Polymeric Amines and Ampholytes Derived from Poly(acryloyl chloride): Synthesis, Influence on Silicic Acid Condensation and Interaction with Nucleic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
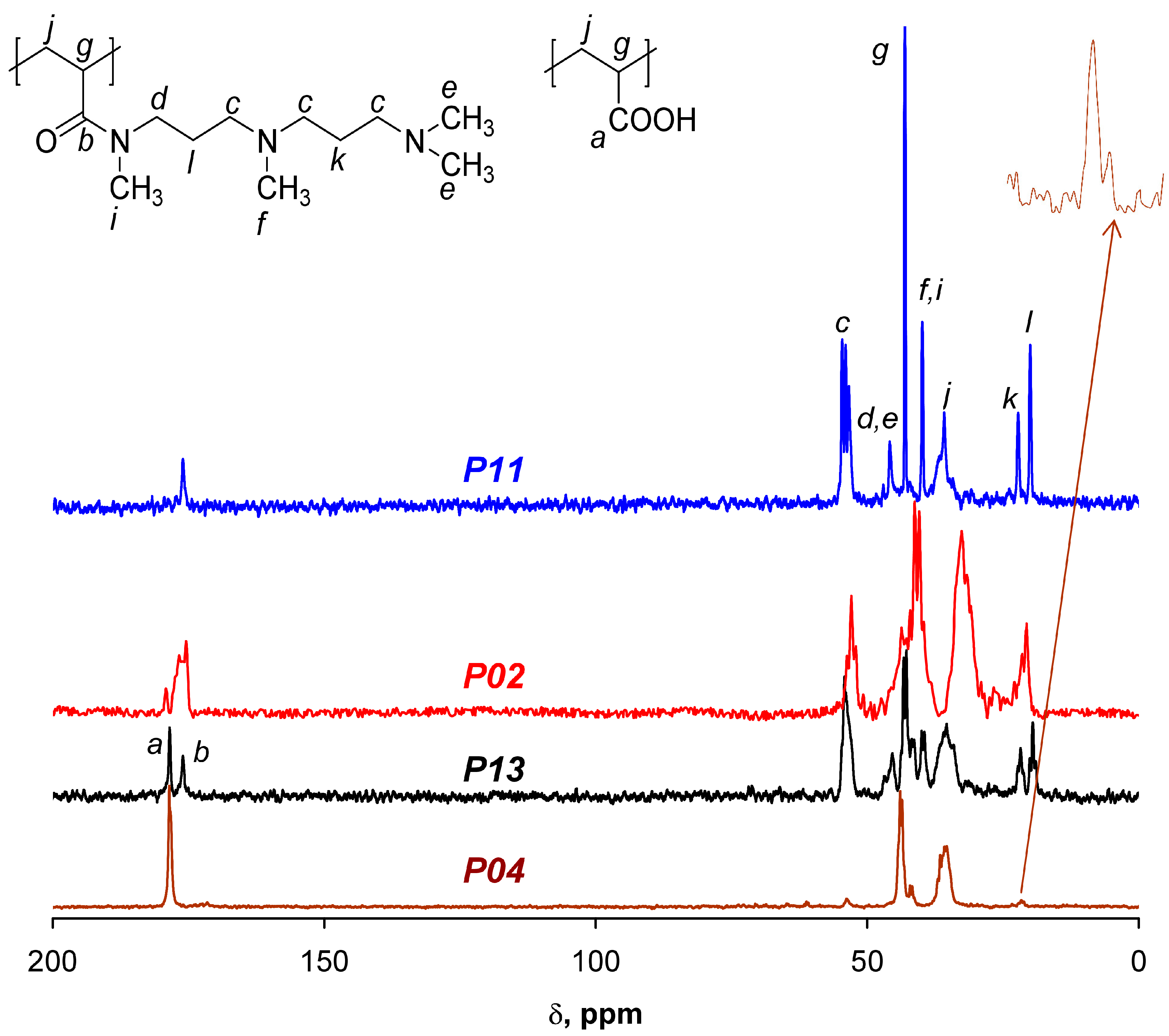
2.2. Characterization of the Copolymers and Composites
2.3. Synthesis of Poly(acryloyl chloride) (PAC)
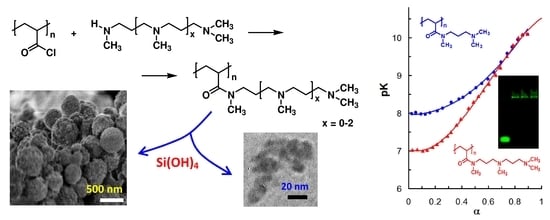
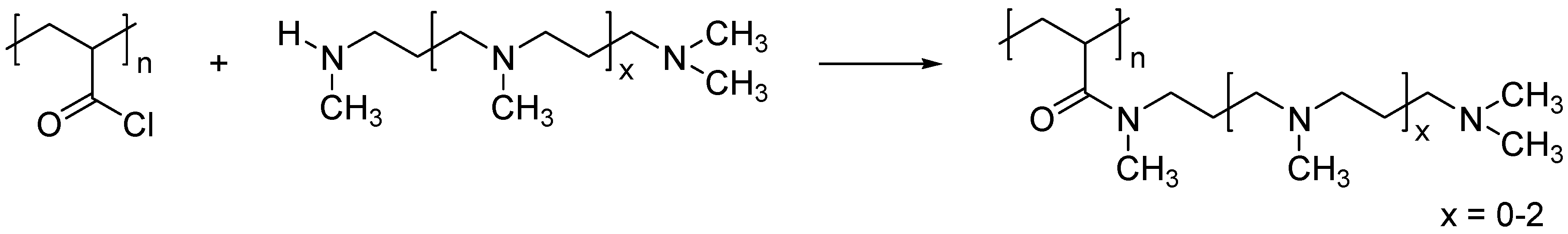
2.4. Reaction of PAC with Polyamines
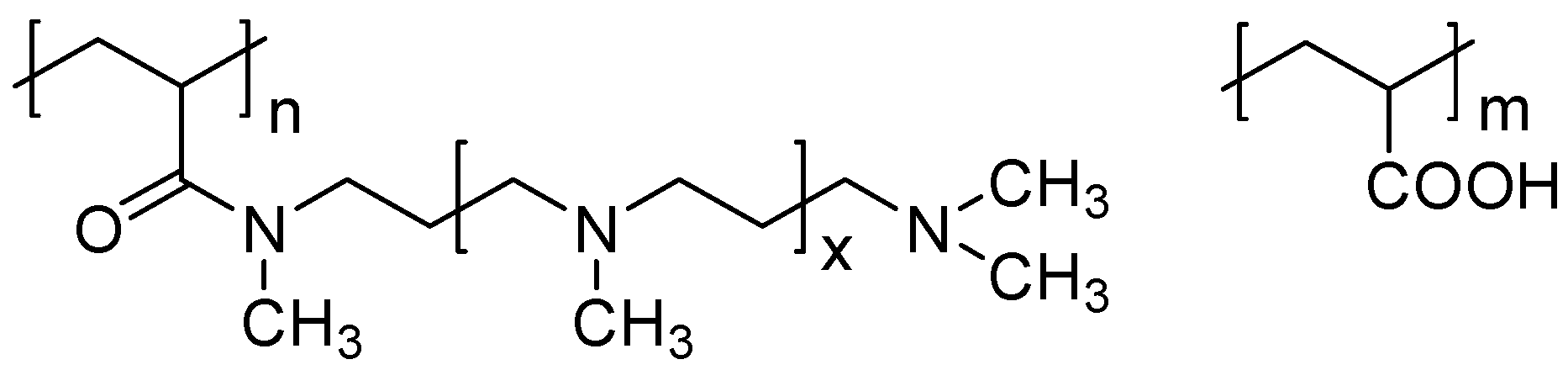
2.5. Study of Silicic Acid Condensation in the Presence of New Polymers and Synthesis of Soluble and Solid Composites
2.6. Synthesis and Electrophoresis of Oligonucleotide Complexes with Polymers and Composite Nanoparticles
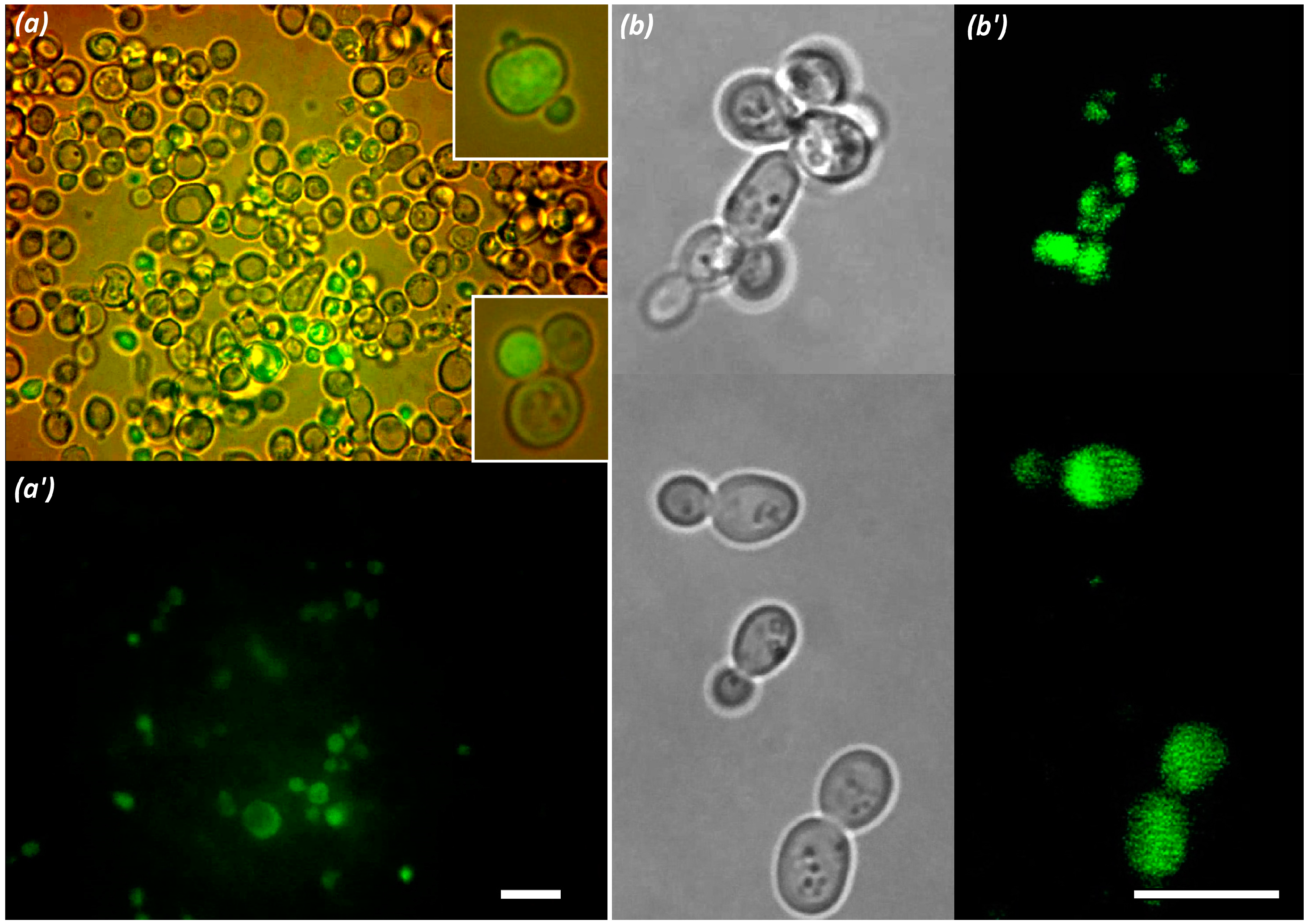
2.7. Study of Oligonucleotide Complex Penetration into Model Yeast Cells
3. Results
- the presence of PSA in the system effectively shifted the charge of the particles from positive in the case of free polymers to neutral and negative (Table 4, #2–5, 7, 13–17 compared with #8–11 and 18–19);
- high content of PSA in the composite decreased or prevented interaction with the oligonucleotide (Table 4, #1–3, 6, and 12) which became apparent in the DNA fluorescence near Rf = 1.
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, Y.; Broekhuis, A.A.; Stuart, M.C.A.; Picchioni, F. Polymeric amines by chemical modifications of alternating aliphatic polyketones. J. Appl. Polym. Sci. 2008, 107, 262–271. [Google Scholar] [CrossRef]
- Butun, V.; Liu, S.; Weaver, J.V.M.; Bories-Azeau, X.; Cai, Y.; Armes, S.P. A brief review of “schizophrenic” Block copolymers. React. Funct. Polym. 2006, 66, 157–165. [Google Scholar] [CrossRef]
- Smedt, S.C.D.; Demeester, J.; Hennink, W.E. Cationic polymer based gene delivery systems. Pharm. Res. 2000, 17, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Pandit, V.; Watson, A.; Ren, L.; Mixon, A.; Kotha, S.P. Multilayered nanoparticles for gene delivery used to reprogram human foreskin fibroblasts to neurospheres. Tissue Eng. Part C 2015, 21, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Miyata, K.; Gouda, N.; Takemoto, H.; Oba, M.; Lee, Y.; Koyama, H.; Yamasaki, Y.; Itake, K.; Nishiyama, N.; Kataoka, K. Enhanced transfection with silica-coated polyplexes loading plasmid DNA. Biomaterials 2010, 31, 4764–4770. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.V.; Singh, A.; Bousbaa, H.; Ferreira, D.; Sarmento, B.; Amiji, M.M. Mad2 checkpoint gene silencing using epidermal growth factor receptor-targeted chitosan nanoparticles in non-small cell lung cancer model. Mol. Pharm. 2014, 11, 3515–3527. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Cho, C.-S.; Cho, M.-H.; Jiang, H.-L. Spermine-alt-poly(ethylene glycol) polyspermine as a safe and efficient aerosol gene carrier for lung cancer therapy. J. Biomed. Mater. Res. Part A 2014, 102, 2230–2237. [Google Scholar] [CrossRef] [PubMed]
- Scholz, C.; Kos, P.; Leclercq, L.; Jin, X.; Cottet, H.; Wagner, E. Correlation of length of linear oligo(ethanamino) amides with gene transfer and cytotoxicity. ChemMedChem 2014, 9, 2104–2110. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.H., Jr.; Day, K.N.; Hemp, S.T.; Long, T.E. Synthesis of folic acid-containing imidazolium copolymers for potential gene delivery applications. Macromol. Chem. Phys. 2013, 214, 797–805. [Google Scholar] [CrossRef]
- Gu, L.; Zhu, S.; Hrymak, A.N.J. Acidic and basic hydrolysis of poly(N-vinylformamide. J. Appl. Polym. Sci. 2002, 86, 3412–3419. [Google Scholar] [CrossRef]
- Buruiana, E.C.; Buruiana, T.; Hahui, L. Preparation and characterization of new optically active poly(N-acryloyl chloride) functionalized with (S)-phenylalanine and pendant pyrene. J. Photochem. Photobiol. A 2007, 189, 65–72. [Google Scholar] [CrossRef]
- Annenkov, V.V.; Zelinskiy, S.N.; Danilovtseva, E.N.; Perry, C.C. Synthesis of biomimetic polyamines. ARKIVOC 2009, 2009, 116–130. [Google Scholar] [CrossRef]
- Kroger, N.; Deutzmann, R.; Sumper, M. Polycationic peptides from diatom biosilica that direct silica nanosphere formation. Science 1999, 286, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Theato, P. Activated ester containing polymers: Opportunities and challenges for the design of functional macromolecules. Chem. Rev. 2016, 116, 1434–1495. [Google Scholar] [CrossRef] [PubMed]
- Kakuchi, R.; Wongsano, K.; Hoven, V.P.; Theato, P. Activation of stable polymeric esters by using organo-activated acyl transfer reactions. J. Polym. Sci. Part A 2014, 52, 1353–1358. [Google Scholar] [CrossRef]
- Yamazaki, S.; Noda, I.; Tsutsumi, A. l3C NMR relaxation of poly(acrylic acid) in aqueous solution. Effects of charge density on local chain dynamics. Polym. J. 2000, 32, 87–89. [Google Scholar] [CrossRef]
- Pavlov, G.M.; Korneeva, E.V.; Ebel, C.; Gavrilova, I.I.; Nesterova, N.A.; Panarin, E.F. Hydrodynamic behavior, molecular mass and conformational parameters of poly(vinylformamide) molecules. Polym. Sci. A 2004, 46, 1063–1067. [Google Scholar]
- Box, M.J. A new method of constrained optimization and a comparison with other methods. Comput. J. 1965, 8, 42–52. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Newman, S.; Krigbaum, W.R.; Laugier, C.; Flory, P.J. Molecular dimensions in relation to intrinsic viscositie. J. Polym. Sci. 1954, 14, 451–462. [Google Scholar] [CrossRef]
- Iler, R. The Chemistry of Silica; Wiley: New York, NY, USA, 1979; p. 896. ISBN 978-0-471-02404-0. [Google Scholar]
- Belton, D.; Paine, G.; Patwardhan, S.V.; Perry, C.C. Towards an understanding of (bio)silicification: The role of amino acids and lysine oligomers in silicification. J. Mater. Chem. 2004, 14, 2231–2241. [Google Scholar] [CrossRef]
- Annenkov, V.V.; Pal’shin, V.A.; Verkhozina, O.N.; Larina, L.I.; Danilovtseva, E.N. Composite nanoparticles: A new way to siliceous materials and a model of biosilica synthesis. Mater. Chem. Phys. 2015, 165, 227–234. [Google Scholar] [CrossRef]
- Gordon, A.J.; Ford, R.A. The Chemist’s Companion: A Handbook of Practical Data. Techniques, and References; Wiley: New York, NY, USA, 1973; p. 560. ISBN 978-0-471-31590-2. [Google Scholar]
- Smith, A.L. Applied Infrared Spectroscopy: Fundamentals, Techniques, and Analytical Problem-Solving; Wiley: New York, NY, USA, 1979; p. 322. ISBN 978-0-471-04378-2. [Google Scholar]
- Strauss, U.P.; Barbieri, U.P.; Wong, G. Analysis of ionization equilibriums of polyacids in terms of species population distributions. Examination of a “two-state” conformational transition. J. Phys. Chem. 1979, 83, 2840–2843. [Google Scholar] [CrossRef]
- Katchalsky, A.; Spitnik, P. Potentiometric titrations of polymethacrylic acid. J. Polym. Sci. 1947, 2, 432–446. [Google Scholar] [CrossRef]
- Katchalsky, A.; Mazur, J.; Spitnik, P. Polybase properties of polyvinylamine. J. Polym. Sci. 1957, 23, 513–532. [Google Scholar] [CrossRef]
- Hall, H.K., Jr. Correlation of base strengths of amines. J. Am. Chem. Soc. 1957, 79, 5441–5444. [Google Scholar] [CrossRef]
- Annenkov, V.V.; Danilovtseva, E.N.; Tenhu, H.; Aseyev, V.; Hirvonen, S.-P.; Mikhaleva, A.I. Copolymers of 1-vinylimidazole and (meth)acrylic acid: Synthesis and polyelectrolyte properties. Eur. Polym. J. 2004, 40, 1027–1032. [Google Scholar] [CrossRef]
- Schärtl, W. Light Scattering from Polymer Solutions and Nanoparticle Dispersions; Springer: Berlin, Germany, 2007; p. 187. ISBN 978-3-540-71951-9. [Google Scholar]
- Annenkov, V.V.; Danilovtseva, E.N.; Pal’shin, V.A.; Verkhozina, O.N.; Zelinskiya, S.N.; Krishnan, U.M. Silicic acid condensation under the influence of water-soluble polymers: From biology to new materials. RSC Adv. 2017, 7, 20995–21027. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: London, UK, 1990; p. 908. ISBN 0-12-134970-5. [Google Scholar]
- Annenkov, V.V.; Danilovtseva, E.N.; Pal’shin, V.A.; Aseyev, V.O.; Petrov, A.K.; Kozlov, A.S.; Patwardhan, S.V.; Perry, C.C. Poly (vinyl amine)—Silica composite nanoparticles: Models of the silicic acid cytoplasmic pool and as a silica precursor for composite materials formation. Biomacromolecules 2011, 12, 1772–1780. [Google Scholar] [CrossRef] [PubMed]
- Demadis, K.D.; Tsistraki, A.; Popa, A.; Iliab, G.; Visa, A. Promiscuous stabilisation behaviour of silicic acid by cationic macromolecules: The case of phosphonium-grafted dicationic ethylene oxide bolaamphiphiles. RSC Adv. 2012, 2, 631–641. [Google Scholar] [CrossRef]
- Spinde, K.; Pachis, K.; Antonakaki, I.; Paasch, S.; Brunner, E.; Demadis, K.D. Influence of polyamines and related macromolecules on silicic acid polycondensation: Relevance to “soluble silicon pools”? Chem. Mater. 2011, 23, 4676–4687. [Google Scholar] [CrossRef]
- Annenkov, V.V.; Danilovtseva, E.N.; Likhoshway, Y.V.; Patwardhan, S.V.; Perry, C.C. Controlled stabilisation of silicic acid below ph 9 using poly(1-vinylimidazole). J. Mater. Chem. 2008, 18, 553–559. [Google Scholar] [CrossRef]
- Morlay, C.; Cromer, M.; Mouginot, Y.; Vittori, O. Potentiometric study of Cu(II) and Ni(II) complexation with two high molecular weight poly(acrylic acids). Talanta 1998, 45, 1177–1188. [Google Scholar] [CrossRef]
- Danilovtseva, E.N.; Pal’shin, V.A.; Likhoshway, Y.V.; Annenkov, V.V. Condensation of silicic acid in the presence of co(1-vinylimidazole–acrylic acid). Adv. Sci. Lett. 2011, 4, 616–621. [Google Scholar] [CrossRef]
- Sumper, M. Biomimetic patterning of silica by long-chain polyamines. Angew. Chem. Int. Ed. 2004, 116, 2301–2304. [Google Scholar] [CrossRef]
- Yang, J.; Liu, H.; Zhang, X. Design, preparation and application of nucleic acid delivery carriers. Biotechnol. Adv. 2014, 32, 804–817. [Google Scholar] [CrossRef] [PubMed]
- Dubruel, P.; Schacht, E. Vinyl polymers as non-viral gene delivery carriers: Current status and prospects. Macromol. Biosci. 2006, 6, 789–810. [Google Scholar] [CrossRef] [PubMed]
- Akinc, A.; Thomas, M.; Klibanov, A.M.; Langer, R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J. Gene Med. 2005, 7, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Behr, J.P. The proton sponge: A trick to enter cells the viruses did not exploit. Chimia 1997, 51, 34–36. [Google Scholar]
- Zhou, D.; Cutlar, L.; Gao, Y.; Wang, W.; O’Keeffe-Ahern, J.; McMahon, S.; Duarte, B.; Larcher, F.; Rodriguez, B.J.; Greiser, U.; et al. The transition from linear to highly branched poly(b-amino ester)s: Branching matters for gene delivery. Sci. Adv. 2016, 2, e1600102. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Huang, J.Y.; O’Keeffe Ahern, J.; Cutlar, L.; Zhou, D.; Lin, F.H.; Wang, W. Highly branched poly(β-amino esters) for non-viral gene delivery: High transfection efficiency and low toxicity achieved by increasing molecular weight. Biomacromolecules 2016, 17, 3640–3647. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, J.; Ren, H.; O’Keeffe-Ahern, J.; Zhou, D.; Zhou, H.; Chen, J.; Guo, T. Multifunctional oligomer incorporation: A potent strategy to enhance the transfection activity of poly(l-lysine). Biomater. Sci. 2016, 4, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Gao, Y.; Aied, A.; Cutlar, L.; Igoucheva, O.; Newland, B.; Alexeeve, V.; Greiser, U.; Uitto, J.; Wang, W. Highly branched poly(β-amino ester)s for skin gene therapy. J. Control. Release 2016, 244, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Sandeli, E.B.; West, T.S. Recommended nomenclature for titrimetric analysis. Pure Appl. Chem. 1969, 18, 427–436. [Google Scholar] [CrossRef]
- Vader, P.; van der Aa, L.J.; Storm, G.; Schiffelers, R.M.; Engbersen, J.F. Polymeric carrier systems for siRNA delivery. Curr. Top. Med. Chem. 2012, 12, 108–119. [Google Scholar] [CrossRef] [PubMed]
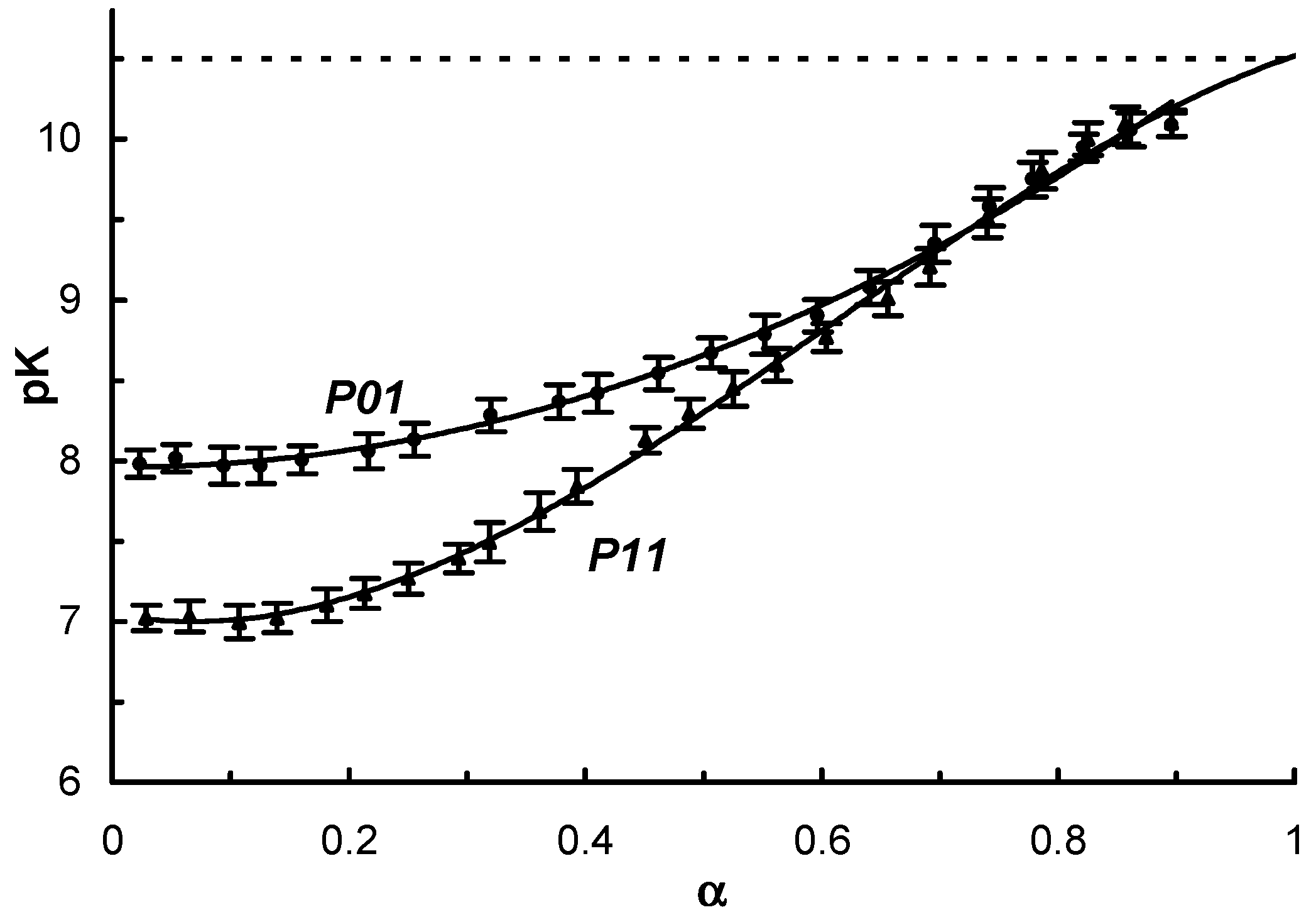



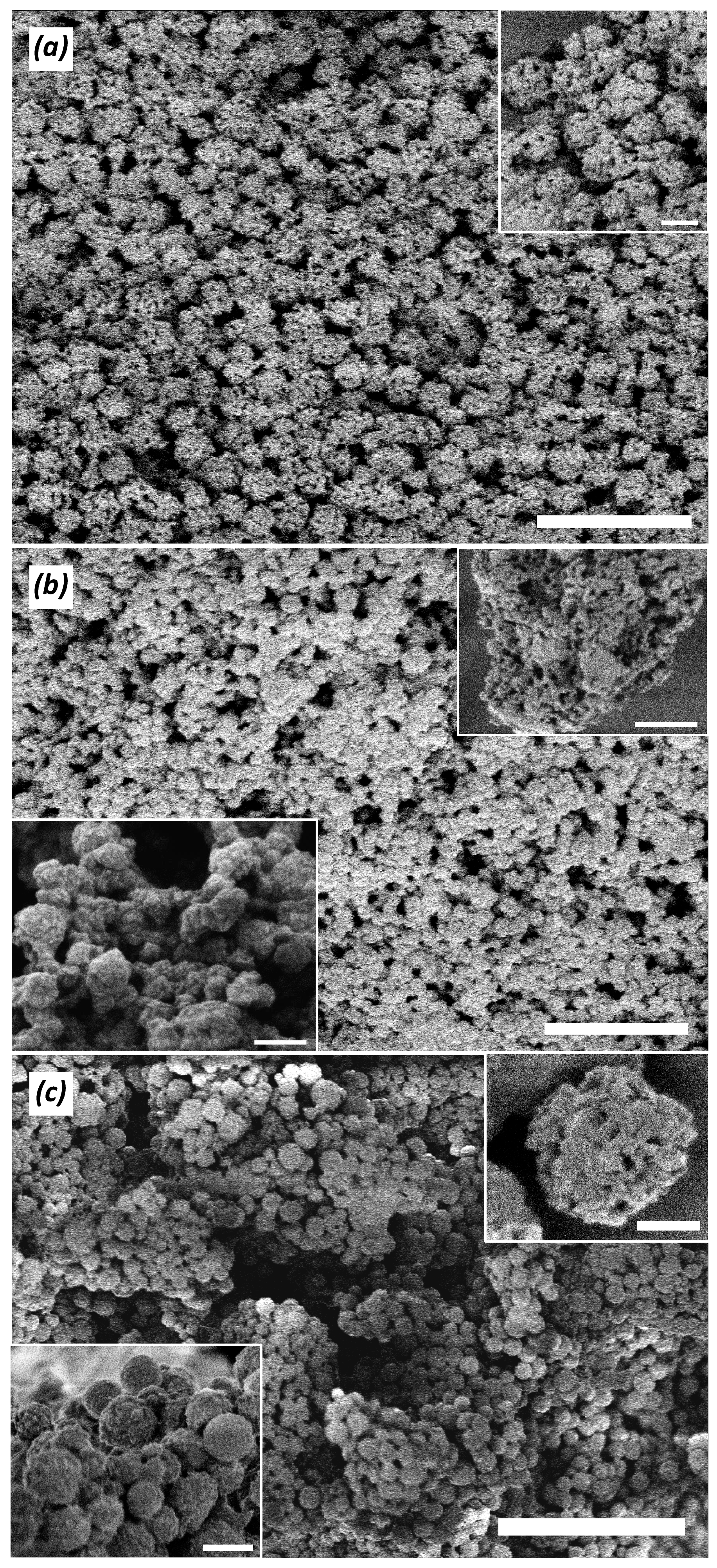
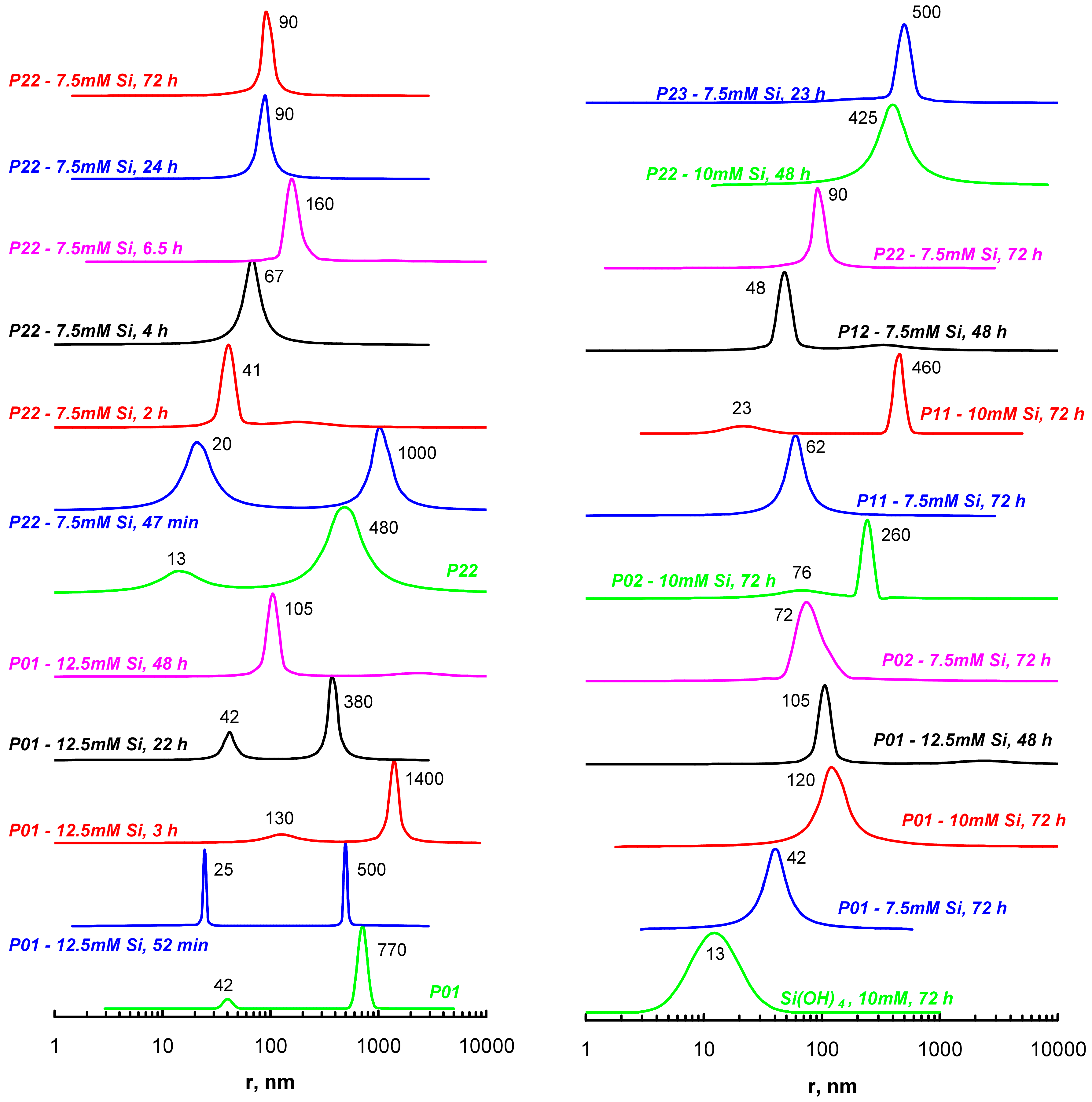
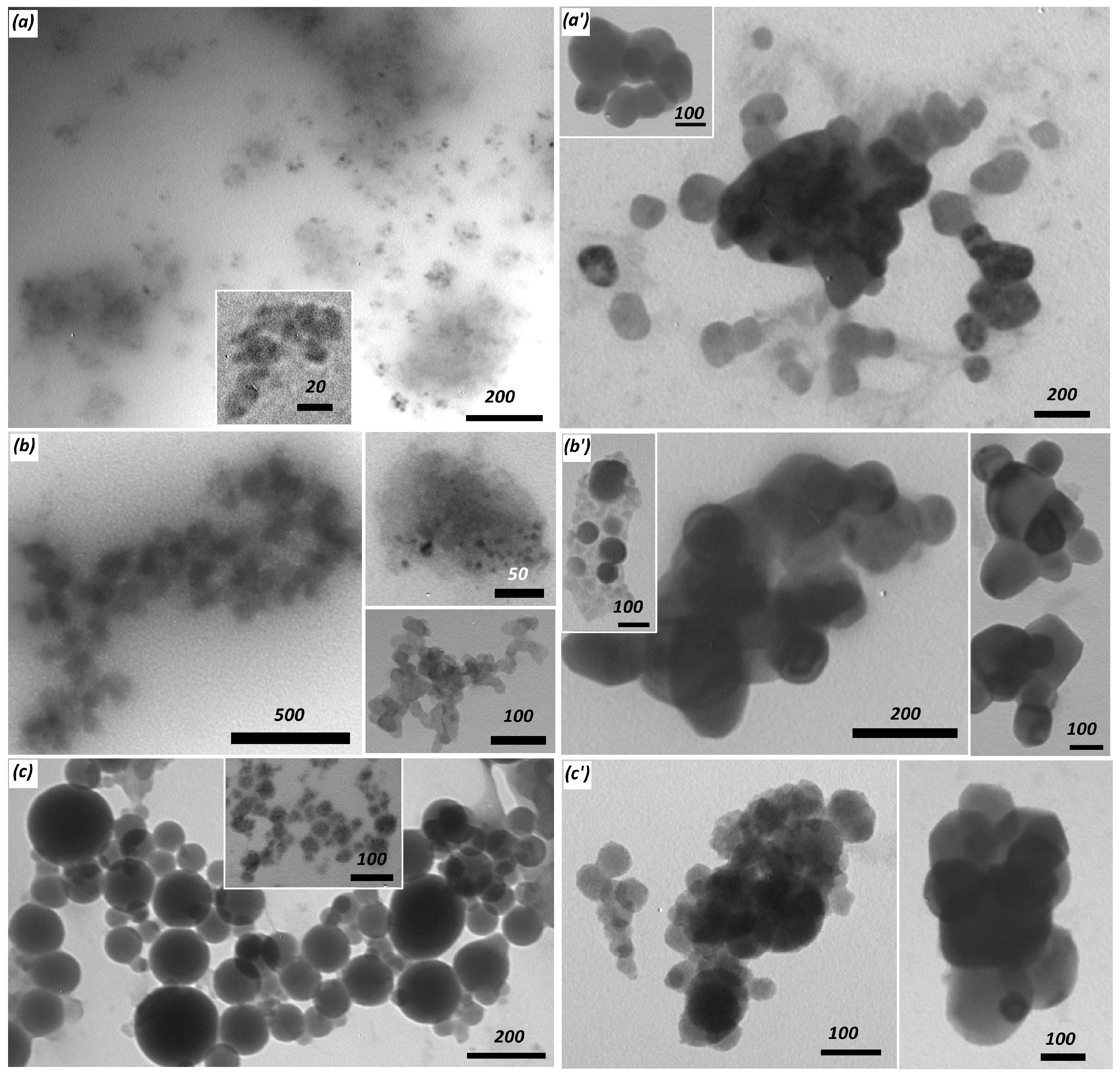

| Polymer # | 1 x | Amine:–COCl Initial Ratio | 1 n:m | Mn, kDa | Mw, kDa | PDI = Mw/Mn | pH Interval of Heterogeneity | |
|---|---|---|---|---|---|---|---|---|
| 2 Polymer | 3 Polymer and Si(OH)4 | |||||||
| P01 | 0 | 1.5:1 | 100:0 | 30.0 | 41.0 | 1.37 | - | - |
| P02 | 0 | 1:1 | 98:2 | 25.8 | 37.9 | 1.47 | - | 2.8–9.8 |
| P03 | 0 | 0.5:1 | 40:60 | 2.7–9.7 | 2.8–9.8 | |||
| P04 | 0 | 0.1:1 | 7:93 | 2.8–7.3 | - | |||
| P11 | 1 | 1.5:1 | 100:0 | 44.4 | 66.6 | 1.50 | - | - |
| P12 | 1 | 1:1 | 95:5 | 44.5 | 66.7 | 1.50 | - | <10 |
| P13 | 1 | 0.5:1 | 36:62 | 30.2 | 47.2 | 1.56 | 7.8–9.9 | 3–10 |
| P14 | 1 | 0.1:1 | 12:88 | 2.7–10.0 | 2.2–5.2 | |||
| P22 | 2 | 1:1 | 90:10 | 57.7 | 78.9 | 1.37 | - | - |
| P23 | 2 | 0.5:1 | 46:54 | 52.8 | 83.3 | 1.58 | - | 6.2–9.2 |
| P24 | 2 | 0.1:1 | 15:85 | 5.6–10.0 | 2.3–5.2 | |||
| Polymer | pH | ||||
|---|---|---|---|---|---|
| 7.4 | 7 | 6.5 | 6 | 5.5 | |
| P01 | 1.6 | 1.3 | 0.8 | 0.4 | 0.06 |
| P02 | 1.2 | 0.9 | 0.6 | 0.3 | 0.1 |
| P11 | 1.7 | 1.8 | 1.8 | 1.5 | 1.0 |
| P12 | 1.8 | 1.7 | 1.5 | 1.2 | 0.9 |
| P22 | 1.5 | 1.5 | 1.5 | 1.3 | 1.2 |
| P23 | 0.9 | 0.9 | 0.9 | 0.9 | 0.9 |
| # | Polymer | Amine Units, mol % | 1 Polymer Conc., g∙L−1 | 2 Rf |
|---|---|---|---|---|
| Free DNA Oligonucleotide | 1 | |||
| 1 | P01 | 100 | 2 | −0.59–0 |
| 2 | P02 | 98 | 2 | −0.43–0 |
| 3 | P03 | 40 | 2 | 1 |
| 4 | P04 | 7 | 2 | 1 |
| 5 | P11 | 100 | 2 | −0.41–−0.03 |
| 6 | P11 | 100 | 1 | −0.20–−0.04 |
| 7 | P11 | 100 | 0.7 | −0.22–0 |
| 8 | P11 | 100 | 0.3 | 0; 0.85 |
| 9 | P11 | 100 | 0.2 | 0; 0–0.86 |
| 10 | P12 | 95 | 2 | −0.25–−0.03 |
| 11 | P12 | 95 | 1 | −0.08–0 |
| 12 | P12 | 95 | 0.7 | 0 |
| 13 | P12 | 95 | 0.3 | 0–0.93 |
| 14 | P12 | 95 | 0.2 | 0–0.96 |
| 15 | P13 | 36 | 2 | −0.13–0 |
| 16 | P22 | 90 | 2 | −0.54–0 |
| 17 | P22 | 90 | 1 | −0.08–−0.02 |
| 18 | P23 | 46 | 2 | −0.45–0 |
| 19 | P23 | 46 | 1 | −0.06–−0.02 |
| 20 | P24 | 12 | 2 | 1 |
| # | Polymer | Amine Units, mol % | 1 Silicate Conc., g∙L−1 | Polymer (Composite): DNA Ratio | 2 Rf |
|---|---|---|---|---|---|
| 1 | P01 | 100 | 12.5 | 2:1 | 1 |
| 2 | P01 | 100 | 10 | 2:1 | 0–0.95 |
| 3 | P01 | 100 | 10 | 4:1 | 0.33–1 |
| 4 | P01 | 100 | 7.5 | 2:1 | 0 |
| 5 | P01 | 100 | 7.5 | 4:1 | 0; 0–0.15 |
| 6 | P02 | 98 | 10 | 2:1 | 0.87–1 |
| 7 | P02 | 98 | 7.5 | 4:1 | 0.22–0.719 |
| 8 | P01 | 100 | Free polymer | 2:1 | −0.35–−0.03 |
| 9 | P01 | 100 | Free polymer | 4:1 | −0.39–−0.03 |
| 10 | P02 | 98 | Free polymer | 2:1 | −0.38–−0.03 |
| 11 | P02 | 98 | Free polymer | 4:1 | −0.34–−0.03 |
| 12 | P11 | 100 | 10 | 4:1 | 0.77–0.92 |
| 13 | P11 | 100 | 7.5 | 4:1 | 0 |
| 14 | P12 | 95 | 7.5 | 4:1 | 0 |
| 15 | P22 | 90 | 10 | 4:1 | 0 |
| 16 | P22 | 90 | 7.5 | 4:1 | −0.08 |
| 17 | P23 | 46 | 7.5 | 4:1 | −0.06 |
| 18 | P11 | 100 | Free polymer | 4:1 | −0.07–−0.03 |
| 19 | P12 | 95 | Free polymer | 4:1 | −0.10–−0.03 |
| 20 | P22 | 90 | Free polymer | 4:1 | −0.07–−0.03 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danilovtseva, E.N.; Maheswari Krishnan, U.; Pal’shin, V.A.; Annenkov, V.V. Polymeric Amines and Ampholytes Derived from Poly(acryloyl chloride): Synthesis, Influence on Silicic Acid Condensation and Interaction with Nucleic Acid. Polymers 2017, 9, 624. https://doi.org/10.3390/polym9110624
Danilovtseva EN, Maheswari Krishnan U, Pal’shin VA, Annenkov VV. Polymeric Amines and Ampholytes Derived from Poly(acryloyl chloride): Synthesis, Influence on Silicic Acid Condensation and Interaction with Nucleic Acid. Polymers. 2017; 9(11):624. https://doi.org/10.3390/polym9110624
Chicago/Turabian StyleDanilovtseva, Elena N., Uma Maheswari Krishnan, Viktor A. Pal’shin, and Vadim V. Annenkov. 2017. "Polymeric Amines and Ampholytes Derived from Poly(acryloyl chloride): Synthesis, Influence on Silicic Acid Condensation and Interaction with Nucleic Acid" Polymers 9, no. 11: 624. https://doi.org/10.3390/polym9110624