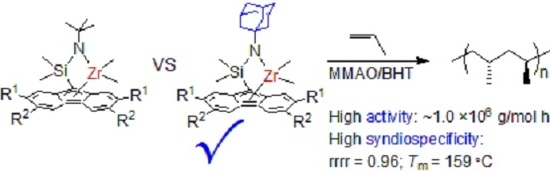
Substituent Effects of Adamantyl Group on Amido Ligand in Syndiospecific Polymerization of Propylene with Ansa-Dimethylsilylene(Fluorenyl)(Amido) Zirconium Complex
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Complexes
2.2.1. Synthesis of [(1-Adamantyl)NSiMe2(2,7-di-t-BuFlu)]ZrMe2 (Zr(1))
2.2.2. Synthesis of [(1-Adamantyl)NSiMe2(3,6-di-t-BuFlu)]ZrMe2 (Zr(2))
2.3. Polymerization Procedure
2.4. Analytical Procedure
3. Results and Discussion
3.1. Molecular Structure of Complexes
3.2. Propylene Polymerization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brintzinger, H.H.; Fischer, D.; Mülhaupt, R.; Rieger, B.; Waymouth, R.M. Stereospecific olefin polymerization with chiral metallocene catalysts. Angew. Chem. Int. Ed. 1995, 34, 1143–1170. [Google Scholar] [CrossRef]
- Resconi, L.; Cavallo, L.; Fait, A.; Piemontesi, F. Selectivity in propene polymerization with metallocene catalysts. Chem. Rev. 2000, 100, 1253–1346. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, W. Olefin polymerization catalyzed by metallocenes. Adv. Catal. 2001, 46, 89–159. [Google Scholar]
- Ewen, J.A.; Jones, R.L.; Razavi, A.; Ferrara, J.D. Syndiospecific propylene polymerizations with group 4 metallocenes. J. Am. Chem. Soc. 1988, 110, 6255–6256. [Google Scholar] [CrossRef] [PubMed]
- Veghini, D.; Henling, L.M.; Burkhardt, T.J.; Bercaw, J.E. Mechanisms of stereocontrol for doubly silylene-bridged Cs- and C1-symmetric zirconocene catalysts for propylene polymerization. Synthesis and molecular structure of Li2[(1,2-Me2Si)2{C5H2–4-(1R,2S,5R-menthyl)}{C5H-3,5-(CHMe2)2)}]·3THF and [(1,2-Me2Si)2{η5-C5H2–4-(1R,2S,5R-menthyl)}{η5-C5H-3,5-(CHMe2)2}]ZrCl2. J. Am. Chem. Soc. 1999, 121, 564–573. [Google Scholar]
- McKnight, A.L.; Waymouth, R.M. Group 4 ansa-cyclopentadienyl-amido catalysts for olefin polymerization. Chem. Rev. 1998, 98, 2587–2598. [Google Scholar] [CrossRef] [PubMed]
- Ittel, S.D.; Johnson, L.K.; Brookhart, M. Late-metal catalysts for ethylene homo- and copolymerization. Chem. Rev. 2000, 100, 1169–1203. [Google Scholar] [CrossRef] [PubMed]
- Gibson, V.C.; Spitzmesser, S.K. Advances in non-metallocene olefin polymerization catalysis. Chem. Rev. 2003, 103, 283–315. [Google Scholar] [CrossRef] [PubMed]
- Makio, H.; Terao, H.; Iwashita, A.; Fujita, T. FI catalysts for olefin polymerization—A comprehensive treatment. Chem. Rev. 2011, 111, 2363–2449. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Pan, L.; Song, D.; Li, Y. Neutral nickel catalysts for olefin homo- and copolymerization: Relationships between catalyst structures and catalytic properties. Chem. Rev. 2015, 115, 12091–12137. [Google Scholar] [CrossRef] [PubMed]
- Coates, G.W. Precise control of polyolefin stereochemistry using single-site metal catalysts. Chem. Rev. 2000, 100, 1223–1252. [Google Scholar] [CrossRef] [PubMed]
- Coates, G.W.; Hustad, P.D.; Reinartz, S. Catalysts for the living insertion polymerization of alkenes: Access to new polyolefin architectures using Ziegler–Natta chemistry. Angew. Chem. Int. Ed. 2002, 41, 2236–2257. [Google Scholar] [CrossRef]
- Xu, G. Copolymerization of ethylene with styrene catalyzed by the [η1:η5-tert-butyl(dimethylfluorenylsilyl)amido]methyltitanium “Cation”. Macromolecules 1998, 31, 2395–2402. [Google Scholar] [CrossRef]
- Irwin, L.J.; Reibenspies, J.H.; Miller, S.A. A sterically expanded “constrained geometry catalyst” for highly active olefin polymerization and copolymerization: An unyielding comonomer effect. J. Am. Chem. Soc. 2004, 126, 16716–16717. [Google Scholar] [CrossRef] [PubMed]
- Schwerdtfeger, E.D.; Miller, S.A. Intrinsic branching effects in syndiotactic copolymers of propylene and higher α-olefins. Macromolecules 2007, 40, 5662–5668. [Google Scholar] [CrossRef]
- Schwerdtfeger, E.D.; Price, C.J.; Chai, J.; Miller, S.A. Tandem catalyst system for linear low-density polyethylene with short and long branching. Macromolecules 2010, 43, 4838–4842. [Google Scholar] [CrossRef]
- Chai, J.; Abboud, K.A.; Miller, S.A. Sterically expanded CGC catalysts: Substituent effects on ethylene and α-olefin polymerization. Dalton Trans. 2013, 42, 9139–9147. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.Y.; Hong, S.D.; Jung, M.W.; Lee, H.; Park, Y.W. Norbornene copolymerization with α-olefins using methylene-bridged ansa-zirconocene. Polyhedron 2005, 24, 1269–1273. [Google Scholar] [CrossRef]
- Kirillov, E.; Razaci, A.; Carpentier, J.F. Syndiotactic-enriched propylene–styrene copolymers using fluorenyl-based half-titanocene catalysts. J. Mol. Catal. A 2006, 249, 230–235. [Google Scholar] [CrossRef]
- Na, S.J.; Wu, C.J.; Yoo, J.; Kim, B.E.; Lee, B.Y. Copolymerization of 5,6-dihydrodicyclopentadiene and ethylene. Macromolecules 2008, 41, 4055–4057. [Google Scholar] [CrossRef]
- Yu, S.T.; Na, S.J.; Lim, T.S.; Lee, B.Y. Preparation of a bulky cycloolefin/ethylene copolymer and its tensile properties. Macromolecules 2010, 43, 725–730. [Google Scholar] [CrossRef]
- Nakayama, Y.; Sogo, Y.; Cai, Z.; Shiono, T. Copolymerization of ethylene with 1,1-disubstituted olefins catalyzed by ansa-(fluorenyl)(cyclododecylamido)dimethyltitanium complexes. J. Polym. Sci. Part A 2013, 51, 1223–1229. [Google Scholar] [CrossRef]
- Razavi, A.; Thewalt, U. Preparation and crystal structures of the complexes (η5-C5H3TMS–CMe2–η5-C13H8)MCl2 and [3,6-ditButC13H6–SiMe2–NtBu]MCl2 (M = Hf, Zr or Ti): Mechanistic aspects of the catalytic formation of a isotactic–syndiotactic stereoblock-type polypropylene. J. Organomet. Chem. 2001, 621, 267–276. [Google Scholar] [CrossRef]
- Busico, V.; Cipullo, R.; Cutillo, F.; Talarico, G.; Razavi, A. Syndiotactic poly(propylene) from [Me2Si(3,6-di-tert-butyl-9-fluorenyl)(N-tert-butyl)]TiCl2–based catalysts: Chain-end or enantiotopic-sites stereocontrol? Macromol. Chem. Phys. 2003, 204, 1269–1274. [Google Scholar] [CrossRef]
- Irwin, L.J.; Miller, S.A. Unprecedented syndioselectivity and syndiotactic polyolefin melting temperature: Polypropylene and poly(4-methyl-1-pentene) from a highly active, sterically expanded η1-Fluorenyl–η1-amido zirconium complex. J. Am. Chem. Soc. 2005, 127, 9972–9973. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Su, H.; Nakayama, Y.; Shiono, T.; Akita, M. Synthesis of C1 symmetrical ansa-cyclopentadienylamidotitanium complexes and their application for living polymerization of propylene. J. Organomet. Chem. 2014, 770, 136–141. [Google Scholar] [CrossRef]
- Tanaka, R.; Chie, Y.; Cai, Z.; Nakayama, Y.; Shinon, T. Structure-stereospecificity relationships of propylene polymerization using substituted ansa-silylene(fluorenyl)(amido) titanium complexes. J. Organomet. Chem. 2016, 804, 95–100. [Google Scholar] [CrossRef]
- Shiono, T. Living polymerization of olefins with ansa-dimethylsilylene(fluorenyl)(amido) dimethyltitanium-based catalysts. Polym. J. 2011, 43, 331–351. [Google Scholar] [CrossRef]
- Cai, Z.; Su, H.; Shiono, T. Precise synthesis of olefin block copolymers using a syndiospecific living polymerization system. Chin. J. Polym. Sci. 2013, 31, 541–549. [Google Scholar] [CrossRef]
- Cai, Z.; Ikeda, T.; Akita, M.; Shiono, T. Substituent effects of tert-butyl groups on fluorenyl ligand in syndiospecific living polymerization of propylene with ansa-fluorenylamidodimethyltitanium complex. Macromolecules 2005, 38, 8135–8139. [Google Scholar] [CrossRef]
- Shiono, T.; Harada, R.; Cai, Z.; Nakayama, Y. A highly active catalyst composed of ansa-fluorenylamidodimethyltitanium derivative for propene polymerization. Top. Catal. 2009, 52, 675–680. [Google Scholar] [CrossRef]
- Cai, Z.; Nakayama, Y.; Shiono, T. Substituent effects of tert-butyl groups on fluorenyl ligand of [t-BuNSiMe2Flu] ZrMe2. Chin. J. Polym. Sci. 2008, 31, 575–578. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, B.; Shiono, T.; Cai, Z. Highly active ansa-(Fluorenyl)(amido)titanium-based catalysts with low load of methylaluminoxane for syndiotactic-specific living polymerization of propylene. Organometallics 2017, 36, 3009–3012. [Google Scholar] [CrossRef]
- Song, X.; Ma, Q.; Yuan, H.; Cai, Z. Synthesis of hydroxy-functionalized ultrahigh molecular weight polyethylene using fluorenylamidotitanium complex. Chin. J. Polym. Sci. 2017. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS: An Empirical Absorption Correction Program for Area Detector Data; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, C71, 3–8. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- SAINT+, version 6.22a; Bruker AXS Inc.: Madison, WI, USA, 2002.
- SAINT+, version v7.68A; Bruker AXS Inc.: Madison, WI, USA, 2009.
- SHELXTL NT/2000, version 6.1; Bruker AXS Inc.: Madison, WI, USA, 2002.
- Tolman, C.A. Formation of three-coordinate nickel(0) complexes by phosphorus ligand dissociation from NiL4. J. Am. Chem. Soc. 1974, 96, 53–60. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.; Taft, R.W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 1991, 91, 165–195. [Google Scholar] [CrossRef]
- Busico, V.; Cipullo, R.; Cutillo, F.; Friederichs, N.; Ronca, S.; Wang, B. Improving the performance of methylalumoxane: A facile and efficient method to trap “free” trimethylaluminum. J. Am. Chem. Soc. 2003, 125, 12402–12403. [Google Scholar] [CrossRef] [PubMed]
- Cipullo, R.; Busico, V.; Fraldi, N.; Pellecchia, R.; Talarico, G. Improving the behavior of bis(phenoxyamine) Group 4 metal catalysts for controlled alkene polymerization. Macromolecules 2009, 42, 3869–3872. [Google Scholar] [CrossRef]
- Cai, Z.; Nakayama, Y.; Shiono, T. Facile synthesis of tailor-made stereoblock polypropylenes via successive variation of monomer pressure. Macromolecules 2008, 41, 6596–6598. [Google Scholar] [CrossRef]


| Complex | Zr(1) | Zr(2) |
|---|---|---|
| Formula | C35H51NSiZr | C35H51NSiZr |
| Formula weight | 605.07 | 605.07 |
| Crystal system | Triclinic | Monoclinic |
| Space group | P | P1 21/c1 |
| a (Å) | 10.5309(10) | 12.673(3) |
| b (Å) | 11.6688(11) | 19.886(4) |
| c (Å) | 14.6617(14) | 14.198(3) |
| Β (deg) | 75.818(2) | 111.899(4) |
| V (Å3) | 1584.3(3) | 3320.0(12) |
| Z | 2 | 4 |
| F(000) | 644 | 1288 |
| Dcalcd. (g.cm−3) | 1268 | 1211 |
| μ (mm−1) | 0.408 | 0.390 |
| Theta range for data collection | 1.924 to 30.715° | 1.842 to 25.499° |
| Reflections collected | 16,281 | 23,033 |
| Independent reflections | 9719 [R(int) = 0.2619] | 6155 [R(int) = 0.0635] |
| Final R indices [I > 2δ(I)] | R1 = 0.0387, wR2 = 0.0905 | R1 = 0.0546, wR2 = 0.1417 |
| Parameters | Zr(1) | Zr(2) | Zr(f) |
|---|---|---|---|
| Zr(1)-C(1) | 2.3960(17) | 2.402(5) | 2.385(3) |
| Zr(1)-C(2) | 2.5443(17) | 2.535(5) | 2.563(3) |
| Zr(1)-C(3) | 2.4948(17) | 2.524(5) | 2.486(3) |
| Zr(1)-C(4) | 2.6871(17) | 2.664(4) | 2.702(3) |
| Zr(1)-C(5) | 2.6706(17) | 2.655(4) | 2.667(3) |
| Zr(1)-N(1) | 2.0536(15) | 2.061(4) | 2.058(3) |
| Zr(1)-Si(1) | 2.9867(6) | 3.005(16) | 2.981(3) |
| θ a = 2/3 (θ1 + θ2 + θ3) | 71.06 | 68.80 | 70.12 |
| Entry | cat. | Cocatalyst | Al/Zr | P (atm) | Temp (°C) | Time (min) | Yield (g) | Activity d (×103) | Mn e (×103) | MWD e | Tm f (°C) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Zr(1) | dMMAO | 400 | 1 | 0 | 30 | 1.29 | 129 | 4.4 | 1.51 | 139 |
| 2 | Zr(2) | dMMAO | 400 | 1 | 0 | 30 | 1.17 | 117 | 3.8 | 1.42 | 155 |
| 3 | Zr(1) | dMMAO | 400 | 1 | 20 | 30 | 3.23 | 323 | 2.5 | 1.67 | 132 |
| 4 | Zr(2) | dMMAO | 400 | 1 | 20 | 30 | 3.56 | 356 | 3.1 | 1.53 | 148 |
| 5 b | Zr(f) | dMMAO | 400 | 1 | 0 | 30 | 0 | 0 | - | - | - |
| 6 b | Zr(g) | dMMAO | 400 | 1 | 0 | 30 | 0 | 0 | - | - | - |
| 7 b | Zr(f) | dMMAO | 400 | 1 | 20 | 30 | 1.77 | 177 | 1.17 | 1.43 | 125 |
| 8 b | Zr(g) | dMMAO | 400 | 1 | 20 | 30 | 1.39 | 139 | 1.39 | 1.67 | 145 |
| 9 | Zr(1) | MMAO/BHT | 400 | 1 | 0 | 30 | 2.12 | 212 | 5.2 | 1.55 | 139 |
| 10 | Zr(1) | MMAO/BHT | 200 | 1 | 0 | 30 | 1.52 | 152 | 4.8 | 1.43 | 138 |
| 11 | Zr(1) | MMAO/BHT | 100 | 1 | 0 | 30 | 0.99 | 99 | 4.1 | 1.5 | 138 |
| 12 | Zr(1) | MMAO/BHT | 50 | 1 | 0 | 30 | 0.95 | 95 | 3.8 | 1.47 | 138 |
| 13 c | Zr(1) | MMAO/BHT | 400 | 8 | 0 | 8 | 1.2 | 900 | 7.4 | 1.81 | 142 |
| 14 c | Zr(2) | MMAO/BHT | 400 | 8 | 0 | 8 | 1.35 | 1012 | 2.8 | 2.32 | 159 |
| Catalyst | Stereosequence Distribution a | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| mmmm | mmmr | rmmr | mmrr | mmrm + rmrr | rmrm | rrrr | mrrr | mrrm | |
| Zr(1) | 0.00 | 0.00 | 0.021 | 0.043 | 0.026 | 0.021 | 0.820 | 0.069 | 0.00 |
| Zr(2) | 0.00 | 0.00 | 0.007 | 0.014 | 0.003 | 0.00 | 0.958 | 0.018 | 0.00 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Li, S.; Shiono, T.; Cai, Z. Substituent Effects of Adamantyl Group on Amido Ligand in Syndiospecific Polymerization of Propylene with Ansa-Dimethylsilylene(Fluorenyl)(Amido) Zirconium Complex. Polymers 2017, 9, 632. https://doi.org/10.3390/polym9110632
Sun Y, Li S, Shiono T, Cai Z. Substituent Effects of Adamantyl Group on Amido Ligand in Syndiospecific Polymerization of Propylene with Ansa-Dimethylsilylene(Fluorenyl)(Amido) Zirconium Complex. Polymers. 2017; 9(11):632. https://doi.org/10.3390/polym9110632
Chicago/Turabian StyleSun, Yanjie, Shuhui Li, Takeshi Shiono, and Zhengguo Cai. 2017. "Substituent Effects of Adamantyl Group on Amido Ligand in Syndiospecific Polymerization of Propylene with Ansa-Dimethylsilylene(Fluorenyl)(Amido) Zirconium Complex" Polymers 9, no. 11: 632. https://doi.org/10.3390/polym9110632