Photo Irradiation-Induced Core Crosslinked Poly(ethylene glycol)-block-poly(aspartic acid) Micelles: Optimization of Block Copolymer Synthesis and Characterization of Core Crosslinked Micelles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
2.3. Synthesis of Bromo Alkylated Chalcone Amide (1a–c)
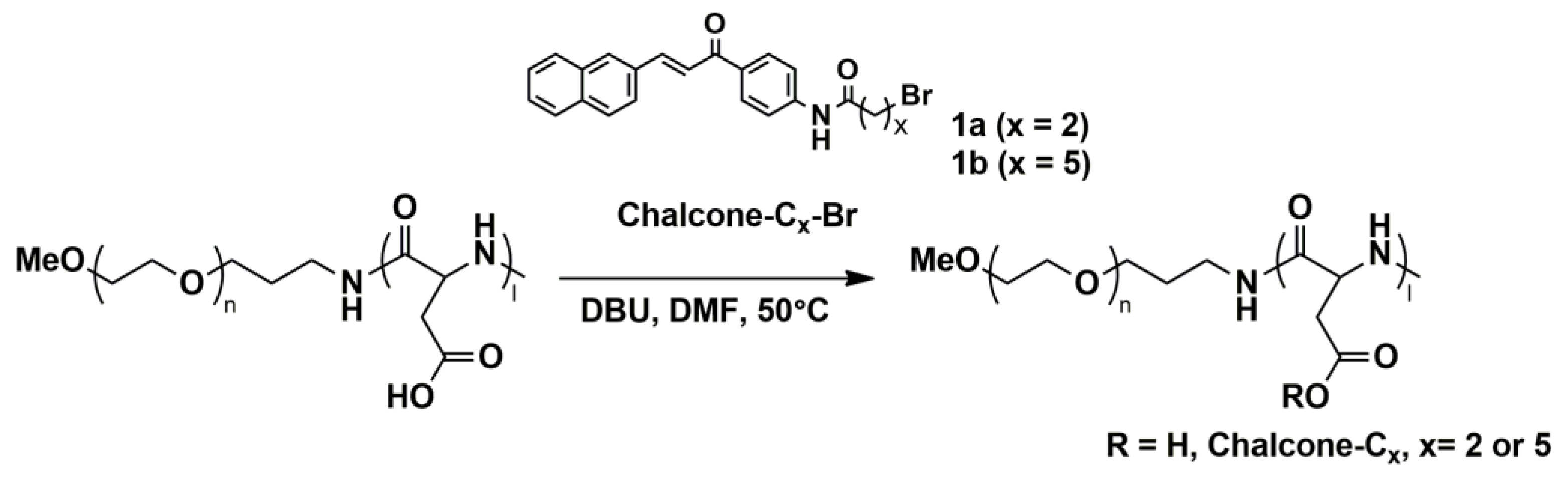
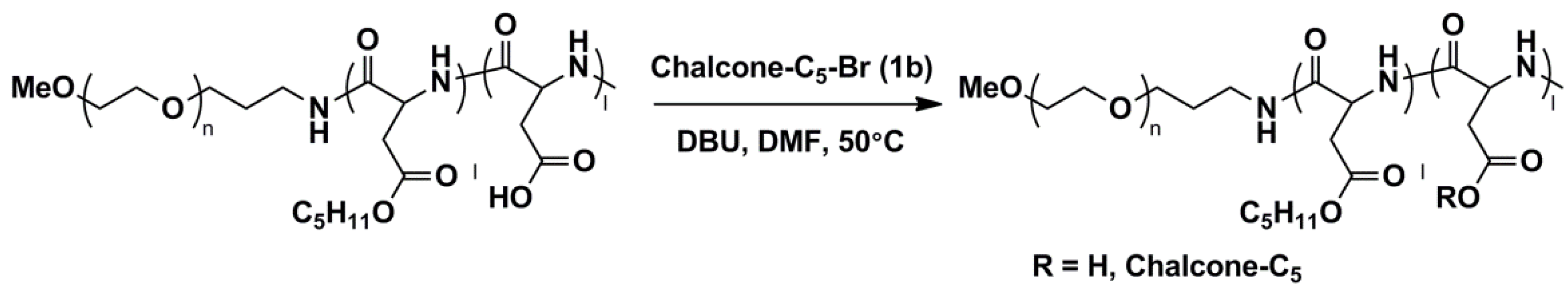
2.4. Esterification of PEG-b-P(Asp)
2.5. Micelle Preparation
2.6. Photo-Crosslinking of Polymeric Micelles
2.7. GPC Elution Measurements of Polymeric Micelles
2.8. Preparation of Adriamycin-Encapsulated Polymeric Micelles
2.9. Drug-Release Experiment
3. Results and Discussion
3.1. Optimization of Photoreactive Chalcone-Conjugated Block Copolymers
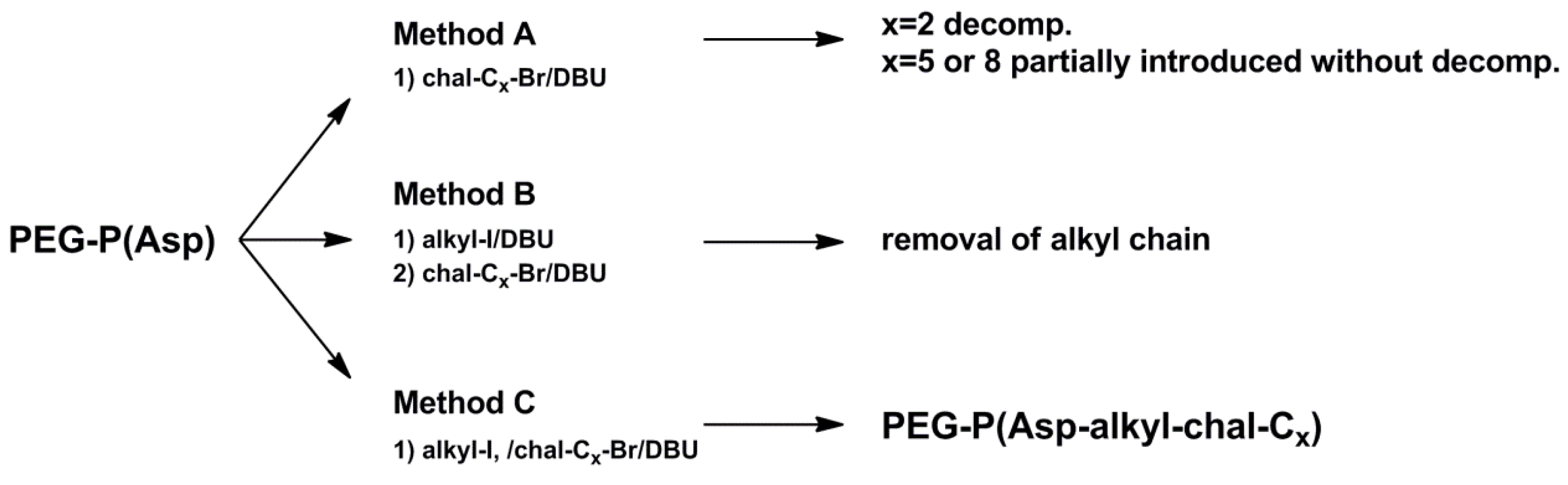
3.1.1. Method A
3.1.2. Method B
3.1.3. Method C
3.2. Photo-Crosslinking of Polymeric Micelles
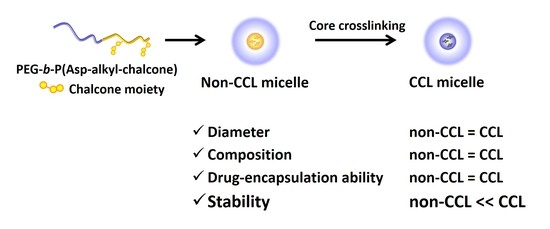
3.3. Measurement of Size and Molecular Weight upon Photo-Irradiation
3.4. DLS Measurements of CCL and Non-CCL Micelles
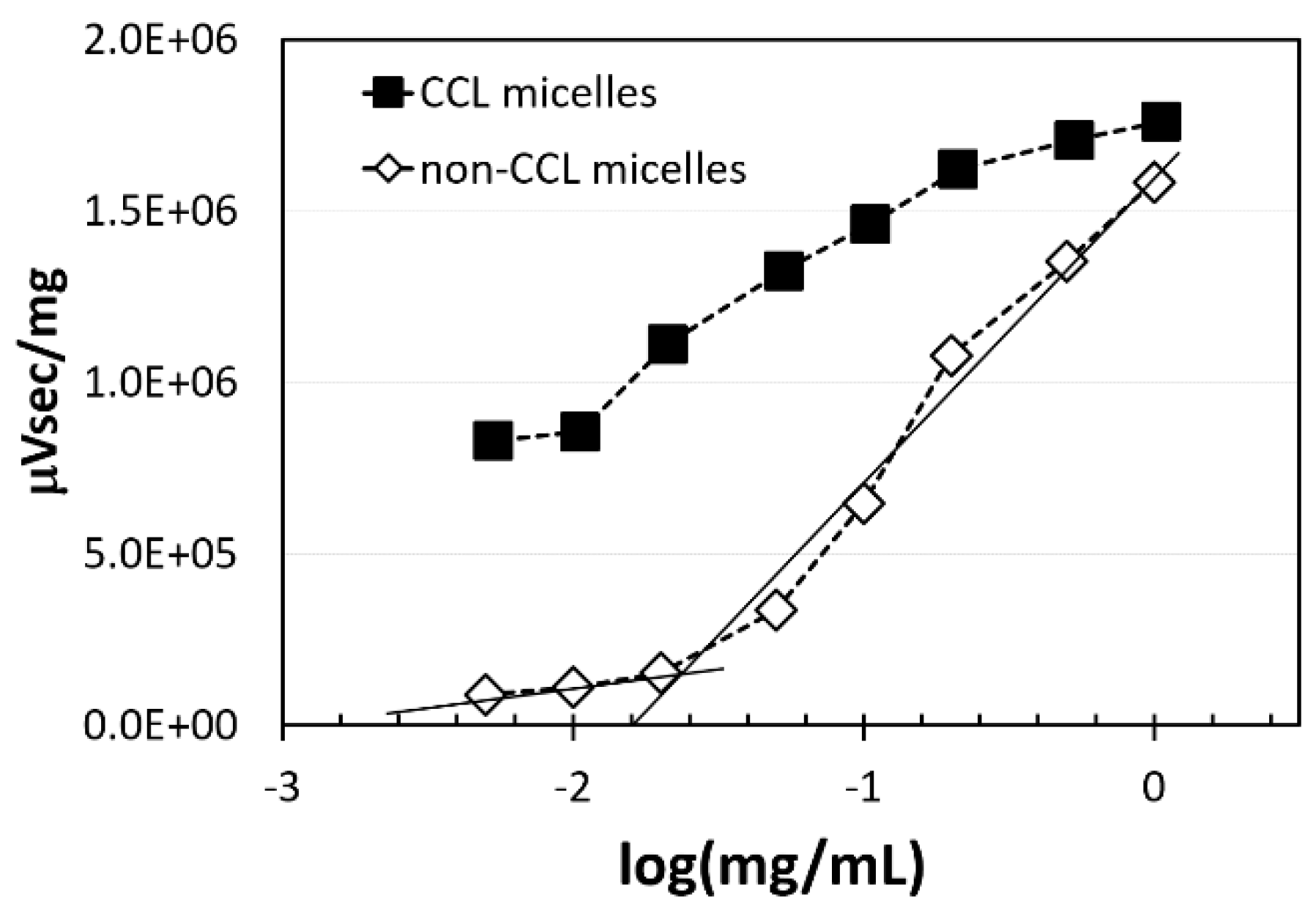
3.5. GPC Measurements of CCL and Non-CCL Micelles for Calculation of CMC
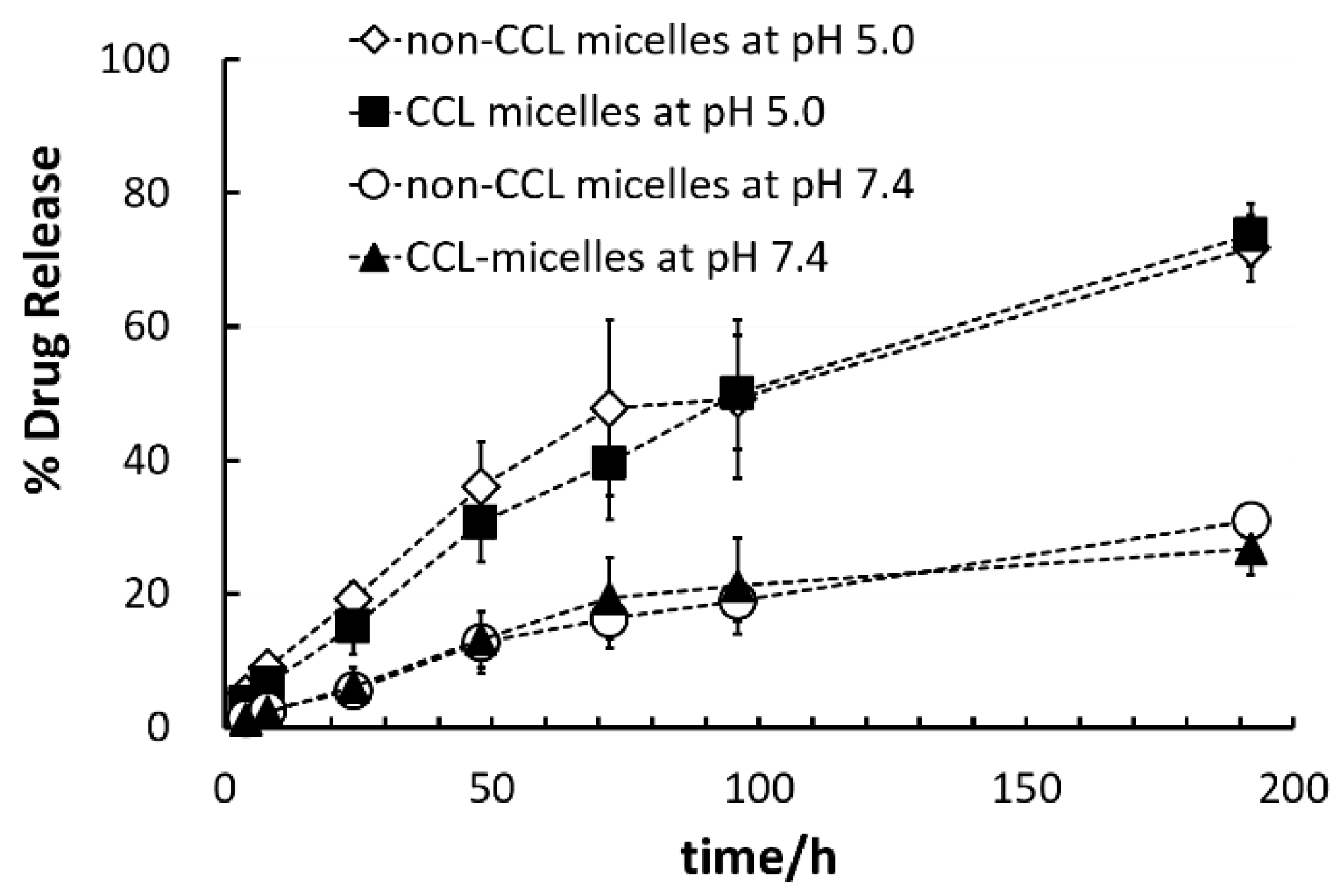
3.6. Comparisons of Two Micelles’ Adriamycin Encapsulation and Release Behaviors
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aliabadi, H.M.; Lavasanifar, A. Polymeric micelles for drug delivery. Expert Opin. Drug Deliv. 2006, 3, 139–162. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M. Polymeric micelles as a new drug carrier system and their required considerations for clinical trials. Expert Opin. Drug Deliv. 2010, 7, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent SMANCS. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Torchilin, V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.M.; Chess, R.B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003, 2, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Veronese, F.M.; Pasut, G. PEGylation, successful approach to drug delivery. Drug Discov. Today 2005, 10, 1451–1458. [Google Scholar] [CrossRef]
- Klibanov, A.L.; Maruyama, K.; Torchilin, V.P.; Huang, L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990, 268, 235–237. [Google Scholar] [CrossRef]
- Yokoyama, M. Polymeric micelles as drug carriers: Their lights and shadows. J. Drug Target. 2014, 22, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, N.; Matsumura, Y.; Kataoka, K. Development of polymeric micelles for targeting intractable cancers. Cancer Sci. 2016, 107, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P.; Omelyanenko, V.G.; Papisov, M.I.; Bogdanov, A.A., Jr.; Trubetskoy, V.S.; Herron, J.N.; Gentry, C.A. Poly(ethylene glycol) on the liposome surface: On the mechanism of polymer-coated liposome longevity. Biochim. Biophys. Acta 1994, 1195, 11–20. [Google Scholar] [CrossRef]
- Yokoyama, M.; Okano, T.; Sakurai, Y.; Ekimoto, H.; Shibazaki, C.; Kataoka, K. Toxicity and antitumor activity against solid tumors of micelle-forming polymeric anticancer drug and its extremely long circulation in blood. Cancer Res. 1991, 51, 3229–3236. [Google Scholar] [PubMed]
- Yokoyama, M.; Okano, T.; Sakurai, Y.; Fukushima, S.; Okamoto, K.; Kataoka, K. Selective delivery of Adriamycin to a solid tumor using a polymeric micelle carrier system. J. Drug Target. 1999, 7, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, K.; Wang, Z.; Kokuryo, D.; Aoki, I.; Yokoyama, M. A polymeric micelle magnetic resonance imaging (MRI) contrast agent reveals blood–brain barrier (BBB) permeability for macromolecules in cerebral ischemia-reperfusion injury. J. Control. Release 2017, 253, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Sanada, Y.; Akiba, I.; Hashida, S.; Sakurai, K.; Shiraishi, K.; Yokoyama, M.; Yagi, N.; Shinohara, Y.; Amemiya, Y. Composition dependence of the micellar architecture made from poly(ethylene glycol)-block-poly(partially benzyl-esterified aspartic acid). J. Phys. Chem. B 2012, 116, 8241–8250. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, K.; Sanada, Y.; Mochizuki, S.; Kawano, K.; Maitani, Y.; Sakurai, K.; Yokoyama, M. Determination of polymeric micelles’ structural characteristics, and effect of the characteristics on pharmacokinetic behaviors. J. Control. Release 2015, 203, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y. Poly (amino acid) micelle nanocarriers in preclinical and clinical studies. Adv. Drug Deliv. Rev. 2008, 60, 899–914. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, T.; Matsumura, Y.; Suzuki, M.; Shimizu, K.; Goda, R.; Nakamura, I.; Nakatomi, I.; Yokoyama, M.; Kataoka, K.; Kakizoe, T. NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxel. Br. J. Cancer 2005, 92, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, F.; Kitagawa, M.; Negishi, T.; Onda, T.; Matsumoto, S.; Hamaguchi, T.; Matsumura, Y. Novel SN-38–incorporating polymeric micelles, NK012, eradicate vascular endothelial growth factor–secreting bulky tumors. Cancer Res. 2006, 66, 10048–10056. [Google Scholar] [CrossRef] [PubMed]
- Dams, E.T.M.; Laverman, P.; Oyen, W.J.; Storm, G.; Scherphof, G.L.; van der Meer, J.W.; Corstens, F.H.; Boerman, O.C. Accelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomes. J. Pharmacol. Exp. Ther. 2000, 292, 1071–1079. [Google Scholar] [PubMed]
- Laverman, P.; Carstens, M.G.; Boerman, O.C.; Dams, E.T.M.; Oyen, W.J.; van Rooijen, N.; Corstens, F.H.; Storm, G. Factors affecting the accelerated blood clearance of polyethylene glycol-liposomes upon repeated injection. J. Pharmacol. Exp. Ther. 2001, 298, 607–612. [Google Scholar] [PubMed]
- Wang, X.Y.; Ishida, T.; Kiwada, H. Anti-PEG IgM elicited by injection of liposomes is involved in the enhanced blood clearance of a subsequent dose of PEGylated liposomes. J. Control. Release 2007, 119, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Koide, H.; Asai, T.; Hatanaka, K.; Urakami, T.; Ishii, T.; Kenjo, E.; Nishihara, M.; Yokoyama, M.; Ishida, T.; Kiwada, H.; Oku, N. Particle size-dependent triggering of accelerated blood clearance phenomenon. Int. J. Pharm. 2008, 362, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Koide, H.; Asai, T.; Kato, H.; Ando, H.; Shiraishi, K.; Yokoyama, M.; Oku, N. Size-dependent induction of accelerated blood clearance phenomenon by repeated injections of polymeric micelles. Int. J. Pharm. 2012, 432, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, K.; Kawano, K.; Maitani, Y.; Aoshi, T.; Ishii, K.J.; Sanada, Y.; Mochizuki, S.; Sakurai, K.; Yokoyama, M. Exploring the relationship between anti-PEG IgM behaviors and PEGylated nanoparticles and its significance for accelerated blood clearance. J. Control. Release 2016, 234, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Kadam, V.S.; Nicol, E.; Gaillard, C. Synthesis of flower-like poly(ethylene oxide) based macromolecular architectures by photo-cross-linking of block copolymers self-assemblies. Macromolecules 2012, 45, 410–419. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Chen, W.; Meng, F.; Wang, Z.; Cheng, R.; Deng, C.; Liu, H.; Zhong, Z. Core-crosslinked pH-sensitive degradable micelles: A promising approach to resolve the extracellular stability versus intracellular drug release dilemma. J. Control. Release 2012, 164, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Shunai, X.; Merdan, T.; Schaper, A.K.; Xi, F.; Kissel, T. Core-cross-linked polymeric micelles as paclitaxel carriers. Bioconj. Chem. 2004, 15, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Iijima, M.; Nagasaki, Y.; Okada, T.; Kato, M.; Kataoka, K. Core-polymerized reactive micelles from heterotelechelic amphiphilic block copolymers. Macromolecules 1999, 32, 1140–1146. [Google Scholar] [CrossRef]
- Yusa, S.; Sugahara, M.; Endo, T.; Morishima, Y. Preparation and characterization of a pH-responsive nanogel based on a photo-cross-linked micelle formed from block copolymers with controlled structure. Langmuir 2009, 25, 5258–5265. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Kim, D. pH-Sensitive micelles with cross-linked cores formed from polyaspartamide derivatives for drug delivery. Langmuir 2011, 27, 12090–12097. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Bertrand, J.; Tong, X.; Zhao, Y. Photo-cross-linkable polymer micelles in hydrogen-bonding-built layer-by-layer films. Langmuir 2009, 25, 13151–13157. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Luo, S.; Armes, S.P.; Shi, W.; Liu, S. UV irradiation-induced shell cross-linked micelles with pH-responsive cores using ABC triblock copolymers. Macromolecules 2006, 39, 5987–5994. [Google Scholar] [CrossRef]
- Jiang, J.; Qi, B.; Lepage, M.; Zhao, Y. Polymer micelles stabilization on demand through reversible photo-cross-linking. Macromolecules 2007, 40, 790–792. [Google Scholar] [CrossRef]
- He, J.; Tong, X.; Zhao, Y. Corona-cross-linked polymer vesicles displaying a large and reversible temperature-responsive volume transition. Macromolecules 2009, 42, 4845–4852. [Google Scholar] [CrossRef]
- Sun, X.; Rossin, R.; Turner, J.L.; Becker, M.L.; Joralemon, M.J.; Welch, M.J.; Wooley, K.L. An assessment of the effects of shell cross-linked nanoparticle size, core composition, and surface PEGylation on in vivo biodistribution. Biomacromolecules 2005, 6, 2541–2554. [Google Scholar] [CrossRef] [PubMed]
- Qi, K.; Ma, Q.; Remsen, E.E.; Clark, C.G.; Wooley, K.L. Determination of the bioavailability of biotin conjugated onto shell cross-linked (SCK) nanoparticles. J. Am. Chem. Soc. 2004, 126, 6599–6607. [Google Scholar] [CrossRef] [PubMed]
- Selvam, P.; Nanjundan, S. Synthesis and characterization of new photoresponsive acrylamide polymers having pendant chalcone moieties. React. Funct. Polym. 2005, 62, 179–193. [Google Scholar] [CrossRef]
- Allcock, H.R.; Cameron, C.G. Synthesis of photo-cross-linkable chalcone-bearing polyphosphazenes. Macromolecules 1994, 27, 3131–3135. [Google Scholar] [CrossRef]
- Zhao, C.L.; Winnik, M.A. Fluorescence probe techniques used to study micelle formation in water-soluble block copolymers. Langmuir 1990, 6, 514–516. [Google Scholar] [CrossRef]
- Kwon, G.; Naito, M.; Yokoyama, M.; Okano, T.; Sakurai, Y.; Kataoka, K. Micelles based on AB block copolymers of poly(ethylene oxide) and poly(β-benzyl l-aspartate). Langmuir 1993, 9, 945–949. [Google Scholar] [CrossRef]
- Kataoka, K.; Matsumoto, T.; Yokoyama, M.; Okano, T.; Sakurai, Y.; Fukushima, S.; Okamoto, K.; Kwon, G.S. Doxorubicin-loaded poly(ethylene glycol)–poly(β-benzyl-l-aspartate) copolymer micelles: Their pharmaceutical characteristics and biological significance. J. Control. Release 2000, 64, 143–153. [Google Scholar] [CrossRef]
- Yokoyama, M.; Fukushima, S.; Uehara, R.; Okamoto, K.; Kataoka, K.; Sakurai, Y.; Okano, T. Characterization of physical entrapment and chemical conjugation of adriamycin in polymeric micelles and their design for in vivo delivery to a solid tumor. J. Control. Release 1998, 50, 79–92. [Google Scholar] [CrossRef]

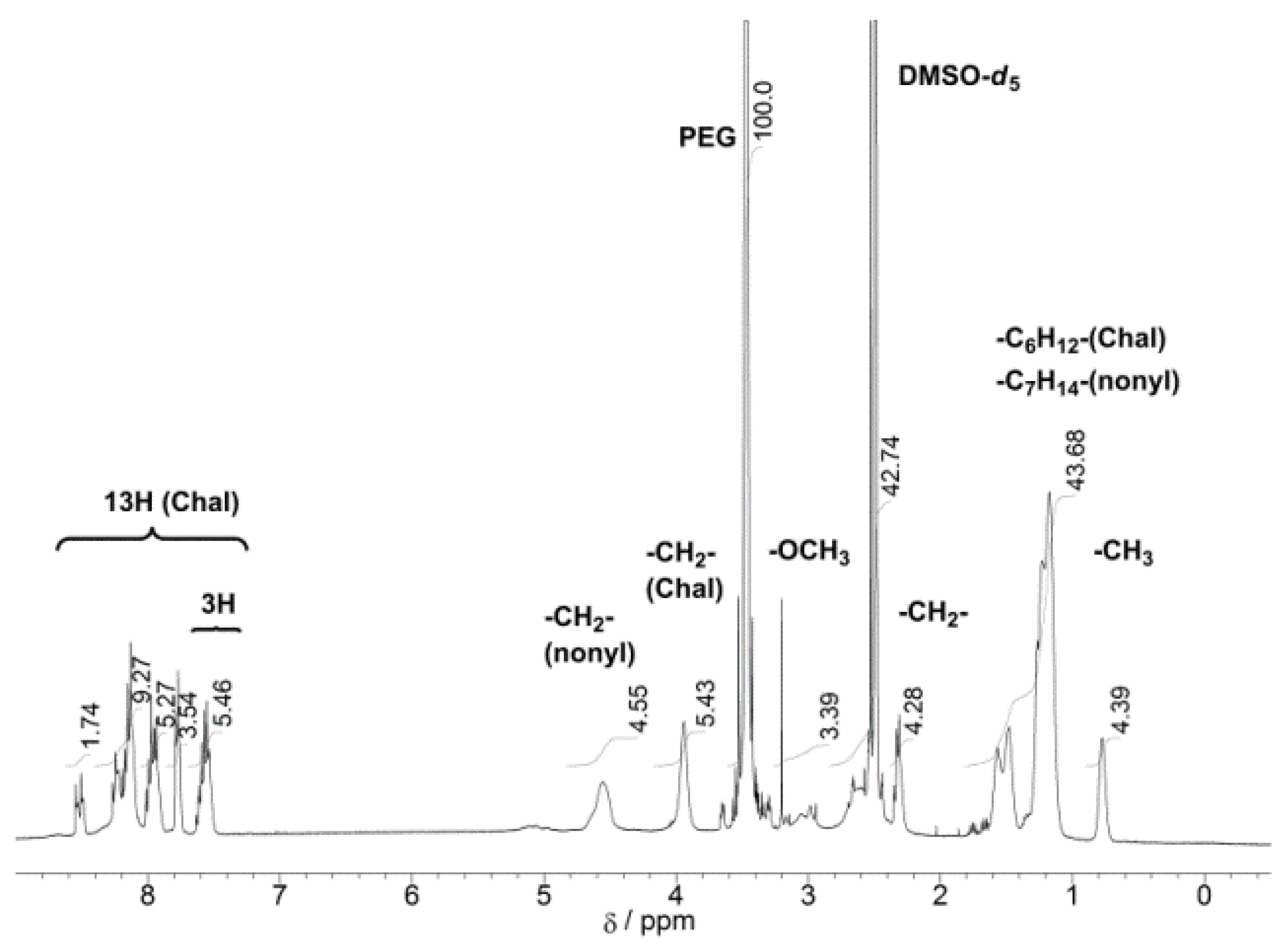
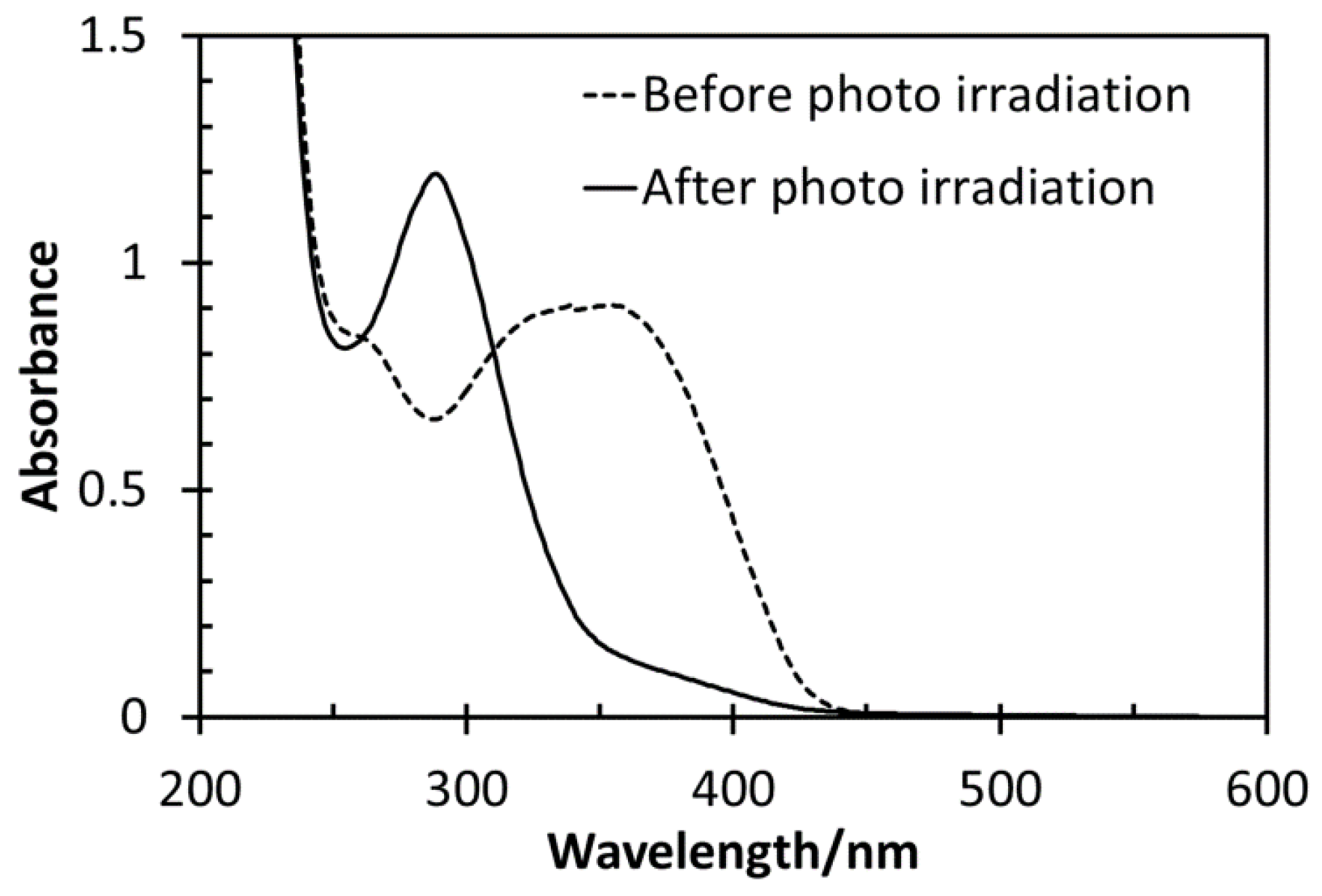
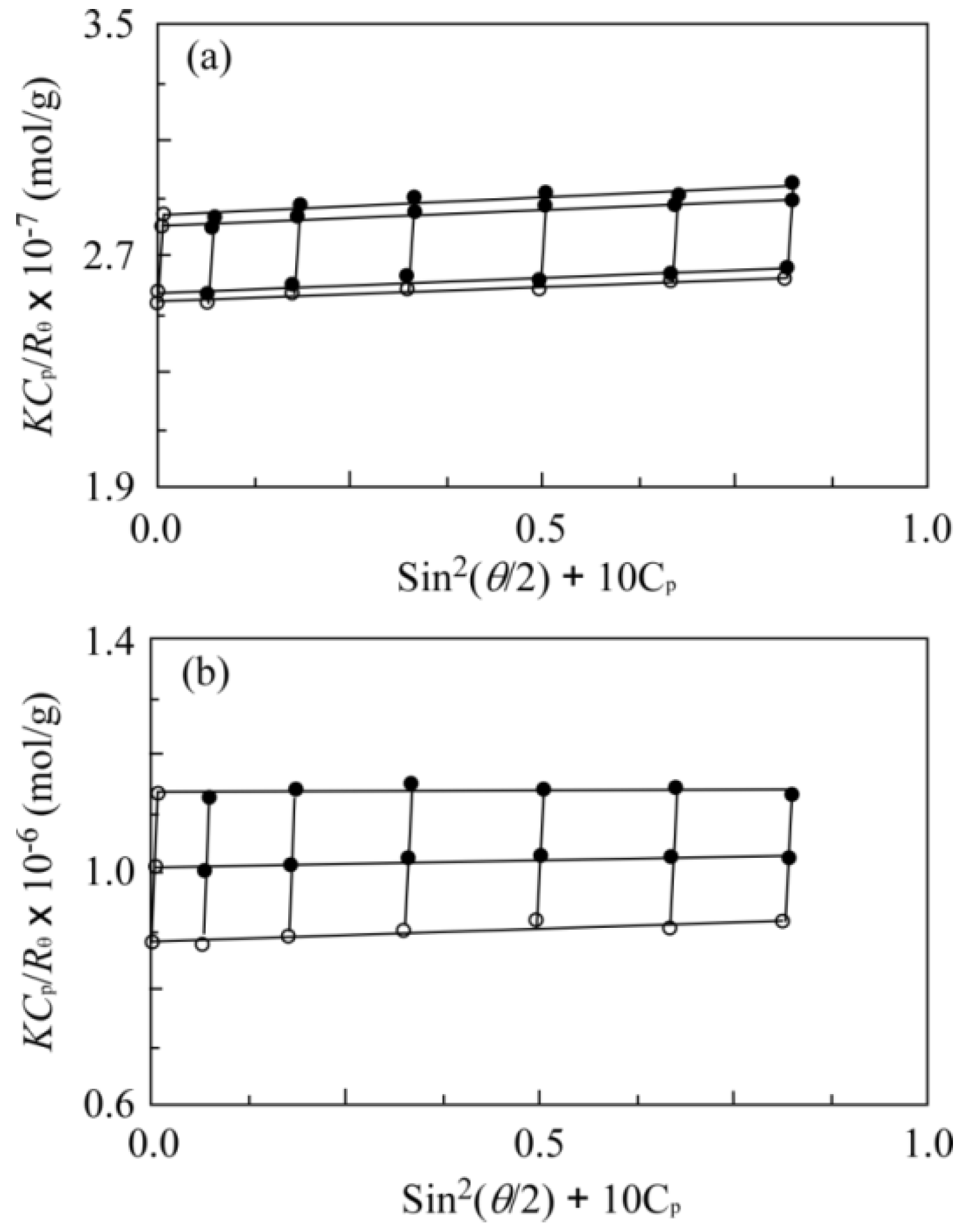
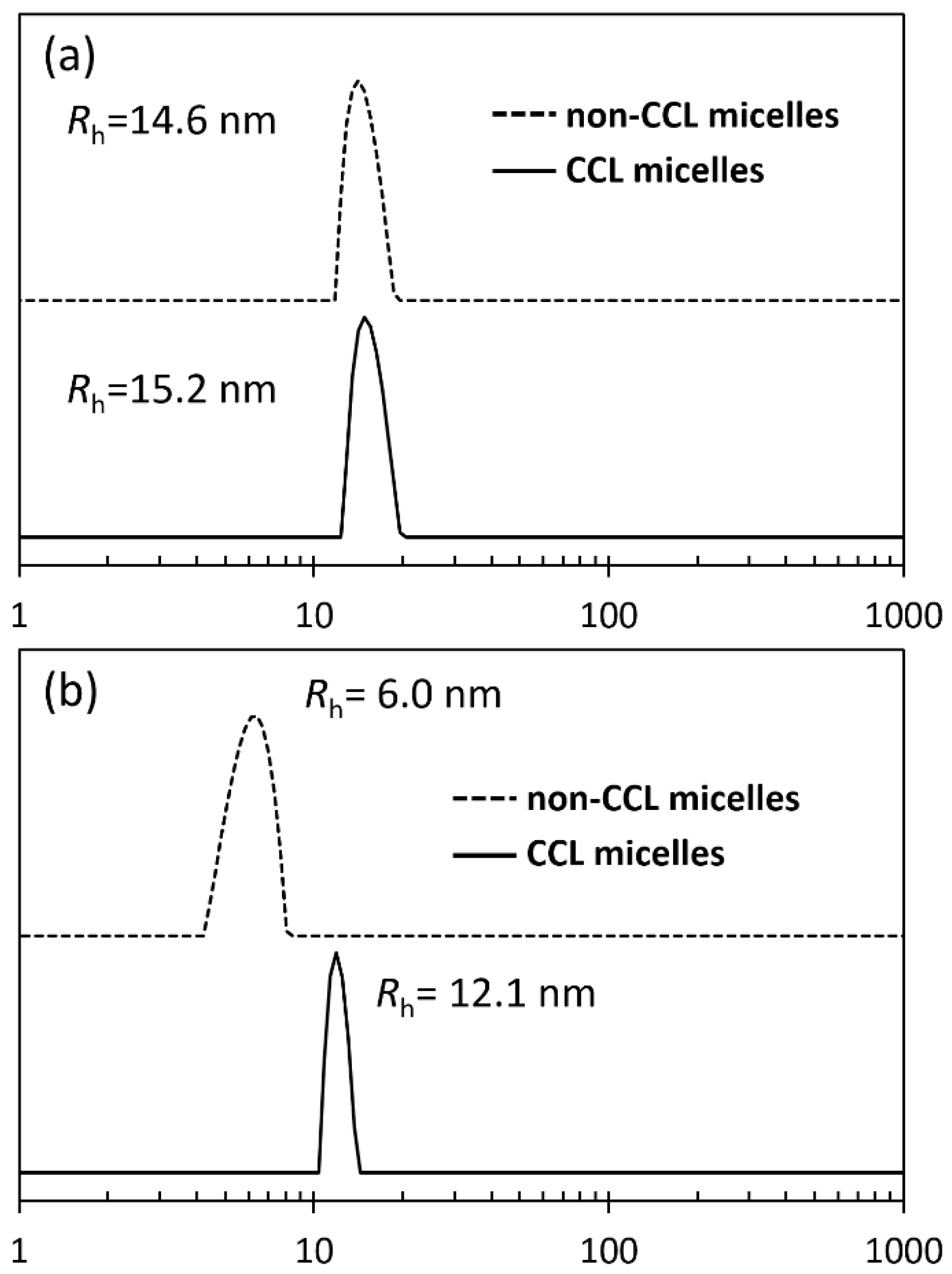
| Run | Chal-C5-Br Feed | DBU Feed | Chal-C5 Found | C5H11 Found | |
|---|---|---|---|---|---|
| Number | Ratio | /eq * | Number | Number | |
| 1 | 31.2 | 1.2 | 1.2 | 2.3 | 2.4 |
| 2 | 26.0 | 1.0 | 1.0 | 2.1 | 2.5 |
| 3 | 20.8 | 0.8 | 0.75 | 1.4 | 3.4 |
| 4 | 15.6 | 0.6 | 0.5 | 0.6 | 7.7 |
| Run | Chal-Cx-Br Feed | CyH2y+1-I Feed | Chal-Cx Found | CyH2y+1 Found | ||||
|---|---|---|---|---|---|---|---|---|
| x | eq * | Number | y | eq * | Number | Number | Number | |
| 1 | 5 | 0.3 | 7.8 | 5 | 1.1 | 28.6 | 2.6 | 8.8 |
| 2 | 5 | 0.3 | 7.8 | 5 | 1.5 | 39.0 | 3.5 | 12.9 |
| 3 | 5 | 0.3 | 7.8 | 5 | 2.0 | 52.0 | 2.7 | 14.2 |
| 4 | 5 | 0.3 | 7.8 | 9 | 1.5 | 39.0 | 0.0 | 13.2 |
| 5 | 8 | 0.3 | 7.8 | 9 | 1.5 | 39.0 | 2.8 | 18.2 |
| 6 | 8 | 0.6 | 15.6 | 9 | 1.2 | 31.2 | 5.6 | 9.3 |
| 7 | 8 | 0.8 | 20.8 | 9 | 1.0 | 26.0 | 6.9 | 7.0 |
| 8 | 8 | 0.8 | 20.8 | 9 | 0.8 | 20.8 | 8.6 | 6.0 |
| 9 | 8 | 0.8 | 20.8 | 9 | 0.6 | 15.6 | 8.8 | 4.4 |
| Run | C9H19/N | Chal-C8/N | Diameter/nm |
|---|---|---|---|
| 1 | 18.2 | 2.8 | 129 *1 |
| 2 | 9.3 | 5.6 | 37 |
| 3 | 7.0 | 6.9 | 17 |
| 4 | 7.0 | 8.6 | 29 |
| Run | DLS Diameter/nm | SLS Mw × 10−6 | A2 (cm3·mol/g2)b × 105 | Rg/Rh | Nagg | Media |
|---|---|---|---|---|---|---|
| Non-CCL micelle | 29 | 3.93 | 1.55 | 0.837 | 310 | in NS 1 |
| CCL micelle | 30 | 1.14 | 1.29 | 0.982 | 90 | Methanol 2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiraishi, K.; Yusa, S.-i.; Ito, M.; Nakai, K.; Yokoyama, M. Photo Irradiation-Induced Core Crosslinked Poly(ethylene glycol)-block-poly(aspartic acid) Micelles: Optimization of Block Copolymer Synthesis and Characterization of Core Crosslinked Micelles. Polymers 2017, 9, 710. https://doi.org/10.3390/polym9120710
Shiraishi K, Yusa S-i, Ito M, Nakai K, Yokoyama M. Photo Irradiation-Induced Core Crosslinked Poly(ethylene glycol)-block-poly(aspartic acid) Micelles: Optimization of Block Copolymer Synthesis and Characterization of Core Crosslinked Micelles. Polymers. 2017; 9(12):710. https://doi.org/10.3390/polym9120710
Chicago/Turabian StyleShiraishi, Kouichi, Shin-ichi Yusa, Masanori Ito, Keita Nakai, and Masayuki Yokoyama. 2017. "Photo Irradiation-Induced Core Crosslinked Poly(ethylene glycol)-block-poly(aspartic acid) Micelles: Optimization of Block Copolymer Synthesis and Characterization of Core Crosslinked Micelles" Polymers 9, no. 12: 710. https://doi.org/10.3390/polym9120710