Effect of Ammonium Polyphosphate to Aluminum Hydroxide Mass Ratio on the Properties of Wood-Flour/Polypropylene Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Flame-Retardant Composites
2.3. Physical-Mechanical Properties
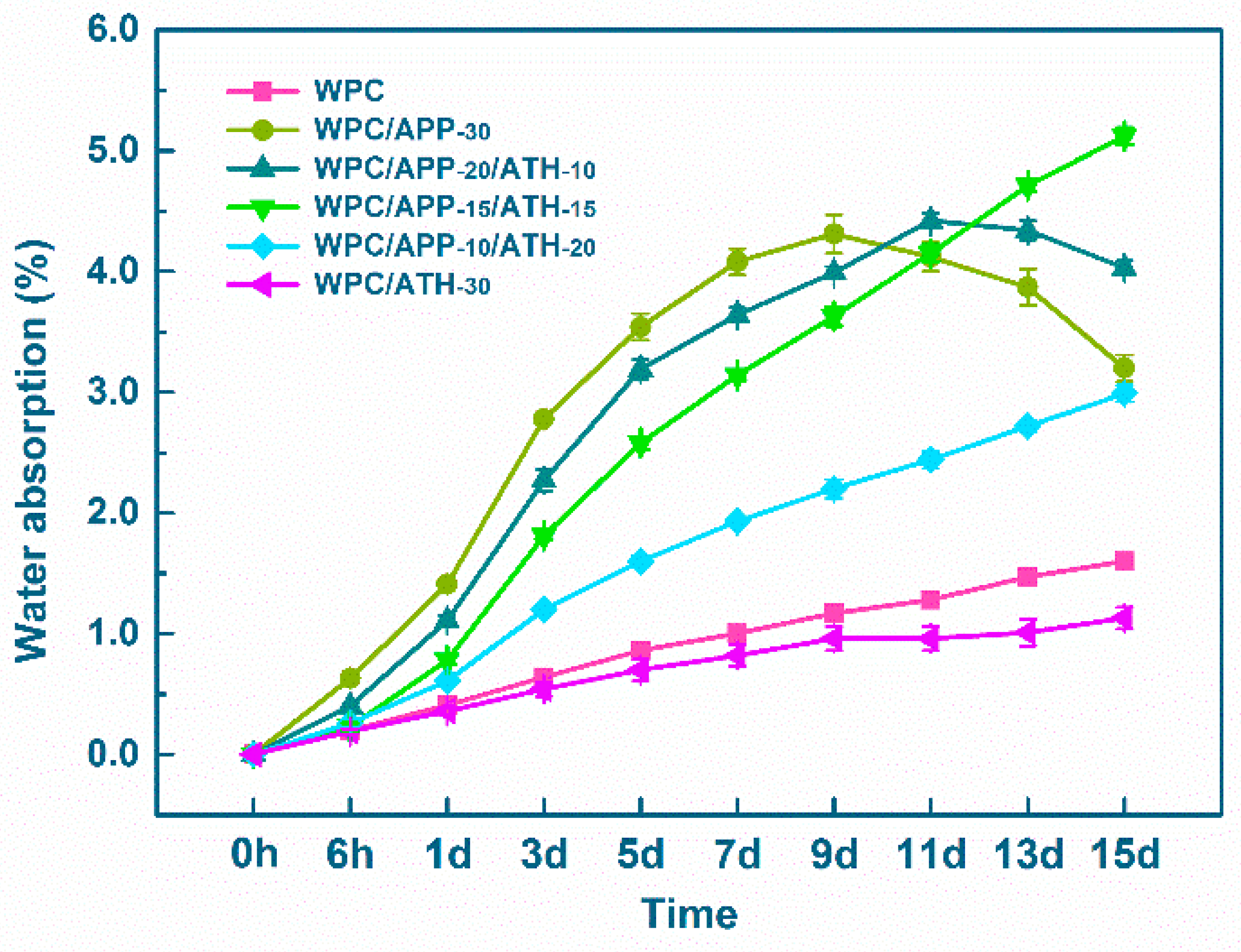
2.3.1. Water Soaking Test
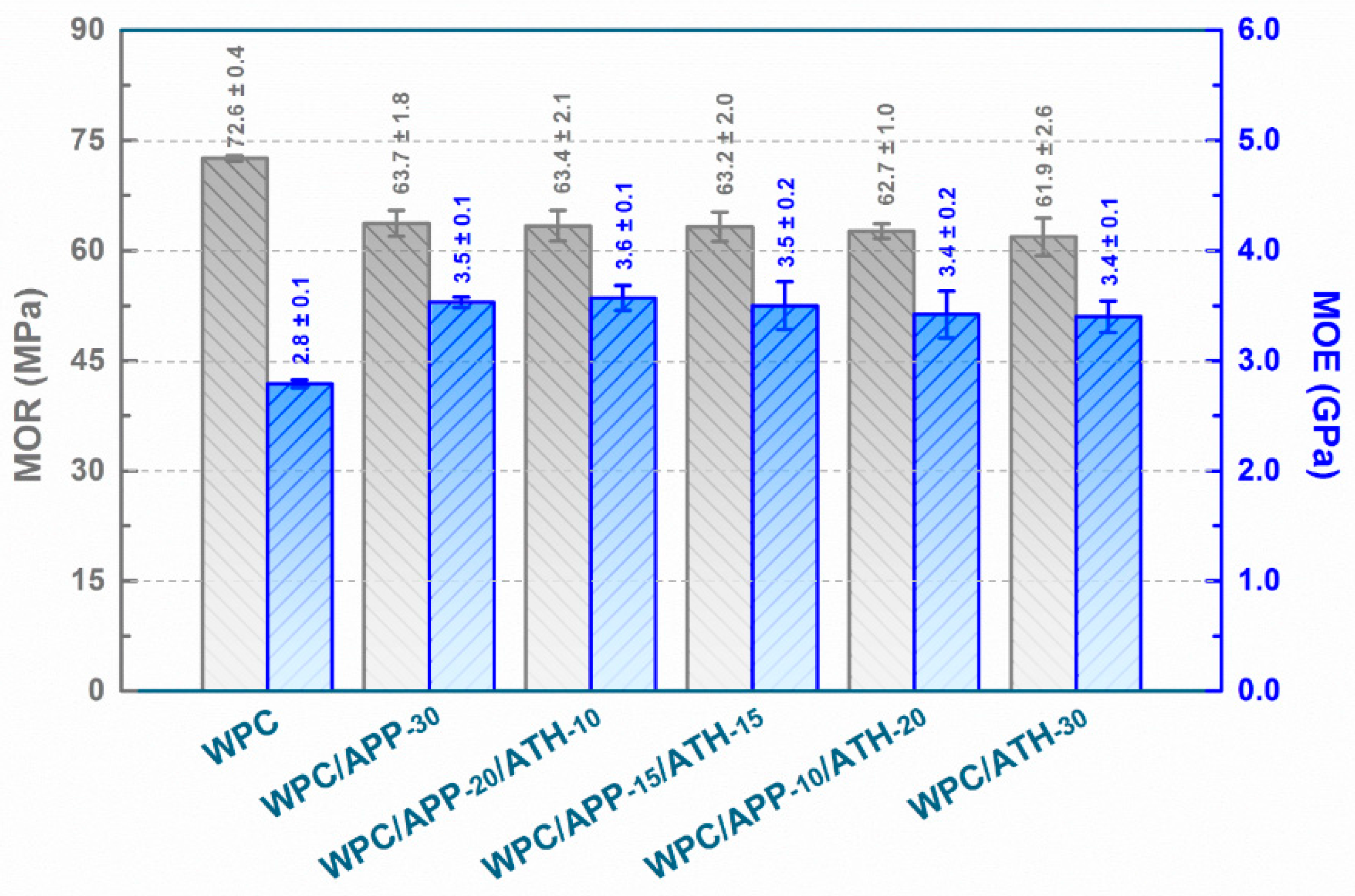
2.3.2. Three-Point Bending Test
2.3.3. Impact Strength
2.4. Thermogravimetric Analysis (TGA)
2.5. Cone Calorimetry
2.6. Scanning Electron Microscopy (SEM)
2.7. Laser Raman Spectroscopy (LRS)
2.8. Fourier Transform Infrared Spectroscopy (FTIR)
2.9. Thermogravimetric Analysis-Infrared Spectrometry (TGA-IR)
3. Results and Discussion
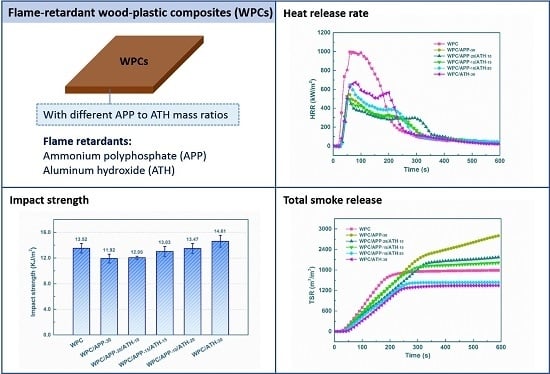
3.1. Physical-Mechanical Performance of WPCs
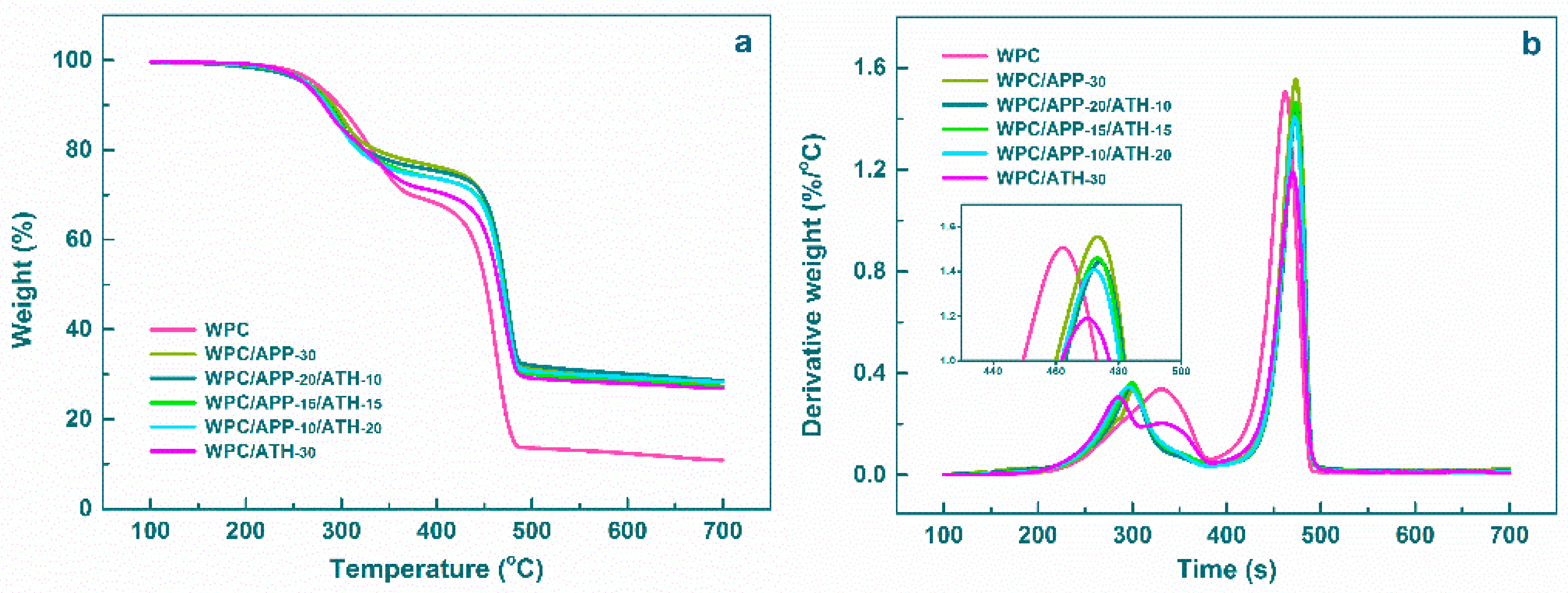
3.2. Thermal Degradation Analysis
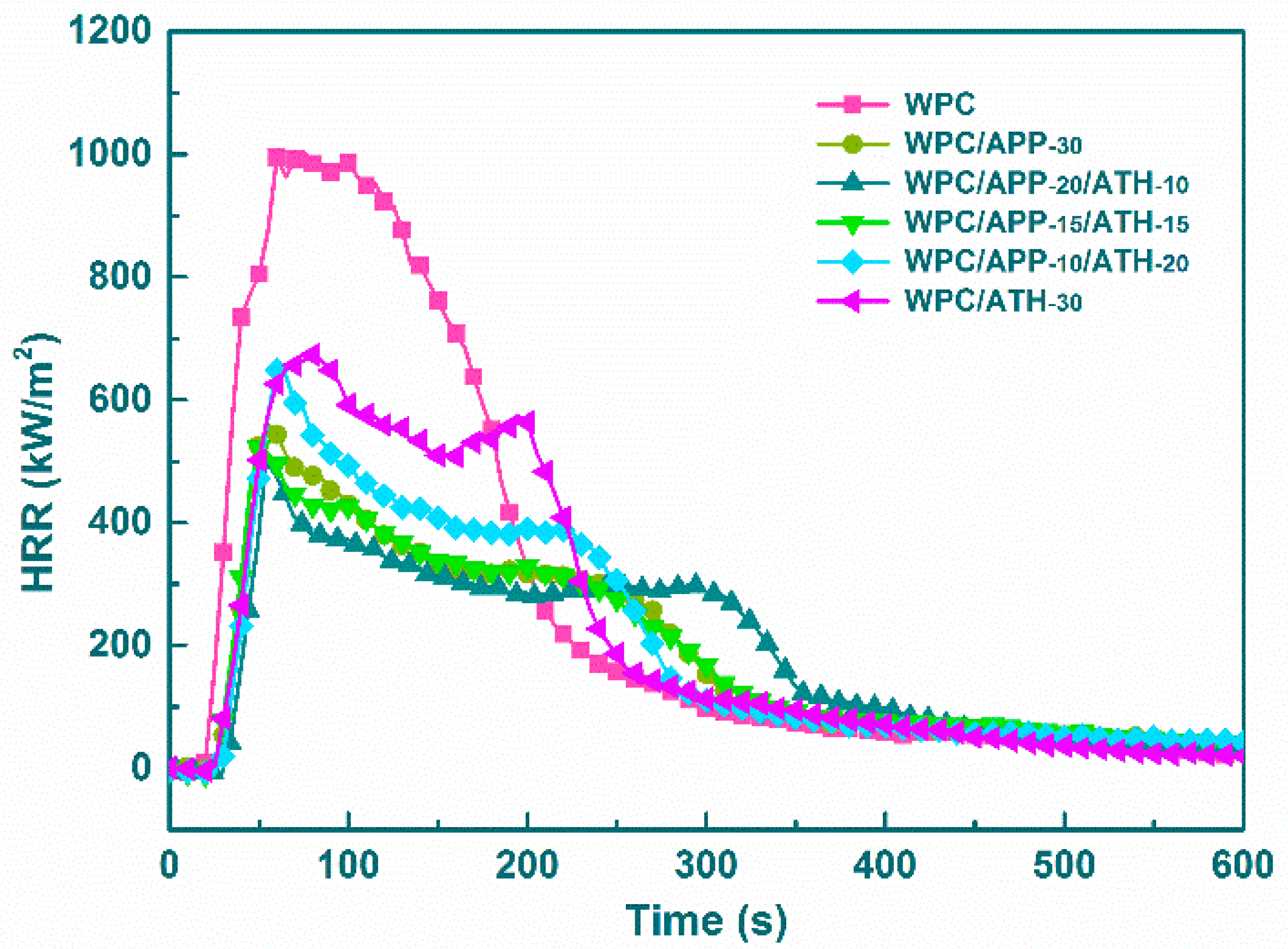
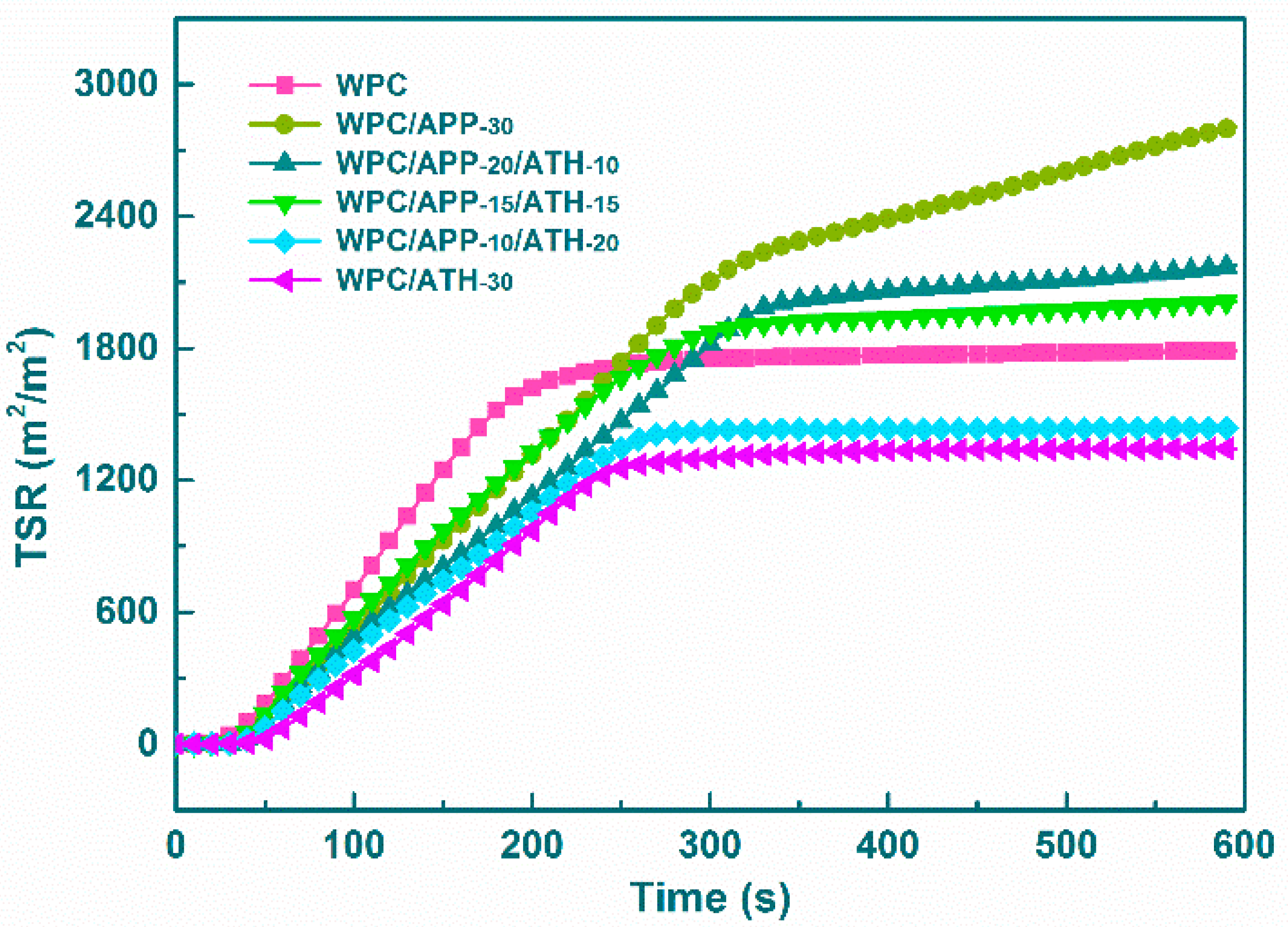
3.3. Cone Calorimetry
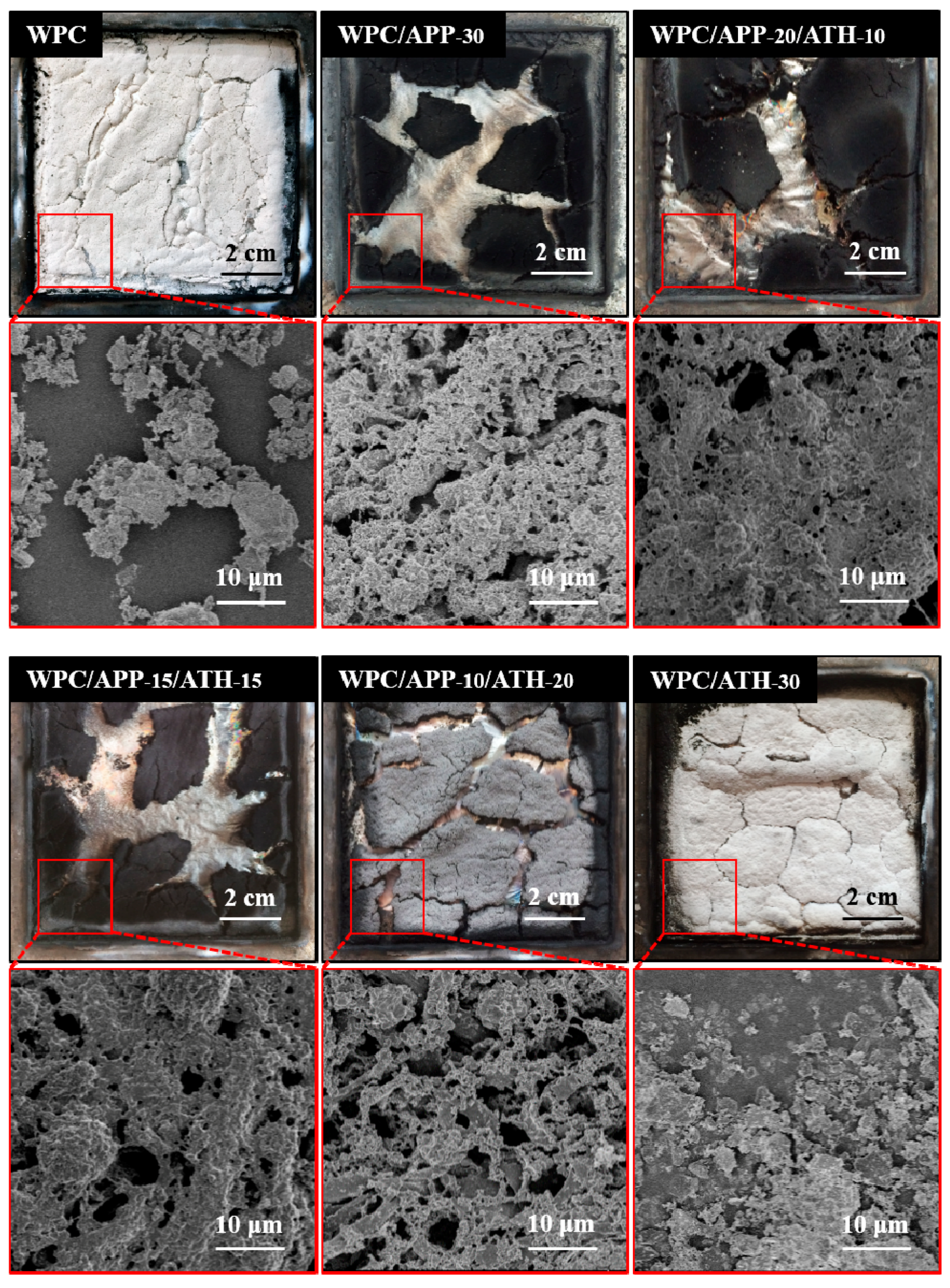
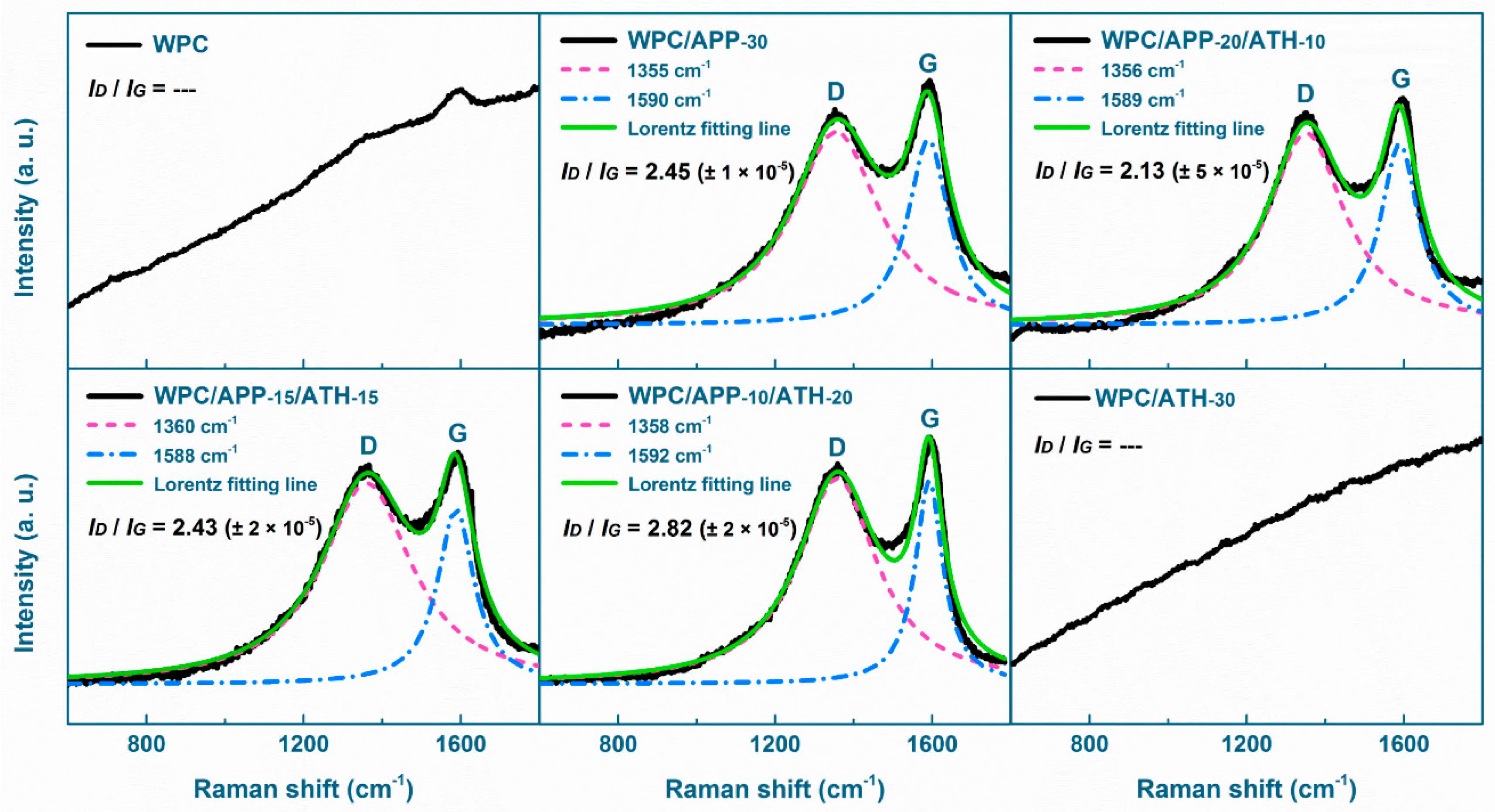
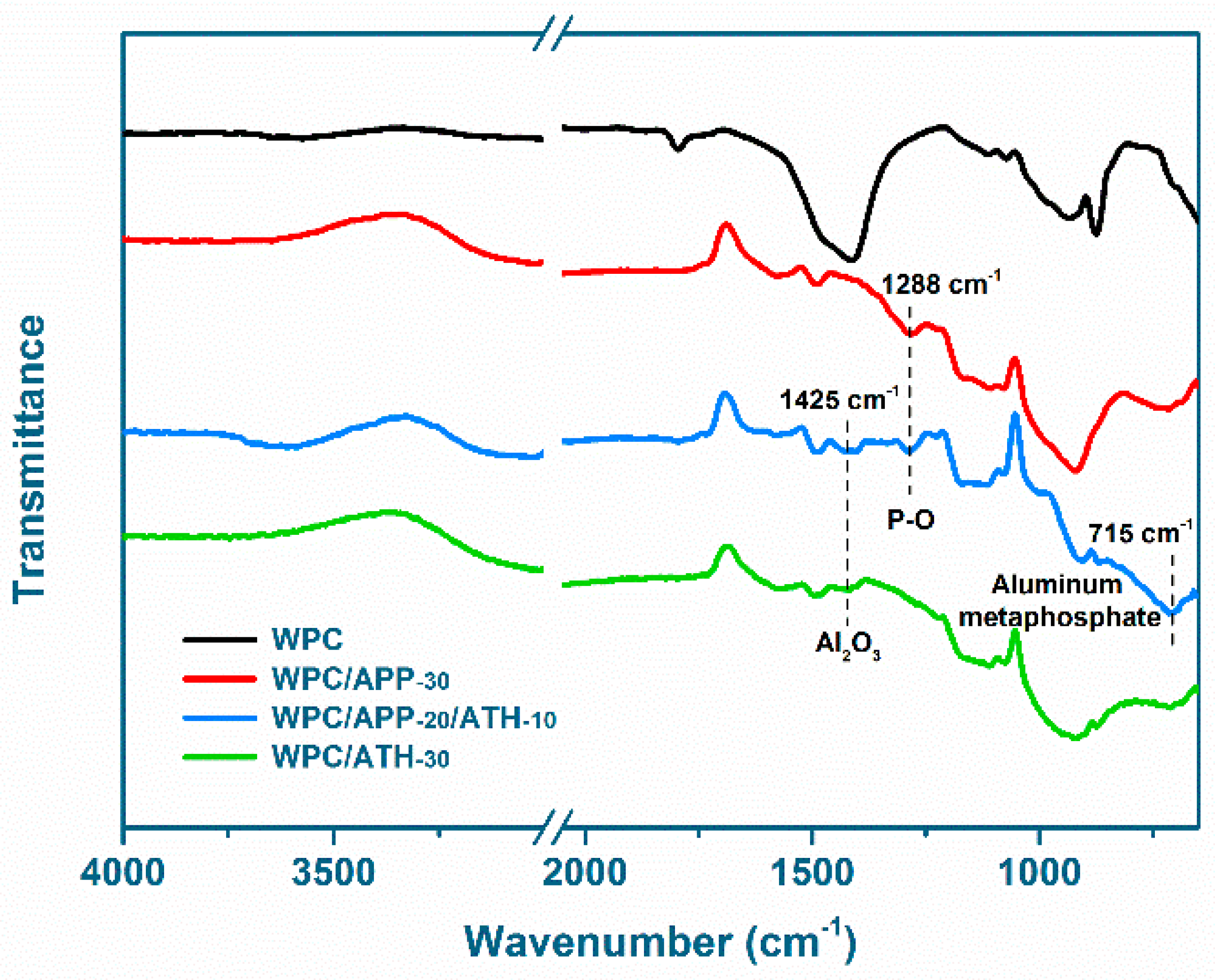
3.4. Residue Morphology and Structure
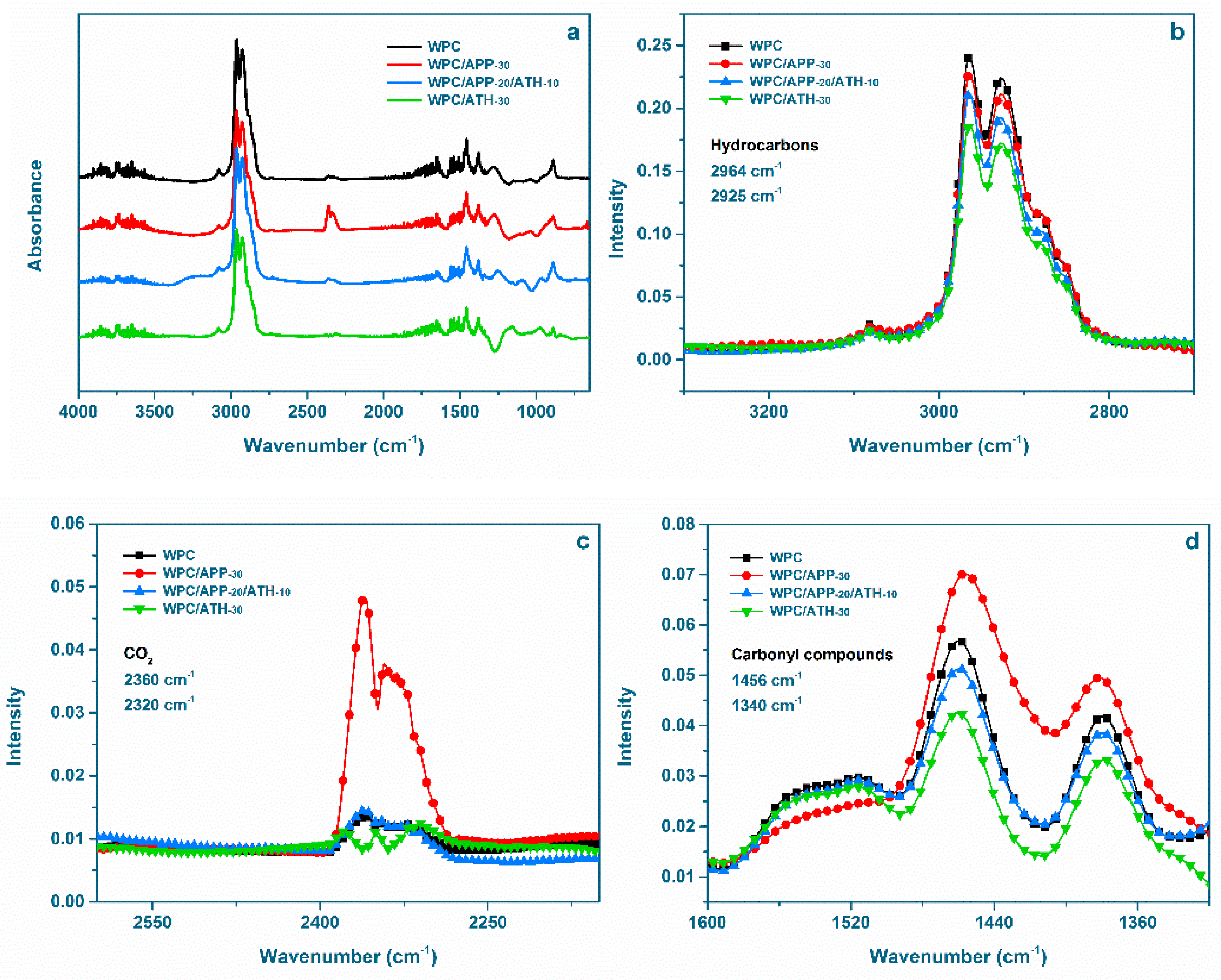
3.5. Evolved Gas Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Afrifah, K.A.; Hickok, R.A.; Matuana, L.M. Polybutene as a matrix for wood plastic composites. Compos. Sci. Technol. 2010, 70, 167–172. [Google Scholar] [CrossRef]
- Zhang, J.J.; Rizvi, G.M.; Park, C.B.; Hasan, M.M. Study on cell nucleation behavior of HDPE-wood composites/supercritical CO2 solution based on rheological properties. J. Mater. Sci. 2011, 46, 3777–3784. [Google Scholar] [CrossRef]
- Binhussain, M.A.; EI-Tonsy, M.M. Palm leave and plastic waste wood composite for out-door structures. Constr. Build. Mater. 2013, 47, 1431–1435. [Google Scholar] [CrossRef]
- Ayrilmis, N.; Kaymakci, A.; Güleç, T. Potential use of decayed wood in production of wood plastic composite. Ind. Crop. Prod. 2015, 74, 279–284. [Google Scholar] [CrossRef]
- Ashori, A. Wood-plastic composites as promising green-composites for automotive industries. Bioresour. Technol. 2008, 99, 4661–4667. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, S.G.; Bajwa, D.S.; Holt, G.; Coffelt, T.; Nakayama, F. Properties of thermoplastic composites with cotton and guayule biomass residues as fiber fillers. Ind. Crop. Prod. 2011, 33, 747–755. [Google Scholar] [CrossRef]
- Alamri, H.; Low, I.M. Effect of water absorption on the mechanical properties of nanoclay filled recycled cellulose fibre reinforced epoxy hybrid nanocomposites. Compos. Part A 2013, 44, 23–31. [Google Scholar] [CrossRef]
- Bazant, P.; Munster, L.; Machovsky, M.; Sedlak, J.; Pastorek, M.; Kozakova, Z.; Kuritka, I. Wood flour modifed by hierachical Ag/ZnO as potential filler for wood-plastic composites with enhanced surface antibacterial performance. Ind. Crop. Prod. 2014, 62, 179–187. [Google Scholar] [CrossRef]
- Fu, S.Y.; Song, P.G.; Yang, H.T.; Jin, Y.M.; Lu, F.Z.; Ye, J.W.; Wu, Q. Effect of carbon nanotubes and its functionalization on the thermal and flammability properites of polypropylene/wood flour composites. J. Mater. Sci. 2010, 45, 3520–3528. [Google Scholar] [CrossRef]
- Hapuarachchi, T.D.; Peigs, T. Multiwalled carbon nanotubes and sepiolite nanoclays as flame retardants for polylactide and its natural fibre reinforced composites. Compos. Part A 2010, 41, 954–963. [Google Scholar] [CrossRef]
- Suardana, N.P.G.; Ku, M.S.; Lim, J.K. Effects of diammonium phosphate on the flammability and mechanical properties of bio-composites. Mater. Des. 2011, 32, 1990–1999. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Zhang, J.; Lu, B.X.; Xin, Z.X.; Kang, C.K.; Kinn, J.K. Effect of flame retardants on mechanical properties, flammability and foamability of PP/wood-fiber composites. Compos. Part. B 2012, 43, 150–158. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, S.F.; Wang, F.; Yan, Y.T.; Li, J.Z.; Zhang, W. Effect of microencapsulated ammonium polyphosphate on flame retardancy and mechanical properties of wood flour/polypropylene composites. Polym. Compos. 2016, 37, 666–673. [Google Scholar] [CrossRef]
- Subasinghe, A.; Bhattacharyya, D. Performance of different intumescent ammonium polyphosphate flame retardants in PP/kenaf fibre composites. Compos. Part A 2014, 65, 91–99. [Google Scholar] [CrossRef]
- Schip, A.; Su, S. Effectiveness of pre-treated wood particles and halogen-free flame retardants used in wood-plastic composites. Polym. Degrad. Stab. 2016, 126, 81–92. [Google Scholar] [CrossRef]
- Faruk, O.; Matuana, L.M. Nanoclay reinforced HDPE as a matric for wood-plastic composites. Compos. Sci. Technol. 2008, 68, 2073–2077. [Google Scholar] [CrossRef]
- Chapple, S.; Anandjiwala, R. Flammability of natural fiber-reinforced composites and strategies for fire retardancy: A review. J. Thermoplast. Compos. Mater. 2010, 23, 871–893. [Google Scholar] [CrossRef]
- Deka, B.K.; Maji, T.K. Effect of TiO2 and nanoclay on the properties of wood polymer nanocompsotes. Compos. Part A 2011, 42, 2117–2125. [Google Scholar] [CrossRef]
- Ren, Y.L.; Wang, Y.L.; Wang, L.J.; Liu, T.T. Evaluation of intumescent fire retardants and synergistic agents for use on wood flour/recycled polypropylene composites. Constr. Build. Mater. 2015, 76, 273–278. [Google Scholar] [CrossRef]
- Mouritz, A.P.; Gibson, A.G. Fire Properties of Polymer Composite Materials; Springer: Dordrecht, The Netherlands, 2006; Volume 143. [Google Scholar]
- Rakotomalala, M.; Wagner, S.; Döring, M. Recent developments in halogen free flame retardants for epoxy resins for electrical and electronic applications. Materials 2010, 3, 4300–4327. [Google Scholar] [CrossRef] [PubMed]
- Winandy, J.E. State of the art paper: Effects of fire-retardant treatments on chemistry and engineering properties of wood. Wood Fiber Sci. 2013, 45, 131–148. [Google Scholar]
- Shao, Z.B.; Deng, C.; Tan, Y.; Chen, M.J.; Chen, L.; Wang, Y.Z. Flame retardation of polypropylene via a novel intumescent flame retardant: Ethylenediamine-modified ammonium polyphosphate. Polym. Degrad. Stab. 2014, 106, 88–96. [Google Scholar] [CrossRef]
- Lim, K.S.; Bee, S.T.; Sin, L.T.; Tee, T.T.; Ratnam, C.T.; Hui, D.; Rahmat, A.R. A review of application of ammonium polyphosphate as intumescent flame retardant in thermoplastic composites. Compos. Part B 2016, 84, 155–174. [Google Scholar] [CrossRef]
- Chen, X.L.; Jiang, Y.F.; Jiao, C.M. Smoke suppression properties of ferrite yellow on flame retardant thermoplastic polyurethane based on ammonium polyphosphate. J. Hazard Mater. 2014, 266, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, B.; Wartig, K.A.; Mülhaupt, R.; Schartel, B. Flame-retardancy properties of intumescent ammonium poly (phosphate) and mineral filler magnesium hydroxide in combination with graphene. Polymers 2014, 6, 2875–2895. [Google Scholar] [CrossRef]
- Zhao, X.L.; Chen, C.K.; Chen, X.L. Effects of carbon fibers on the flammability and smoke emission characteristics of halogen-free thermoplastic polyurethane/ammonium polyphosphate. J. Mater. Sci. 2016, 51, 3762–3771. [Google Scholar] [CrossRef]
- Stark, N.M.; White, R.H.; Mueller, S.A.; Osswald, T.A. Evaluation of various fire retardants for use in wood flour-polyethylene composites. Polym. Degrad. Stab. 2010, 95, 1903–1910. [Google Scholar] [CrossRef]
- Seefeldt, H.; Braun, U.; Wagner, M.H. Residue stabilization in the fire retardancy of wood-plastic composites: Combination of ammonium polyphosphate, expandable graphite, and red phosphorus. Macromol. Mater. Eng. 2012, 213, 2370–2377. [Google Scholar] [CrossRef]
- Shukor, F.; Hassan, A.; Islam, M.S.; Mokhtar, M.; Hasan, M. Effect of ammonium polyphosphate on flame retardancy, thermal stability and mechanical properties of alkali treated kenaf fiber filled PLA biocomposites. Mater. Des. 2014, 54, 425–429. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, W.; Zhang, S.F.; Li, J.Z. Preparation and characterization of microencapsulated ammonium polyphosphate with UMF and its application in WPCs. Constr. Build. Mater. 2014, 65, 151–158. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, W.; Chen, H.; Zhang, S.F.; Li, J.Z. Synergistic effect of synthetic zeolites on flame-retardant wood-flour/polypropylene composites. Constr. Build. Mater. 2015, 79, 337–344. [Google Scholar] [CrossRef]
- Carosio, F.; Alongi, J.; Malucelli, G. Layer by Layer ammonium polyphosphate-based coatings for flame retardancy of polyester-cotton blends. Carbohydr. Polym. 2012, 88, 1460–1469. [Google Scholar] [CrossRef]
- Ramazani, S.A.A.; Rahimi, A.; Frounchi, M.; Radman, S. Investigation of flame retardancy and physical-mechanical properties of zinc borate and alumnium hydroxide propylene composites. Mater. Des. 2008, 29, 1051–1056. [Google Scholar] [CrossRef]
- Witkowski, A.; Stec, A.A.; Hull, T.R. The influence of metal hydroxide fire retardants and nanoclay on the thermal decomposition of EVA. Polym. Degrad. Stab. 2012, 97, 2231–2240. [Google Scholar] [CrossRef]
- Liang, J.Z.; Zhang, Y.J. A study of the flame-retardant properties of polypropylene/Al(OH)3/Mg(OH)2 composites. Polym. Int. 2010, 59, 539–542. [Google Scholar] [CrossRef]
- Hippi, U.; Mattila, J.; Korhonen, M.; Seppälä, J. Compatibilization of polyethylene/aluminum hydroxide (PE/ATH) and polyethylene/magnesium hydroxide (PE/MH) composites with functionalized polyethylenes. Polymers 2003, 44, 1193–1201. [Google Scholar] [CrossRef]
- Zhang, L.; Li, C.Z.; Zhou, Q. Aluminum hydroxide filled ethylene vinyl acetate (EVA) composites: Effect of the interfacial compatibilizer and the particle size. J. Mater. Sci. 2007, 42, 4227–4232. [Google Scholar] [CrossRef]
- Jiao, C.M.; Chen, X.L. Flame retardant synergism of hydroxy silicone oil and Al(OH)3 in EVA Composite. Polym. Plast. Technol. Eng. 2009, 48, 665–670. [Google Scholar] [CrossRef]
- Cárdenas, M.A.; García-López, D.; Gobernado-Mitre, I.; Merino, J.C.; Pastor, J.M.; de D. Martinez, J.; Barbeta, J.; Calveras, D. Mechanical and fire retardant properties of EVA/clay/ATH nanocomposites—Effect of particle size and surface treatment of ATH filler. Polym. Degrad. Stab. 2008, 93, 2032–2037. [Google Scholar]
- Yen, Y.Y.; Wang, H.T.; Guo, W.J. Synergistic flame retardant effect of metal hydroxide and nanoclay in EVA composites. Polym. Degrad. Stab. 2012, 97, 863–869. [Google Scholar] [CrossRef]
- Castrovinci, A.; Gamino, G.; Dreyelle, C.; Duquesne, S.; Maginiez, C.; Vouters, M. Ammonium polyphosphate-aluminum trihydroxide antagonism in fire retarded butadiene-styrene block copolymer. Eur. Polym. J. 2005, 41, 2023–2033. [Google Scholar] [CrossRef]
- Qin, Z.L.; Li, D.H.; Li, Q.; Yang, R.J. Effect of nano-aluminum hydroxide on mechanical properties, flame retardancy and combustion behavior of intumescent flame retarded polypropylene. Mater. Des. 2016, 89, 988–995. [Google Scholar] [CrossRef]
- Bras, M.L.; Bugajny, M.; Lefebvre, J.M.; Bourbigot, S. Use of polyurethanes as char-forming agents in polypropylene intumescent formulations. Polym. Int. 2015, 49, 1115–1124. [Google Scholar] [CrossRef]
- Bourbigot, S.; Bras, M.L.; Duquesne, S.; Rochery, M. Recent advances for intumescent polymers. Macromol. Mater. Eng. 2004, 289, 499–511. [Google Scholar] [CrossRef]
- Cérard, C.; Fontaine, G.; Bellayer, S.; Bourbigot, S. Reaction to fire of an intumescent epoxy resin: Protection mechanisms and synergy. Polym. Degrad. Stab. 2012, 97, 1366–1386. [Google Scholar]
- Zammarano, M.; Franceschi, M.; Bellayer, S.; Gilman, J.W.; Meriani, S. Preparation and flame retardance properties of revolutionary self-extinguishing epoxy nanocomposites based on layered double hydroxides. Polymers 2005, 46, 9314–9328. [Google Scholar] [CrossRef]
- Ashori, A.; Sheshmani, S. Hybrid composites made from recycled materials: Moisture absorption and thickness swelling behavior. Bioresour. Technol. 2010, 101, 4717–4720. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.L.; Sain, M.; Cooper, P. Performance of natural-fiber-plastic composites under stress for outdoor applications: Effect of moisture, temperature, and ultraviolet light exposure. J. Appl. Polym. Sci. 2006, 99, 2570–2577. [Google Scholar] [CrossRef]
- Ayrilmis, N.; Benthien, J.T.; Thoemen, H.; White, R.H. Effects of fire-retardants on physical, mechanical, and fire properties of flat-pressed WPCs. Eur. J. Wood Wood Prod. 2012, 70, 215–224. [Google Scholar] [CrossRef]
- Wang, W.; Peng, Y.; Zhang, W.; Li, J.Z. Effect of pentaerythritol on the properties of wood-flour/polypropylene/ammonium polyphosphate composites system. BioResources 2015, 10, 6917–6927. [Google Scholar] [CrossRef]
- Mareri, P.; Bastide, S.; Binda, N.; Crespy, A. Mechanical behavior of polypropylene composites containing fine mineral filler: Effect of filler surface treatment. Compos. Sci. Technol. 1998, 58, 747–752. [Google Scholar] [CrossRef]
- Hirschler, M.M.; Shakir, S. Comparison of the fire performance of various upholstered furniture composite combinations (fabric/foam) in two rate of heat release calorimeters: Cone and Ohio State University instruments. J. Fire Sci. 1991, 9, 223–248. [Google Scholar] [CrossRef]
- Modesti, M.; Lorenzetti, A.; Simioni, F.; Checchin, M. Influence of different flame retardants on fire behaviour of modified PIR/PUR polymers. Polym. Degrad. Stab. 2001, 74, 475–479. [Google Scholar] [CrossRef]
- Hull, T.R.; Witkowski, A.; Hollingbery, L. Fire retardant action of mineral fillers. Polym. Degrad. Stab. 2011, 96, 1462–1469. [Google Scholar] [CrossRef]
- Subasingle, A.; Das, R.; Bhattacharyya, D. Study of thermal, flammability and mechanical properties of intumescent flame retardant PP/kenaf nanocomposites. Int. J. Smart Nano Mater. 2016, 7, 202–220. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Yu, B.; Duan, L.J.; Gui, Z.; Wang, B.B.; Hu, Y.; Yuen, R.K.K. Graphitic carbon nitride/phosphorus-rich aluminum phosphinates hybrids as smoke suppressants and flame retardants for polystyrene. J. Hazard Mater. 2017, 332, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Tai, Q.; Hu, Y.; Yuen, R.K.K.; Song, L.; Lu, H.D. Synthesis, structure-property relationship of polyphosphoramides with high char residues. J. Mater. Chem. 2011, 21, 6621. [Google Scholar] [CrossRef]
- Bras, M.L.; Bourbigot, S.; Tallec, Y.L.; Laureyns, J. Synergy in intumescence-application to β-cyclodextrin carbonization agent in intumescent addtives for fire retardant polyethylene formulations. Polym. Degrad. Stab. 1997, 56, 11–21. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.F.; Wang, W.; Pan, Y.; Song, L.; Hu, Y.; Liew, K.M. Formation of layer-by-layer assembled titanate nanotubes filled coating on flexible polyurethane foam with improved flame retardant and smoke suppression properties. ACS Appl. Mater. Interfaces 2015, 7, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Zhong, H.W.; Wei, Z.M.; Ning, H. FT-IR spectrometric analysis of aluminum triphosphate. Phys. Test. Chem. Anal. Part B 2009, 45, 765–771. [Google Scholar]
- Dong, Y.Y.; Gui, Z.; Hu, Y.; Wu, Y.; Jiang, S.H. The influence of titanate nanotube on the improved thermal properties and the smoke suppression in poly (methyl methacrylate). J. Hazard Mater. 2012, 209–210, 34–39. [Google Scholar] [CrossRef] [PubMed]

| Samples | PP (wt %) | WF (wt %) | PP-g-MAH (wt %) | APP (wt %) | ATH (wt %) |
|---|---|---|---|---|---|
| WPC | 55 | 40 | 5 | - | - |
| WPC/APP-30 | 37 | 28 | 5 | 30 | - |
| WPC/APP-20/ATH-10 | 37 | 28 | 5 | 20 | 10 |
| WPC/APP-15/ATH-15 | 37 | 28 | 5 | 15 | 15 |
| WPC/APP-10/ATH-20 | 37 | 28 | 5 | 10 | 20 |
| WPC/ATH-30 | 37 | 28 | 5 | - | 30 |
| Samples | Tmax1 a (°C) | Tmax2 b (°C) | Tmax3 c (°C) | Residue (wt %) |
|---|---|---|---|---|
| WPC | 330 | 462 | - | 10.8 |
| WPC/APP-30 | 303 | 473 | - | 27.3 |
| WPC/APP-20/ATH-10 | 299 | 474 | - | 28.6 |
| WPC/APP-15/ATH-15 | 300 | 473 | - | 28.2 |
| WPC/APP-10/ATH-20 | 296 | 472 | - | 27.3 |
| WPC/ATH-30 | 285 | 331 | 470 | 26.8 |
| Samples | IT | PHRR | AHRR | THR | Residue |
|---|---|---|---|---|---|
| (s) | (kW/m2) | (kW/m2) | (MJ/m2) | (wt %) | |
| WPC | 15 ± 2 | 1003 ± 3 | 292 ± 4 | 170 ± 3 | 6 ± 1 |
| WPC/APP-30 | 23 ± 2 | 560 ± 3 | 164 ± 2 | 124 ± 2 | 19 ± 1 |
| WPC/APP-20/ATH-10 | 29 ± 2 | 498 ± 3 | 164 ± 2 | 115 ± 2 | 27 ± 2 |
| WPC/APP-15/ATH-15 | 24 ± 2 | 522 ± 4 | 162 ± 1 | 119 ± 3 | 25 ± 1 |
| WPC/APP-10/ATH-20 | 24 ± 1 | 595 ± 3 | 173 ± 3 | 128 ± 2 | 25 ± 2 |
| WPC/ATH-30 | 24 ± 2 | 673 ± 2 | 184 ± 3 | 136 ± 1 | 22 ± 1 |
| Samples | Yco | Yco2 | CO/CO2 | TSR |
|---|---|---|---|---|
| (kg/kg) | (kg/kg) | Weight Ratio | (m2/m2) | |
| WPC | 0.071 ± 0.001 | 3.91 ± 0.01 | 0.018 ± 0.001 | 1789 ± 11 |
| WPC/APP-30 | 0.227 ± 0.007 | 2.56 ± 0.02 | 0.089 ± 0.003 | 2808 ± 15 |
| WPC/APP-20/ATH-10 | 0.222 ± 0.018 | 2.57 ± 0.01 | 0.086 ± 0.007 | 2176 ± 16 |
| WPC/APP-15/ATH-15 | 0.216 ± 0.011 | 2.75 ± 0.01 | 0.078 ± 0.004 | 2012 ± 13 |
| WPC/APP-10/ATH-20 | 0.103 ± 0.002 | 3.13 ± 0.03 | 0.033 ± 0.001 | 1437 ± 19 |
| WPC/ATH-30 | 0.053 ± 0.001 | 3.38 ± 0.02 | 0.016 ± 0.001 | 1344 ± 13 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Peng, Y.; Zammarano, M.; Zhang, W.; Li, J. Effect of Ammonium Polyphosphate to Aluminum Hydroxide Mass Ratio on the Properties of Wood-Flour/Polypropylene Composites. Polymers 2017, 9, 615. https://doi.org/10.3390/polym9110615
Wang W, Peng Y, Zammarano M, Zhang W, Li J. Effect of Ammonium Polyphosphate to Aluminum Hydroxide Mass Ratio on the Properties of Wood-Flour/Polypropylene Composites. Polymers. 2017; 9(11):615. https://doi.org/10.3390/polym9110615
Chicago/Turabian StyleWang, Wen, Yao Peng, Mauro Zammarano, Wei Zhang, and Jianzhang Li. 2017. "Effect of Ammonium Polyphosphate to Aluminum Hydroxide Mass Ratio on the Properties of Wood-Flour/Polypropylene Composites" Polymers 9, no. 11: 615. https://doi.org/10.3390/polym9110615