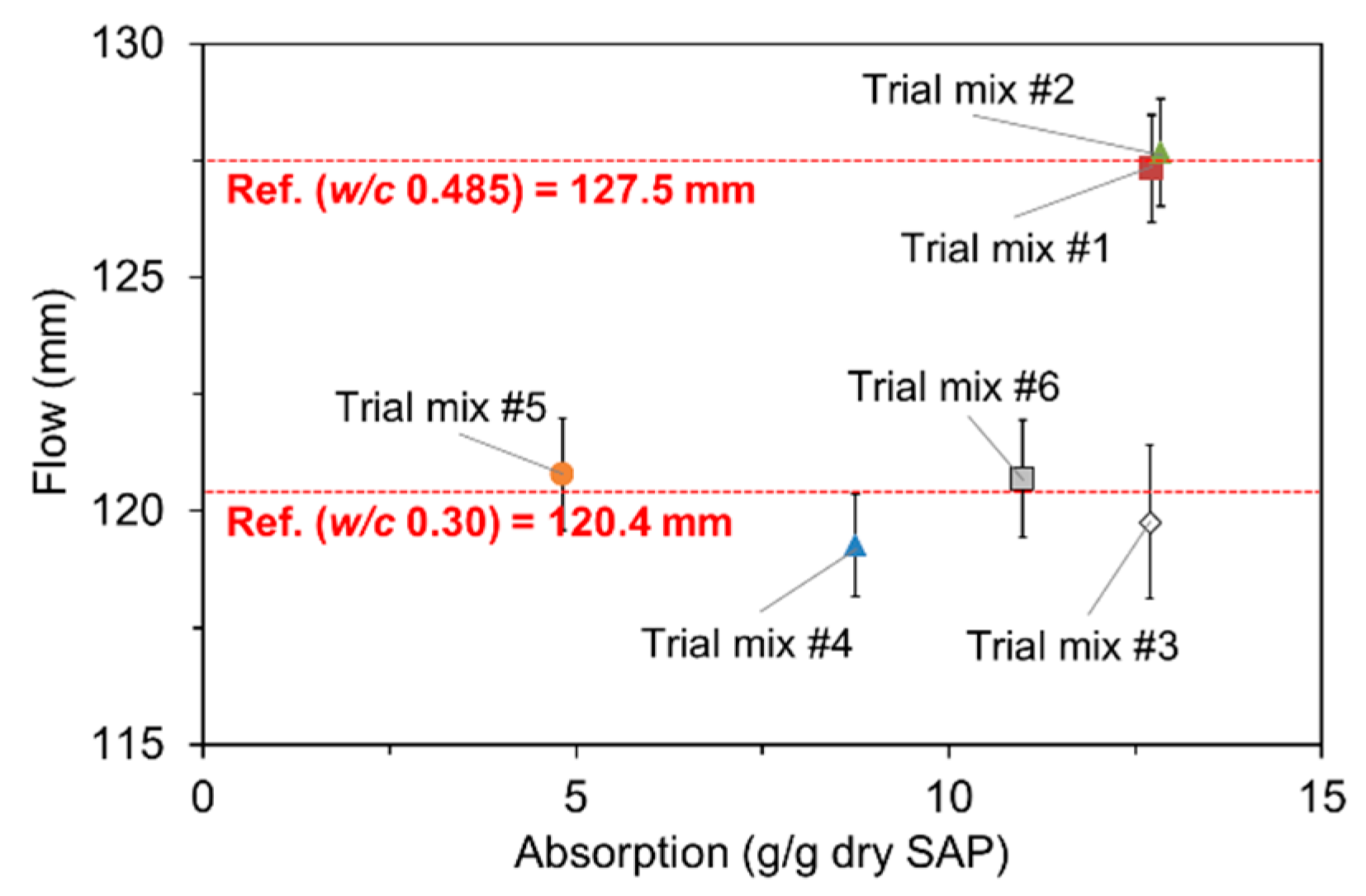
3.4. Absorption Capacity in Fresh Mortar
3.5. Semi-Adiabatic Temperature Rise
3.6. Mechanical Properties
4. Conclusions
- While the free absorptivity measured in distilled water reached a nearly constant level that measured in high-ionic cement filtrate gradually decreased due to the neutralization of SAP.
- The absorption capacity of SAP under both load-free and actual mixing conditions increased with decreased cross-linking density and increased particle size. The absorption rate was greater for finer SAP.
- The greater the cross-linking density of SAP, the shorter the desorption time. The rate of water release was higher for finer SAP due to weaker intermolecular attraction forces.
- The sorption isotherm showed that most of the absorbed water was released above 85 to 90% RH for all SAPs, demonstrating a potential benefit of SAPs in internal curing.
- The flow- and hydration heat-based methods were found to be feasible to evaluate the actual absorptivity of SAP under mixing conditions.
- SAP additions overall reduced the mechanical properties of mortar. While the flexural strength and elastic modulus were reduced by about 8 to 20% compared to the reference mixture, the compressive strength was reduced by about 18 to 36%. This indicates that the SAP additions more affected the compressive strength than flexural strength and elastic modulus.
- Whereas the early-age mechanical properties were more related with the gel strength of swollen SAP particulates, the later-age mechanical properties were more concerned with the moisture retention capacity and spatial distribution of SAP.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Snoeck, D.; Jensen, O.M.; De Belie, N. The influence of superabsorbent polymers on the autogenous shrinkage properties of cement pastes with supplementary cementitious materials. Cem. Concr. Res. 2015, 74, 59–67. [Google Scholar] [CrossRef]
- Ramazani-Harandi, M.J.; Zohuriaan-Mehr, M.J.; Yousefi, A.A.; Ershad-Langroudi, A.; Kabiri, K. Rheological determination of the swollen gel strength of superabsorbent polymer hydrogels. Polym. Test. 2006, 25, 470–474. [Google Scholar] [CrossRef]
- Wang, F.; Yang, J.; Cheng, H.; Wu, J.; Liang, X. Study on mechanism of desorption behavior of saturated superabsorbent polymers in concrete. ACI Mater. J. 2015, 112, 463–470. [Google Scholar] [CrossRef]
- Mignon, A.; Snoeck, D.; Dubruel, P.; Van Vlierberghe, S.; De Belie, N. Crack mitigation in concrete: Superabsorbent polymers as key to success? Materials 2017, 10, 237. [Google Scholar] [CrossRef] [PubMed]
- Jensen, O.M.; Hansen, P.F. Water-entrained cement-based materials I. Principles and theoretical background. Cem. Concr. Res. 2002, 31, 647–654. [Google Scholar] [CrossRef]
- Jensen, O.M.; Hansen, P.F. Water-entrained cement-based materials II. Experimental observations. Cem. Concr. Res. 2002, 32, 973–978. [Google Scholar] [CrossRef]
- Schröfl, C.; Mechtcherine, V.; Gorges, M. Relation between the molecular structure and the efficiency of superabsorbent polymers (SAP) as concrete admixture to mitigate autogenous shrinkage. Cem. Concr. Res. 2012, 42, 865–873. [Google Scholar] [CrossRef]
- Song, C.; Choi, Y.C.; Choi, S. Effect of internal curing by superabsorbent polymers–internal relative humidity and autogenous shrinkage of alkali-activated slag mortars. Constr. Build. Mater. 2016, 123, 198–206. [Google Scholar] [CrossRef]
- Azarijafari, H.; Kazemian, A.; Rahimi, M.; Yahia, A. Effects of pre-soaked super absorbent polymers on fresh and hardened properties of self-consolidating lightweight concrete. Constr. Build. Mater. 2016, 113, 215–220. [Google Scholar] [CrossRef]
- Shen, D.; Wang, X.; Cheng, D.; Zhang, J.; Jiang, G. Effect of internal curing with super absorbent polymers on autogenous shrinkage of concrete at early age. Constr. Build. Mater. 2016, 106, 512–522. [Google Scholar] [CrossRef]
- Wyrzykowski, M.; Hu, Z.; Ghourchian, S.; Scrivener, K.; Lura, P. Corrugated tube protocol for autogenous shrinkage measurements: Review and statistical assessment. Mater. Struct. 2017, 50, 57. [Google Scholar] [CrossRef]
- Lam, H.; Hooton, R.D. Effects of internal curing methods on restrained shrinkage and permeability. In Proceedings of 4th International Seminar on Self-Desiccation and Its Importance in Concrete Technology; Persson, B., Bentz, D., Nilsson, L.-O., Eds.; Lund University: Lund, Sweden, 2005; pp. 210–228. [Google Scholar]
- Lura, P.; Durand, F.; Loukili, A.; Kovler, K.; Jensen, O.M. Compressive strength of cement pastes and mortars with suberabsorbent polymers. In RILEM Proceedings of PRO 52, Volume Changes of Hardening Concrete: Testing and Mitigation; Jensen, O.M., Lura, P., Kovler, K., Eds.; RILEM Publications SARL: Bagneux, France, 2006; pp. 20–23. [Google Scholar]
- Esteves, L.P.; Cachim, P.; Ferreira, V.M. Mechanical properties of cement mortars with superabsorbent polymers. Adv. Constr. Mater. 2007, 451–462. [Google Scholar] [CrossRef]
- Snoeck, D.; Schaubroeck, D.; Dubruel, P.; De Belie, N. Effect of high amounts of superabsorbent polymers and additional water on the workability, microstructure and strength of mortars with a water-to-cement ratio of 0.50. Constr. Build. Mater. 2014, 72, 148–157. [Google Scholar] [CrossRef]
- Mechtcherine, V.; Secrieru, E.; Schröfl, C. Effect of superabsorbent polymers (SAPs) on rheological properties of fresh cement-based mortars—Development of yield stress and plastic viscosity over time. Cem. Concr. Res. 2015, 67, 52–65. [Google Scholar] [CrossRef]
- Secrieru, E.; Mechtcherine, V.; Schröfl, C.; Borin, D. Rheological characterisation and prediction of pumpability of strainhardening cement-based-composites (SHCC) with and without addition of superabsorbent polymers (SAP) at various temperatures. Constr. Build. Mater. 2016, 112, 581–594. [Google Scholar] [CrossRef]
- Bessaies-Bey, H.; Baumann, R.; Schmitz, M.; Radler, M.; Roussel, N. Effect of polyacrylamide on rheology of fresh cement pastes. Cem. Concr. Res. 2015, 76, 98–106. [Google Scholar] [CrossRef]
- Lee, H.X.D.; Wong, H.S.; Buenfeld, N. Potential of superabsorbent polymer for self-sealing cracks in concrete. Adv. Appl. Ceram. 2010, 109, 296–302. [Google Scholar] [CrossRef]
- Snoeck, D.; Steuperaert, S.; Van Tittelboom, K.; Dubruel, P.; De Belie, N. Visualization of water penetration in cementitious materials with superabsorbent polymers by means of neutron radiography. Cem. Concr. Res. 2012, 42, 1113–1121. [Google Scholar] [CrossRef]
- Lee, H.X.D.; Wong, H.S.; Buenfeld, N. Self-sealing of cracks in concrete using superabsorbent polymers. Cem. Concr. Res. 2016, 79, 194–208. [Google Scholar] [CrossRef]
- Hong, G.; Choi, S. Rapid self-sealing of cracks in cementitious materials incorporating superabsorbent polymers. Constr. Build. Mater. 2017, 143, 366–375. [Google Scholar] [CrossRef]
- Snoeck, D.; Van Tittelboom, K.; Steuperaert, S.; Dubruel, P.; De Belie, N. Self-healing cementitious materials by the combination of microfibres and superabsorbent polymers. J. Intell. Mater. Syst. Struct. 2014, 25, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Shen, D.; Shi, H.; Tang, X.; Ji, Y.; Jiang, G. Effect of internal curing with super absorbent polymers on residual stress development and stress relaxation in restrained concrete ring specimens. Constr. Build. Mater. 2016, 120, 309–320. [Google Scholar] [CrossRef]
- Assmann, A.; Reinhardt, H.W. Tensile creep and shrinkage of SAP modified concrete. Cem. Concr. Res. 2014, 58, 179–185. [Google Scholar] [CrossRef]
- Xin, J.; Huang, D.; Li, J.; Lin, C. Effect of SAP on the tensile creep of early-age concrete using ring specimens. Eur. J. Environ. Civ. Eng. 2016. [Google Scholar] [CrossRef]
- Wyrzykowski, M.; Lura, P. Controlling the coefficient of thermal expansion of cementitious materials—A new application for superabsorbent polymers. Cem. Concr. Compos. 2013, 35, 49–58. [Google Scholar] [CrossRef]
- Mönnig, S.; Lura, P. Superabsorbent Polymers—An Additive to Increase Freeze-Thaw Resistance of High Strength Concrete. In Advances in Construction Materials; Grosse, C.U., Ed.; Springer: Berlin, Germany, 2007; pp. 351–358. [Google Scholar]
- Laustsen, S.; Hasholt, M.T.; Jensen, O.M. Void structure of concrete with superabsorbent polymers and its relation to frost resistance of concrete. Mater. Struct. 2015, 48, 357–368. [Google Scholar] [CrossRef]
- Mönnig, S. Superabsorbing Additions in Concrete—Applications, Modelling and Comparison of Different Internal Water Sources. Ph.D. Thesis, University of Stuttgart, Stuttgart, Germany, 2009. [Google Scholar]
- Esteves, L.P. Superabsorbent polymers: On their interaction with water and pore fluid. Cem. Concr. Compos. 2011, 33, 717–724. [Google Scholar] [CrossRef]
- Kang, S.-H.; Hong, S.-G.; Moon, J. Absorption kinetics of superabsorbent polymers (SAP) in various cement-based solutions. Cem. Concr. Res. 2017, 97, 73–83. [Google Scholar] [CrossRef]
- NWSP. Polyacrylate Superabsorbent Powders—Determination of the Free Swell Capacity in Saline by Gravimetric Measurement; NWSP 240.0.R2 (15) ISO 17190-5; NWSP: Białystok, Poland, 2001. [Google Scholar]
- Wang, F.; Yang, J.; Hu, S.; Li, X.; Cheng, H. Influence of superabsorbent polymers on the surrounding cement paste. Cem. Concr. Res. 2016, 81, 112–121. [Google Scholar] [CrossRef]
- Johansen, N.A.; Millard, M.J.; Mezencevova, A.; Garas, V.Y.; Kurtis, K.E. New method for determination of absorption capacity of internal curing agents. Cem. Concr. Res. 2009, 39, 65–68. [Google Scholar] [CrossRef]
- Castro, J.; Keiser, L.; Golias, M.; Weiss, J. Absorption and desorption properties of fine lightweight aggregate for application to internally cured concrete mixtures. Cem. Concr. Compos. 2011, 33, 1001–1008. [Google Scholar] [CrossRef]
- Wyrzykowski, M.; Ghourchian, S.; Sinthupinyo, S.; Chitvoranund, N.; Chintana, T.; Lura, P. Internal curing of high performance mortars with bottom ash. Cem. Concr. Compos. 2016, 71, 1–9. [Google Scholar] [CrossRef]
- Hasholt, M.T.; Jespersen, M.H.S.; Jensen, O.M. Mechanical properties of concrete with SAP. Part I: Development of compressive strength. In Proceedings of the RILEM Proc. PRO 74 “Use of Superabsorbent Polymers and Other New Additives in Concrete”; Jensen, O.M., Hasholt, M.T., Laustsen, S., Eds.; RILEM Publications SARL: Bagneux, France, 2010; pp. 117–126. [Google Scholar]
- Trtik, P.; Muench, B.; Weiss, W.J.; Herth, G.; Kaestner, A.; Lehmann, E.; Lura, P. Neutron Tomography Measurements of Water Release from Superabsorbent Polymers in Cement Paste. In International Conference on Material Science and 64th RILEM Annual Week; RILEM Publications SARL: Aachen, Germany, 2010; pp. 175–185. [Google Scholar]

| Chemical Nomenclature | Chemical Formula | Molar Mass (g/mol) | Constitutional Formula | Density (g/cm3) | Appearance |
|---|---|---|---|---|---|
| Poly (sodium prop-2-enoate) | (C3H3NaO2)n | Variable | 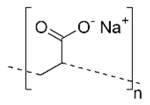 | 1.22 |  |
| Material | Weight Per Unit Volume (kg/m3) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control 0.485 | Control 0.30 | AC a 0 g/g | AC 7.5 g/g | AC 15 g/g | Trial Mix 1 (SAP A 0.2%) | Trial Mix 2 (SAP A 0.4%) | Trial Mix 3 (SAP A 0.4%) | Trial Mix 4 (SAP B 0.4%) | Trial Mix 5 (SAP C 0.4%) | Trial Mix 6 (SAP D 0.4%) | |
| Cement | 527.1 | 696.5 | 527.1 | 527.1 | 527.1 | 527.1 | 527.1 | 696.5 | 696.5 | 696.5 | 696.5 |
| Water | 255.6 | 209.0 | 255.6 | 263.5 | 271.4 | 269.0 | 282.4 | 244.3 | 233.3 | 222.4 | 239.6 |
| Sand | 1449.5 | 1462.7 | 1449.5 | 1449.5 | 1449.5 | 1449.5 | 1449.5 | 1462.7 | 1462.7 | 1462.7 | 1462.7 |
| SAP | 0 | 0 | 1.054 | 1.054 | 1.054 | 1.054 | 2.108 | 2.786 | 2.786 | 2.786 | 2.786 |
| Specimen | Weight Per Unit Volume (kg/m3) | Total w/c b | ||||
|---|---|---|---|---|---|---|
| Cement | Water | Sand | SAP | IC Water a | ||
| Control | 527.1 | 255.6 | 1449.5 | 0 | 0 | 0.485 |
| SAP A-0.2 | 519.9 | 252.2 | 1429.7 | 1.04 | 13.21 | 0.510 |
| SAP A-0.4 | 512.9 | 248.8 | 1410.5 | 2.05 | 26.06 | 0.536 |
| SAP A-0.6 | 506.1 | 245.5 | 1391.8 | 3.04 | 38.56 | 0.561 |
| SAP B-0.2 | 522.1 | 253.2 | 1449.5 | 1.04 | 9.14 | 0.503 |
| SAP B-0.4 | 517.3 | 250.9 | 1422.6 | 2.07 | 18.11 | 0.520 |
| SAP B-0.6 | 512.5 | 248.6 | 1409.4 | 3.08 | 26.91 | 0.538 |
| SAP C-0.2 | 524.3 | 254.3 | 1441.8 | 1.05 | 5.05 | 0.495 |
| SAP C-0.4 | 521.6 | 253.0 | 1434.4 | 2.09 | 10.06 | 0.504 |
| SAP C-0.6 | 518.9 | 251.7 | 1427.0 | 3.11 | 15.01 | 0.514 |
| SAP D-0.2 | 520.9 | 252.6 | 1432.5 | 1.04 | 11.45 | 0.507 |
| SAP D-0.4 | 514.8 | 249.7 | 1415.7 | 2.06 | 22.63 | 0.529 |
| SAP D-0.6 | 508.9 | 246.8 | 1399.5 | 3.05 | 33.56 | 0.551 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, K.-K.; Kim, K.-K.; Choi, W.; Yeon, J.H. Hygral Behavior of Superabsorbent Polymers with Various Particle Sizes and Cross-Linking Densities. Polymers 2017, 9, 600. https://doi.org/10.3390/polym9110600
Yun K-K, Kim K-K, Choi W, Yeon JH. Hygral Behavior of Superabsorbent Polymers with Various Particle Sizes and Cross-Linking Densities. Polymers. 2017; 9(11):600. https://doi.org/10.3390/polym9110600
Chicago/Turabian StyleYun, Kyong-Ku, Kwan-Kyu Kim, Wonchang Choi, and Jung Heum Yeon. 2017. "Hygral Behavior of Superabsorbent Polymers with Various Particle Sizes and Cross-Linking Densities" Polymers 9, no. 11: 600. https://doi.org/10.3390/polym9110600





