Four Mixed-Ligand Zn(II) Three-Dimensional Metal-Organic Frameworks: Synthesis, Structural Diversity, and Photoluminescent Property
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Physical Techniques
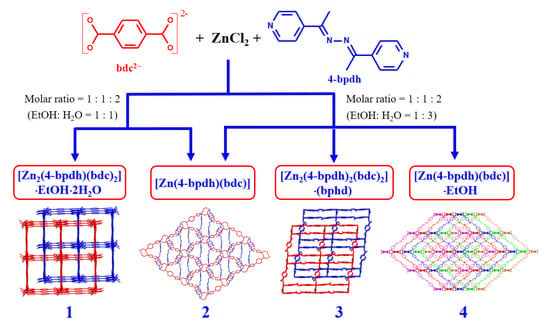
2.2. Synthesis of {[Zn2(bdc)2(4-bpdh)]·C2H5OH·2H2O}n (1) and [Zn(bdc)(4-bpdh)]n (2)
2.3. Synthesis of [Zn(bdc)(4-bpdh)]n (2), {[Zn2(bdc)2(4-bpdh)2]·(4-bpdh)}n (3) and {[Zn(bdc)(4-bpdh)]·C2H5OH}n (4)
2.4. Crystallographic Data Collection and Refinements
2.5. In Situ X-ray Powder Diffraction
2.6. Spectral Measurement
3. Results and Discussion
3.1. Syntheses and Characterization of Compounds 1–4
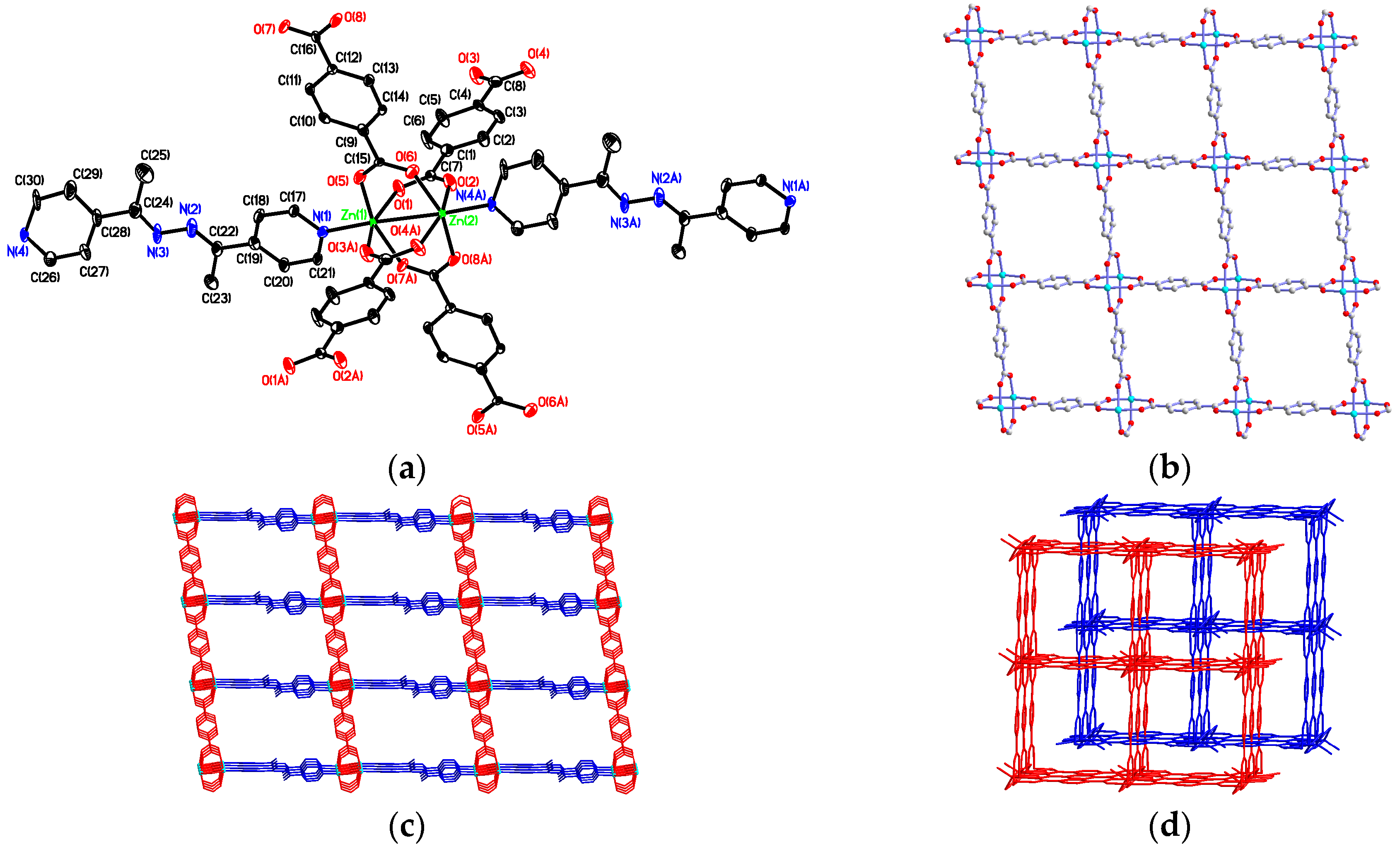
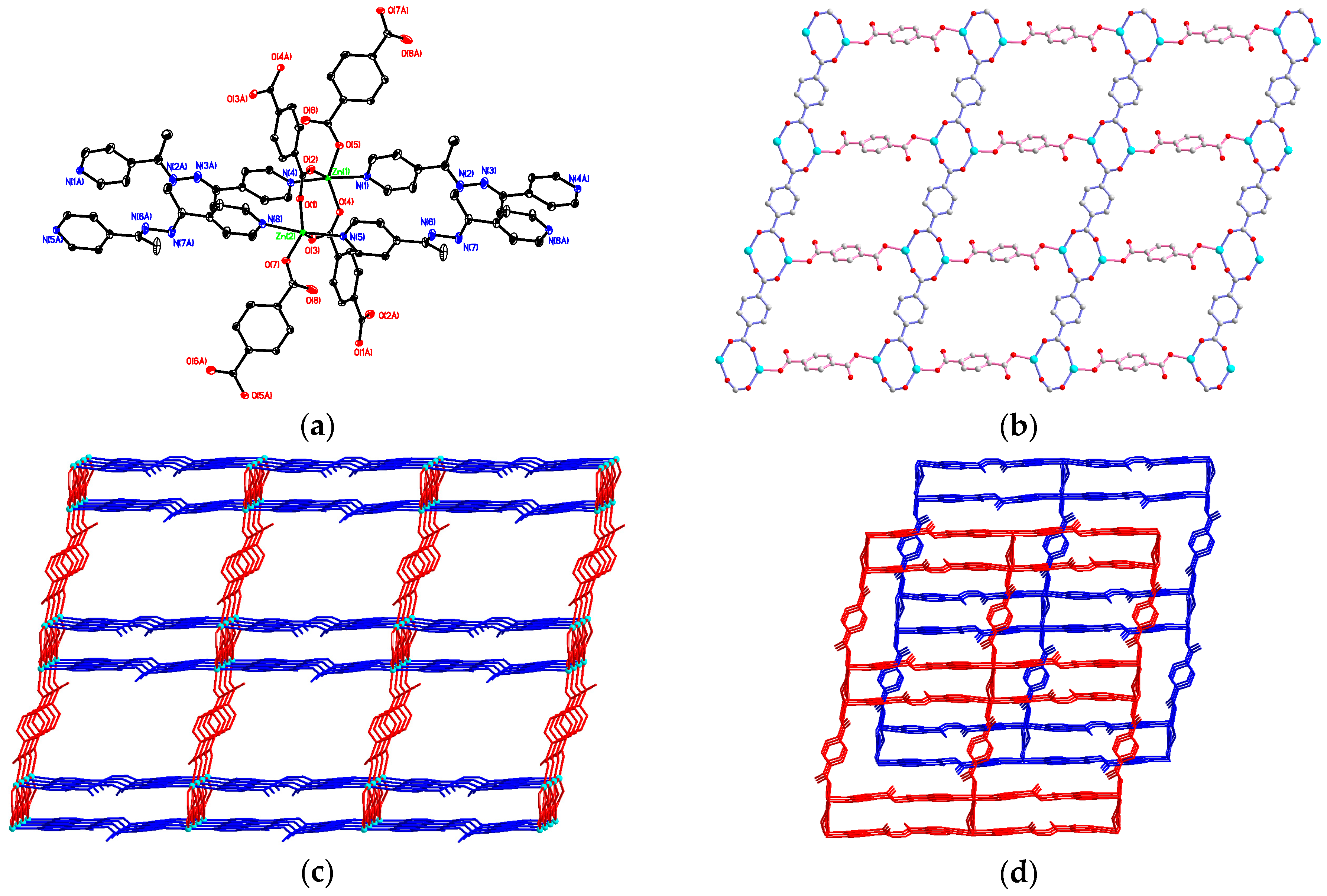
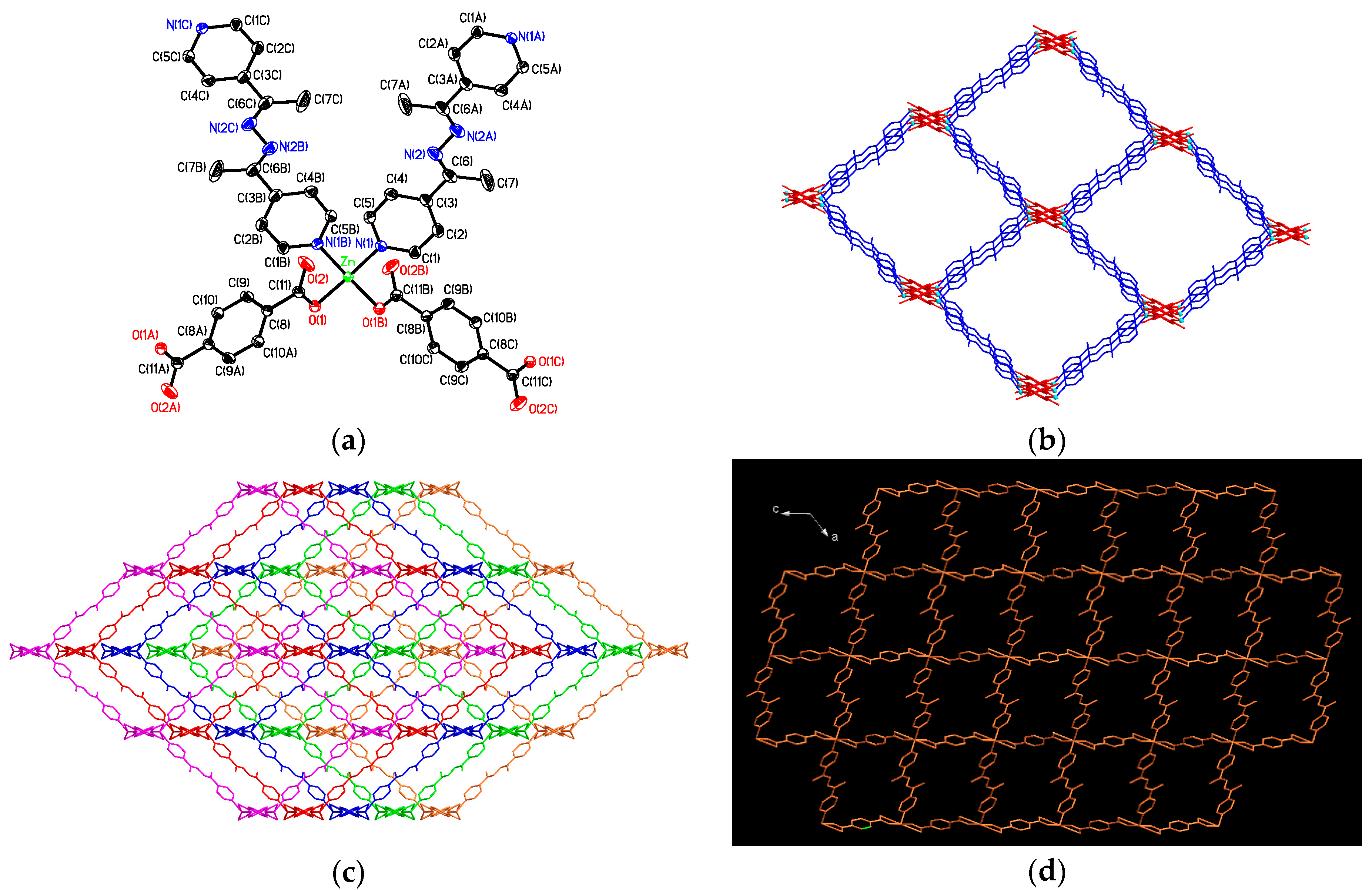
3.2. Structural Description of {[Zn2(bdc)2(4-bpdh)]·C2H5OH·2H2O}n (1)
3.3. Structural Description of [Zn(bdc)(4-bpdh)]n (2)
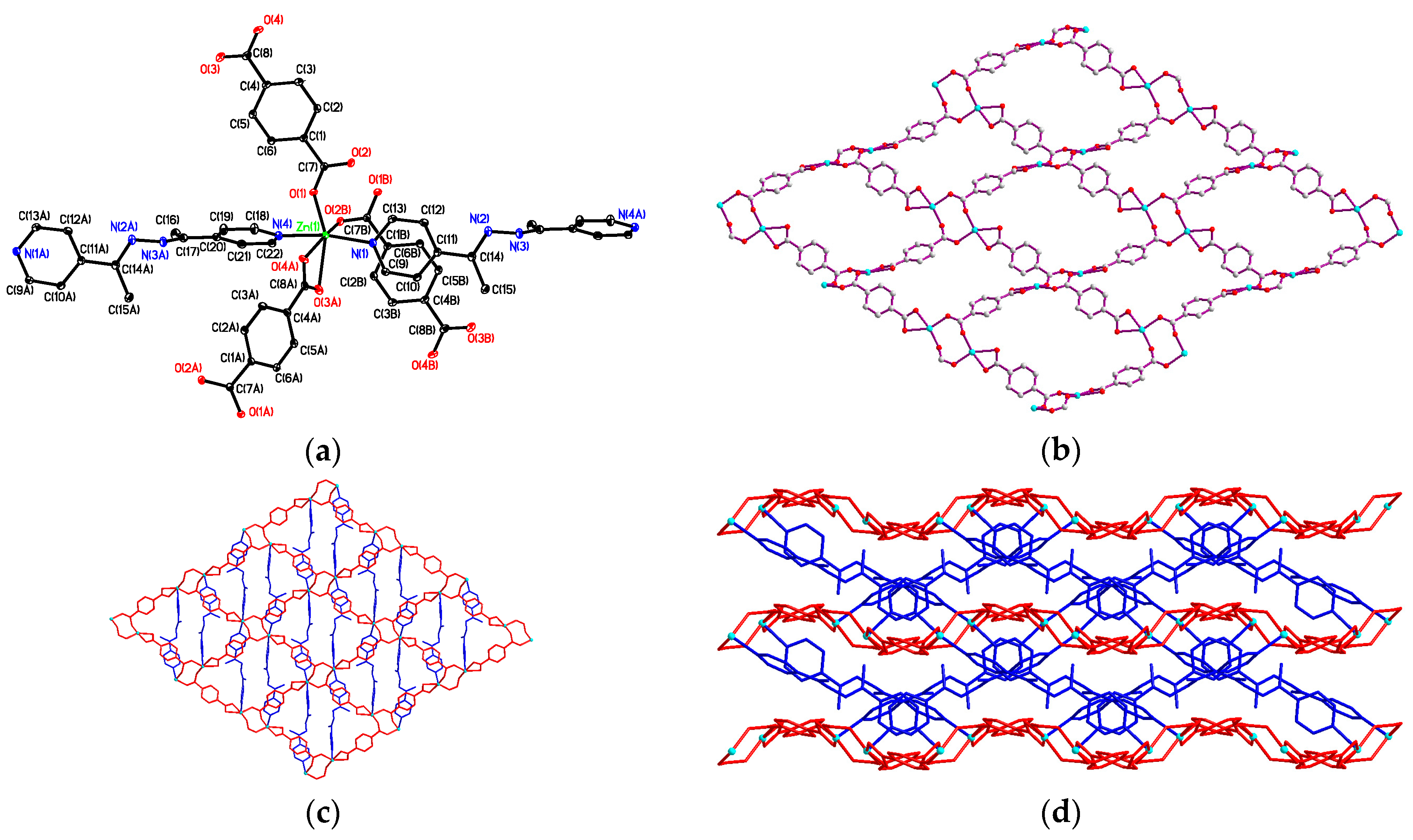
3.4. Structural Description of {[Zn2(bdc)2(4-bpdh)2]·(4-bpdh)}n (3)
3.5. Structural Description of {[Zn(bdc)(4-bpdh)]·C2H5OH}n (4)
3.6. Thermal Stability of CPs 1–4
3.7. Factors Affecting the Structural Diversity and Interpenetrating of CPs 1–4
3.8. Gas-Sorption Studies of CPs 1–4
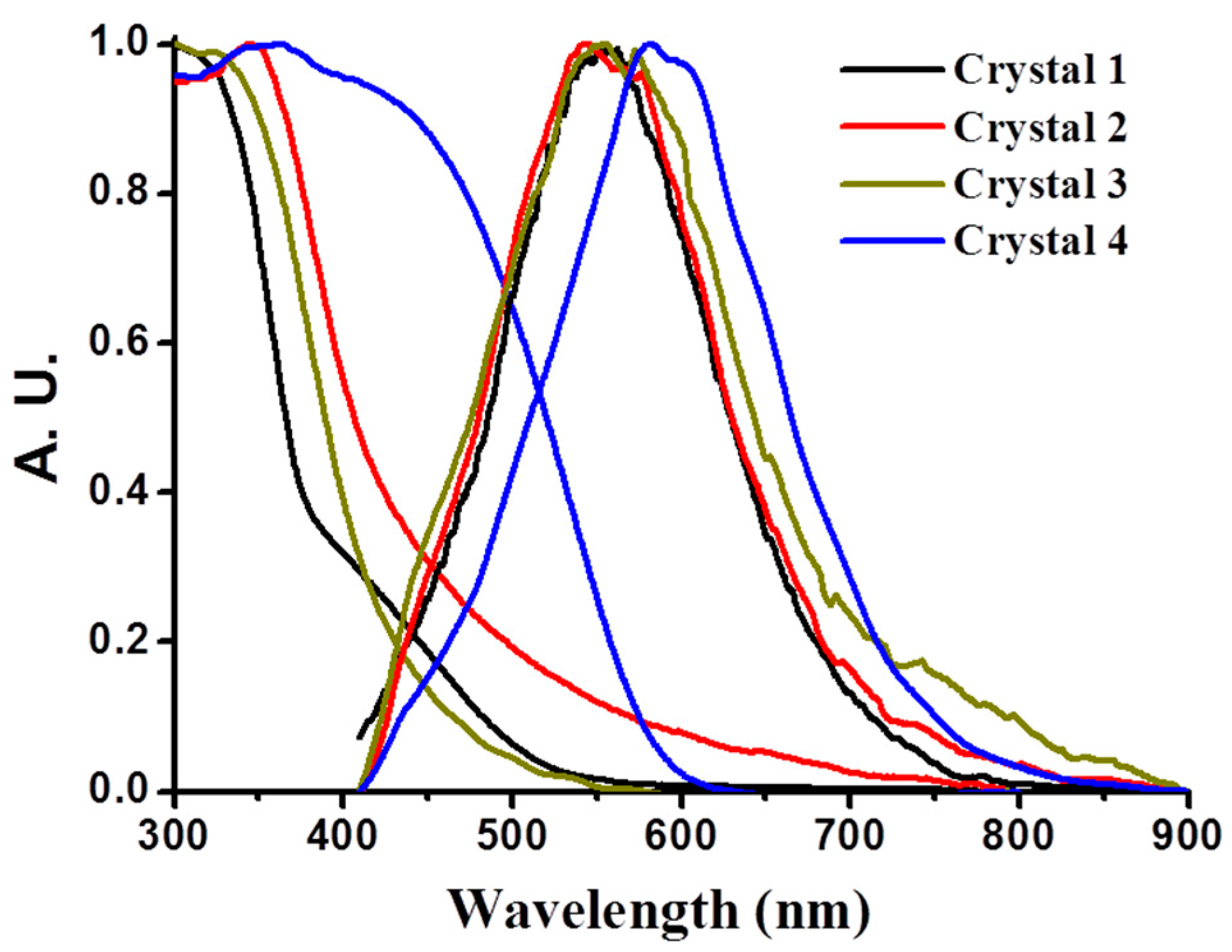
3.9. Luminescence Property of CPs 1–4
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Batten, S.R.; Champness, N.R.; Chen, X.M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Suh, M.P.; Reedijk, J. Coordination polymers, metal–organic frameworks and the need for terminology guidelines. CrystEngComm 2012, 14, 3001–3004. [Google Scholar] [CrossRef]
- Batten, S.R.; Champness, N.R.; Chen, X.M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Suh, M.P.; Reedijk, J. Terminology of metal–organic frameworks and coordination polymers. Pure Appl. Chem. 2013, 85, 1715–1724. [Google Scholar] [CrossRef]
- Li, B.; Chrzanowski, M.; Zhang, Y.; Ma, S. Applications of metal–organic frameworks featuring multi-functional sites. Coord. Chem. Rev. 2016, 307, 106–129. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Hu, Z.; Wang, G.; Uvdal, K. Coordination polymers for energy transfer: Preparations, properties, sensing applications, and perspectives. Coord. Chem. Rev. 2015, 284, 206–235. [Google Scholar] [CrossRef]
- Bradshaw, D.; Claridge, J.B.; Cussen, E.J.; Prior, T.J.; Rosseinsky, M.J. Design, Chirality, and Flexibility in Nanoporous Molecule-Based Materials. Acc. Chem. Res. 2015, 38, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.; Vilela, S.M.F.; Tome, J.P.C.; Paz, F.A.A. Multifunctional metal–organic frameworks: From academia to industrial applications. Chem. Soc. Rev. 2015, 44, 6774–6803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Huo, F. Metal–organic framework composites: From fundamentals to applications. Nanoscale 2015, 7, 7482–7501. [Google Scholar] [CrossRef] [PubMed]
- Janiak, C.; Vieth, J.K. MOFs, MILs and more: Concepts, properties and applications for porous coordination networks (PCNs). New J. Chem. 2010, 34, 2366–2388. [Google Scholar] [CrossRef]
- He, Y.; Li, B.; O’Keeffe, M.; Chen, B. Multifunctional metal–organic frameworks constructed from meta-benzenedicarboxylate units. Chem. Soc. Rev. 2014, 43, 5618–5656. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.J.; Lu, J.; Hong, M.; Cao, R. Metal–organic frameworks based on flexible ligands (FL-MOFs): Structures and applications. Chem. Soc. Rev. 2014, 43, 5867–5895. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Meng, W.; Wu, J.; Ding, J.; Hou, H.; Fan, Y. Crystalline central-metal transformation in metal-organic frameworks. Coord. Chem. Rev. 2016, 307, 130–146. [Google Scholar] [CrossRef]
- Burtch, N.C.; Walton, K.S. Modulating Adsorption and Stability Properties in Pillared Metal–Organic Frameworks: A Model System for Understanding Ligand Effects. Acc. Chem. Res. 2015, 48, 2850–2857. [Google Scholar] [CrossRef] [PubMed]
- De Voorde, B.V.; Bueken, B.; Denayer, J.; De Vos, D. Adsorptive separation on metal–organic frameworks in the liquid phase. Chem. Soc. Rev. 2014, 43, 5766–5788. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Deibert, B.J.; Li, J. Luminescent metal–organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef] [PubMed]
- Canivet, J.; Fateeva, A.; Guo, Y.; Coasnecd, B.; Farrusseng, D. Water adsorption in MOFs: Fundamentals and applications. Chem. Soc. Rev. 2014, 43, 5594–5617. [Google Scholar] [CrossRef] [PubMed]
- Burtch, N.C.; Jasuja, H.; Walton, K.S. Water Stability and Adsorption in Metal–Organic Frameworks. Chem. Rev. 2014, 114, 10575–10612. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.B.; Liu, S.Y.; Ye, J.W.; Li, X.Y.; Zhang, J.P. Photoluminescent Metal–Organic Frameworks for Gas Sensing. Adv. Sci. 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, D.; O’Keeffe, M.; Yaghi, O.M. Topological Analysis of Metal–Organic Frameworks with Polytopic Linkers and/or Multiple Building Units and the Minimal Transitivity Principle. Chem. Rev. 2014, 114, 1343–1370. [Google Scholar] [CrossRef] [PubMed]
- Broker, G.A.; Tiekink, E.R.T. Co-crystal formation between 2,2′-dithiodibenzoic acid and each of 4,4′-bipyridine, trans-1,2-bis(4-pyridyl) ethane and 1,2-bis(4-pyridyl)ethane. CrystEngComm 2007, 9, 1096–1109. [Google Scholar] [CrossRef]
- Lan, Y.Q.; Jiang, H.L.; Li, S.L.; Xu, Q. Solvent-Induced Controllable Synthesis, Single-Crystal to Single-Crystal Transformation and Encapsulation of Alq3 for Modulated Luminescence in (4,8)-Connected Metal–Organic Frameworks. Inorg. Chem. 2012, 51, 7484–7491. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.R.; Wang, X.L.; Shao, K.Z.; Yang, G.S.; Su, Z.M.; Yuan, G. Remarkable solvent-size effects in constructing novel porous 1,3,5-benzenetricarboxylate metal–organic frameworks. CrystEngComm 2012, 14, 5596–5603. [Google Scholar] [CrossRef]
- Guo, M.; Sun, Z.M. Solvents control over the degree of interpenetration in metal–organic frameworks and their high sensitivities for detecting nitrobenzene at ppm level. J. Mater. Chem. 2012, 22, 15939–15946. [Google Scholar] [CrossRef]
- Karmakar, A.; Rúbio, G.M.D.M.; Guedes da Silva, M.F.C.; Hazra, S.; Pombeiro, A.J.L. Solvent-Dependent Structural Variation of Zinc(II) Coordination Polymers and Their Catalytic Activity in the Knoevenagel Condensation Reaction. Cryst. Growth Des. 2015, 15, 4185–4197. [Google Scholar] [CrossRef]
- Roesky, H.W.; Andruh, M. The interplay of coordinative, hydrogen bonding and π–π stacking interactions in sustaining supramolecular solid-state architectures: A study case of bis(4-pyridyl)- and bis(4-pyridyl-N-oxide) tectons. Coord. Chem. Rev. 2003, 236, 91–119. [Google Scholar] [CrossRef]
- Barnett, S.A.; Champness, N.R. Structural diversity of building-blocks in coordination framework synthesis—Combining M(NO3)2 junctions and bipyridyl ligands. Coord. Chem. Rev. 2003, 246, 145–168. [Google Scholar] [CrossRef]
- Pan, L.; Olson, D.H.; Ciemnolonski, L.R.; Heddy, R.; Li, J. Separation of Hydrocarbons with a Microporous Metal Organic Framework. Angew. Chem. Int. Ed. 2006, 45, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Qin, C.; Wang, E.B.; Xu, L.; Su, Z.M.; Hu, C.W. Interlocked and Interdigitated Architectures from Self-Assembly of Long Flexible Ligands and Cadmium Salts. Angew. Chem. Int. Ed. 2004, 43, 5036–5040. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Chun, H.; Kim, G.H.; Lee, H.S.; Kim, K. Vapor phase inclusion of ferrocene and its derivative in a microporous metal–organic porous material and its structural characterization by single crystal X-ray diffraction. Chem. Commun. 2006, 2759–2761. [Google Scholar] [CrossRef]
- Wu, H.; Yang, J.; Su, Z.M.; Batten, S.R.; Ma, J.F. An Exceptional 54-Fold Interpenetrated Coordination Polymer with 103-srs Network Topology. J. Am. Chem. Soc. 2011, 133, 11406–11409. [Google Scholar] [CrossRef] [PubMed]
- Rowsell, J.L.C.; Yaghi, O.M. Strategies for hydrogen storage in metal–organic frameworks. Angew. Chem. Int. Ed. 2005, 44, 4670–4679. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.; Broadbelt, L.J.; Snurr, R.Q. Is catenation beneficial for hydrogen storage in metal–organic frameworks? Chem. Commun. 2008, 4132–4134. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Eckert, J.; Forster, P.M.; Yoon, J.W.; Hwang, Y.K.; Chang, J.S.; Collier, C.D.; Parise, J.B.; Zhou, H.C. Further Investigation of the Effect of Framework Catenation on Hydrogen Uptake in Metal–Organic Frameworks. J. Am. Chem. Soc. 2008, 130, 15896–15902. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Sun, D.; Ambrogio, M.; Fillinger, J.A.; Parkin, S.; Zhou, H.C. Framework-Catenation Isomerism in Metal–Organic Frameworks and Its Impact on Hydrogen Uptake. J. Am. Chem. Soc. 2007, 129, 1858–1859. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.L.; Tatsu, Y.; Lu, Z.H.; Xu, Q. Non-, Micro-, and Mesoporous Metal–Organic Framework Isomers: Reversible Transformation, Fluorescence Sensing, and Large Molecule Separation. J. Am. Chem. Soc. 2010, 132, 5586–5587. [Google Scholar] [CrossRef] [PubMed]
- Bureekaew, S.; Sato, H.; Matsuda, R.; Kubota, Y.; Hirose, R.; Kim, J.; Kato, K.; Takata, M.; Kitagawa, S. Control of Interpenetration for Tuning Structural Flexibility Influences Sorption Properties. Angew. Chem. Int. Ed. 2010, 49, 7660–7664. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.M.; Farha, O.K.; Sarjeant, A.A.; Hupp, J.T.; Scheidt, K.A. Two Azolium Rings Are Better Than One: A Strategy for Controlling Catenation and Morphology in Zn and Cu Metal–Organic Frameworks. Cryst. Growth Des. 2011, 11, 4747–4750. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Zhuang, C.F.; Tang, Y.; Su, C.Y. Guest Inclusion and Interpenetration Tuning of Cd(II)/Mn(II) Coordination Grid Networks Assembled from a Rigid Linear Diimidazole Schiff Base Ligand. Inorg. Chem. 2009, 48, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Lin, W. Chirality-Controlled and Solvent-Templated Catenation Isomerism in Metal–Organic Frameworks. J. Am. Chem. Soc. 2008, 130, 13834–13835. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.J.; Sun, D.; Liu, F.J.; Huang, R.B.; Zheng, L.S. Discrete Octamer Water Cluster and 1D T5(2) Water Tape Trapped in Two Luminescent Zn(II)/1,2-Bis(imidazol-1′-yl)ethane/Dicarboxylate Hosts: From 2D (4,4) Net to 3D 5-Fold Interpenetrated Diamond Network. Cryst. Growth Des. 2011, 11, 5475–5482. [Google Scholar] [CrossRef]
- Nijem, N.; Veyan, J.F.; Kong, L.; Li, K.; Pramanik, S.; Zhao, Y.; Li, J.; Langreth, D.; Chabal, Y.J. Interaction of Molecular Hydrogen with Microporous Metal Organic Framework Materials at Room Temperature. J. Am. Chem. Soc. 2010, 132, 1654–1664. [Google Scholar] [CrossRef] [PubMed]
- Curtin, D.M.; Dong, Y.B.; Smith, M.D.; Barclay, T.; zur Loye, H.C. Two Versatile N,N′-Bipyridine-Type Ligands for Preparing Organic–Inorganic Coordination Polymers: New Cobalt- and Nickel-Containing Framework Materials. Inorg. Chem. 2001, 40, 2825–2834. [Google Scholar] [CrossRef]
- Shi, Y.J.; Li, L.H.; Xu, Y.; Chen, X.T.; Xue, Z.; You, X.Z. Syntheses and structures of two- and one-dimensional organic–inorganic hybrid compounds assembled from PbI2 and 1,4-bis(4-pyridyl)-2,3-diaza-1,3-butadiene. Inorg. Chem. Commun. 2002, 5, 1090–1094. [Google Scholar] [CrossRef]
- Shen, L. Synthesis, crystal structure and magnetic property of a novel sheet-like polymer [Mn(bpd)(NCS)2(CH3OH)2]n (bpd = 1,4-bis(4-pyridyl)-2,3-diaza-1,3-butadiene). Inorg. Chem. Commun. 2003, 6, 1133–1136. [Google Scholar] [CrossRef]
- Patra, G.K.; Goldberg, I. Supramolecular Design of Coordination Complexes of Silver(I) with Polyimine Ligands: Synthesis, Materials Characterization, and Structure of New Polymeric and Oligomeric Materials. Cryst. Growth. Des. 2003, 3, 321–329. [Google Scholar] [CrossRef]
- Dong, Y.B.; Zhao, X.; Huang, R.Q.; Smith, M.D.; zur Loye, H.C. New Ag(I)-Containing Coordination Polymers Generated from Multidentate Schiff-Base Ligands. Inorg. Chem. 2004, 43, 5603–5612. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.H.; Liu, Y.Q.; Fang, R.B. Synthesis, crystal structures and magnetic properties of a coordination polymer containing the [Mn5(μ1,1-N3)8(N3)2(bpd)5(H2O)2] repeat cluster units (bpd = 1,4-bis(4-pyridyl)-2,3-diaza-1,3-butadiene). Inorg. Chem. Commun. 2006, 9, 699–702. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, G.; Ma, J.S. Anion Control of the Self-Assembly of One-Dimensional Molecular Ladders vs. Three-Dimensional Cross-like Arrays Based on a Bidentate Schiff Base Ligand. Cryst. Growth Des. 2006, 6, 1897–1902. [Google Scholar] [CrossRef]
- Granifo, J.; Garland, M.T.; Baggio, R. The effect in the assembly and node nuclearity of the long and rigid character of bis-pyridyl exo-bidentate spacers when react with zinc acetate: Crystal structures of the high nuclearity coordination polymers [Zn7(μ4-O)2(OAc)10(3pdb)]n (3pdb = 1,4-bis(3-pyridyl)-2,3-diaza-1,3-butadiene) and [Zn7(μ4-O)2(OAc)10(4pdb)]n (4pdb = 1,4-bis(4-pyridyl)-2,3-diaza-1,3-butadiene). Polyhedron 2006, 25, 2277–2283. [Google Scholar]
- Wang, C.C.; Lin, W.Z.; Huang, W.T.; Ko, M.J.; Lee, G.H.; Ho, M.L.; Lin, C.W.; Shih, C.W.; Chou, P.T. New supramolecular isomers with 2D 44 square-grid and 3D 65·8 frameworks in a one-pot synthesis; reversible solvent uptake and intriguing luminescence properties. Chem. Commun. 2008, 1299–1301. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Lee, G.H.; Wang, H.T. Synthesis, Structural Characterization and Thermal Stability of [Mn(3-bpd)2(NCS)2(H2O)2]·2H2O (1) and {[Mn(bpe)(NCS)2(H2O)2]·(3-bpd)·(bpe)·H2O}n (2) from One-Pot Crystallization. J. Chin. Chem. Soc. 2009, 56, 709–717. [Google Scholar] [CrossRef]
- Liu, C.Y.; Wang, H.T.; Chung, W.C.; Cheng, Y.T.; Chen, Y.T.; Ho, M.L.; Wang, C.C.; Lee, G.H.; Sheu, H.S. Assembly of Three 2D Metal–Organic Frameworks (MOFs) Derived from Flexible Ligands, 1,4-bis(3-pyridyl)-2,3-diaza-1,3-butadiene (3-bpd) and/or 1,2-bis(4-pyridyl)ethane (dpe). J. Chin. Chem. Soc. 2012, 59, 1070–1079. [Google Scholar] [CrossRef]
- Dong, Y.B.; Smith, M.D.; Layland, R.C.; zur Loye, H.C. A Novel Noninterpenetrating Polycyclohexane Network: A New Inorganic/Organic Coordination Polymer Structural Motif Generated by Self-Assembly of “T-Shaped” Moieties. Chem. Mater. 2000, 12, 1156–1161. [Google Scholar] [CrossRef]
- Dong, Y.B.; Smith, M.D.; zur Loye, H.C. New Inorganic/Organic Coordination Polymers Generated from Bidentate Schiff-Base Ligands. Inorg. Chem. 2000, 39, 4927–4935. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.B.; Smith, M.D.; zur Loye, H.C. New Inorganic–Organic Coordination Polymers Generated from Rigid or Flexible Bidentate Ligands and Co(NCS)2·xH2O. J. Solid State Chem. 2000, 155, 143–153. [Google Scholar] [CrossRef]
- Parshamoni, S.; Konar, S. Selective CO2 adsorption in four Zinc(II)-based metal organic frameworks constructed using a rigid N,N′-donor linker and various dicarboxylate ligands. CrystEngComm 2016, 18, 4395–4404. [Google Scholar] [CrossRef]
- Parshamoni, S.; Sanda, S.; Jena, H.S.; Konar, S. Tuning CO2 Uptake and Reversible Iodine Adsorption in Two Isoreticular MOFs through Ligand Functionalization. Chem. Asian J. 2015, 10, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi, E.; Masoomi, M.Y.; Yamini, Y.; Morsali, A. Application of Mechanosynthesized Azine-Decorated Zinc(II) Metal–Organic Frameworks for Highly Efficient Removal and Extraction of Some Heavy-Metal Ions from Aqueous Samples: A Comparative Study. Inorg. Chem. 2015, 54, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, B.; Maity, D.K.; Mondal, R.; Colacio, E.; Ghoshal, D. Two Series of Isostructural Coordination Polymers with Isomeric Benzenedicarboxylates and Different Azine Based N,N′-Donor Ligands: Syntheses, Characterization and Magnetic Properties. Cryst. Growth Des. 2015, 15, 4427–4437. [Google Scholar] [CrossRef]
- Manna, B.; Singh, S.; Karmakar, A.; Desai, A.V.; Ghosh, S.K. Selective Anion Exchange and Tunable Luminescent Behaviors of Metal–Organic Framework Based Supramolecular Isomers. Inorg. Chem. 2015, 54, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, B.; Haldar, R.; Maity, D.K.; Maji, T.K.; Ghoshal, D. Pillared-bilayer porous coordination polymers of Zn(II): Enhanced hydrophobicity of pore surface by changing the pillar functionality. CrystEngComm 2015, 17, 3478–3486. [Google Scholar] [CrossRef]
- Safarifard, V.; Beheshti, S.; Morsali, A. An interpenetrating amine-functionalized metal–organic framework as an efficient and reusable catalyst for the selective synthesis of tetrahydro-chromenes. CrystEngComm 2015, 17, 1680–1685. [Google Scholar] [CrossRef]
- Maity, D.K.; Bhattacharya, B.; Mondal, R.; Ghoshal, D. Five diverse bivalent metal coordination polymers based on benzene dicarboxylate and bent dipyridyl ligands: Syntheses, structures, and photoluminescent properties. CrystEngComm 2014, 16, 8896–8909. [Google Scholar] [CrossRef]
- Bhattacharya, B.; Haldar, R.; Dey, R.; Maji, T.K.; Ghoshal, D. Porous coordination polymers based on functionalized Schiff base linkers: Enhanced CO2uptake by pore surface modification. Dalton Trans. 2014, 43, 2272–2282. [Google Scholar] [CrossRef] [PubMed]
- Dey, R.; Bhattacharya, B.; Pachfule, P.; Banerjee, R.; Ghoshal, D. Flexible dicarboxylate based pillar-layer metal organic frameworks: Differences in structure and porosity by tuning the pyridyl based N,N′ linkers. CrystEngComm 2014, 16, 2305–2316. [Google Scholar] [CrossRef]
- Masoomi, M.Y.; Stylianou, K.C.; Morsali, A.; Retailleau, P.; Maspoch, D. Selective CO2 Capture in Metal–Organic Frameworks with Azine-Functionalized Pores Generated by Mechanosynthesis. Cryst. Growth Des. 2014, 14, 2092–2096. [Google Scholar] [CrossRef]
- Parshamoni, S.; Sanda, S.; Jena, H.S.; Tomar, K.; Konar, S. Exploration of Structural Topologies in Metal–Organic Frameworks Based on 3-(4-Carboxyphenyl)propionic Acid, Their Synthesis, Sorption, and Luminescent Property Studies. Cryst. Growth Des. 2014, 14, 2022–2033. [Google Scholar] [CrossRef]
- Zhou, J.; Du, L.; Qiao, Y.F.; Hu, Y.; Li, B.; Li, L.; Wang, X.Y.; Yang, J.; Xie, M.J.; Zhao, Q.H. Intriguing Architectures Generated from 1,4-Bis(3- or 4-pyridyl)-2,3-diaza-1,3-butadiene with Aromatic Dicarboxylates: Syntheses, Crystal Structures, and Properties. Cryst. Growth Des. 2014, 14, 1175–1183. [Google Scholar] [CrossRef]
- Bhattacharya, B.; Maity, D.K.; Pachfule, P.; Colacio, E.; Ghoshal, D. Syntheses, X-ray structures, catalytic activity and magnetic properties of two new coordination polymers of Co(II) and Ni(II) based on benzenedicarboxylate and linear N,N′-donor Schiff base linkers. Inorg. Chem. Front. 2014, 1, 414–425. [Google Scholar] [CrossRef]
- Bhattacharya, B.; Dey, R.; Maity, D.K.; Ghoshal, D. Formation of three new metal organic hybrids of Cd(II) with N,N′ donor spacer: An in situ perchlorate to chloride transformation. CrystEngComm 2013, 15, 9457–9464. [Google Scholar] [CrossRef]
- Sanda, S.; Parshamoni, S.; Adhikary, A.; Konar, S. A Family of Metal–Organic Frameworks Based on Carboxylates and a Neutral, Long, and Rigid Ligand: Their Structural Revelation, Magnetic, and Luminescent Property Study. Cryst. Growth Des. 2013, 13, 5442–5449. [Google Scholar] [CrossRef]
- Bhattacharya, B.; Dey, R.; Pachfule, P.; Banerjee, R.; Ghoshal, D. Four 3D Cd(II)-Based Metal Organic Hybrids with Different N,N′-Donor Spacers: Syntheses, Characterizations, and Selective Gas Adsorption Properties. Cryst. Growth Des. 2013, 13, 731–739. [Google Scholar] [CrossRef]
- Zhang, S.-Q.; Jiang, F.-L.; Wu, M.-Y.; Ma, J.; Bu, Y.; Hong, M.-C. Assembly of Discrete One-, Two-, and Three-Dimensional Zn(II) Complexes Containing Semirigid V-Shaped Tricarboxylate Ligands. Cryst. Growth Des. 2012, 12, 1452–1463. [Google Scholar] [CrossRef]
- Siemens Analytical Instruments Division. SMART V 4.043 Software for CCD Detector System; Siemens Analytical Instruments Division: Madison, WI, USA, 1995. [Google Scholar]
- Siemens Analytical Instruments Division. SAINT V 4.035 Software for CCD Detector System; Siemens Analytical Instruments Division: Madison, WI, USA, 1995. [Google Scholar]
- Sheldrick, G.M. Program for the Refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 1993. [Google Scholar]
- Siemens Analytical Instruments Division. SHELXTL 5.03 (PC-Version), Program Liberary for Structure Solution and Molecular Graphics; Siemens Analytical Instruments Division: Madison, WI, USA, 1995. [Google Scholar]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- O’Keeffe, M.; Peskov, M.A.; Ramsden, S.J.; Yaghi, O.M. The Reticular Chemistry Structure Resource (RCSR) Database of, and Symbols for, Crystal Nets. Acc. Chem. Res. 2008, 41, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Zhang, J.P.; Huang, X.C.; Chen, X.M. Supramolecular isomerism in coordination polymers. Chem. Soc. Rev. 2009, 38, 2385–2396. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Sheu, G.B.; Ke, S.Y.; Shih, C.Y.; Cheng, Y.J.; Chen, Y.T.; Cho, C.H.; Ho, M.L.; Chen, W.T.; Liao, R.H.; et al. A 3D porous supramolecular architecture via π–π assembly of 2D metal–organic frameworks (MOFs): Structure-versus-luminescence reversibility and gas adsorption properties. CrystEngComm 2015, 17, 1264–1272. [Google Scholar] [CrossRef]
- Nagaraja, C.M.; Ugale, B.; Chanthapally, A. Construction of a 2D Interwoven and 3D Interpenetrated Metal–Organic Frameworks of Zn(II) by Varying N,N′-Donor Spacers. CrystEngComm 2014, 16, 4805–4815. [Google Scholar] [CrossRef]

| Compound | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| empirical formula | C32H22N4O11Zn2 | C22H18N4O4Zn1 | C51H43.5N10O8Zn2 | C26H30N4O6Zn1 |
| formula mass (g mol−1) | 769.28 | 467.77 | 1055.20 | 559.91 |
| crystal system | Triclinic | Monoclinic | Triclinic | Monoclinic |
| space group | P-1 | P21/c | P-1 | C2/c |
| a (Å) | 10.893(2) | 7.4958(5) | 10.4787(5) | 27.020(2) |
| b (Å) | 10.899(2) | 13.5492(9) | 14.7264(7) | 6.0013(4) |
| c (Å) | 18.088(3) | 19.512(1) | 15.6046(7) | 20.136(1) |
| α (deg) | 83.619(3) | 90.00 | 98.494(1) | 90.00 |
| β (deg) | 81.716(3) | 90.927(2) | 92.658(1) | 128.095(1) |
| γ (deg) | 88.729(3) | 90.00 | 104.9995(9) | 90.00 |
| V (Å3) | 2112.0(5) | 1981.4(2) | 2291.4(2) | 2569.7(3) |
| Z | 2 | 4 | 2 | 4 |
| T (K) | 150(2) | 150(2) | 200(2) | 150(2) |
| Dcalcd (g cm−3) | 1.210 | 1.568 | 1.529 | 1.447 |
| μ (mm−1) | 1.187 | 1.278 | 1.116 | 1.004 |
| θ range (deg) | 1.88–25.0 | 1.83–27.5 | 1.32–27.5 | 1.92–27.5 |
| total no. of data collected | 22,177 | 18,016 | 29,841 | 9404 |
| no. of unique data | 7422 | 4561 | 10,488 | 2939 |
| no. of obsd data (I > 2σ(I)) | 5511 | 3594 | 8875 | 2588 |
| Rint | 0.0884 | 0.0588 | 0.0398 | 0.0355 |
| refine params | 456 | 282 | 612 | 181 |
| R1, wR2 1 (I > 2σ(I)) | 0.1073, 0.2718 | 0.0446, 0.1025 | 0.0477, 0.1307 | 0.0588, 0.1620 |
| R1, wR2 1 (all data) | 0.1367, 0.2881 | 0.0621, 0.1103 | 0.0577, 0.1414 | 0.0653, 0.1684 |
| GOF 2 | 1.153 | 1.018 | 1.070 | 1.053 |
| Compound | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| C. N. of Zn(II) | 5 | 6 | 5 | 4 |
| Geometry of Zn(II) center | SP | Oh | TBP | Td |
| Coordination mode of bdc2− | bis-bidentate | chelating/bidentate | bis-bidentate & bis-monodentate | bis-monodentate |
| Interpenetration | Two-fold | None | Two-fold | Five-fold |
| Guest molecule | EtOH, H2O | None | 4-bpdh | EtOH |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-C.; Ke, S.-Y.; Cheng, C.-W.; Wang, Y.-W.; Chiu, H.-S.; Ko, Y.-C.; Sun, N.-K.; Ho, M.-L.; Chang, C.-K.; Chuang, Y.-C.; et al. Four Mixed-Ligand Zn(II) Three-Dimensional Metal-Organic Frameworks: Synthesis, Structural Diversity, and Photoluminescent Property. Polymers 2017, 9, 644. https://doi.org/10.3390/polym9120644
Wang C-C, Ke S-Y, Cheng C-W, Wang Y-W, Chiu H-S, Ko Y-C, Sun N-K, Ho M-L, Chang C-K, Chuang Y-C, et al. Four Mixed-Ligand Zn(II) Three-Dimensional Metal-Organic Frameworks: Synthesis, Structural Diversity, and Photoluminescent Property. Polymers. 2017; 9(12):644. https://doi.org/10.3390/polym9120644
Chicago/Turabian StyleWang, Chih-Chieh, Szu-Yu Ke, Chia-Wen Cheng, Yu-Wen Wang, Hsiao-Shan Chiu, Yu-Chien Ko, Ning-Kuei Sun, Mei-Lin Ho, Chung-Kai Chang, Yu-Chun Chuang, and et al. 2017. "Four Mixed-Ligand Zn(II) Three-Dimensional Metal-Organic Frameworks: Synthesis, Structural Diversity, and Photoluminescent Property" Polymers 9, no. 12: 644. https://doi.org/10.3390/polym9120644