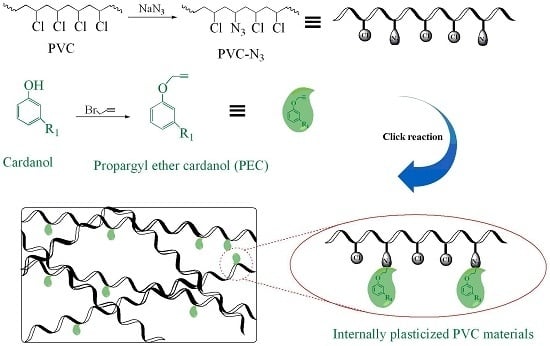
Cardanol Groups Grafted on Poly(vinyl chloride)—Synthesis, Performance and Plasticization Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Propargyl Ether Cardanol
2.3. Synthesis of Azide-Functionalized PVC (PVC-N3)
2.4. Synthesis of Internally Plasticized PVC Materials
2.5. Preparation of PVC Films and PVC–DOP Films
2.6. Characterization
2.6.1. Elemental Analysis
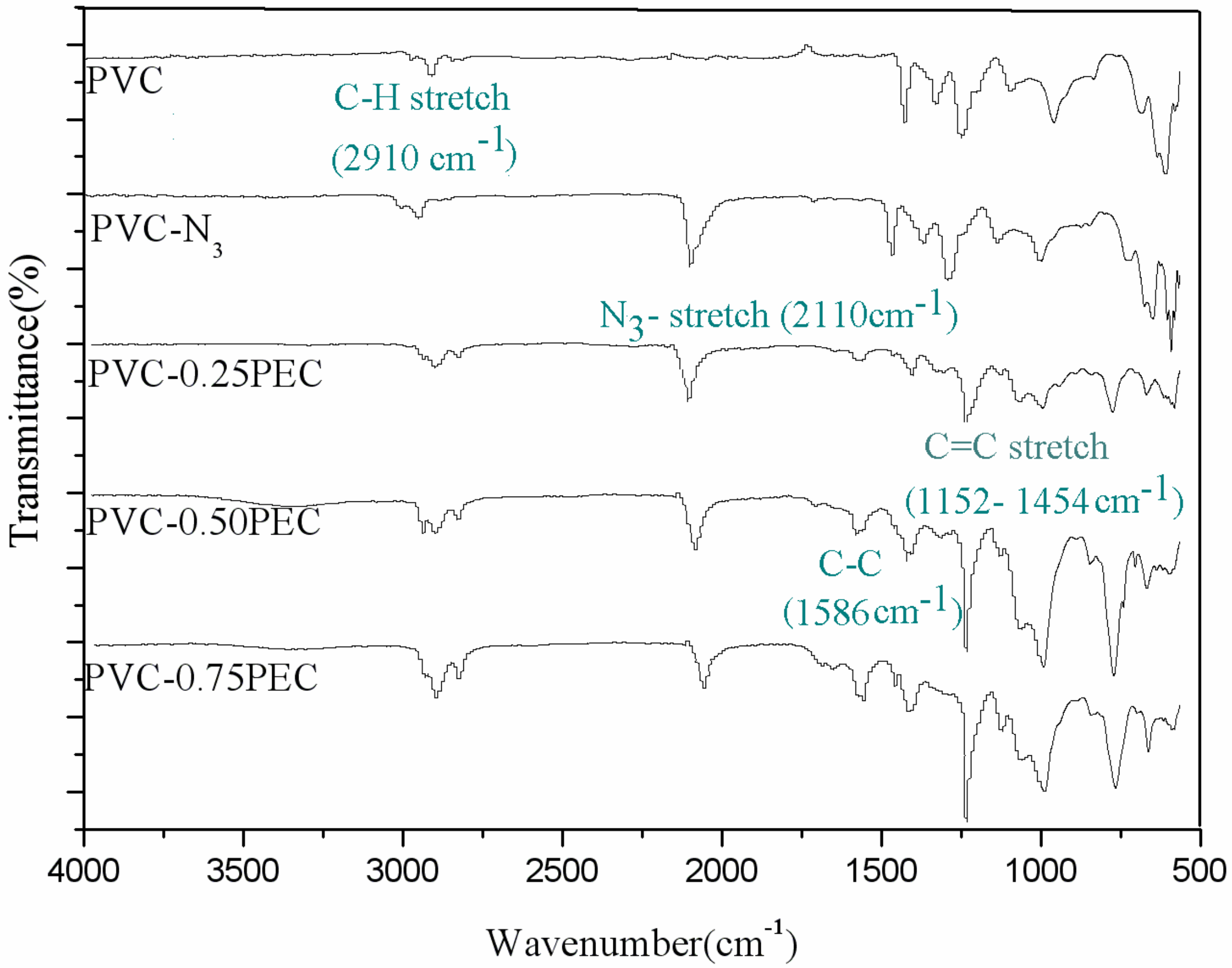
2.6.2. Fourier-Transform Infrared (FT-IR)
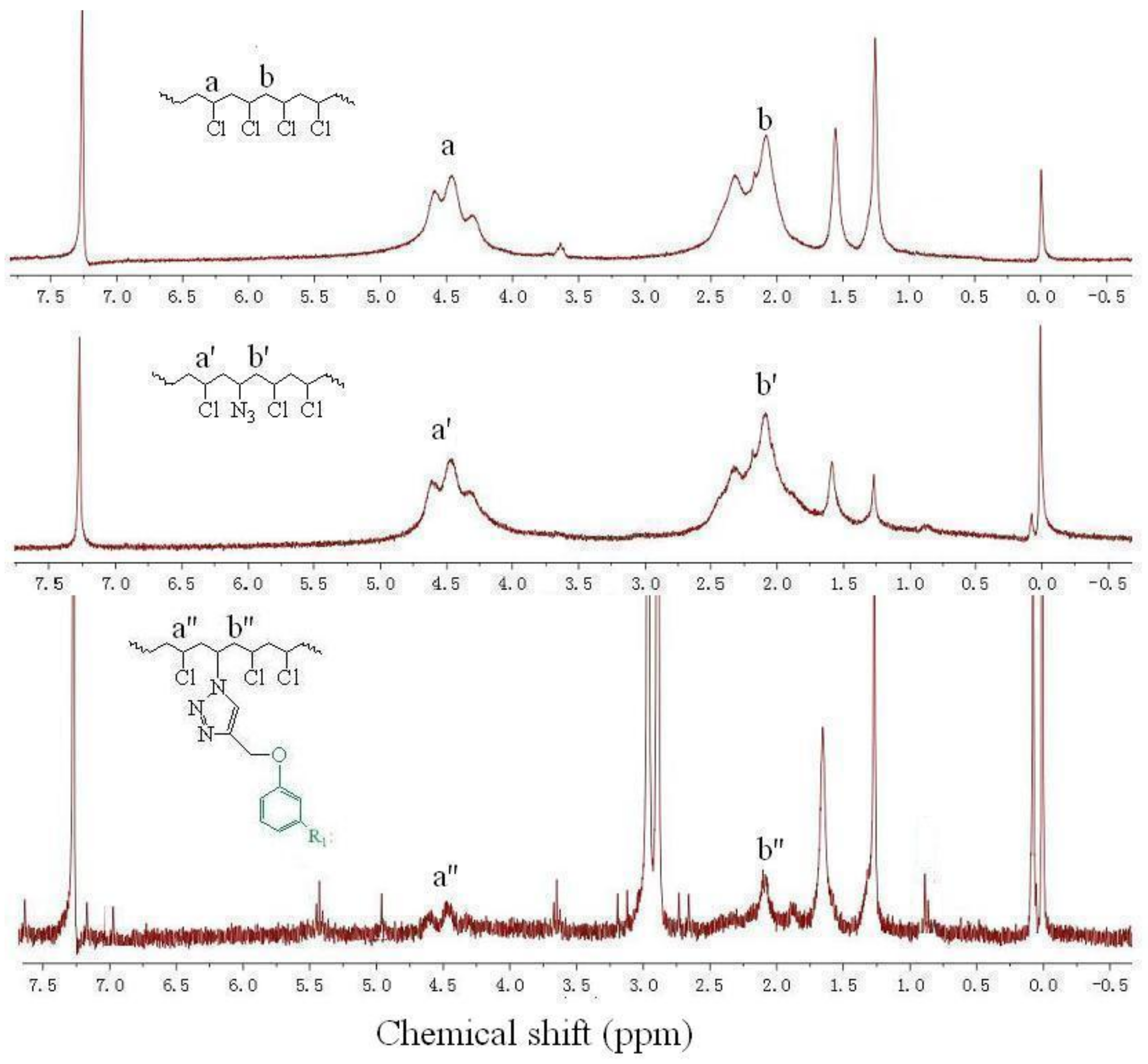
2.6.3. 1H Nuclear Magnetic Resonance (NMR)
2.6.4. Gel Permeation Chromatography (GPC)
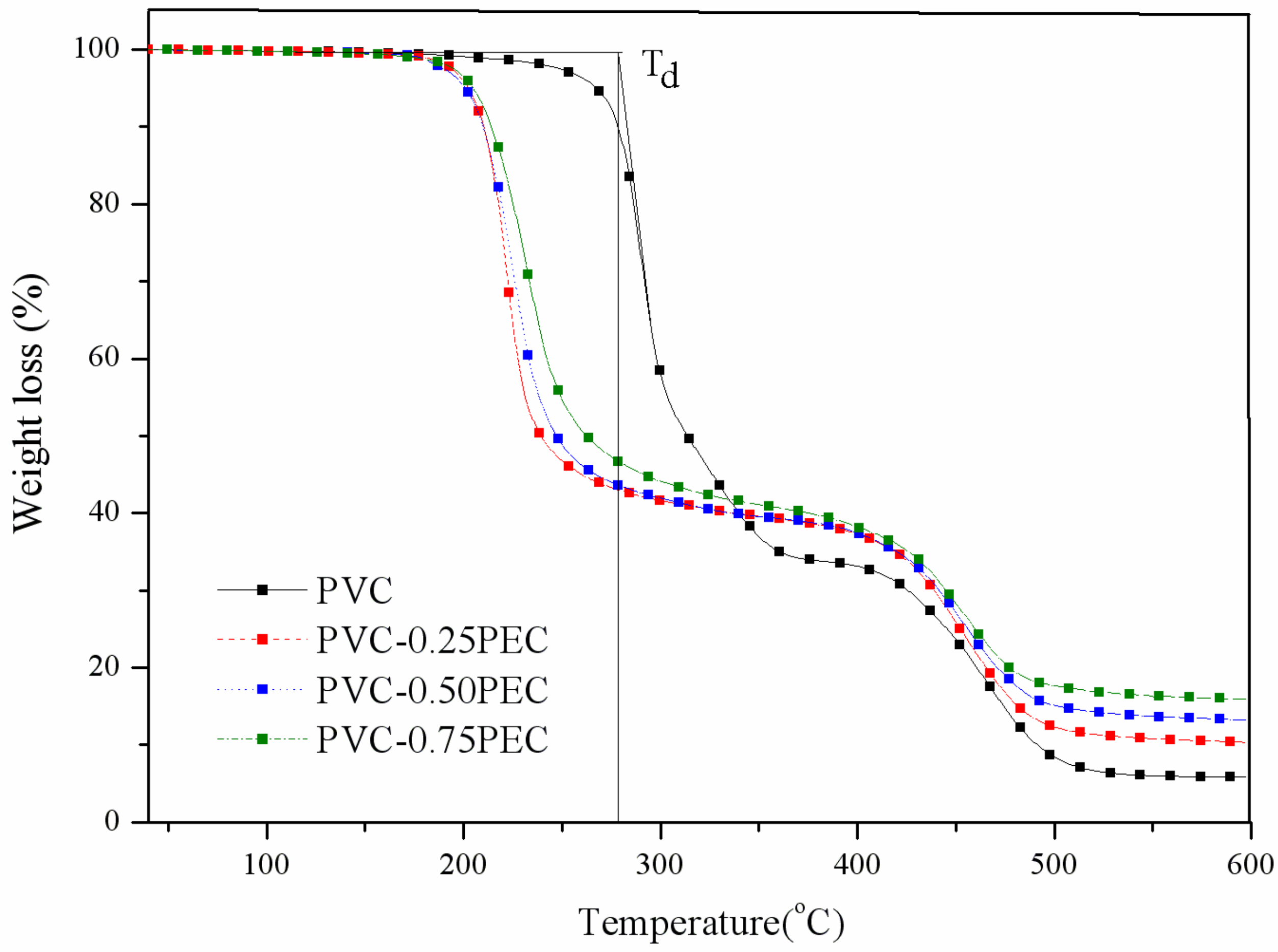
2.6.5. Thermogravimetric Analysis (TGA)
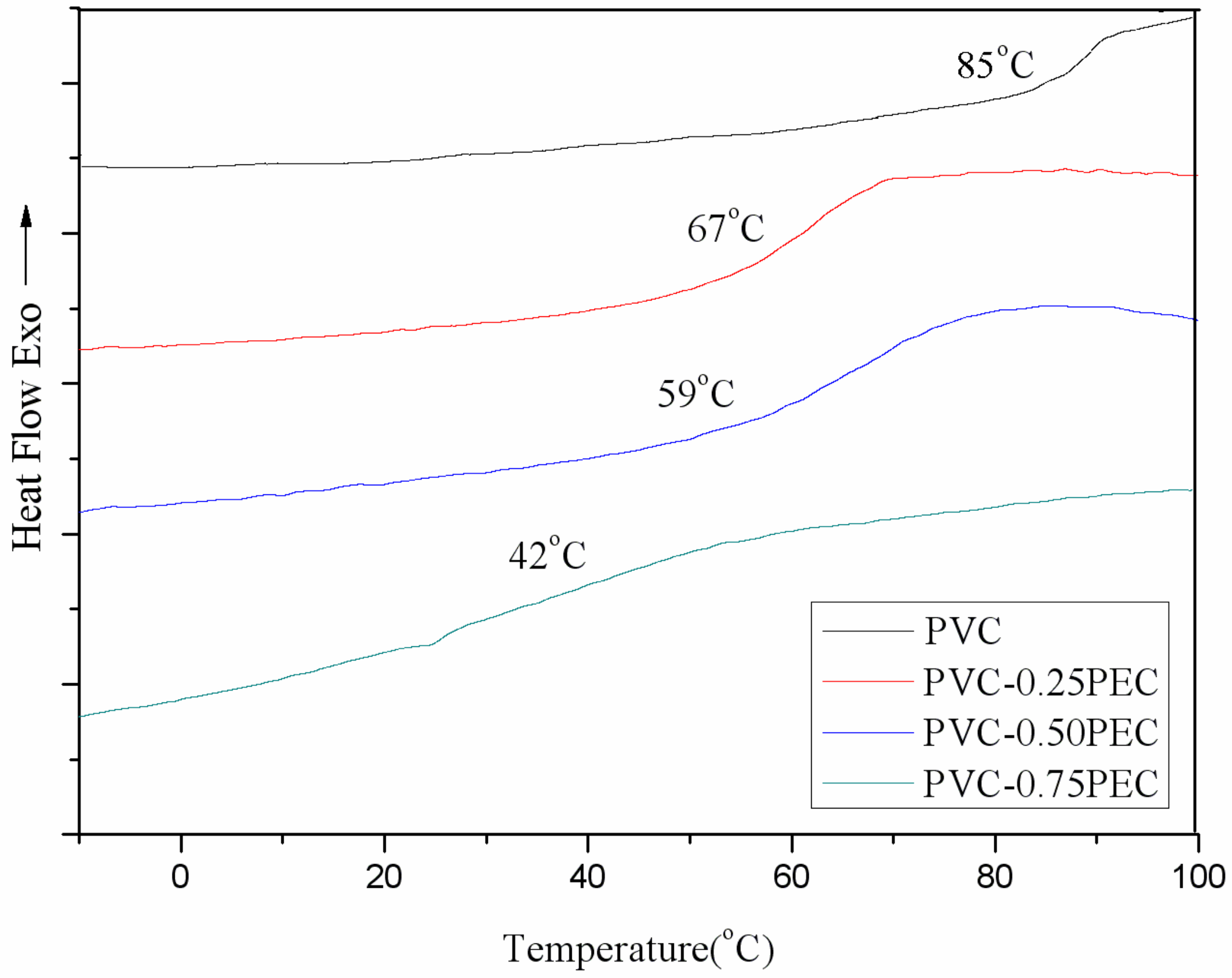
2.6.6. Differential Scanning Calorimetry (DSC)
2.6.7. Leaching Tests
3. Results
3.1. Chemical Structure of PEC
3.2. Chemical Structure of Internally Plasticized PVC Materials
3.3. Performances of Internally Plasticized PVC Materials
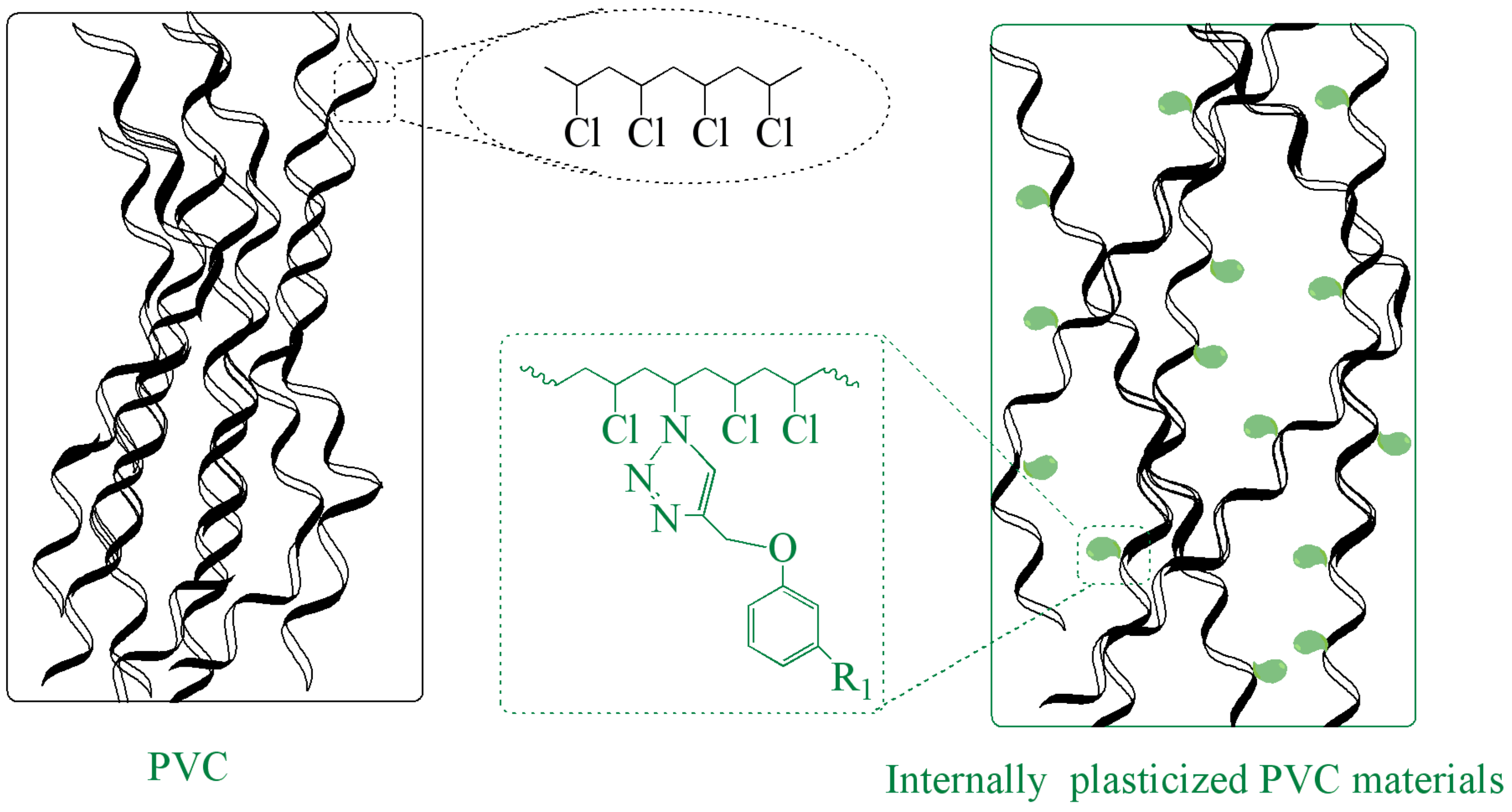
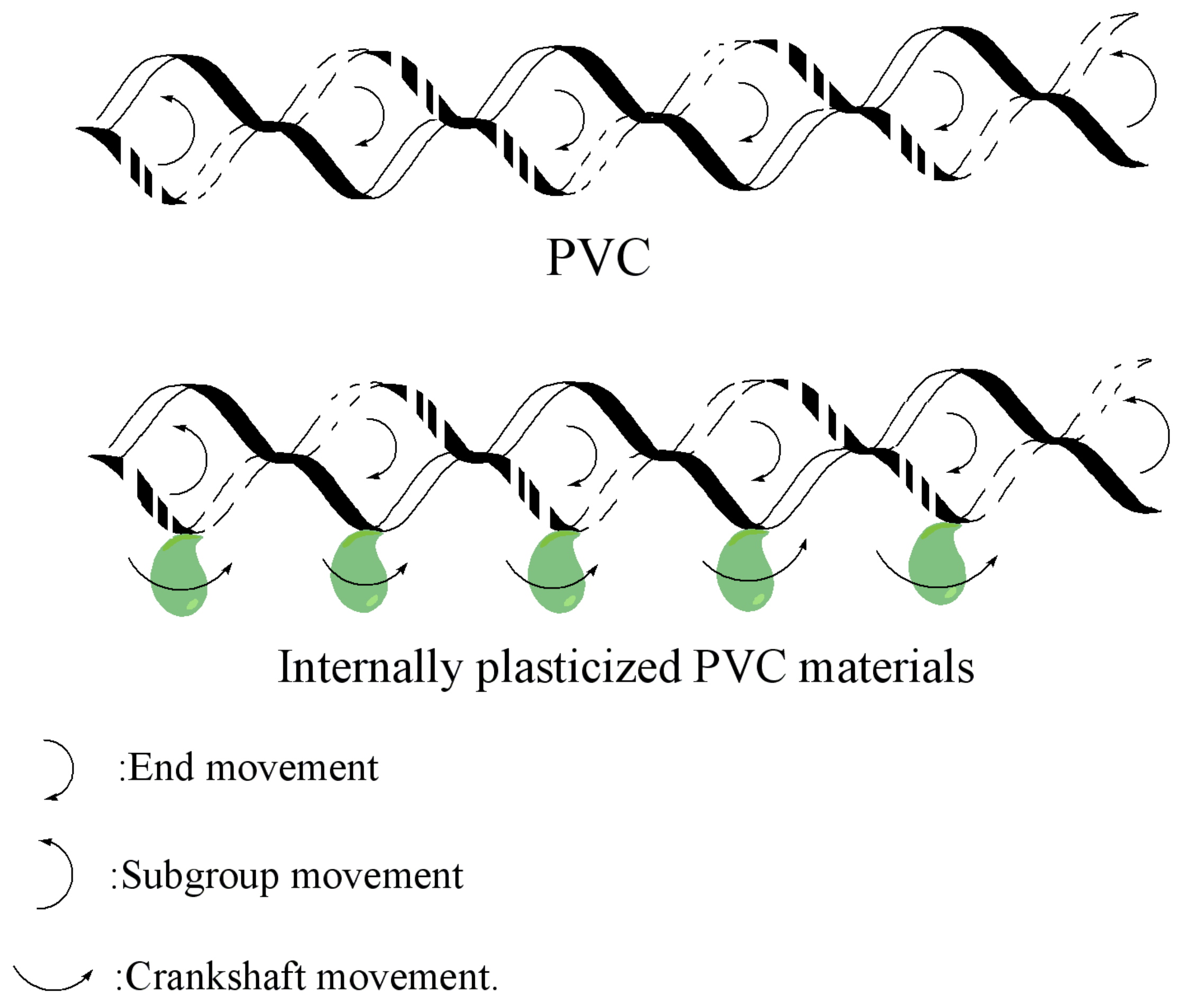
3.4. Mechanism of Internal Plasticization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bocque, M.; Voirin, C.; Lapinte, V.; Caillol, S.; Robin, J.J. Petro-based and bio-based plasticizers: Chemical structures to plasticizing properties. J. Polym. Sci. Part B Polym. Chem. 2016, 54, 11–13. [Google Scholar] [CrossRef]
- Boisvert, A.; Jones, S.; Issop, L.; Erythropel, H.C.; Papadopoulos, V.; Culty, M. In vitro functional screening as a means to identify new plasticizers devoid of reproductive toxicity. Environ. Res. 2016, 150, 496–512. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.T.; Giovanoulis, G.; Cousins, A.P.; Magnér, J.; Cousins, I.T.; Wit, C.C. Human exposure, hazard and risk of alternative plasticizers to phthalate esters. Sci. Total 2016, 541, 451–467. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Albaladejo, E.; Fernandes, D.; Lacorte, S.; Porte, C. Comparative toxicity, oxidative stress and endocrine disruption potential of plasticizers in JEG-3 human placental cells. Toxicol. In Vitro 2017, 38, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Chieng, B.W.; Ibrahim, N.A.; Then, Y.Y.; Loo, Y.Y. Epoxidized jatropha oil as a sustainable plasticizer to poly(lactic acid). Polymers 2017, 9, 204. [Google Scholar] [CrossRef]
- Greco, A.; Maffezzoli, A. Cardanol derivatives as innovative bio-plasticizers for poly-(lactic acid). Polym. Degrad. Stabil. 2016, 132, 213–219. [Google Scholar] [CrossRef]
- Jarray, A.; Gerbaud, V.; Hemati, M. Polymer-plasticizer compatibility during coating formulation: A multi-scale investigation. Prog. Org. Coat. 2016, 101, 195–206. [Google Scholar] [CrossRef]
- Jia, P.Y.; Zhang, M.; Hu, L.; Feng, G.; Bo, C.; Zhou, Y. Design and synthesis of a castor oil based plasticizer containing THEIC and diethyl phosphate groups for the preparation of flame-retardant PVC materials. RSC Adv. 2017, 7, 897–903. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, S.; Guo, W.; Wang, P.; Xing, W.; Song, L.; Hu, Y. Renewable cardanol-based phosphate as a flame retardant toughening agent for epoxy resins. ACS Sustain. Chem. Eng. 2017, 5, 3409–3416. [Google Scholar] [CrossRef]
- Navarro, R.; Perrino, M.P.; Tardajos, M.G.; Reinecke, H. Phthalate Plasticizers Covalently Bound to PVC: Plasticization with suppressed migration. Macromolecules 2010, 43, 2377–2381. [Google Scholar] [CrossRef]
- Lee, K.W.; Chung, J.W.; Kwak, S. Structurally enhanced self-plasticization of poly(vinyl chloride) via click grafting of hyperbranched polyglycerol. Macromol. Rapid Commum. 2016, 37, 2045. [Google Scholar] [CrossRef] [PubMed]
- Navarro, R.; Perrino, M.P.; García, C.; Elvira, C.; Gallardo, A.; Reinecke, H. Highly flexible PVC materials without plasticizer migration as obtained by efficient one-pot procedure using trichlorotriazine chemistry. Macromolecules 2016, 49, 2224–2227. [Google Scholar] [CrossRef]
- Jia, P.Y.; Hu, L.; Feng, G.; Bo, C.; Zhang, M.; Zhou, Y. PVC materials without migration obtained by chemical modification of azide-functionalized PVC and triethyl citrate plasticizer. Mater. Chem. Phys. 2017, 190, 25–30. [Google Scholar] [CrossRef]
- Jia, P.Y.; Hu, L.; Zhang, M.; Feng, G.; Zhou, Y. Phosphorus containing castor oil based derivatives: Potential non-migratory flame retardant plasticizer. Eur. Polym. J. 2017, 87, 209–220. [Google Scholar] [CrossRef]
- Fouquet, T.; Fetzer, L.; Mertz, G.; Puchot, L.; Verge, P. Photoageing of cardanol: Characterization, circumvention by side chain methoxylation and application for photocrosslinkable polymers. RSC Adv. 2015, 5, 54899–54912. [Google Scholar] [CrossRef]
- Gour, R.S.; Kodgire, V.V.; Badiger, M.V.J. Toughening of epoxy novolac resin using cardanol based flexibilizers. Appl. Polym. Sci. 2016, 133, 43318. [Google Scholar] [CrossRef]
- Perdriau, S.; Harder, S.; Heeres, H.J.; de Vries, J.G. Selective conversion of polyenes to monoenes by RuCl3-catalyzed transfer hydrogenation: The case of cashew nutshell liquid. ChemSusChem 2012, 5, 2427–2434. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Yang, J.; Sun, H.; Fan, H.; Chen, Y.; Wang, F.; Shi, B. Novel environmentally sustainable cardanol-based plasticizer covalently bound to PVC via click chemistry: Synthesis and properties. RSC Adv. 2015, 5, 16980–16985. [Google Scholar] [CrossRef]
- Jia, P.Y.; Hu, L.H.; Shang, Q.Q.; Wang, R.; Zhang, M.; Zhou, Y. Self-Plasticization of PVC materials via chemical modification of mannich base of cardanol butyl ether. ACS Sustain. Chem. Eng. 2017, 5, 6665–6673. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, J.; Chen, S.; Zhou, Y. Synthesis and fire properties of rigid polyurethane foams made from a polyol derived from melamine and cardanol. Polym. Degrad. Stabil. 2014, 110, 27–34. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Z.; Jiang, J.; Nie, X.; Zhou, Y.; Murray, R.E. A novel biobased plasticizer of epoxidized cardanol glycidyl ether: Synthesis and application in soft poly(vinyl chloride)films. RSC Adv. 2015, 5, 56171–56180. [Google Scholar] [CrossRef]
- Yao, L.L.; Chen, C.; Xu, W.; Ye, Z.; Shen, Z.; Chen, M. Preparation of cardanol based epoxy plasticizer by click chemistry and its action on poly(vinyl chloride). J. Appl. Polym. Sci. 2017. [Google Scholar] [CrossRef]
- Lafarge, J.; Kebir, N.; Schapman, D.; Gadenne, V.; Burel, F. Design of bacteria repellent PVC surfaces using the click chemistry. Cellulose 2013, 20, 2779–2790. [Google Scholar] [CrossRef]
- Kiskan, B.; Demiray, G.; Yagci, Y. Thermally curable polyvinylchloride via click chemistry. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3512–3518. [Google Scholar] [CrossRef]
- Akat, H.; Ozkan, M. Synthesis and characterization of poly (vinylchloride) type macrophotoinitiator comprising side-chain thioxanthone via click chemistry. eXPRESS Polym. Lett. 2011, 5, 318–326. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, J.; Knothe, G.; Nie, X.; Jiang, J. Synthesis of epoxidized cardanol and its antioxidative properties for vegetable oils and biodiesel. ACS Sustain. Chem. Eng. 2016, 4, 901–906. [Google Scholar] [CrossRef]
- Chen, J.; Nie, X.; Liu, Z.; Mi, Z.; Zhou, Y. Synthesis and application of polyepoxide cardanol glycidyl ether as biobased polyepoxide reactive diluent for epoxy resin. ACS Sustain. Chem. Eng. 2015, 3, 1164–1171. [Google Scholar] [CrossRef]
- Huang, K.; Zhang, Y.; Li, M.; Lian, J.; Yang, X.; Xia, J. Preparation of a light color cardanol-based curing agent and epoxy resin composite: Cure-induced phase separation and its effect on properties. Prog. Org. Coat. 2012, 74, 240–247. [Google Scholar] [CrossRef]
- Bowers, B.F.; Huang, B.; Shu, X.; Miller, B.C. Investigation of reclaimed asphalt pavement blending efficiency through GPC and FTIR. Constr. Build. Mater. 2014, 50, 517–523. [Google Scholar] [CrossRef]
- Geng, J.; Li, H.; Sheng, Y. Changing regularity of SBS in the aging process of polymer modified asphalt binder based on GPC analysis. Int. J. Pavement Res. Technol. 2014, 7, 77–82. [Google Scholar]
- Mauritz, K.A.; Storey, R.F.; Wilson, B.S. Efficiency of plasticization of PVC by higher-order di-alkyl phthalates and survey of mathematical models for prediction of polymer/diluent blend Tg’s. J. Vinyl Technol. 1990, 12, 165–1173. [Google Scholar] [CrossRef]
- Caiying, B.; Lihong, H.; Puyou, J.; Bingchuan, L.; Jing, Z.; Yonghong, Z. Structure and thermal properties of phosphoruscontaining polyol synthesized from cardanol. RSC Adv. 2015, 5, 106651–106660. [Google Scholar]
- Jie, C.; Xiaoying, L.; Yigang, W.; Ke, L.; Jinrui, W.; Jianchun, J.; Xiaoan, N. Synthesis and application of a novel environmental plasticizer based on cardanol for poly(vinyl chloride). J. Taiwan Inst. Chem. Eng. 2016, 65, 488–497. [Google Scholar]
- Xiaoying, L.; Xiaoan, N.; Jie, C.; Yigang, W.; Ke, L. Synthesis and Application of a Novel Epoxidized Plasticizer Based on Cardanol for Poly(vinyl chloride). J. Renew. Mater. 2017, 5, 154–164. [Google Scholar]
- Daniels, P.H. A brief overview of theories of PVC plasticization and methods used to evaluate PVC-plasticizer interaction. J. Vinyl Addit. Technol. 2009, 15, 219–223. [Google Scholar] [CrossRef]
- Cadogan, D.F.; Howick, C.J. Plasticizers in Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley and Sons: New York, NY, USA, 1996. [Google Scholar]
- Clark, F.W. Plasticizer. Chem. Ind. 1941, 60, 225–230. [Google Scholar]
- Wancong, S.; Zhibo, S.; Pingping, J. Plasticizers and Their Applications, 2nd ed.; Chemical Industry Press: Beijing, China, 2004; pp. 536–538. [Google Scholar]
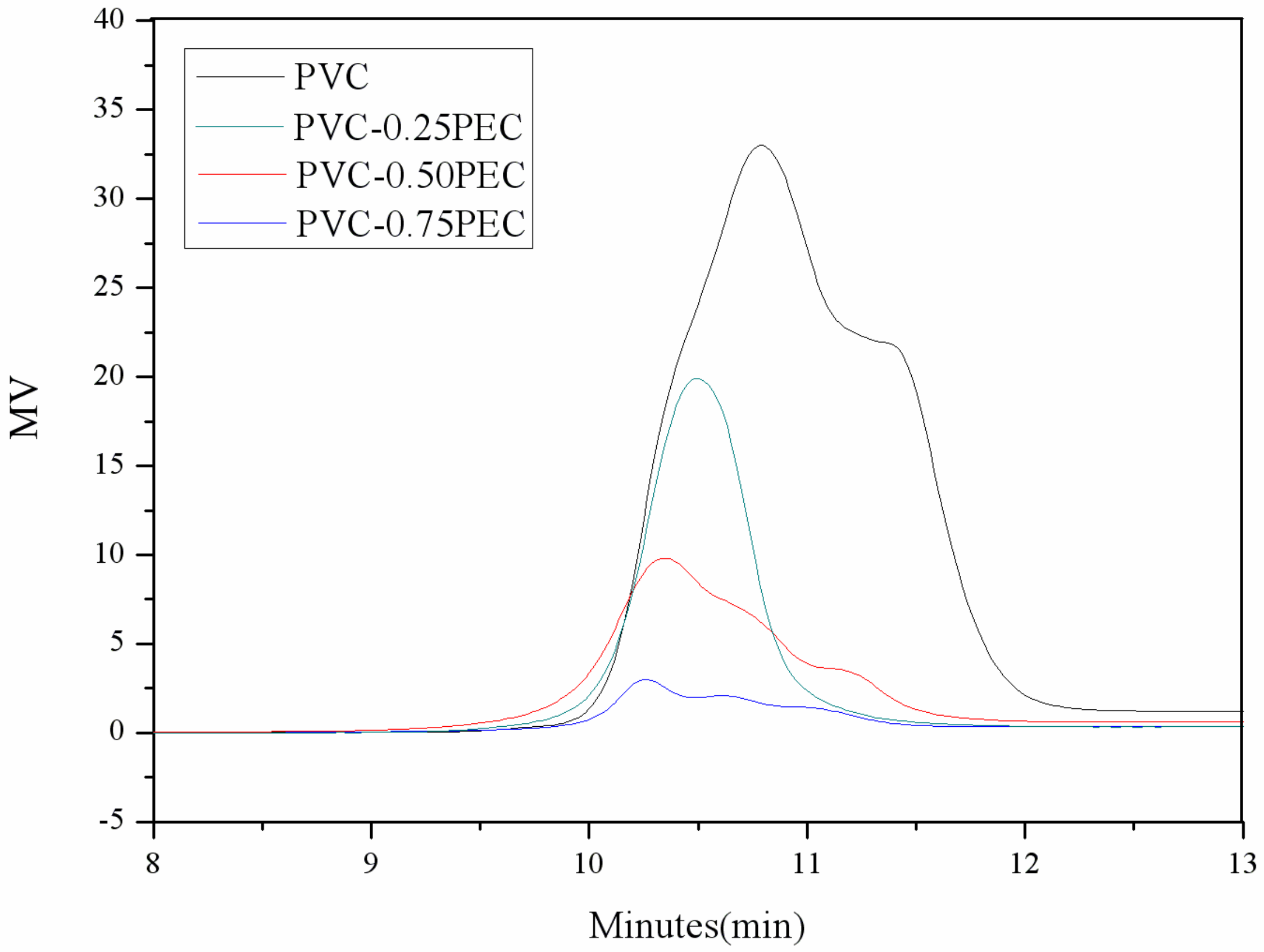
| PVC materials | Number average molecular weight (Mn/g·mol−1) | Weight-average molecular weight (MW/g·mol−1) | Polydispersity indices |
|---|---|---|---|
| PVC | 15,100 | 18,900 | 1.2 |
| PVC-0.25PEC | 21,200 | 26,700 | 1.2 |
| PVC-0.50PEC | 23,800 | 30,200 | 1.3 |
| PVC-0.75PEC | 25,400 | 31,900 | 1.3 |
| PVC materials | Td (°C) | Tg (°C) | Char residue (%) |
|---|---|---|---|
| PVC | 278.4 | 85 | 5.8 |
| PVC-0.25PEC | 211.3 | 67 | 10.0 |
| PVC-0.50PEC | 209.4 | 59 | 16.5 |
| PVC-0.75PEC | 208.6 | 42 | 17.6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, P.; Zhang, M.; Hu, L.; Wang, R.; Sun, C.; Zhou, Y. Cardanol Groups Grafted on Poly(vinyl chloride)—Synthesis, Performance and Plasticization Mechanism. Polymers 2017, 9, 621. https://doi.org/10.3390/polym9110621
Jia P, Zhang M, Hu L, Wang R, Sun C, Zhou Y. Cardanol Groups Grafted on Poly(vinyl chloride)—Synthesis, Performance and Plasticization Mechanism. Polymers. 2017; 9(11):621. https://doi.org/10.3390/polym9110621
Chicago/Turabian StyleJia, Puyou, Meng Zhang, Lihong Hu, Rui Wang, Chao Sun, and Yonghong Zhou. 2017. "Cardanol Groups Grafted on Poly(vinyl chloride)—Synthesis, Performance and Plasticization Mechanism" Polymers 9, no. 11: 621. https://doi.org/10.3390/polym9110621