A Novel Human-Like Collagen Hydrogel Scaffold with Porous Structure and Sponge-Like Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of HLCS and HLCC Hydrogels
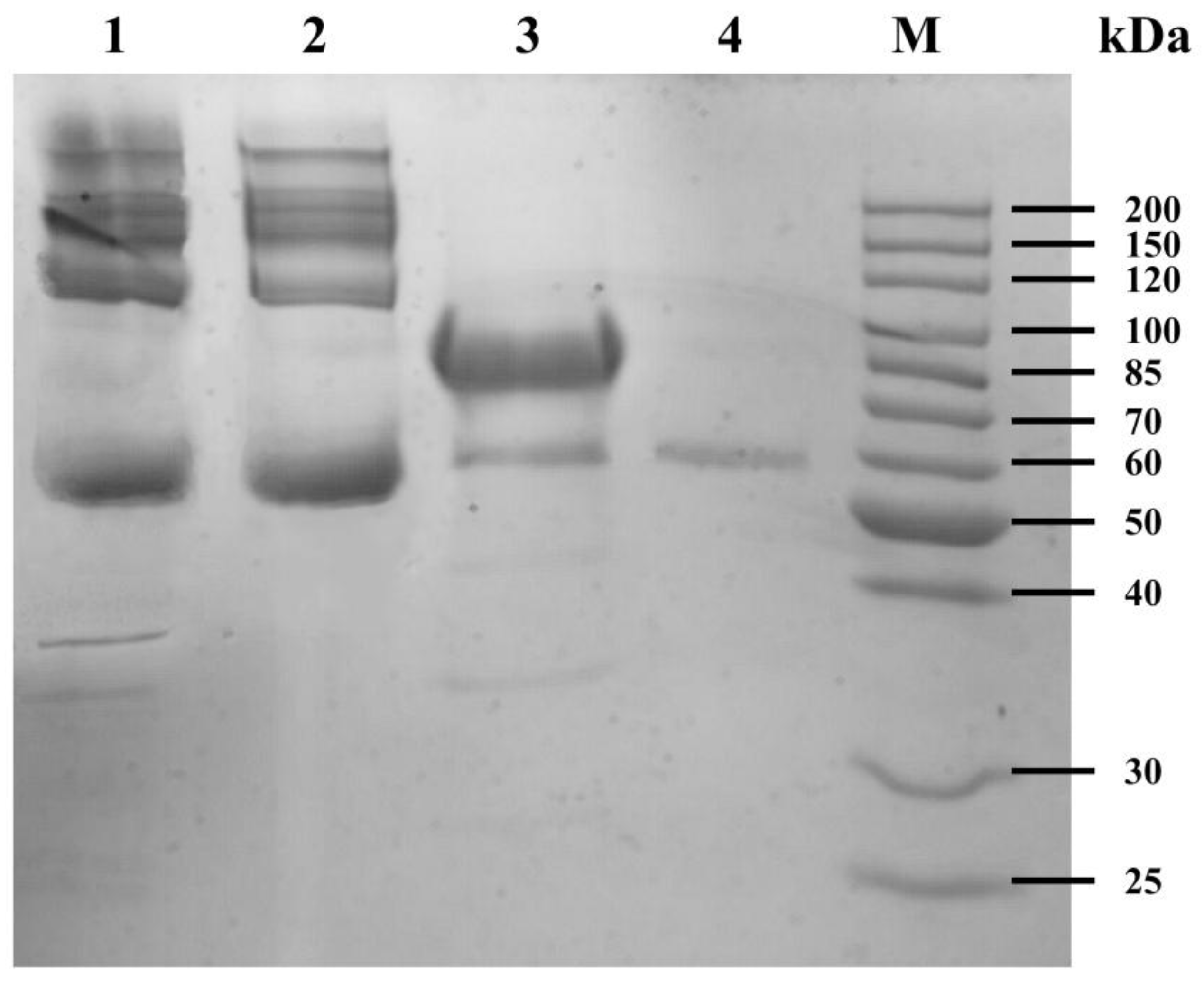
2.3. SDS-PAGE of the Water Extract of HLCS Hydrogel
2.4. Morphology of Hydrogels
2.5. Density and Porosity of Hydrogels
2.6. Swelling Behavior of Hydrogels
2.7. Compressive Mechanical Properties of Hydrogels
2.8. Cell Culture and Viability Analysis
2.9. Cell Attachment and Proliferation Analysis
2.10. Animal Implantation and Histological Evaluation
2.11. Statistical Analysis
3. Results and Discussion
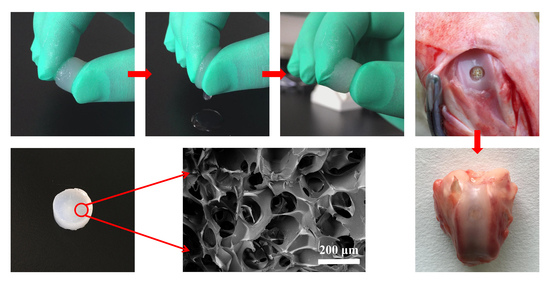
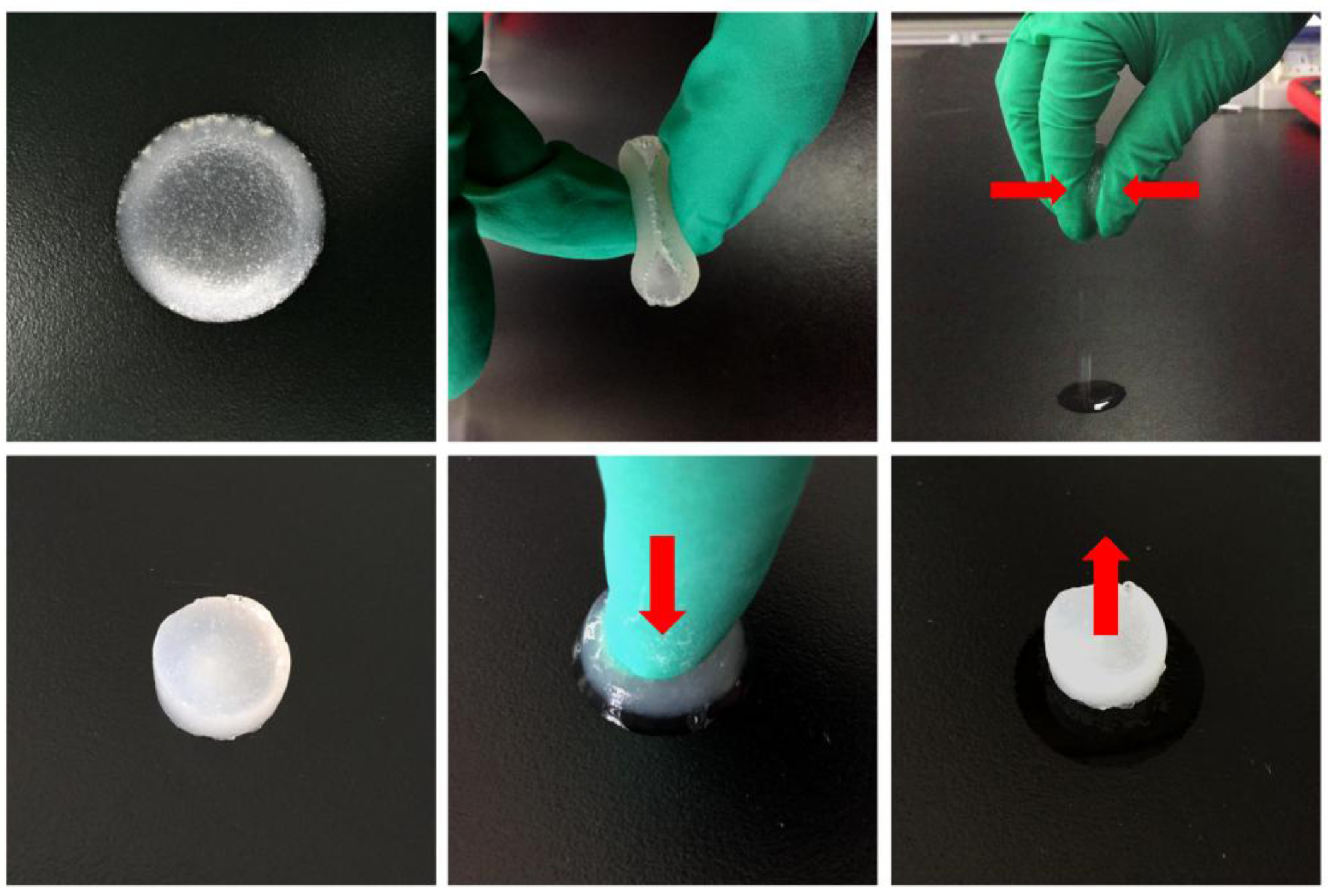
3.1. Preparation of the HLCS Hydrogel
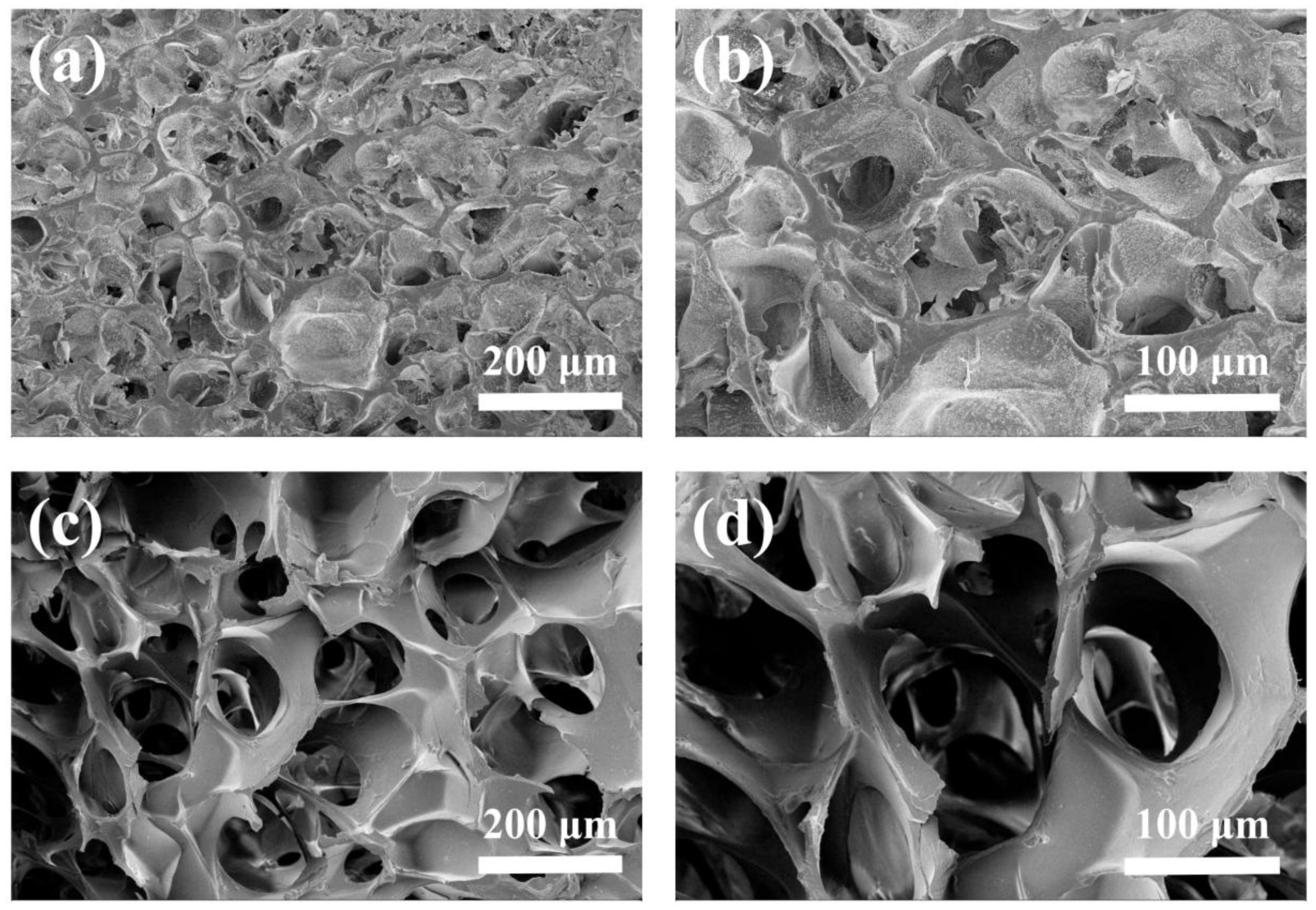
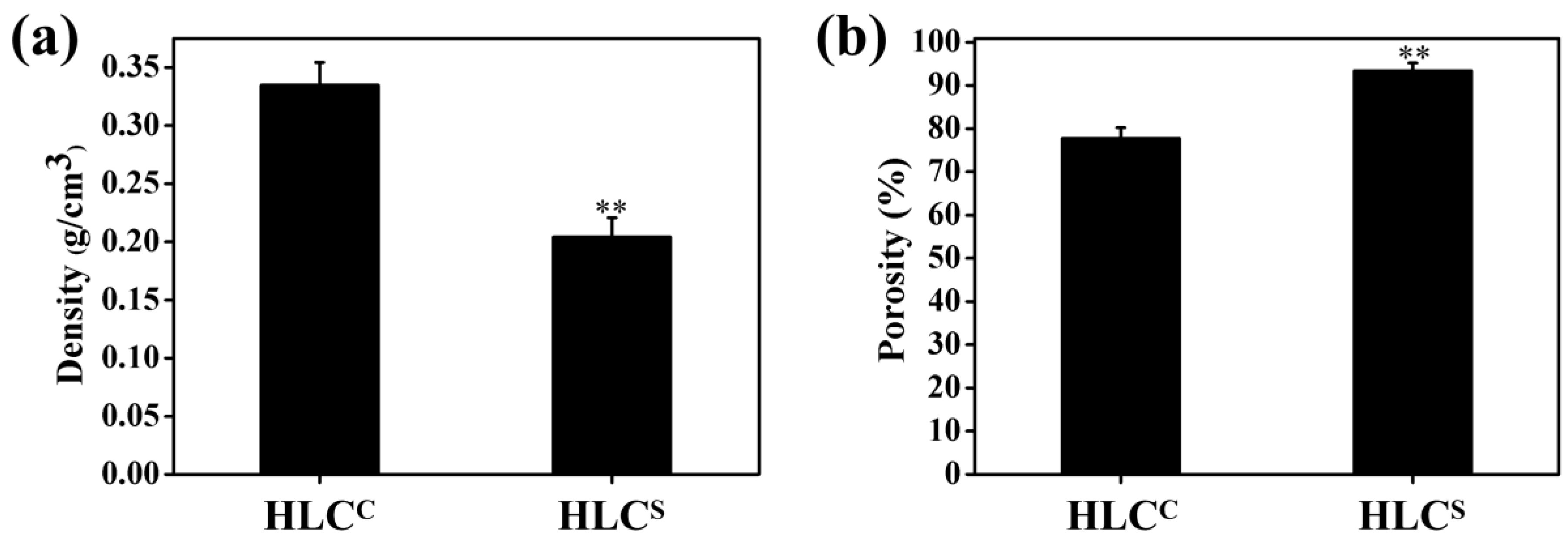
3.2. Morphology, Density, and Porosity of Hydrogels
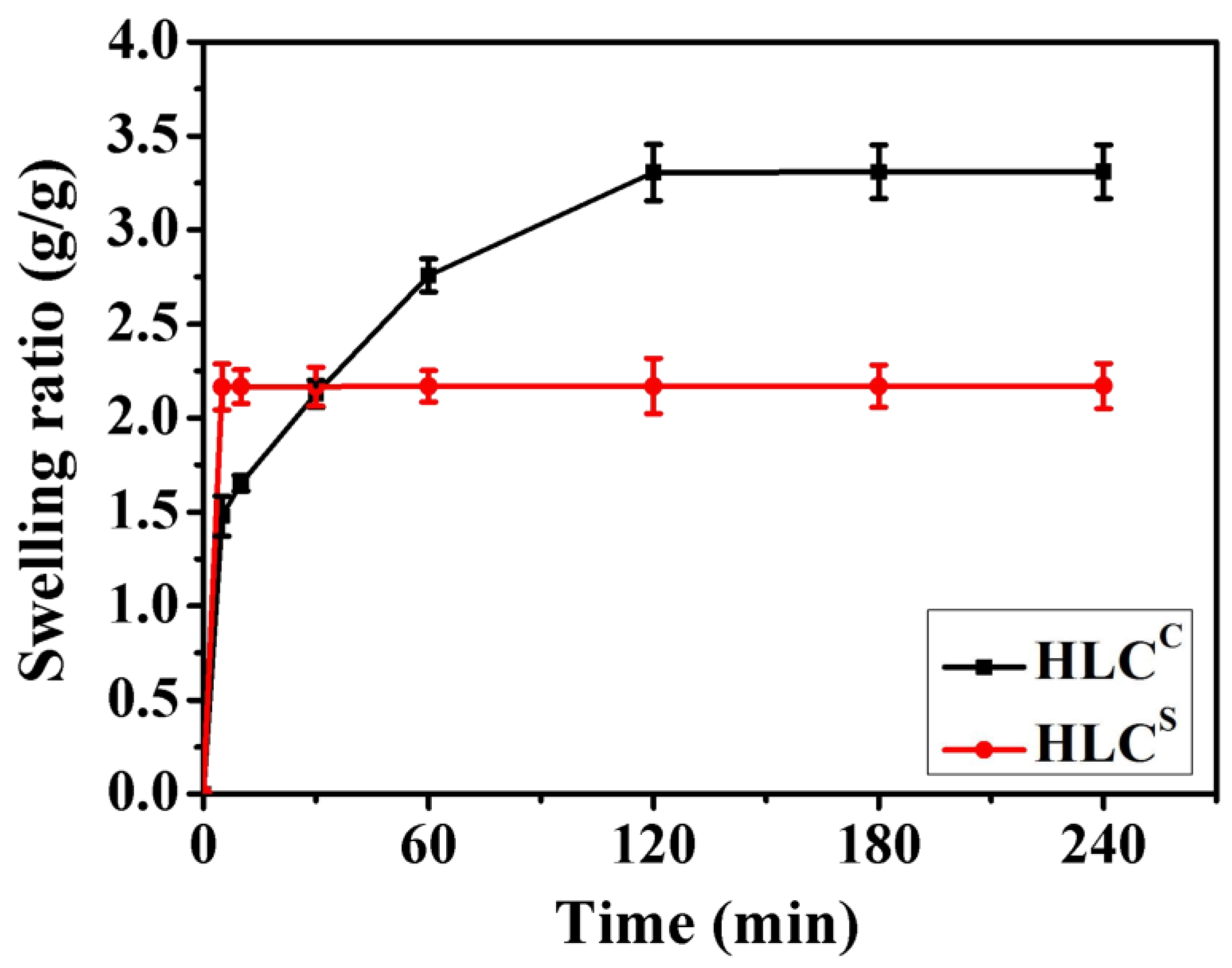
3.3. Swelling Ratio of Hydrogels
3.4. Compressive Mechanical Properties of Hydrogels
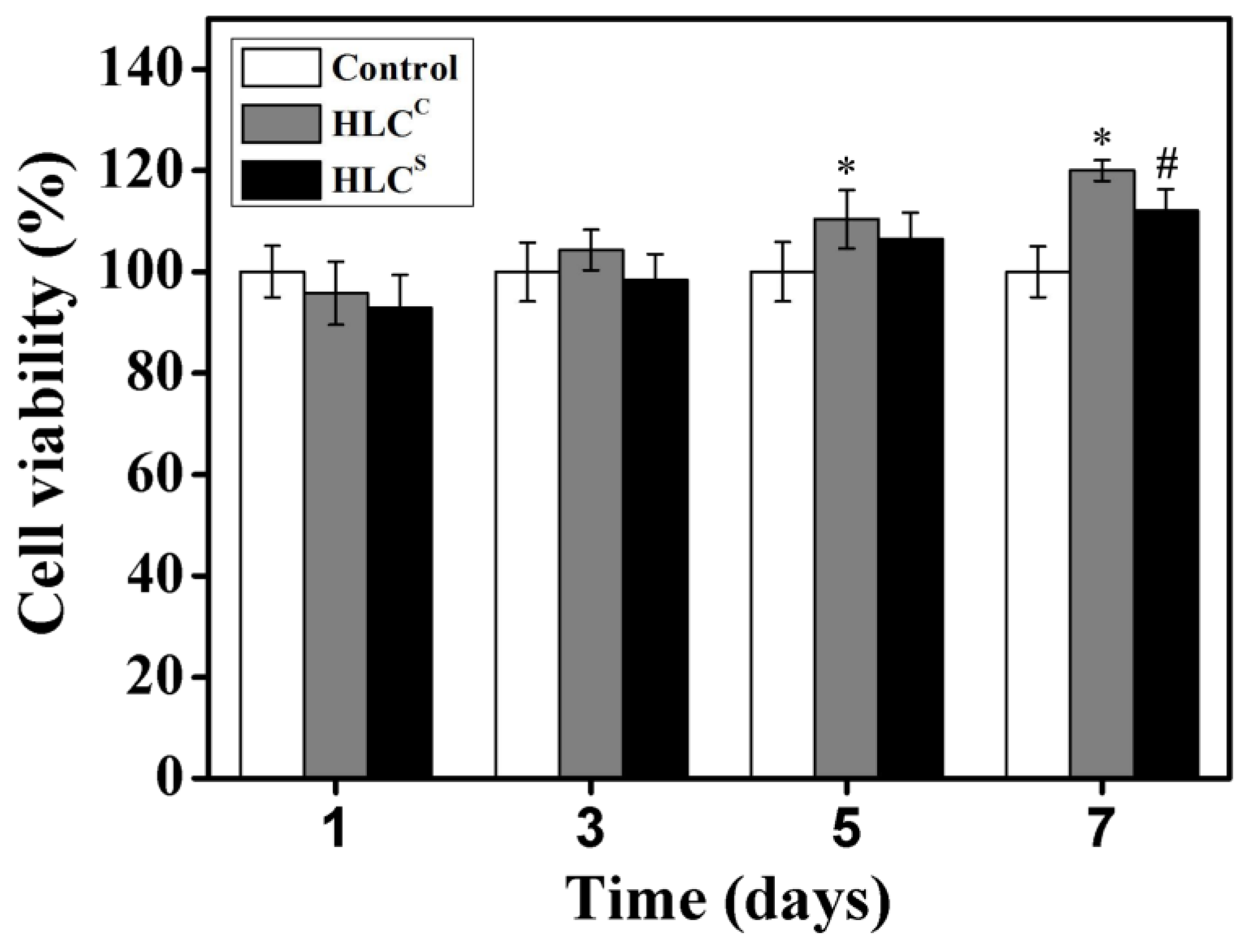
3.5. Cell Viability Analysis
3.6. Adhesion and Proliferation of Chondrocytes on the Hydrogels
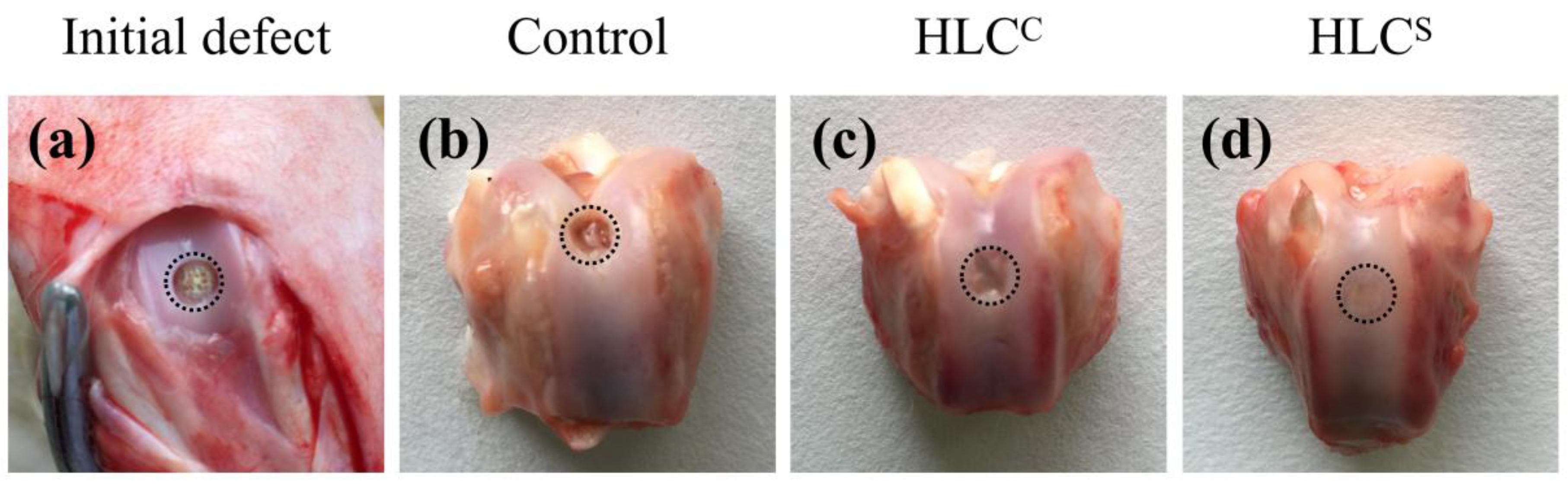
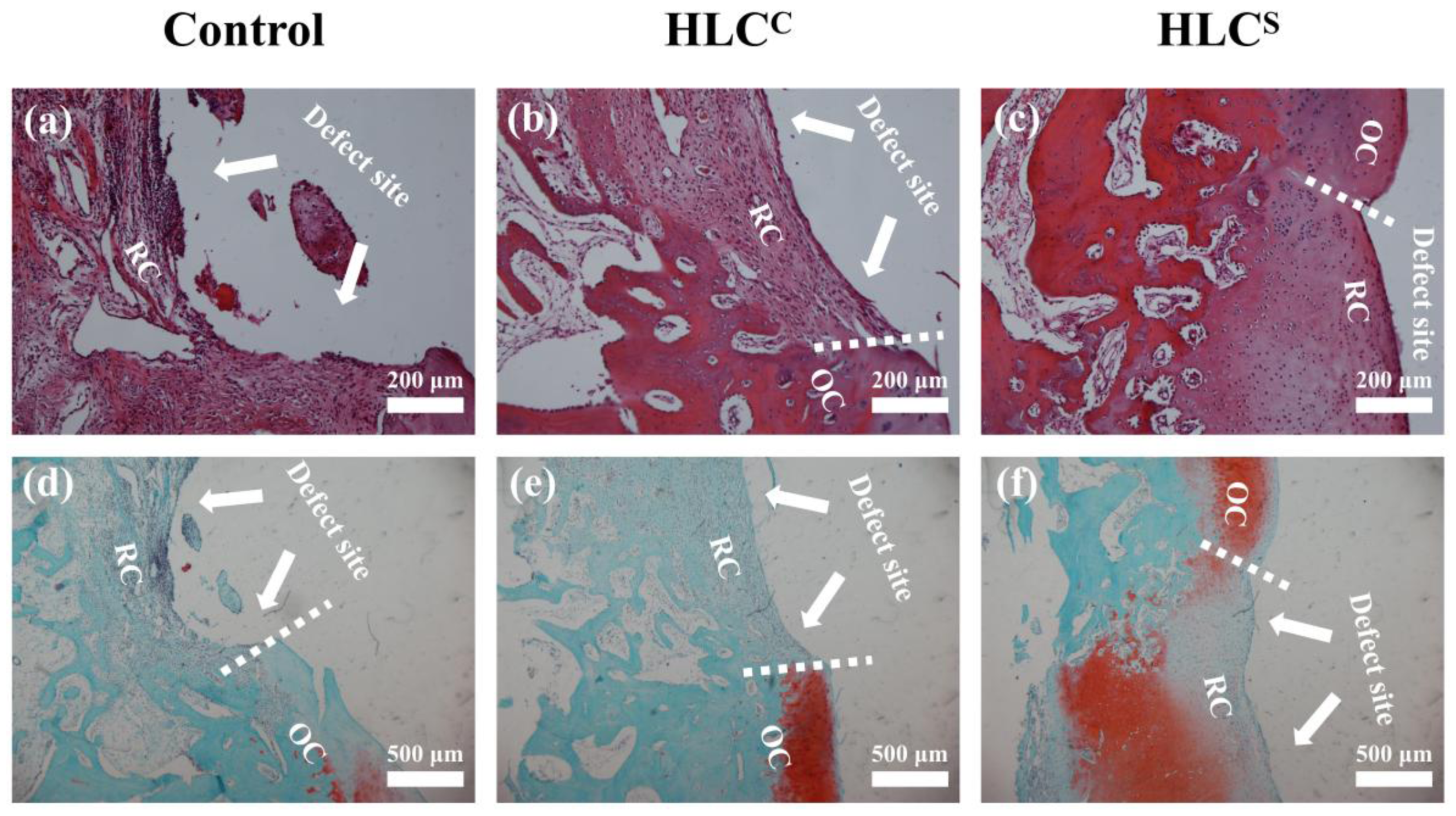
3.7. Observation and Histological Evaluation of Cartilage Repair
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ducheyn, P.; Mauck, R.L.; Smith, D.H. Biomaterials in the repair of sports injuries. Nat. Mater. 2012, 11, 652–654. [Google Scholar] [CrossRef] [PubMed]
- Rahman, R.A.; Sukri, N.M.; Nazir, N.M.; Sha’Ban, M. Tissue engineering of articular cartilage: From bench to bed-side. Tissue Eng. Regen. Med. 2015, 12, 1–11. [Google Scholar] [CrossRef]
- Ochi, M.; Adachi, N.; Nobuto, H.; Yanada, S.; Ito, Y.; Agung, M. Articular cartilage repair using tissue engineering technique-novel approach with minimally invasive procedure. Artif. Organs 2004, 28, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Castrogiovanni, P.; Mazzone, V.; Szychlinska, M.; Castorina, S.; Loreto, C. Histochemistry as a unique approach for investigating normal and osteoarthritic cartilage. Eur. J. Histochem. 2014, 58, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Castrogiovanni, P.; Leonardi, R.; Trovato, F.M.; Szychlinska, M.A.; Di Giunta, A.; Loreto, C.; Castorina, S. New perspectives for articular cartilage repair treatment through tissue engineering: A contemporary review. World J. Orthop. 2014, 5, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Unlike bone, cartilage regeneration remains elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yu, X.; Huang, F.; Cen, S.; Zhong, G.; Xiang, Z. Tissue engineering stratified scaffolds for articular cartilage and subchondral bone defects repair. Orthopedics 2013, 36, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Makris, E.A.; Gomoll, A.H.; Malizos, K.N.; Hu, J.C.; Athanasiou, K.A. Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 2015, 11, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Salick, M.R.; Cordie, T.; Mi, H.Y.; Peng, X.F.; Turng, L.S. Electrospinning homogeneous nanofibrous poly(propylene carbonate)/gelatin composite scaffolds for tissue engineering. Ind. Eng. Chem. Res. 2014, 53, 9391–9400. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Janik, H.; Marzec, M. A review: Fabrication of porous polyurethane scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 48, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Freed, L.E.; Vunjaknovakovic, G.; Biron, R.J.; Eagles, D.B.; Lesnoy, D.C.; Barlow, S.K.; Langer, R. Biodegradable polymer scaffolds for tissue engineering. Nat. Biotechnol. 1994, 12, 689–693. [Google Scholar] [CrossRef]
- Freed, L.E.; Marquis, J.C.; Nohria, A.; Emmanual, J.; Mikos, A.G.; Langer, R. Neocartilage formation in vitro and in vivo using cells cultured on synthetic biodegradable polymers. J. Biomed. Mater. Res. 1993, 27, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for bone tissue engineering: State of the art and new perspectives. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Celikkin, N.; Rinoldi, C.; Costantini, M.; Trombetta, M.; Rainer, A.; Swieszkowski, W. Naturally derived proteins and glycosaminoglycan scaffolds for tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 1277–1299. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Yang, K.C.; Lin, K.H.; Liu, H.C.; Lin, F.H. A highly organized three-dimensional alginate scaffold for cartilage tissue engineering prepared by microfluidic technology. Biomaterials 2011, 32, 7118–7126. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Kuboyama, N. A biodegradable porous composite scaffold of PGA/β-TCP for bone tissue engineering. Bone 2010, 46, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, P.; Schweller, R.M.; Khademhosseini, A.; West, J.L.; Bashir, R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu. Rev. Biomed. Eng. 2014, 16, 247–276. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Annabi, N.; Khademhosseini, A.; Dehghani, F. Fabrication of porous chitosan scaffolds for soft tissue engineering using dense gas CO2. Acta Biomater. 2011, 7, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.P.; Su, C.H. Surface modification of electrospun PLLA nanofibers by plasma treatment and cationized gelatin immobilization for cartilage tissue engineering. Acta Biomater. 2011, 7, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xia, Z.; Czernuszka, J. Design and development of three-dimensional scaffolds for tissue engineering. Chem. Eng. Res. Des. 2007, 85, 1051–1064. [Google Scholar] [CrossRef]
- Woodfield, T.B.; Malda, J.; De Wijn, J.; Peters, F.; Riesle, J.; van Blitterswijk, C.A. Design of porous scaffolds for cartilage tissue engineering using a three-dimensional fiber-deposition technique. Biomaterials 2004, 25, 4149–4161. [Google Scholar] [CrossRef] [PubMed]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Yuan, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol. Adv. 2017, 35, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Luo, Y.E.; Fan, D.D.; Guo, L.; Xi, J.F.; Mi, Y.; Ma, P. Improving the production of human-like collagen by pulse-feeding glucose during the fed-batch culture of recombinant Escherichia coli. Biotechnol. Appl. Biochem. 2012, 59, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, X.; Fan, D.; Zhu, C.; Deng, J.; Hui, J.; Ma, P. Synthesis and characterization of hyaluronic acid/human-like collagen hydrogels. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 43, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xue, W.; Liu, Y.; Fan, D.; Zhu, C.; Ma, X. Novel multifunctional PB and PBH hydrogels as soft filler for tissue engineering. J. Mater. Chem. B 2015, 3, 4742–4755. [Google Scholar] [CrossRef]
- Duan, Z.; Fan, D.; Zhu, C.; Ma, X.; Hui, J. Hemostatic efficacy of human-like collagen sponge in arterioles and liver injury model. Afr. J. Microbiol. Res. 2012, 6, 2543–2551. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, Y.; Fan, D.; Zhu, C.; Liu, L.; Duan, Z. A novel human-like collagen hemostatic sponge with uniform morphology, good biodegradability and biocompatibility. J. Biomater. Appl. 2017, 31, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Fan, D.; Wang, Y. Human-like collagen/hyaluronic acid 3D scaffolds for vascular tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 34, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, X.; Zhao, J.; Ma, S.; Ma, X.; Fan, D.; Zhu, C.; Liu, Y. A novel smart injectable hydrogel prepared by microbial transglutaminase and human-like collagen: Its characterization and biocompatibility. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Esra, Ç.; Eallen, F. Combining protein micro-phase separation and protein-polysaccharide segregative phase separation to produce gel structures. Food Hydrocoll. 2011, 25, 1538–1546. [Google Scholar] [CrossRef]
- Elbert, D.L. Liquid-liquid two phase systems for the production of porous hydrogels and hydrogel microspheres for biomedical applications: A tutorial review. Acta Biomater. 2011, 7, 31–56. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Wang, C.F.S.; Tian, Z.; Zhang, N.; Sheng, H.; Dai, W.; Qian, F. Elucidating the weak protein-protein interaction mechanisms behind the liquid-liquid phase separation of a mab solution by different types of additives. Eur. J. Pharm. Biopharm. 2017, 120, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.C.; Dey, M.; Dutta, A.K.; Basu, B. Competent processing techniques for scaffolds in tissue engineering. Biotechnol. Adv. 2017, 35, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, L.; Fan, D.; Xue, W.; Zhu, C.; Li, X.; Liu, Y.; Liu, W.; Ma, P.; Wang, Y. Physicochemical properties and biological behavior of injectable crosslinked hydrogels composed of pullulan and recombinant human-like collagen. J. Mater. Sci. 2017, 52, 3771–3785. [Google Scholar] [CrossRef]
- Dado, D.; Levenberg, S. Cell–scaffold mechanical interplay within engineered tissue. Semin. Cell Dev. Biol. 2009, 20, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Naseri, N.; Poirier, J.M.; Girandon, L.; Fröhlich, M.; Oksman, K.; Mathew, A.P. 3-Dimensional porous nanocomposite scaffolds based on cellulose nanofibers for cartilage tissue engineering: Tailoring of porosity and mechanical performance. RSC Adv. 2016, 6, 5999–6007. [Google Scholar] [CrossRef]
- Stratton, S.; Shelke, N.B.; Hoshino, K.; Rudraiah, S.; Kumbar, S.G. Bioactive polymeric scaffolds for tissue engineering. Bioact. Mater. 2016, 1, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Daniele, M.A.; Adams, A.A.; Naciri, J.; North, S.H.; Ligler, F.S. Interpenetrating networks based on gelatin methacrylamide and PEG formed using concurrent thiol click chemistries for hydrogel tissue engineering scaffolds. Biomaterials 2014, 35, 1845–1856. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ritchie, A.C.; Everitt, N.M. Comparison of glutaraldehyde and procyanidin cross-linked scaffolds for soft tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 263–273. [Google Scholar] [CrossRef] [PubMed]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Zhu, C.; Fan, D.; Mi, Y.; Li, X.; Fu, R.Z.; Duan, Z.; Wang, Y.; Feng, R.R. A Novel Human-Like Collagen Hydrogel Scaffold with Porous Structure and Sponge-Like Properties. Polymers 2017, 9, 638. https://doi.org/10.3390/polym9120638
Song X, Zhu C, Fan D, Mi Y, Li X, Fu RZ, Duan Z, Wang Y, Feng RR. A Novel Human-Like Collagen Hydrogel Scaffold with Porous Structure and Sponge-Like Properties. Polymers. 2017; 9(12):638. https://doi.org/10.3390/polym9120638
Chicago/Turabian StyleSong, Xi, Chenhui Zhu, Daidi Fan, Yu Mi, Xian Li, Rong Zhan Fu, Zhiguang Duan, Ya Wang, and Rui Rui Feng. 2017. "A Novel Human-Like Collagen Hydrogel Scaffold with Porous Structure and Sponge-Like Properties" Polymers 9, no. 12: 638. https://doi.org/10.3390/polym9120638