Aggregation Behavior of Nano-Silica in Polyvinyl Alcohol/Polyacrylamide Hydrogels Based on Dissipative Particle Dynamics
Abstract
:1. Introduction
2. Modeling and Methods
2.1. Dissipative Particle Dynamics Method
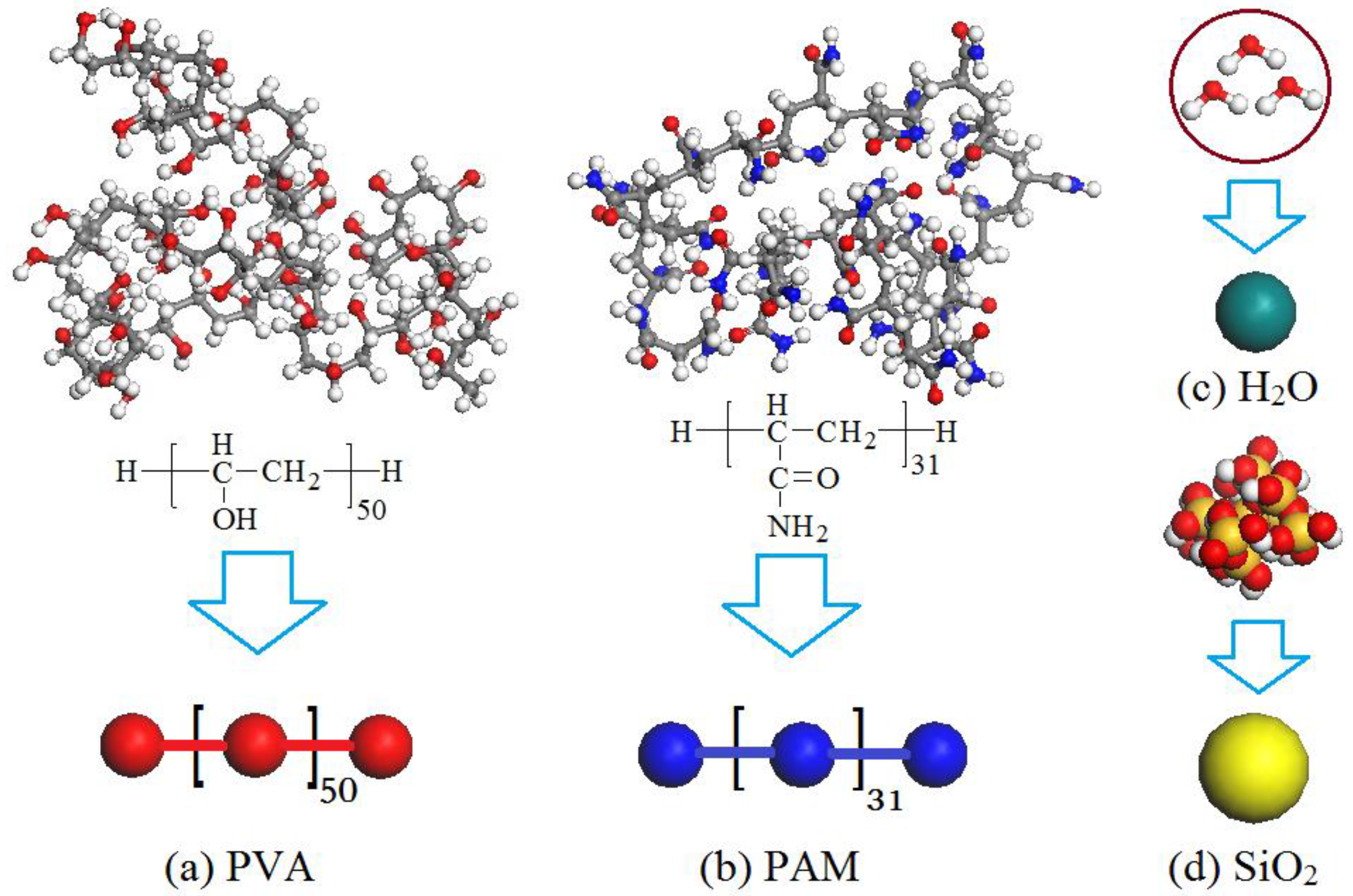
2.2. Models and Interaction Parameters
3. Results and Discussion
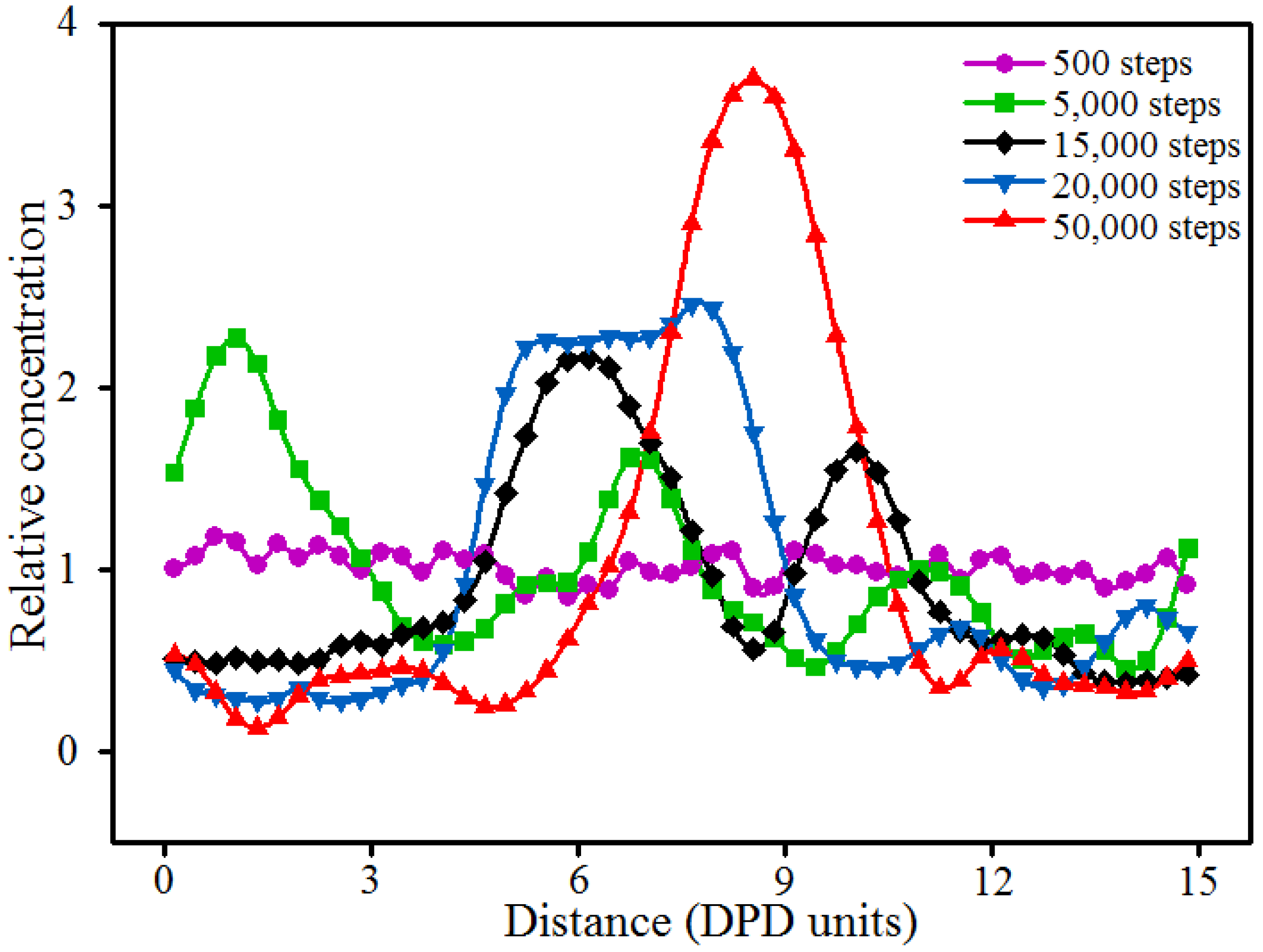
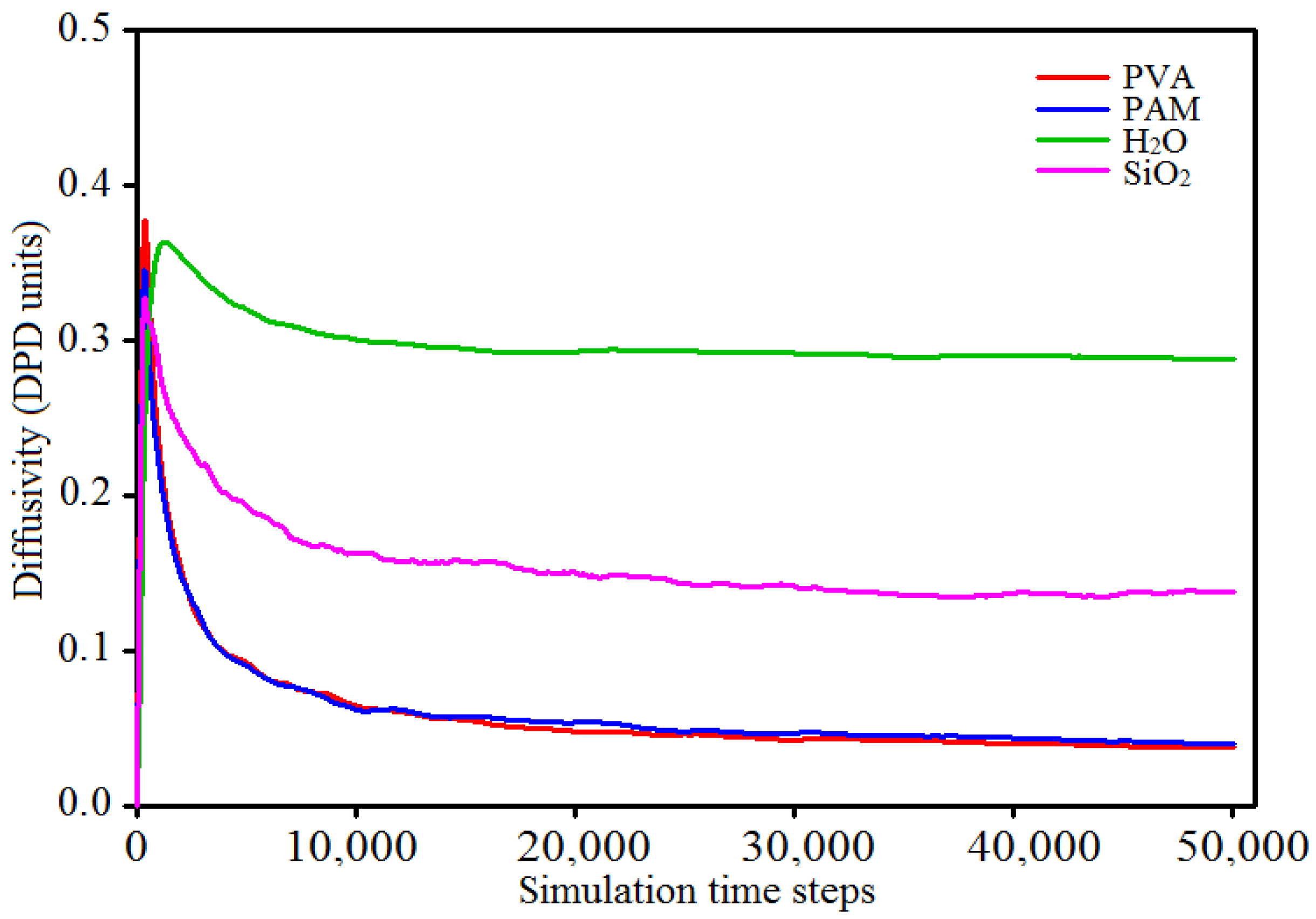
3.1. Parametiric Study on the Time Step Used in Simulations
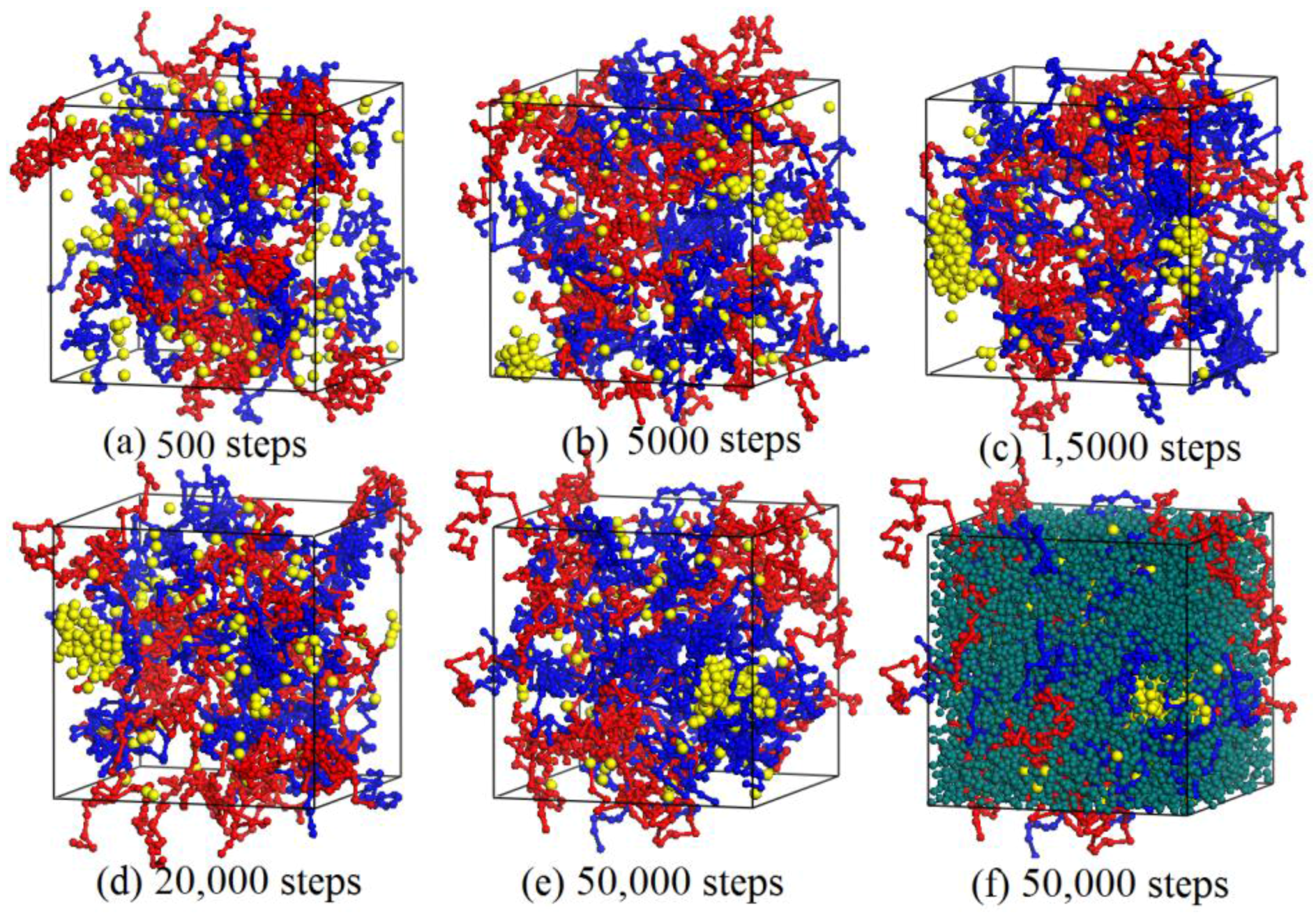
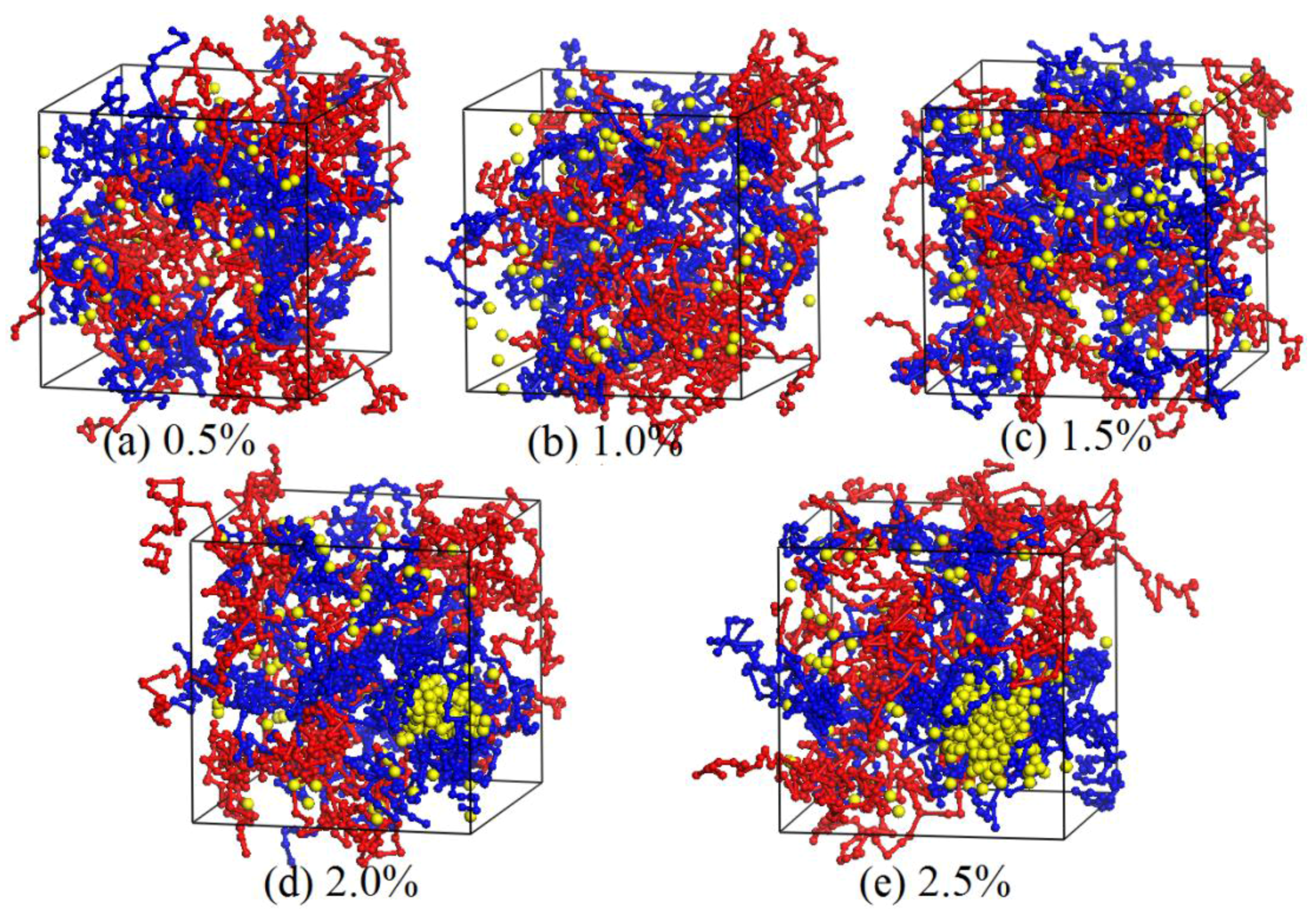
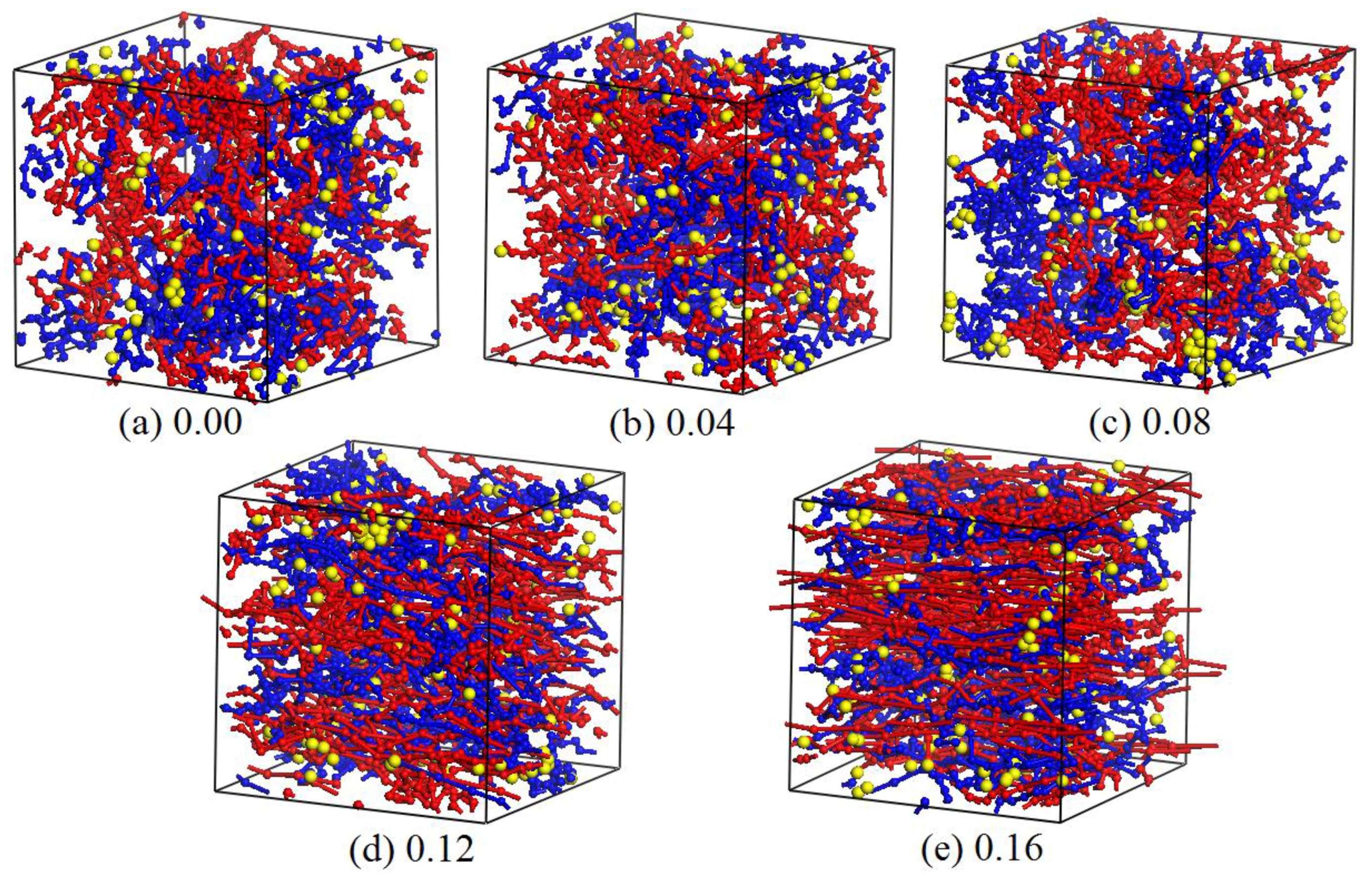
3.2. Dynamics Process of the Aggregation of Nano-Silica and Equilibrium
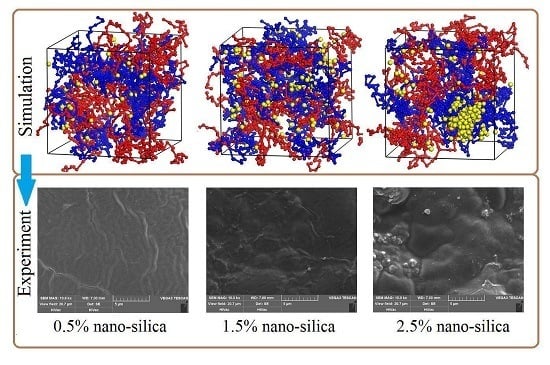
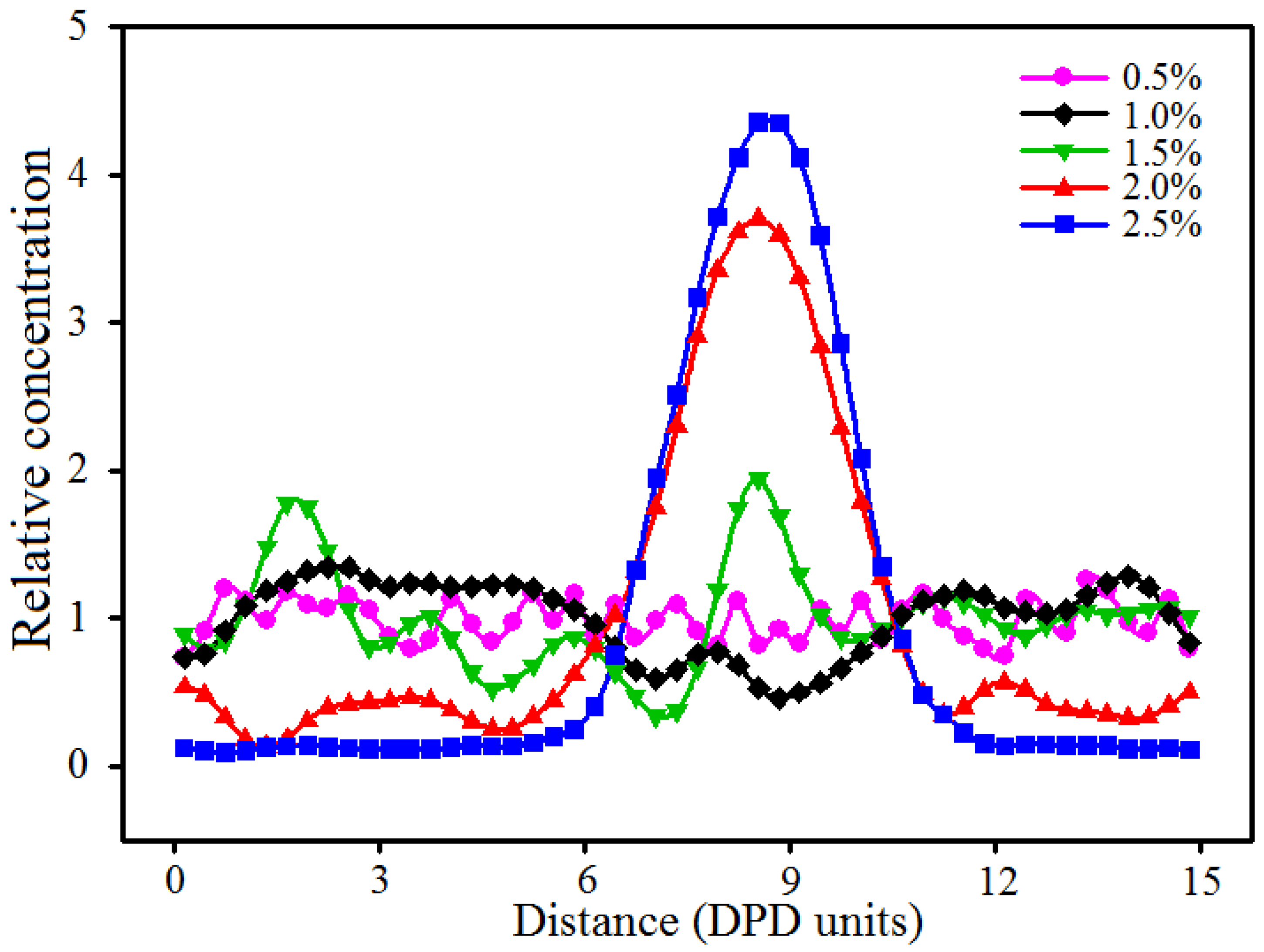
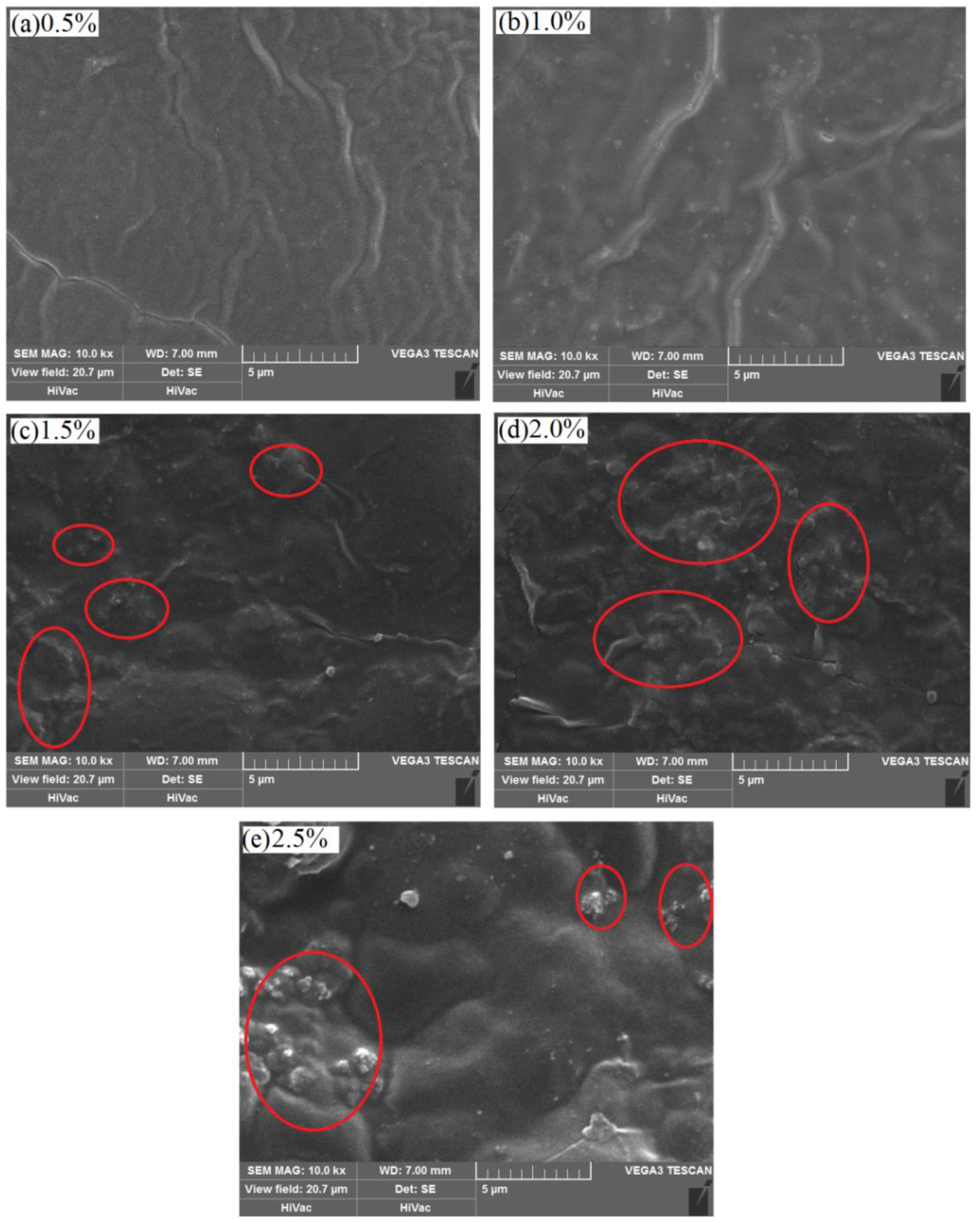
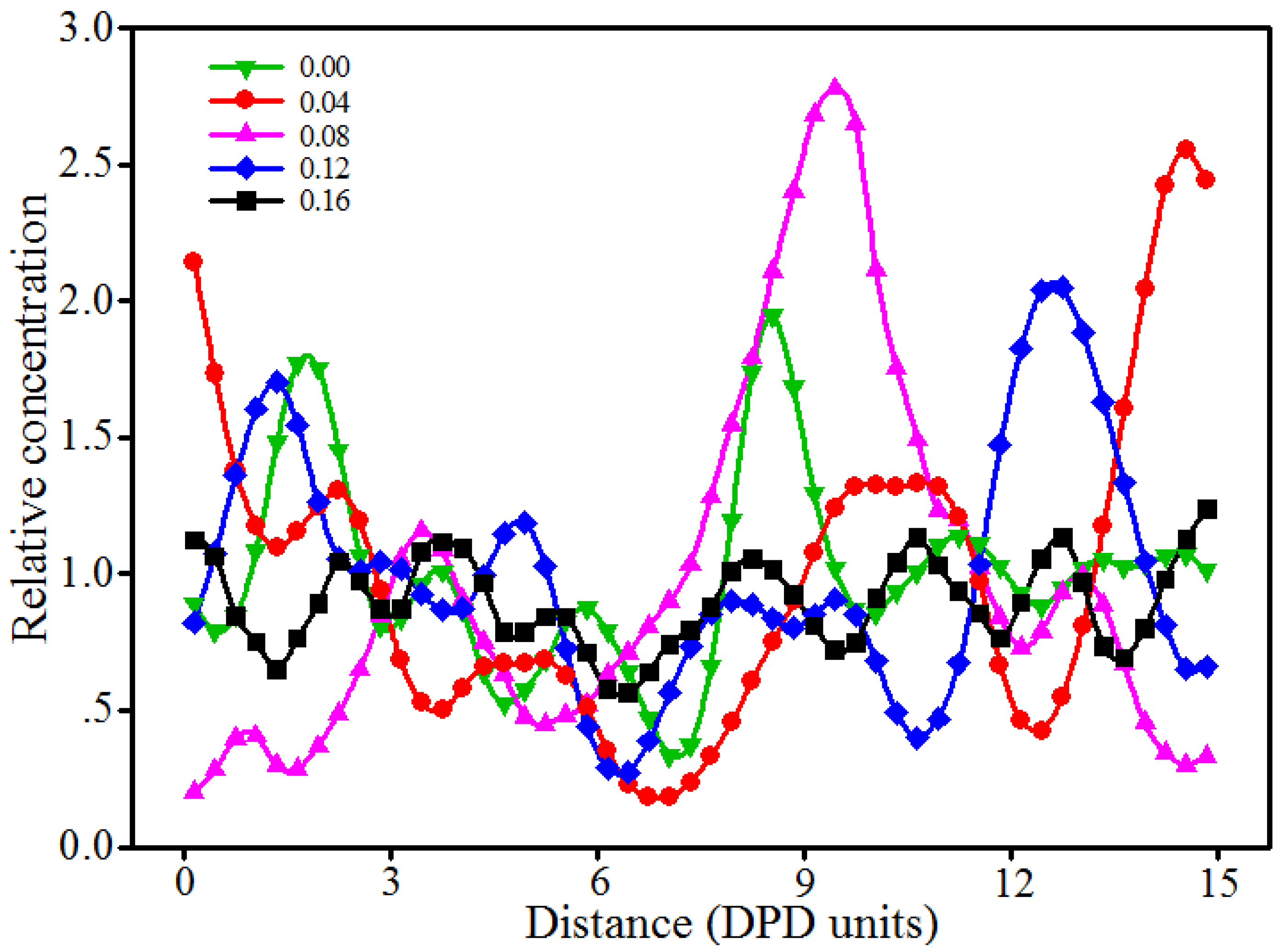
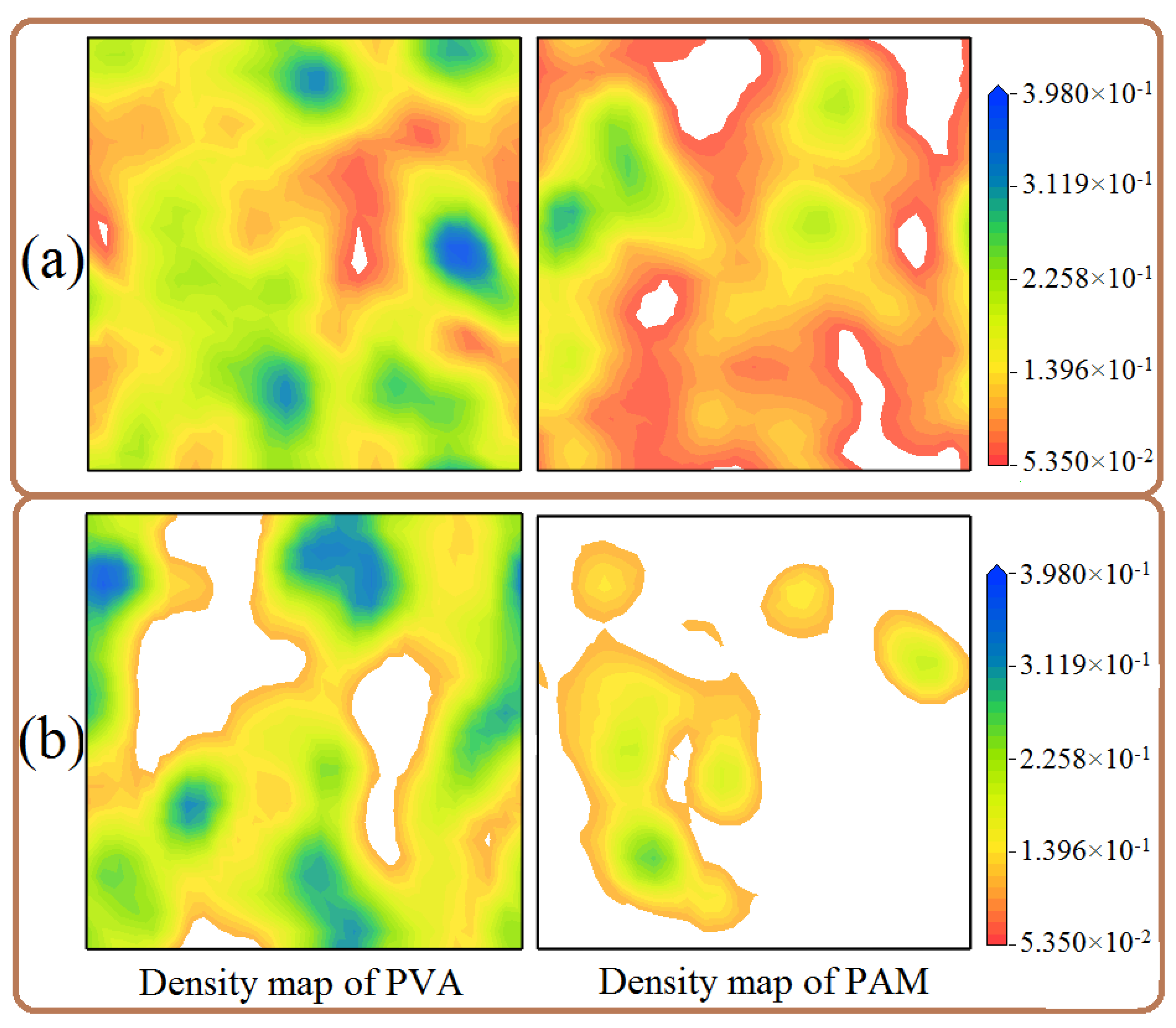
3.3. Effect of Nano-Silica Content
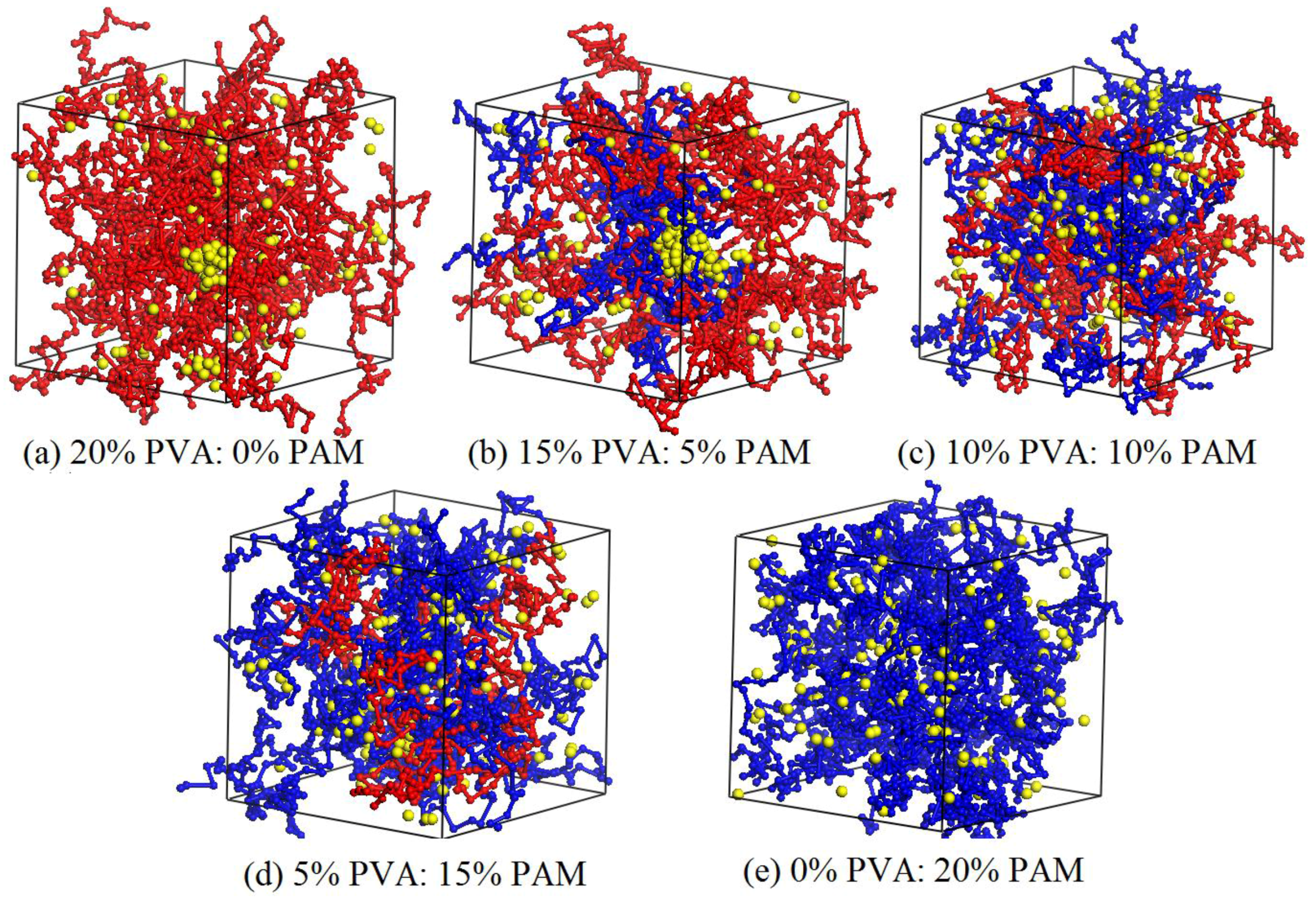
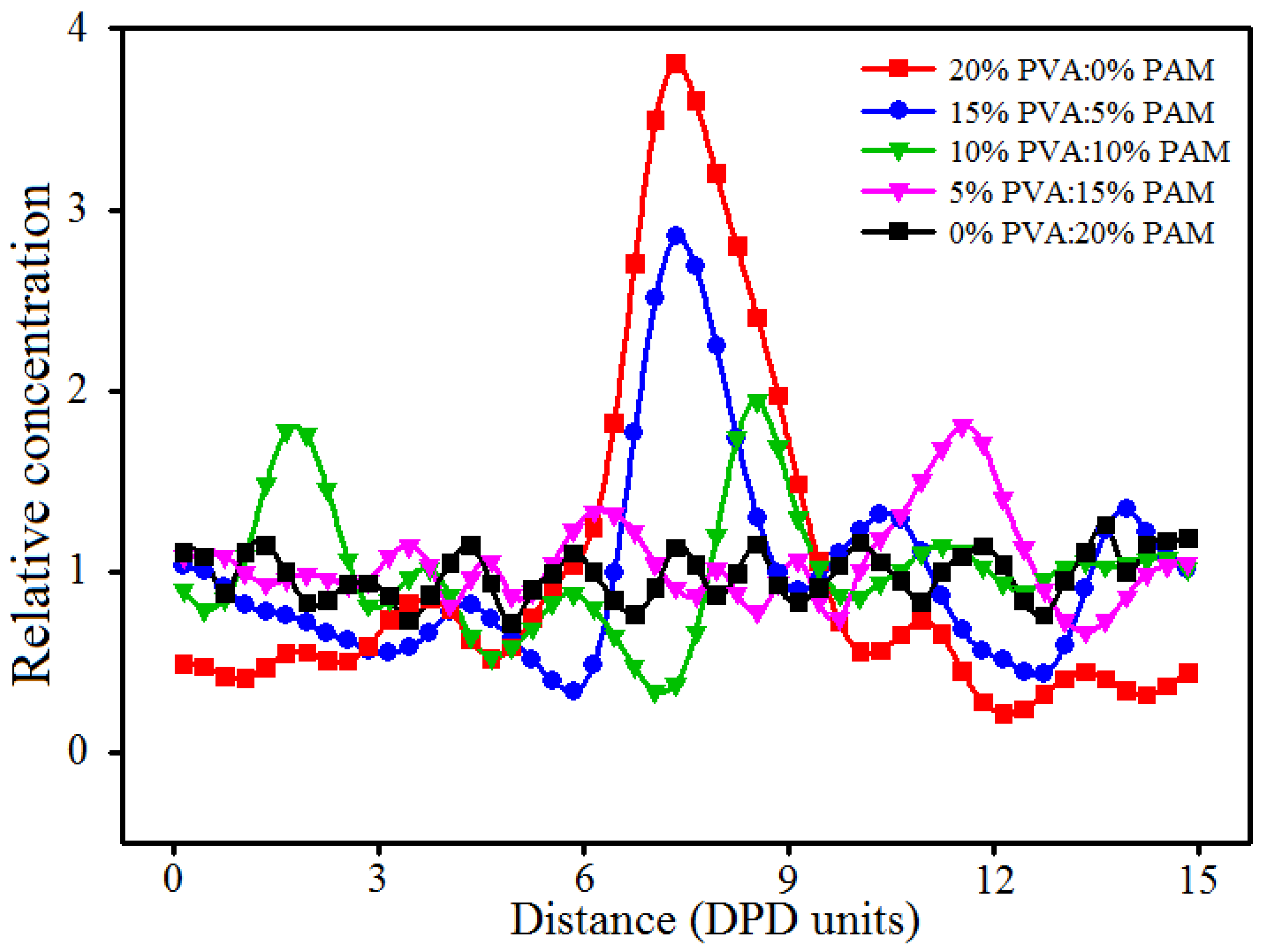
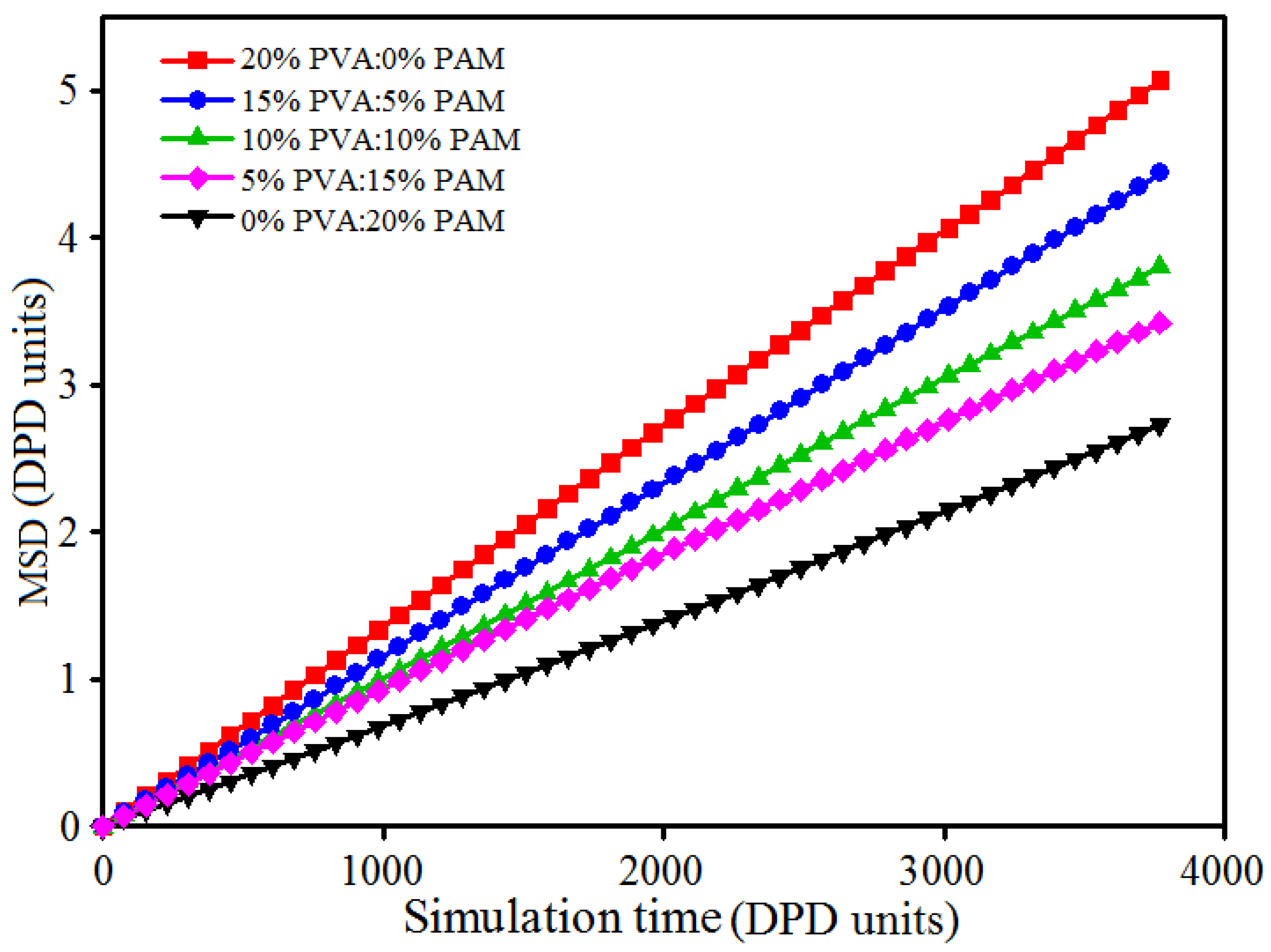
3.4. Effect of Polymer Component Ratio
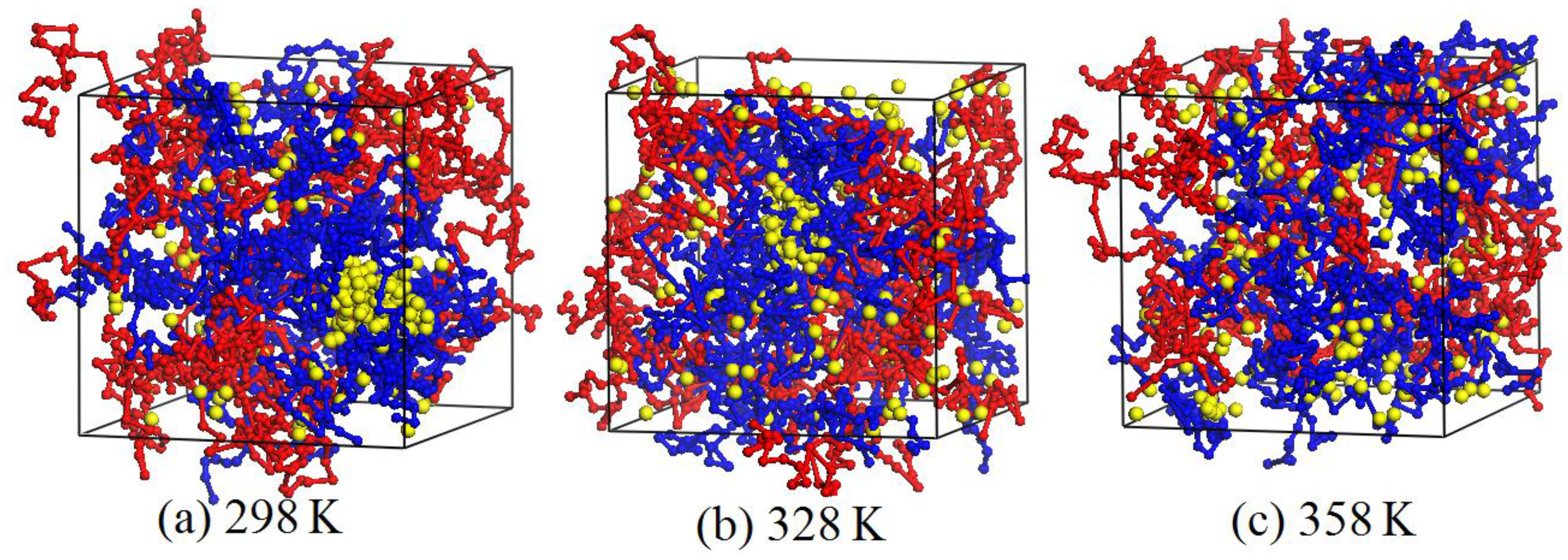
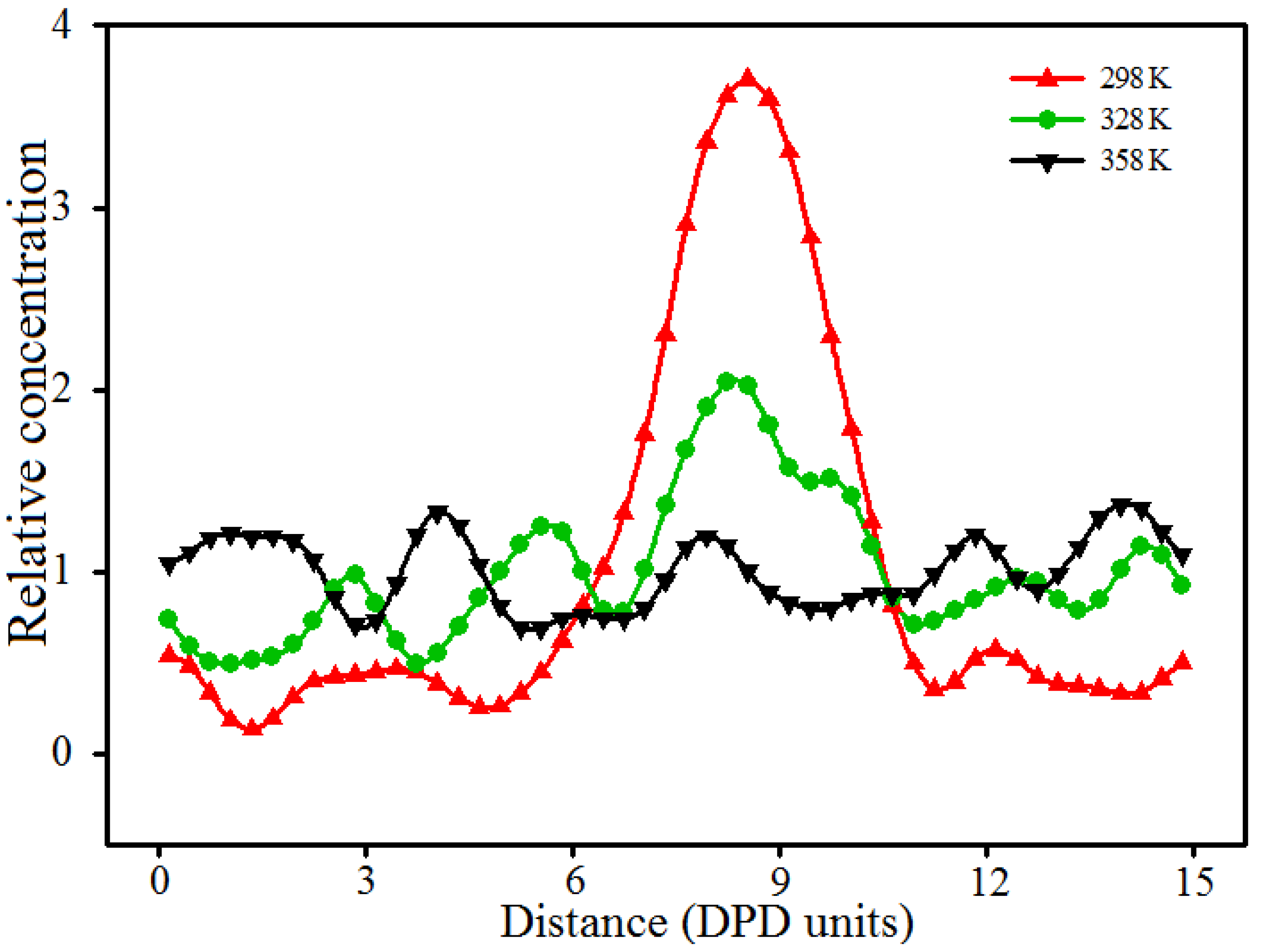
3.5. Effect of Temperature
3.6. Effect of Shear Rate
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, J.; Li, Z.; Gu, X.; Feng, L.; Zhang, C.; Hu, G. A dissipative particle dynamics study on the compatibilizing process of immiscible polymer blends with graft copolymers. Polymer 2012, 53, 4448–4454. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, Y.; Wang, Y.; Chai, W.; Yang, M. Measurement and modeling of the effect of composition ratios on the properties of poly(vinyl alcohol)/poly(vinyl pyrrolidone) membranes. Mater. Des. 2016, 103, 249–258. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, Y.; Chai, W.; Wang, T.; Zhang, Y. Effects of composition ratio on the properties of poly(vinyl alcohol)/poly (acrylic acid) blend membrane: A molecular dynamics simulation study. Mater. Des. 2016, 89, 848–855. [Google Scholar] [CrossRef]
- Yao, Q.; Cosme, J.G.L.; Xu, T.; Miszuk, J.M. Three dimensional electrospun PCL/PLA blend nanofibrous scaffolds with significantly improved stem cells osteogenic differentiation and cranial bone formation. Biomaterials 2017, 115, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Izadifar, M.; Kelly, M.E.; Chen, X. Regulation of sequential release of growth factors using bi-layer polymeric nanoparticles for cardiac tissue engineering. Nanomedicine 2016, 11, 3237–3259. [Google Scholar] [CrossRef] [PubMed]
- Izadifar, M.; Kelly, M.E.; Chen, X. Optimization of Nanoparticles for Cardiovascular Tissue Engineering. Nanotechnology 2015, 26, 235301. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.; Sureshkumar, M.B. Preparation of PAM/PVA blending films by solution-cast technique and its characterization: A spectroscopic study. Iran. Polym. J. 2014, 23, 153–162. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, Y.; Che, Y.; Yang, M.; Li, X.; Zhang, Y. Molecular mechanisms in compatibility and mechanical properties of polyacrylamide/polyvinyl alcohol blends. J. Mech. Behav. Biomed. Mater. 2017, 65, 565–573. [Google Scholar] [CrossRef] [PubMed]
- El-Zawawy, W.K.; Ibrahim, M.M. Preparation and Characterization of Novel Polymer Hydrogel from Industrial Waste and Copolymerization of Poly(vinyl alcohol) and Polyacrylamide. J. Appl. Polym. Sci. 2012, 124, 4362–4370. [Google Scholar] [CrossRef]
- Olubamiji, A.D.; Izadifar, Z.; Si, J.L.; Cooper, D.M.L.; Eames, B.F.; Chen, D.X.B. Modulating mechanical behaviour of 3D-printed cartilage-mimetic PCL scaffolds: Influence of molecular weight and pore geometry. Biofabrication 2016, 8, 025020. [Google Scholar] [CrossRef] [PubMed]
- Chen, X. Dispensed-Based Bio-Manufacturing Scaffolds for Tissue Engineering Applications. Int. J. Eng. Appl. 2014, 2, 1. [Google Scholar]
- Little, C.J.; Bawolin, N.K.; Chen, X. Mechanical Properties of Natural Cartilage and Tissue-Engineered Constructs. Tissue Eng. Part B Rev. 2011, 17, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Corona-Gomez, J.; Chen, X.; Yang, Q. Effect of Nanoparticle Incorporation and Surface Coating on Mechanical Properties of Bone Scaffolds: A Brief Review. J. Funct. Biomater. 2016, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Kothapalli, C.R.; Shaw, M.T.; Wei, M. Biodegradable HA-PLA 3-D porous scaffolds: Effect of nano-sized filler content on scaffold properties. Acta Biomater. 2005, 1, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Mehrasa, M.; Asadollahi, M.A.; Nasri-Nasrabadi, B.; Ghaedi, K.; Salehi, H.; Dolatshahi-Pirouz, A.; Arpanaei, A. Incorporation of mesoporous silica nanoparticles into random electrospun PLGA and PLGA/gelatin nanofibrous scaffolds enhances mechanical and cell proliferation properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 66, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Arcos, D.; Vallet-Regi, M. Sol-gel silica-based biomaterials and bone tissue regeneration. Acta Biomater. 2010, 6, 2874–2888. [Google Scholar] [CrossRef] [PubMed]
- Pablo, G.; Gustavo, Á.; Juan, E.F.; Francisco, M.; Francisco, O.; Wang, H. Clinical and histologic comparison of two different composite grafts for sinus augmentation: A pilot clinical trial. Clin. Oral Implants Res. 2008, 19, 755–759. [Google Scholar]
- Padial-Molina, M.; Galindo-Moreno, P.; Avila-Ortiz, G. Biomimetic ceramics in implant dentistry. Min. Biotechnol. 2009, 21, 173–186. [Google Scholar]
- Von Wilmowsky, C.; Vairaktaris, E.; Pohle, D.; Rechtenwald, T.; Lutz, R.; Munstedt, H.; Koller, G.; Schmidt, M.; Neukam, F.W.; Schlegel, K.A.; et al. Effects of bioactive glass and beta-TCP containing three-dimensional laser sintered polyetheretherketone composites on osteoblasts in vitro. J. Biomed. Mater. Res. 2008, 87A, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Ghanaati, S.M.; Thimm, B.W.; Unger, R.E.; Orth, C.; Kohler, T.; Barbeck, M.; Muller, R.; Kirkpatrick, CJ. Collagen-embedded hydroxylapatite-beta-tricalcium phosphate–silicon dioxide bone substitute granules assist rapid vascularization and promote cell growth. Biomed. Mater. 2010, 5, 025004. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; Liu, X.; Huang, Q.; He, W.; Zhang, R.; Feng, Q.; Benayahu, D. The stimulatory effect of silica nanoparticles on osteogenic differentiation of human mesenchymal stem cells. Biomed. Mater. 2017, 12, 015001. [Google Scholar] [CrossRef] [PubMed]
- Horie, M.; Nishio, K.; Kato, H.; Endoh, S.; Fujita, K.; Nakamura, A.; Hagihara, Y.; Yoshida, Y.; Iwahashi, H. Evaluation of cellular effects of silicon dioxide nanoparticles. Toxicol. Mech. Methods 2014, 24, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liu, H.; Zhou, H.; Zhang, Y.; Gao, X.; Mai, Y. On adhesive properties of nano-silica/epoxy bonded single-lap joints. Mater. Des. 2016, 95, 212–218. [Google Scholar] [CrossRef]
- Vaziri, H.S.; Omaraei, I.A.; Abadyan, M.; Mortezaei, M. Thermophysical and rheological behavior of polystyrene/silica nanocomposites: Investigation of nanoparticle content. Mater. Des. 2011, 32, 4537–4542. [Google Scholar] [CrossRef]
- Luo, Z.; Jiang, J. pH-sensitive drug loading/releasing in amphiphilic copolymer PAE–PEG: Integrating molecular dynamics and dissipative particle dynamics simulations. J. Contr. Release 2012, 162, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, Y.; Lu, Z.; Sun, C. Effect of molecular architecture on the morphology diversity of the multicompartment micelles: A dissipative particle dynamics simulation study. Polymer 2008, 49, 4899–4909. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, X.; Lin, X.; Lu, X.; Zhou, Y.; Tang, Y. Polycaprolactone/chitosan blends: Simulation and experimental design. Mater. Des. 2016, 90, 396–402. [Google Scholar] [CrossRef]
- Tang, Y.; He, Y.; Wang, X. Investigation on the membrane formation process of polymer–diluent system via thermally induced phase separation accompanied with mass transfer across the interface: Dissipative particle dynamics simulation and its experimental verification. J. Membr. Sci. 2015, 474, 196–206. [Google Scholar] [CrossRef]
- Gai, J.; Li, H.; Schrauwen, C.; Hu, G. Dissipative particle dynamics study on the phase morphologies of the ultrahigh molecular weight polyethylene/polypropylene/poly(ethylene glycol) blends. Polymer 2009, 50, 336–346. [Google Scholar] [CrossRef]
- Shi, K.; Lian, C.; Bai, Z.; Zhao, S. Dissipative particle dynamics study of the water/benzene/caprolactam system in the absence or presence of non-ionic surfactants. Chem. Eng. Sci. 2015, 122, 185–196. [Google Scholar] [CrossRef]
- Dai, X.; Ding, H.; Yin, Q.; Wan, G.; Shi, X.; Qiao, Y. Dissipative particle dynamics study on self-assembled platycodin structures: The potential biocarriers for drug delivery. J. Mol. Graph. Model. 2015, 57, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guo, C.; Hu, G.; Liu, H.; Liang, X.; Wang, J.; Ma, J.; Zheng, L. Dissipative particle dynamics simulation of gold nanoparticles stabilization by PEO–PPO–PEO block copolymer micelles. Colloid Polym. Sci. 2007, 285, 1543–1552. [Google Scholar] [CrossRef]
- Groot, R.D.; Warren, P.B. Dissipative particle dynamics: Bridging the gap between atomistic and mesoscopic simulation. J. Chem. Phys. 1997, 107, 4423–4435. [Google Scholar] [CrossRef]
- Rao, Z.; Huoa, Y.; Liu, X. Dissipative particle dynamics and experimental study of alkane-based nanoencapsulated phase change material for thermal energy storage. RSC Adv. 2014, 4, 20797–20803. [Google Scholar] [CrossRef]
- Guo, X.D.; Tan, J.P.K.; Kim, S.H.; Zhang, L.J.; Zhang, Y.; Hedrick, J.L.; Yang, Y.; Qian, Y. Computational studies on self-assembled paclitaxel structures: Templates for hierarchical block copolymer assemblies and sustained drug release. Biomaterials 2009, 30, 6556–6563. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Wang, Y.; Li, X.; Yang, M.; Chai, W.; Wang, K.; Zhang, Y. Study the bonding mechanism of binders on hydroxyapatite surface and mechanical properties for 3DP fabrication bone scaffolds. J. Mech. Behav. Biomed. Mater. 2016, 57, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Wang, Y.; Wang, S.; Zhang, Y.; Chen, X. Investigating the properties and interaction mechanism of nano-silica in polyvinyl alcohol/polyacrylamide blends at an atomic level. J. Mech. Behav. Biomed. Mater. 2017, 75, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Wang, Y.; Chai, W.; Zhang, Y.; Chen, X. Molecular dynamics simulation and experimental study of the bonding properties of polymer binders in 3D powder printed hydroxyapatite bioceramic bone scaffolds. Ceram. Int. 2017, 43, 13702–13709. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, Y.; Wang, Y.; Yang, M. A molecular dynamic simulation method to elucidate the interaction mechanism of nano-SiO2 in polymer blends. J. Mater. Sci. 2017, 52, 12889–12901. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Gao, P.; Gong, X.; Wang, J. Molecular dynamics and dissipative particle dynamics simulations of the miscibility and mechanical properties of GAP/DIANP blending systems. RSC Adv. 2014, 4, 41934–41941. [Google Scholar] [CrossRef]
- Sun, D.; Zhou, J. Dissipative particle dynamics simulation on messoscopic structures of nafion and PVA/nafion blend membranes. Acta Phys. Chim. Sin. 2012, 28, 909–916. [Google Scholar]
- Groot, R.D.; Rabone, K.L. Mesoscopic simulation of cell membrane damage, morphology change and rupture by nonionic surfactants. Biophys. J. 2001, 81, 725–736. [Google Scholar] [CrossRef]
- Allen, M.P.; Tildesley, D.J. Computer Simulation of Liquids; Clarendon Press/Oxford Science Publications: Oxford, UK, 1987. [Google Scholar]
- Babarao, R.; Jiang, J.W. Diffusion and separation of CO2 and CH4 in silicalite, C168 schwarzite, and IRMOF-1: A comparative study from molecular dynamics simulation. Langmuir 2008, 24, 5474–5484. [Google Scholar] [CrossRef] [PubMed]

| Bead Pair | 298 K | 328 K | 358 K | |||
|---|---|---|---|---|---|---|
| PVA–PVA | 0.00 | 25.00 | 0.00 | 27.52 | 0.00 | 30.03 |
| PVA–H2O | 0.23, 0.22 [41] | 25.75 | 0.20 | 28.17 | 0.18 | 30.61 |
| PVA–PAM | 0.15 | 25.49 | 0.14 | 27.98 | 0.12 | 30.42 |
| PVA–SiO2 | 4.17 | 38.65 | 3.62 | 39.35 | 3.12 | 40.23 |
| PAM–PAM | 0.00 | 25.00 | 0.00 | 27.52 | 0.00 | 30.03 |
| PAM–H2O | 0.57 | 26.88 | 0.49 | 29.12 | 0.42 | 31.41 |
| PAM–SiO2 | 2.48 | 33.11 | 2.25 | 34.88 | 2.01 | 36.60 |
| H2O–SiO2 | 6.35 | 45.75 | 5.39 | 45.15 | 4.38 | 44.36 |
| H2O–H2O | 0.00 | 25.00 | 0.00 | 27.52 | 0.00 | 30.03 |
| SiO2–SiO2 | −0.44 | 23.55 | −0.52 | 26.82 | −0.61 | 28.04 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Q.; Wang, Y.; Zhang, Y.; Chen, X. Aggregation Behavior of Nano-Silica in Polyvinyl Alcohol/Polyacrylamide Hydrogels Based on Dissipative Particle Dynamics. Polymers 2017, 9, 611. https://doi.org/10.3390/polym9110611
Wei Q, Wang Y, Zhang Y, Chen X. Aggregation Behavior of Nano-Silica in Polyvinyl Alcohol/Polyacrylamide Hydrogels Based on Dissipative Particle Dynamics. Polymers. 2017; 9(11):611. https://doi.org/10.3390/polym9110611
Chicago/Turabian StyleWei, Qinghua, Yanen Wang, Yingfeng Zhang, and Xiongbiao Chen. 2017. "Aggregation Behavior of Nano-Silica in Polyvinyl Alcohol/Polyacrylamide Hydrogels Based on Dissipative Particle Dynamics" Polymers 9, no. 11: 611. https://doi.org/10.3390/polym9110611