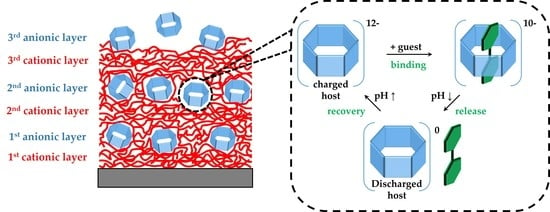
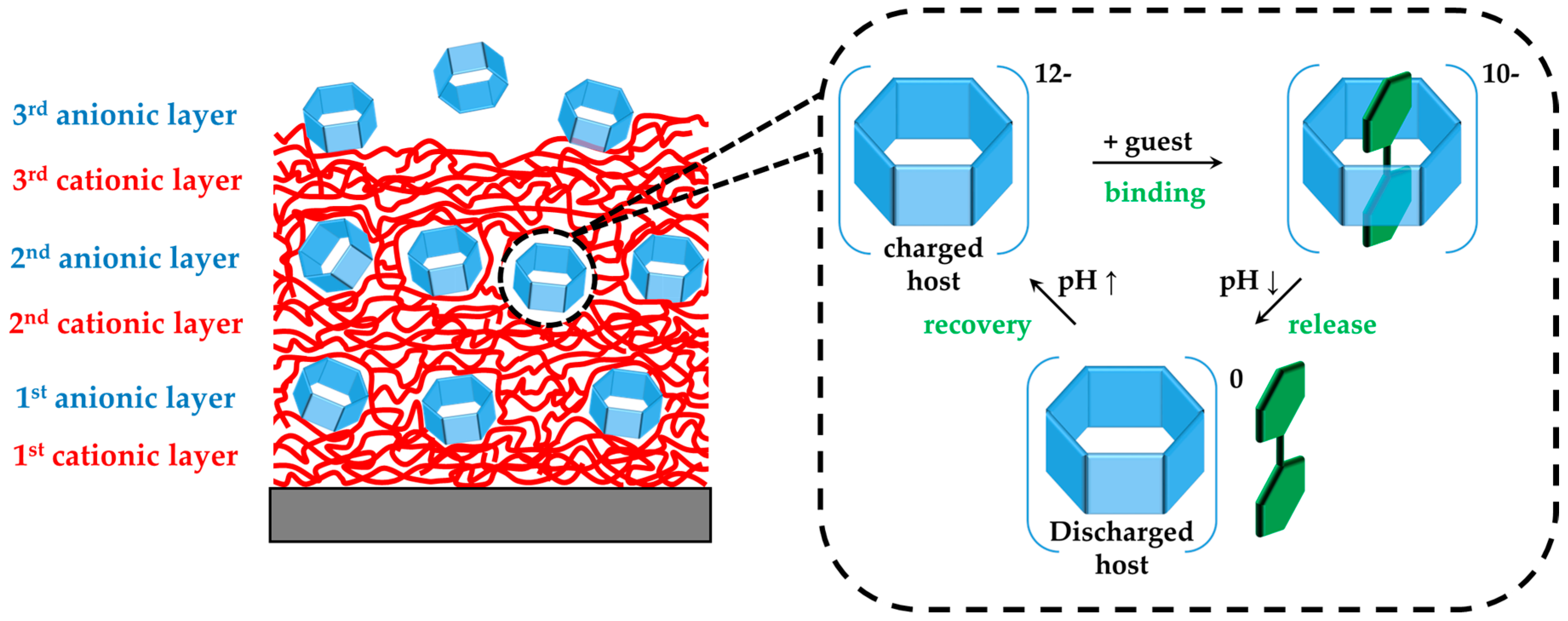
pH-Responsive Host–Guest Complexation in Pillar[6]arene-Containing Polyelectrolyte Multilayer Films
Abstract
:1. Introduction
2. Materials and Methods
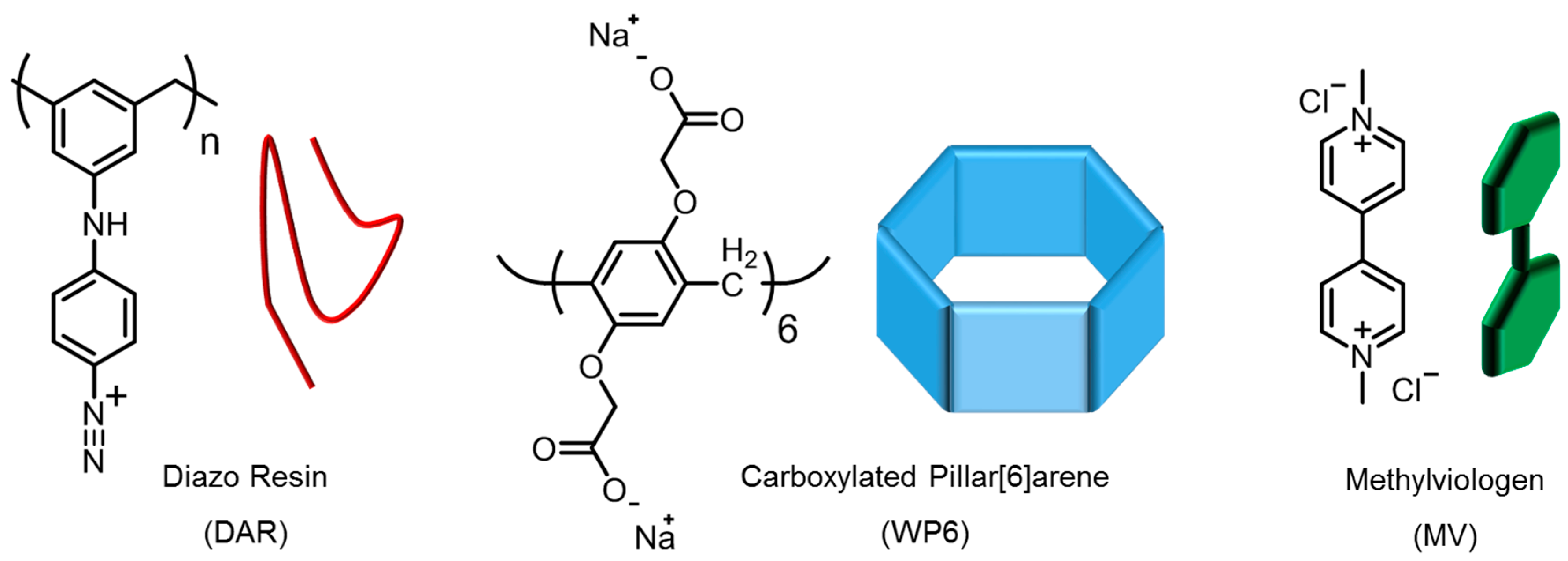
2.1. Materials
2.2. Solutions
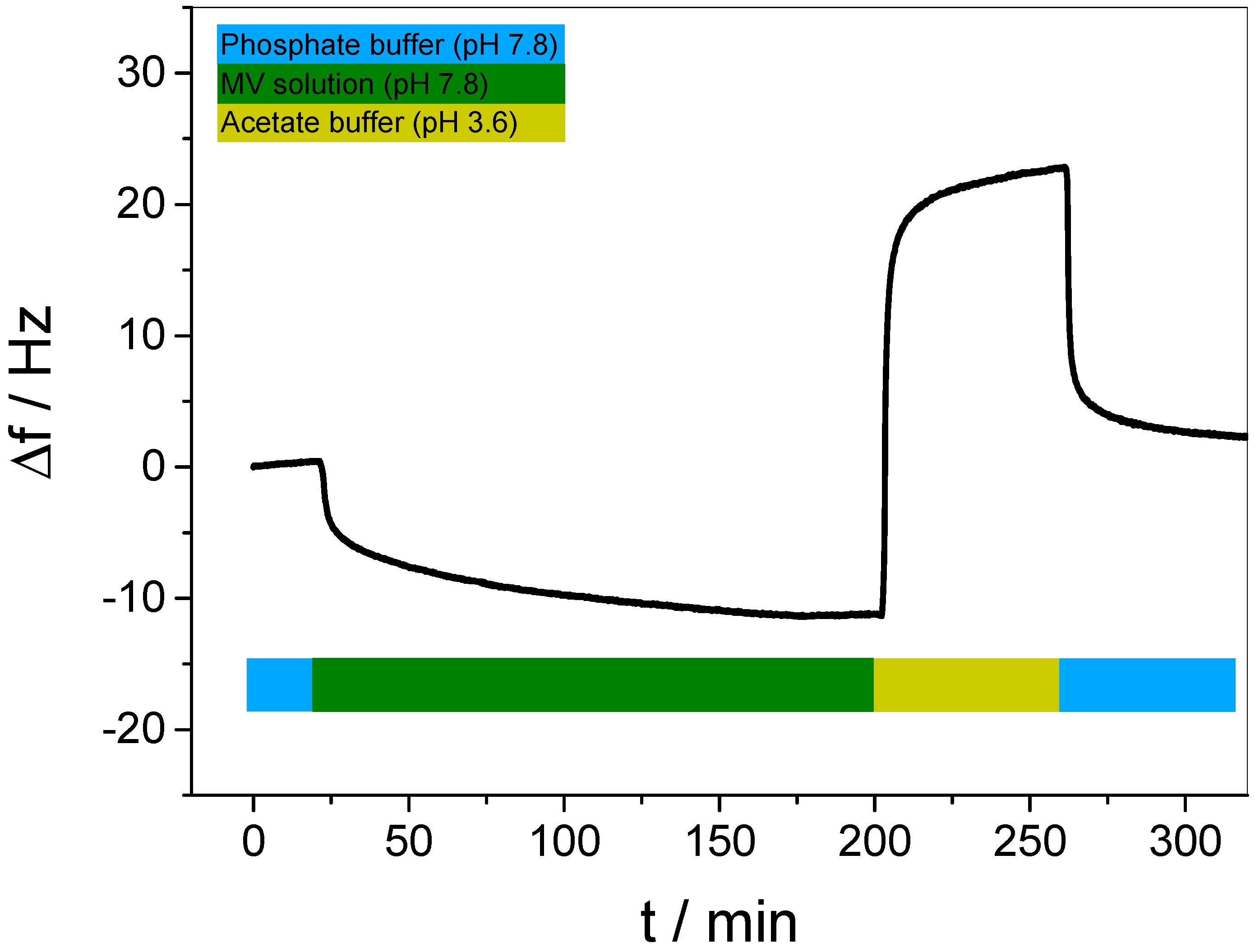
2.3. Characterization of the MV–WP6 Complex
2.4. Substrate Cleaning
2.5. In Situ Preparation of Multilayer Films and Monitoring by QCM-D
2.6. Dip-Coating of Multilayer Films
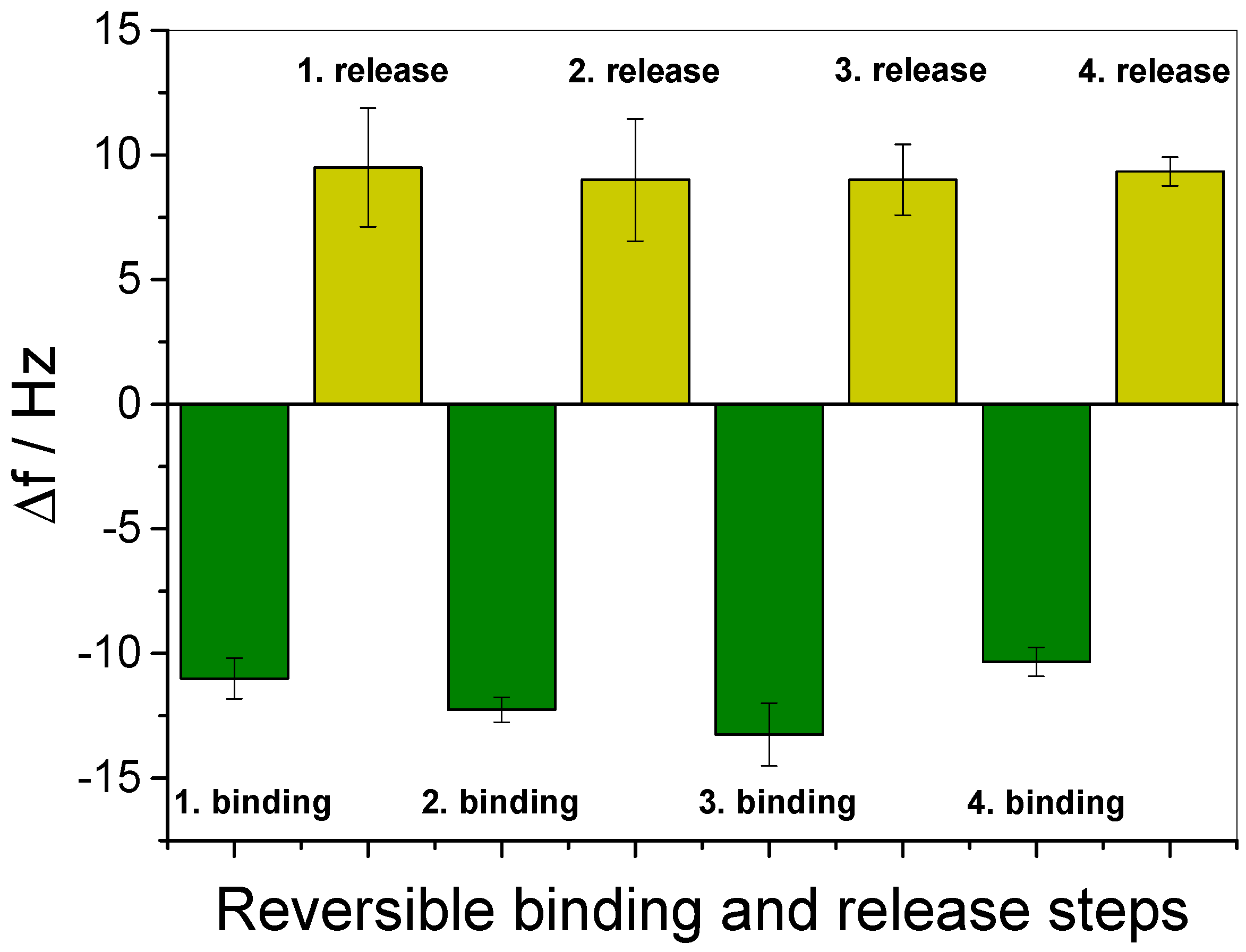
2.7. Guest Molecule Binding and Release
3. Results
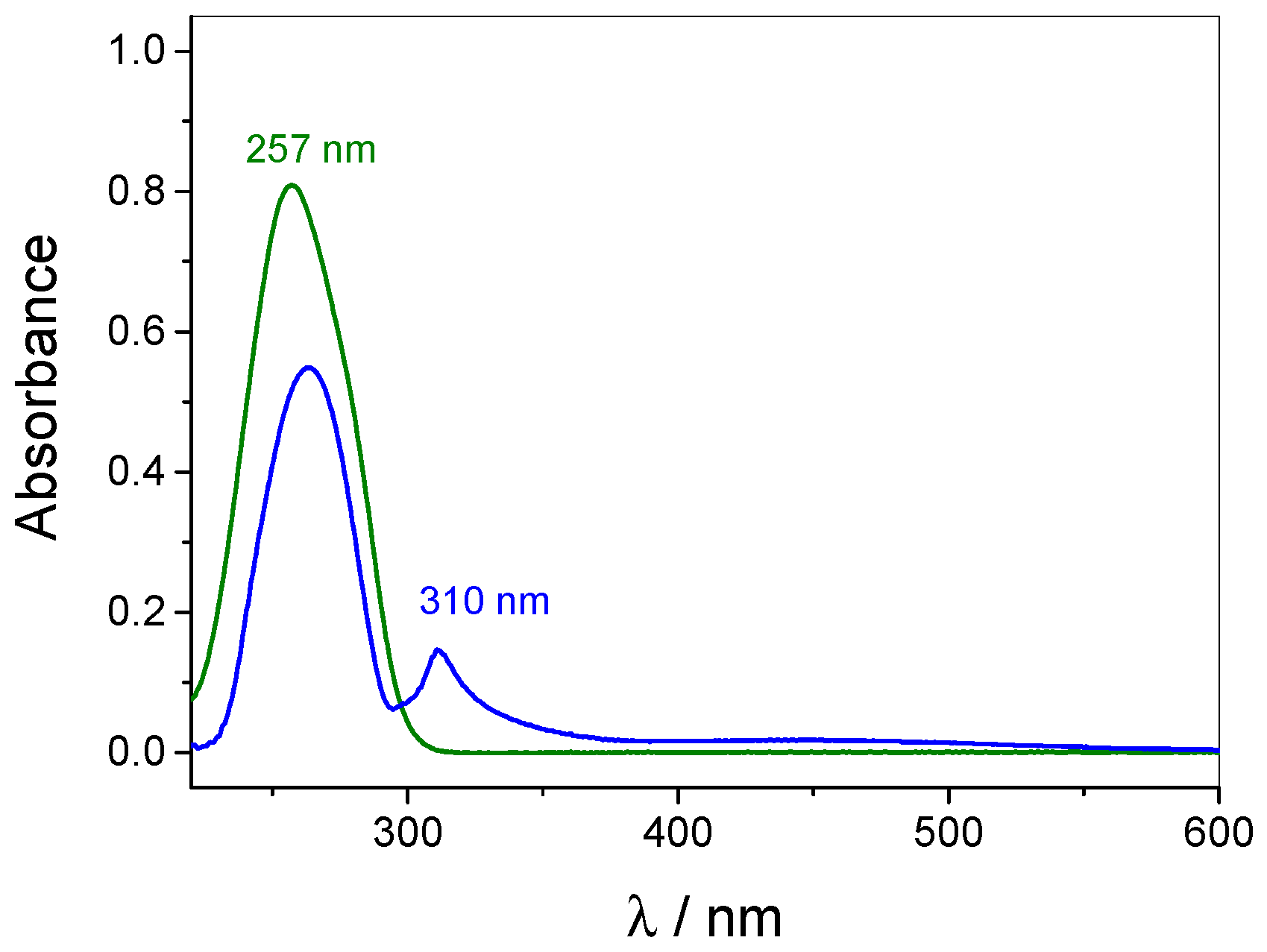
3.1. pH-Tunable Host–Guest Chemistry in Aqueous Solution
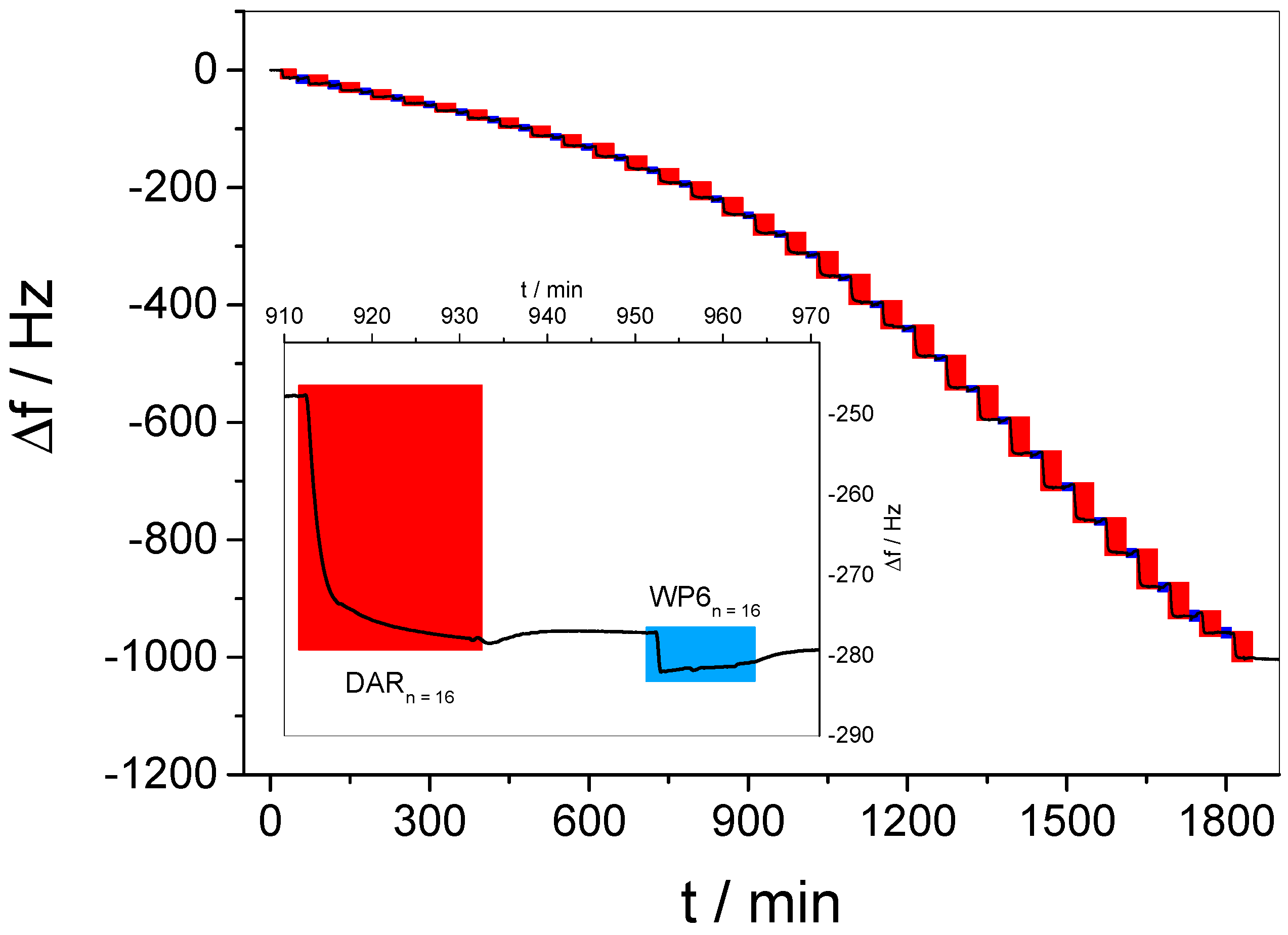
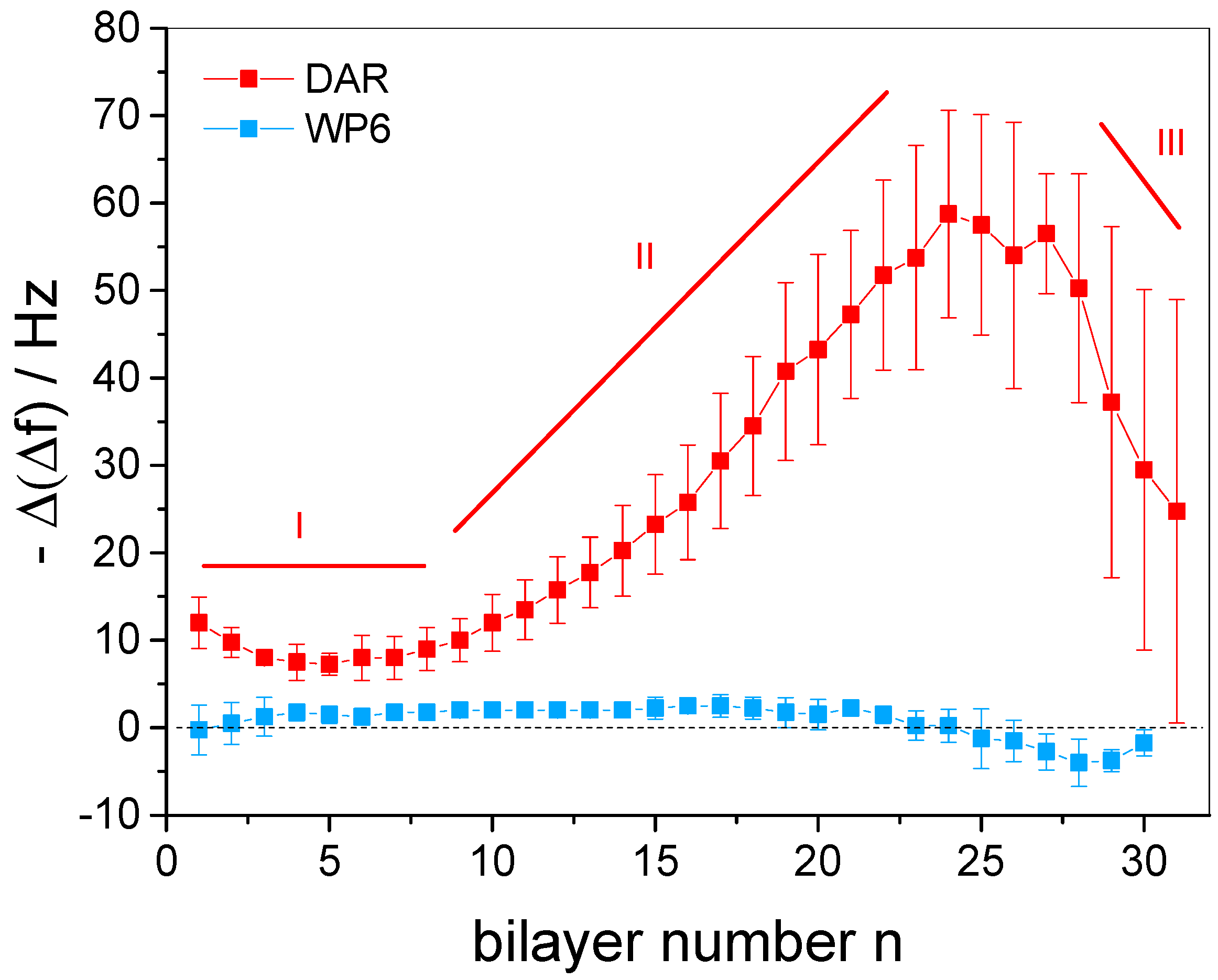
3.2. In Situ QCM-D Study of Multilayer Formation
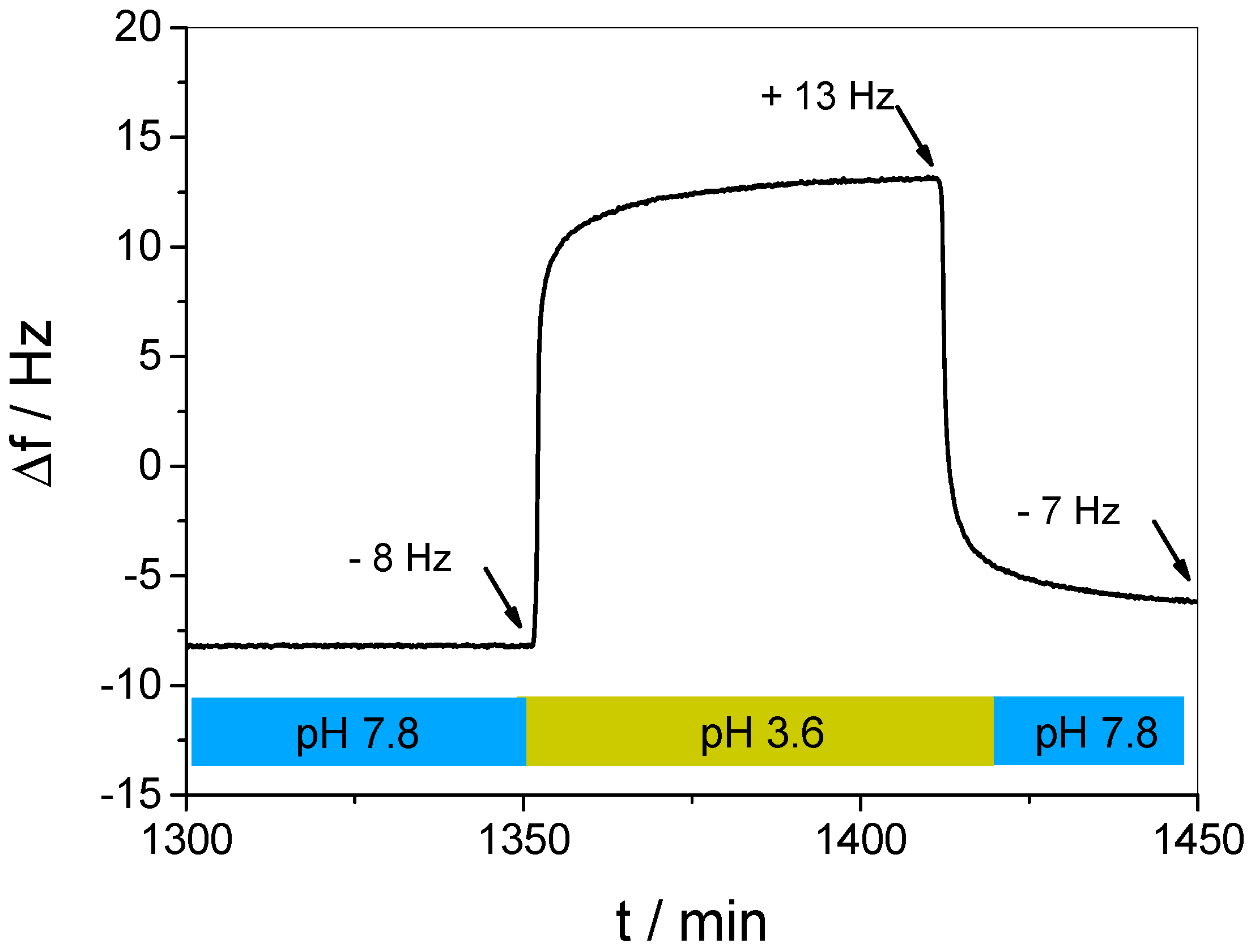
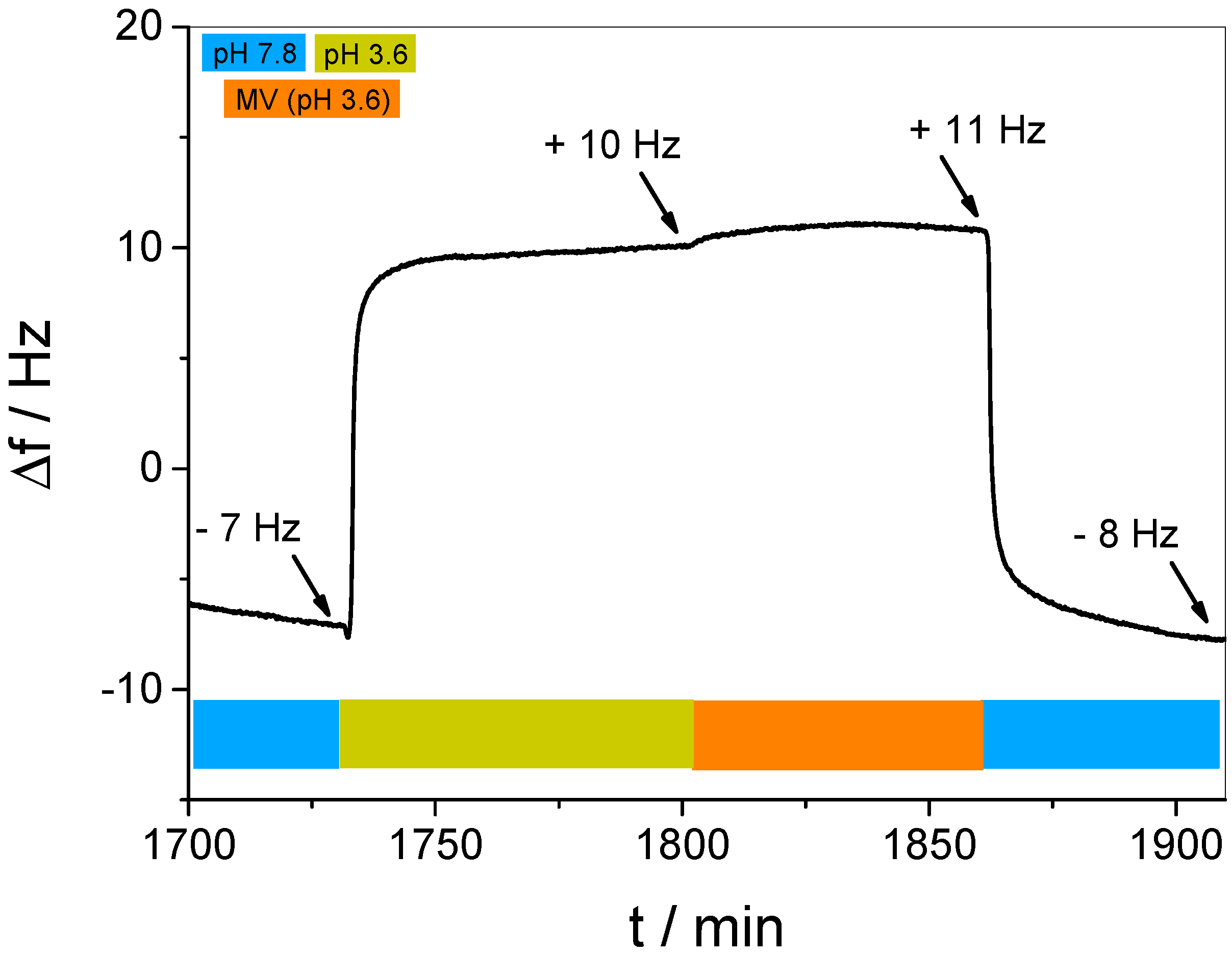
3.3. In Situ Investigation of Guest Binding and Release
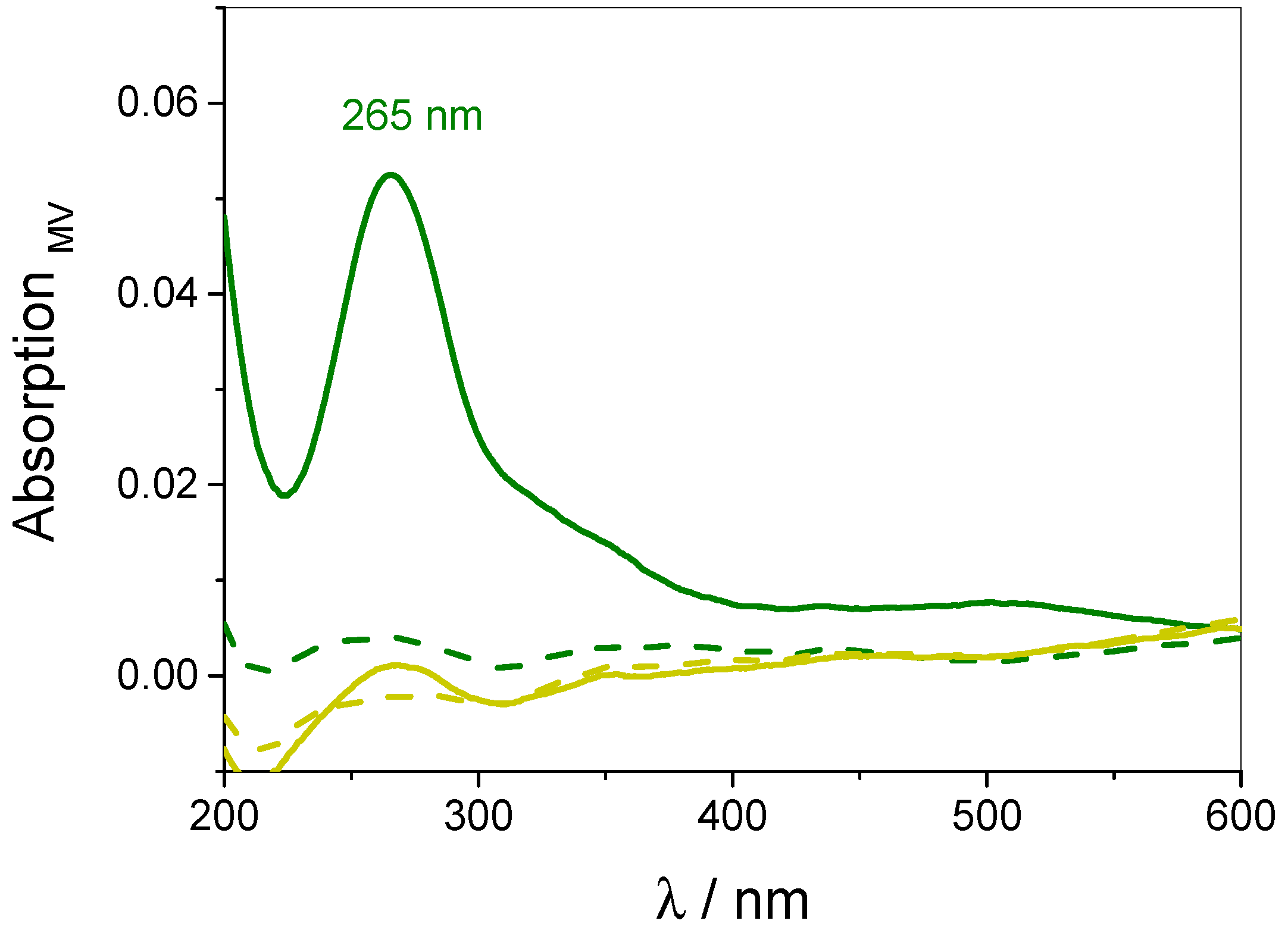
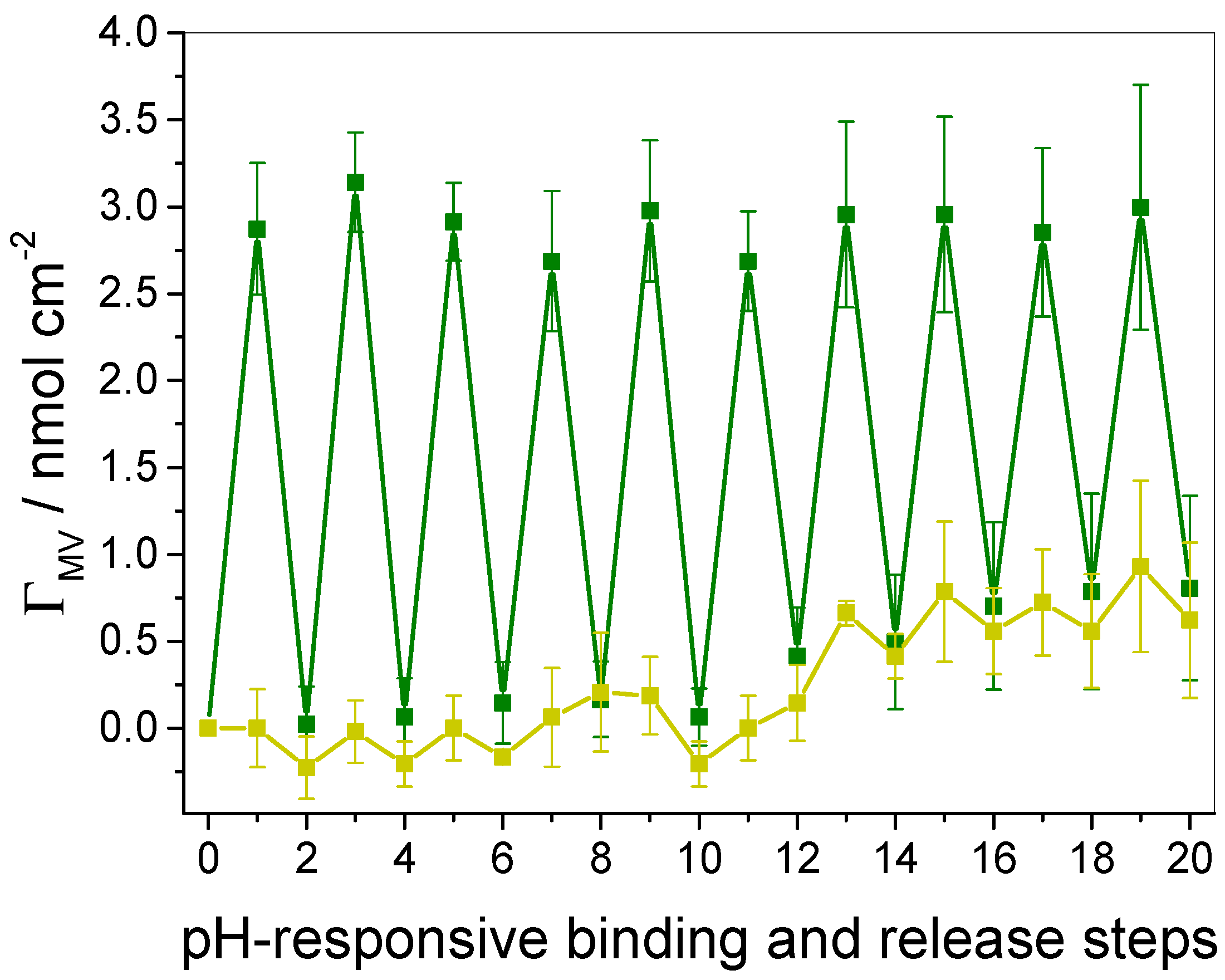
3.4. Quantification of Guest Surface Coverage by UV-Vis Spectra
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Masson, E.; Ling, X.; Joseph, R.; Kyeremeh-Mensah, L.; Lu, X. Cucurbituril chemistry: A tale of supramolecular success. RSC Adv. 2012, 2, 1213–1247. [Google Scholar] [CrossRef]
- Barrow, S.J.; Kasera, S.; Rowland, M.J.; Del Barrio, J.; Scherman, O.A. Cucurbituril-based molecular recognition. Chem. Rev. 2015, 115, 12320–12406. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Jie, K.; Huang, F. Supramolecular amphiphiles based on host-guest molecular recognition motifs. Chem. Rev. 2015, 115, 7240–7303. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Wang, H.; Shao, N.M.; Qiao, S.N.; Cheng, Y.Y. Host-guest chemistry of dendrimer-cyclodextrin conjugates: Selective encapsulations of guests within dendrimer or cyclodextrin cavities revealed by NOE NMR techniques. J. Phys. Chem. B 2012, 116, 11217–11224. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Y.M.; Sun, H.L.; Chang, X.Y.; Liu, Y. Multistimuli-responsive supramolecular assembly of cucurbituril/cyclodextrin pairs with an azobenzene-containing bispyridinium guest. Chemistry 2014, 20, 15108–15115. [Google Scholar] [CrossRef] [PubMed]
- Isenbügel, K.; Gehrke, Y.; Ritter, H. Photo-switchable behavior of azobenzene-dye-modified silica nanoparticles and their assembly with cyclodextrin derivatives. Macromol. Chem. Phys. 2012, 213, 227–233. [Google Scholar] [CrossRef]
- Karakhanov, E.A.; Maximov, A.L. Molecular imprinting technique for the design of cyclodextrin based materials and their application in catalysis. Curr. Org. Chem. 2010, 14, 1284–1295. [Google Scholar] [CrossRef]
- Ravoo, B.J.; Darcy, R. Cyclodextrin bilayer vesicles. Angew. Chem. Int. Ed. 2000, 39, 4324–4326. [Google Scholar] [CrossRef]
- Behrend, R.; Meyer, E.; Rusche, F.I. Ueber condensationsproducte aus glycoluril und formaldehyd. Eur. J. Org. Chem. 1905, 339, 1–37. [Google Scholar] [CrossRef]
- Freeman, W.A.; Mock, W.L.; Shih, N.Y. Cucurbituril. J. Am. Chem. Soc. 1981, 103, 7367–7368. [Google Scholar] [CrossRef]
- Mock, W.L.; Shih, N.Y. Structure and selectivity in host-guest complexes of cucurbituril. J. Org. Chem. 1986, 51, 4440–4446. [Google Scholar] [CrossRef]
- Isaacs, L. Stimuli responsive systems constructed using cucurbit[N]uril-type molecular containers. Acc. Chem. Res. 2014, 47, 2052–2062. [Google Scholar] [CrossRef] [PubMed]
- Appel, E.A.; Biedermann, F.; Rauwald, U.; Jones, S.T.; Zayed, J.M.; Scherman, O.A. Supramolecular cross-linked networks via host-guest complexation with cucurbit[8]uril. J. Am. Chem. Soc. 2010, 132, 14251–14260. [Google Scholar] [CrossRef] [PubMed]
- Rauwald, U.; Scherman, O.A. Supramolecular block copolymers with cucurbit[8]uril in water. Angew. Chem. Int. Ed. 2008, 47, 3950–3953. [Google Scholar] [CrossRef] [PubMed]
- Ogoshi, T.; Yamagishi, T. Pillararenes: Versatile synthetic receptors for supramolecular chemistry. Eur. J. Org. Chem. 2013, 2013, 2961–2975. [Google Scholar] [CrossRef]
- Ogoshi, T.; Kanai, S.; Fujinami, S.; Yamagishi, T.A.; Nakamoto, Y. Para-bridged symmetrical pillar[5]arenes: Their lewis acid catalyzed synthesis and host-guest property. J. Am. Chem. Soc. 2008, 130, 5022–5023. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.R.; Meier, H. Synthesis of pillar[6]arenes and their host-guest complexes. Synthesis-Stuttgart 2015, 47, 1041–1056. [Google Scholar] [CrossRef]
- Ogoshi, T.; Yamagishi, T. New synthetic host pillararenes: Their synthesis and application to supramolecular materials. Bull. Chem. Soc. Jpn. 2013, 86, 312–332. [Google Scholar] [CrossRef]
- Strutt, N.L.; Zhang, H.C.; Schneebeli, S.T.; Stoddart, J.F. Functionalizing pillar[n]arenes. Acc. Chem. Res. 2014, 47, 2631–2642. [Google Scholar] [CrossRef] [PubMed]
- Ogoshi, T.; Ueshima, N.; Sakakibara, F.; Yamagishi, T.; Haino, T. Conversion from pillar[5]arene to pillar[6-15]arenes by ring expansion and encapsulation of c-60 by pillar[n]arenes with nanosize cavities. Org. Lett. 2014, 16, 2896–2899. [Google Scholar] [CrossRef] [PubMed]
- Ogoshi, T.; Hashizume, M.; Yamagishi, T.A.; Nakamoto, Y. Synthesis, conformational and host-guest properties of water-soluble pillar[5]arene. Chem. Commun. 2010, 46, 3708–3710. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.L.; Yang, Y.W. Molecular recognition and self-assembly of pillarenes. J. Incl. Phenom. Macrocycl. Chem. 2015, 81, 13–33. [Google Scholar] [CrossRef]
- Hu, X.Y.; Jia, K.K.; Cao, Y.; Li, Y.; Qin, S.; Zhou, F.; Lin, C.; Zhang, D.M.; Wang, L.Y. Dual photo- and ph-responsive supramolecular nanocarriers based on water-soluble pillar[6]arene and different azobenzene derivatives for intracellular anticancer drug delivery. Chem. Eur. J. 2015, 21, 1208–1220. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.Z.; Jiang, H.J.; Chen, R.; Qiu, F.L.; Dai, G.L.; Han, D.M. A triply-responsive pillar[6]arene-based supramolecular amphiphile for tunable formation of vesicles and controlled release. Chem. Commun. 2014, 50, 10658–10660. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Hu, X.Y.; Li, Y.; Zou, X.C.; Xiong, S.H.; Lin, C.; Shen, Y.Z.; Wang, L.Y. Multistimuli-responsive supramolecular vesicles based on water-soluble pillar[6]arene and saint complexation for controllable drug release. J. Am. Chem. Soc. 2014, 136, 10762–10769. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.C.; Zhou, X.R.; Zhang, Z.B.; Han, C.Y.; Mao, Z.W.; Gao, C.Y.; Huang, F.H. Pillar[6]arene/paraquat molecular recognition in water: High binding strength, ph-responsiveness, and application in controllable self-assembly, controlled release, and treatment of paraquat poisoning. J. Am. Chem. Soc. 2012, 134, 19489–19497. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yao, Y.; Xue, M. A novel fluorescent probe for detecting paraquat and cyanide in water based on pillar[5]arene/10-methylacridinium iodide molecular recognition. Chem. Commun. 2014, 50, 5064–5067. [Google Scholar] [CrossRef] [PubMed]
- Ogoshi, T.; Kida, K.; Yamagishi, T. Photoreversible switching of the lower critical solution temperature in a photoresponsive host-guest system of pillar[6]arene with triethylene oxide substituents and an azobenzene derivative. J. Am. Chem. Soc. 2012, 134, 20146–20150. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.T.; Yang, J.; Yu, G.C.; He, J.M.; Abliz, Z.; Huang, F.H. Synthesis of a water-soluble pillar[9]arene and its ph-responsive binding to paraquat. Chem. Commun. 2014, 50, 2841–2843. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Q.; Jia, X.S.; Li, C.J. A pillar[6]arene-[2]pseudorotaxane based ph-sensitive molecular switch. Chin. J. Chem. 2015, 33, 343–345. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J.D.; Schmitt, J. Buildup of ultrathin multilayer films by a self-assembly process: 3. Consecutively alternating adsorption of anionic and cationic polyelectrolytes on charged surfaces. Thin Solid Films 1992, 210–211, 831–835. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Jaber, J.A.; Schlenoff, J. Recent developments in the properties and applications of polyelectrolyte multilayers. Curr. Opin. Colloid Interface Sci. 2006, 11, 324–329. [Google Scholar] [CrossRef]
- Klitzing, R.V. Internal structure of polyelectrolyte multilayer assemblies. Phys. Chem. Chem. Phys. 2006, 8, 5012–5033. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.P.; Schönhoff, M.; Zhang, X. Unconventional layer-by-layer assembly: Surface molecular imprinting and its applications. Small 2012, 8, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Wulff, G. Molecular imprinting in cross-linked materials with the aid of molecular templates—A way towards artificial antibodies. Angew. Chem. Int. Ed. 1995, 34, 1812–1832. [Google Scholar] [CrossRef]
- Wulff, G. Molecular imprinting in crosslinked polymers—The role of the binding sites. Mol. Cryst. Liq. Cryst. 1996, 276, 1–6. [Google Scholar] [CrossRef]
- Shi, F.; Liu, Z.; Wu, G.L.; Zhang, M.; Chen, H.; Wang, Z.Q.; Zhang, X.; Willner, I. Surface imprinting in layer-by-layer nanostructured films. Adv. Funct. Mater. 2007, 17, 1821–1827. [Google Scholar] [CrossRef]
- Gauczinski, J.; Liu, Z.; Zhang, X.; Schönhoff, M. Mechanism of surface molecular imprinting in polyelectrolyte multilayers. Langmuir 2010, 26, 10122–10128. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cheng, M.; Zhu, X.; Zhang, Y.; An, Q.; Shi, F. A facile method to prepare molecularly imprinted layer-by-layer nanostructured multilayers using postinfiltration and a subsequent photo-cross-linking strategy. ACS Appl. Mater. Interfaces 2013, 5, 8308–8313. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Shi, F.; Liu, Z.; Wang, Z.Q.; Zhang, X. Reversible disulfide cross-linking in layer-by-layer films: Preassembly enhanced loading and ph/reductant dually controllable release. Langmuir 2007, 23, 6377–6384. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Liu, R.; Wu, M.; Li, Z.; Liu, B.; Wang, Z.; Gao, D.; Zhang, Z. Protein-building molecular recognition sites by layer-by-layer molecular imprinting on colloidal particles. Analyst 2009, 134, 1880–1886. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.W.; Liu, Y.L.; Wu, G.L.; Schönhoff, M.; Zhang, X. Bolaform supramolecular amphiphiles as a novel concept for the buildup of surface-imprinted films. Langmuir 2011, 27, 10370–10375. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Liu, Z.; Fu, L.; Shi, F.; Ma, H.; Ozaki, Y.; Zhang, X. Surface-imprinted nanostructured layer-by-layer film for molecular recognition of theophylline derivatives. Langmuir 2008, 24, 11988–11994. [Google Scholar] [CrossRef] [PubMed]
- Gauczinski, J.; Liu, Z.H.; Zhang, X.; Schönhoff, M. Surface molecular imprinting in layer-by-layer films on silica particles. Langmuir 2012, 28, 4267–4273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.W.; Liu, Y.L.; Yuan, B.; Wang, Z.Q.; Schönhoff, M.; Zhang, X. Multilayer films with nanocontainers: Redox-controlled reversible encapsulation of guest molecules. Chem. Eur. J. 2012, 18, 14968–14973. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, H.; Yuan, B.; Zhang, X.; Schönhoff, M. Cucurbit[8]uril-containing multilayer films for the photocontrolled binding and release of a guest molecule. Langmuir 2016, 32, 2410–2418. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, H.; Yuan, B.; Zhang, J.; Zhang, X.; Schönhoff, M. Cucurbit[8]uril as nanocontainer in a polyelectrolyte multilayer film: A quantitative and kinetic study of guest uptake. Langmuir 2015, 31, 10734–10742. [Google Scholar] [CrossRef] [PubMed]
- Bian, Q.; Jin, M.M.; Chen, S.; Xu, L.P.; Wang, S.T.; Wang, G.J. Visible-light-responsive polymeric multilayers for trapping and release of cargoes via host-guest interactions. Polym. Chem. 2017, 8, 5525–5532. [Google Scholar] [CrossRef]
- Xuan, H.Y.; Ren, J.Y.; Zhang, J.H.; Ge, L.Q. Novel highly-flexible, acid-resistant and self-healing host-guest transparent multilayer films. Appl. Surf. Sci. 2017, 411, 303–314. [Google Scholar] [CrossRef]
- Xu, G.; Pranantyo, D.; Xu, L.Q.; Neoh, K.G.; Kang, E.T.; Teo, S.L.M. Antifouling, antimicrobial, and antibiocorrosion multilayer coatings assembled by layer-by-layer deposition. Involving host-guest interaction. Ind. Eng. Chem. Res. 2016, 55, 10906–10915. [Google Scholar] [CrossRef]
- Yuan, B.; Xu, J.F.; Sun, C.L.; Nicolas, H.; Schönhoff, M.; Yang, Q.Z.; Zhang, X. Pillar[6]arene containing multilayer films: Reversible uptake and release of guest molecules with methyl viologen moieties. ACS Appl. Mater. Interfaces 2016, 8, 3679–3685. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Q.; Wu, T.; Liu, F.; Wang, Z.Q.; Zhang, X.; Shen, J.C. Covalently attached multilayer assemblies by sequential adsorption of polycationic diazo-resins and polyanionic poly(acrylic acid). Langmuir 2000, 16, 4620–4624. [Google Scholar] [CrossRef]
- Morgan, G.T.; Alcock, M. The colour and constitution of diazonium salts. Part I. J. Chem. Soc. 1909, 95, 1319–1329. [Google Scholar] [CrossRef]
- Ostendorf, A.; Cramer, C.; Decher, G.; Schönhoff, M. Humidity-tunable electronic conductivity of polyelectrolyte multilayers containing gold nanoparticles. J. Phys. Chem. C 2015, 119, 9543–9549. [Google Scholar] [CrossRef]
- Parveen, N.; Schönhoff, M. Quantifying and controlling the cation uptake upon hydrated ionic liquid-induced swelling of polyelectrolyte multilayers. Soft Matter 2017, 13, 1988–1997. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Okuhara, M. Frequency-shifts of piezoelectric quartz crystals immersed in organic liquids. Anal. Chim. Acta 1982, 142, 281–284. [Google Scholar] [CrossRef]
- Nicolas, H.; Yuan, B.; Zhang, J.W.; Zhang, X.; Schönhoff, M. Correction to “Cucurbit[8]uril as nanocontainer in a polyelectrolyte multilayer film: A quantitative and kinetic study of guest uptake”. Langmuir 2017, 33, 4879. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, H.; Yuan, B.; Zhang, X.; Schönhoff, M. Correction to “Cucurbit[8]uril-containing multilayer films for the photocontrolled binding and release of a guest molecule”. Langmuir 2017, 33, 5098. [Google Scholar] [CrossRef] [PubMed]
| pH | ka/M−1 | n | ΔH/kJ mol−1 | ΔS/J mol−1 K−1 |
|---|---|---|---|---|
| 7.8 | (1.53 ± 0.39) × 106 | 0.92 ± 0.07 | −18.2 ± 1.0 | 57.2 ± 1.6 |
| 4.8 | (1.64 ± 0.42) × 105 | 1.01 ± 0.02 | −17.5 ± 1.0 | 40.9 ± 5.4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolas, H.; Yuan, B.; Xu, J.; Zhang, X.; Schönhoff, M. pH-Responsive Host–Guest Complexation in Pillar[6]arene-Containing Polyelectrolyte Multilayer Films. Polymers 2017, 9, 719. https://doi.org/10.3390/polym9120719
Nicolas H, Yuan B, Xu J, Zhang X, Schönhoff M. pH-Responsive Host–Guest Complexation in Pillar[6]arene-Containing Polyelectrolyte Multilayer Films. Polymers. 2017; 9(12):719. https://doi.org/10.3390/polym9120719
Chicago/Turabian StyleNicolas, Henning, Bin Yuan, Jiangfei Xu, Xi Zhang, and Monika Schönhoff. 2017. "pH-Responsive Host–Guest Complexation in Pillar[6]arene-Containing Polyelectrolyte Multilayer Films" Polymers 9, no. 12: 719. https://doi.org/10.3390/polym9120719