A Biodegradable Microneedle Cuff for Comparison of Drug Effects through Perivascular Delivery to Balloon-Injured Arteries
Abstract
:1. Introduction
2. Methods
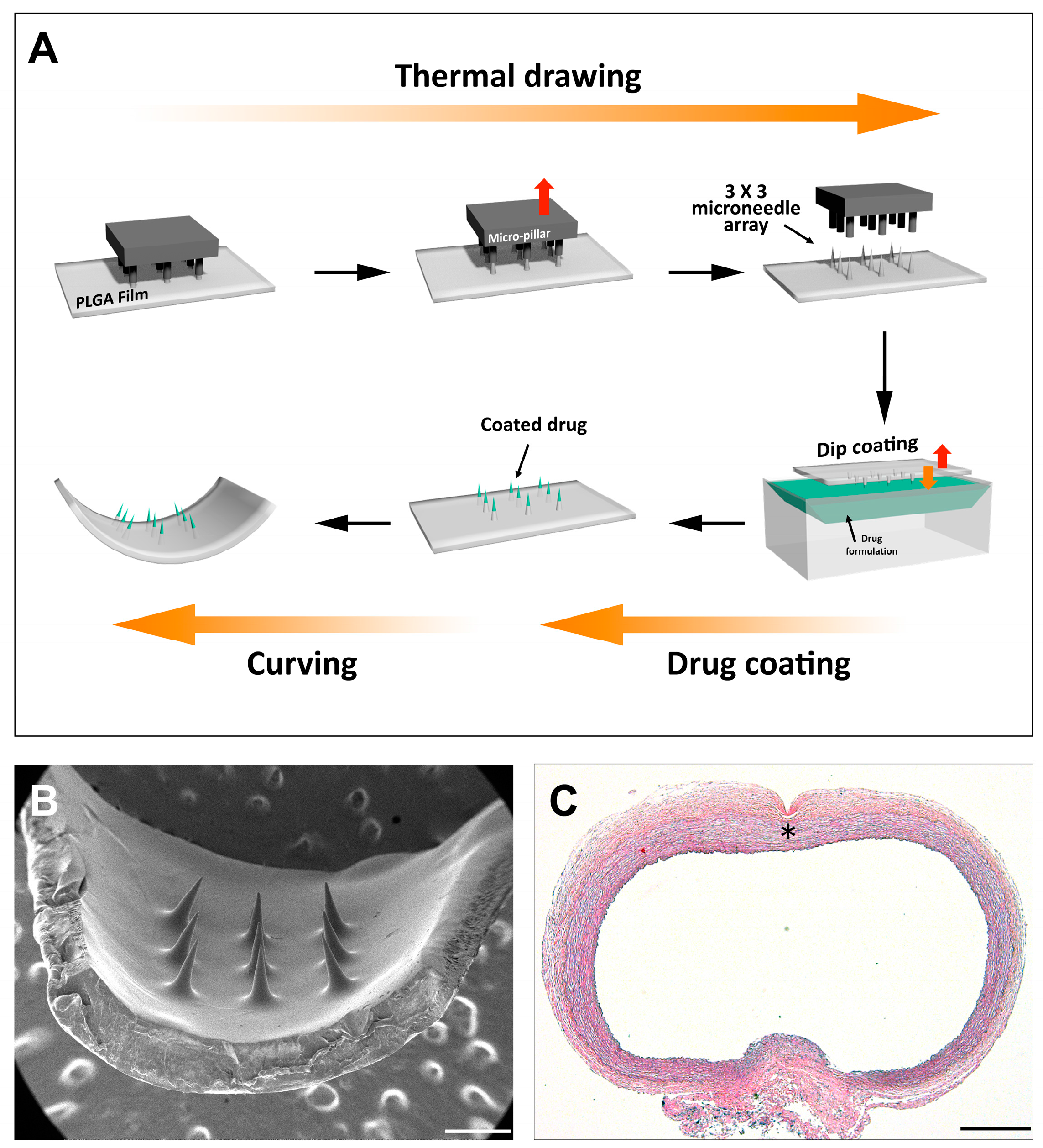
2.1. MN Device Fabrication
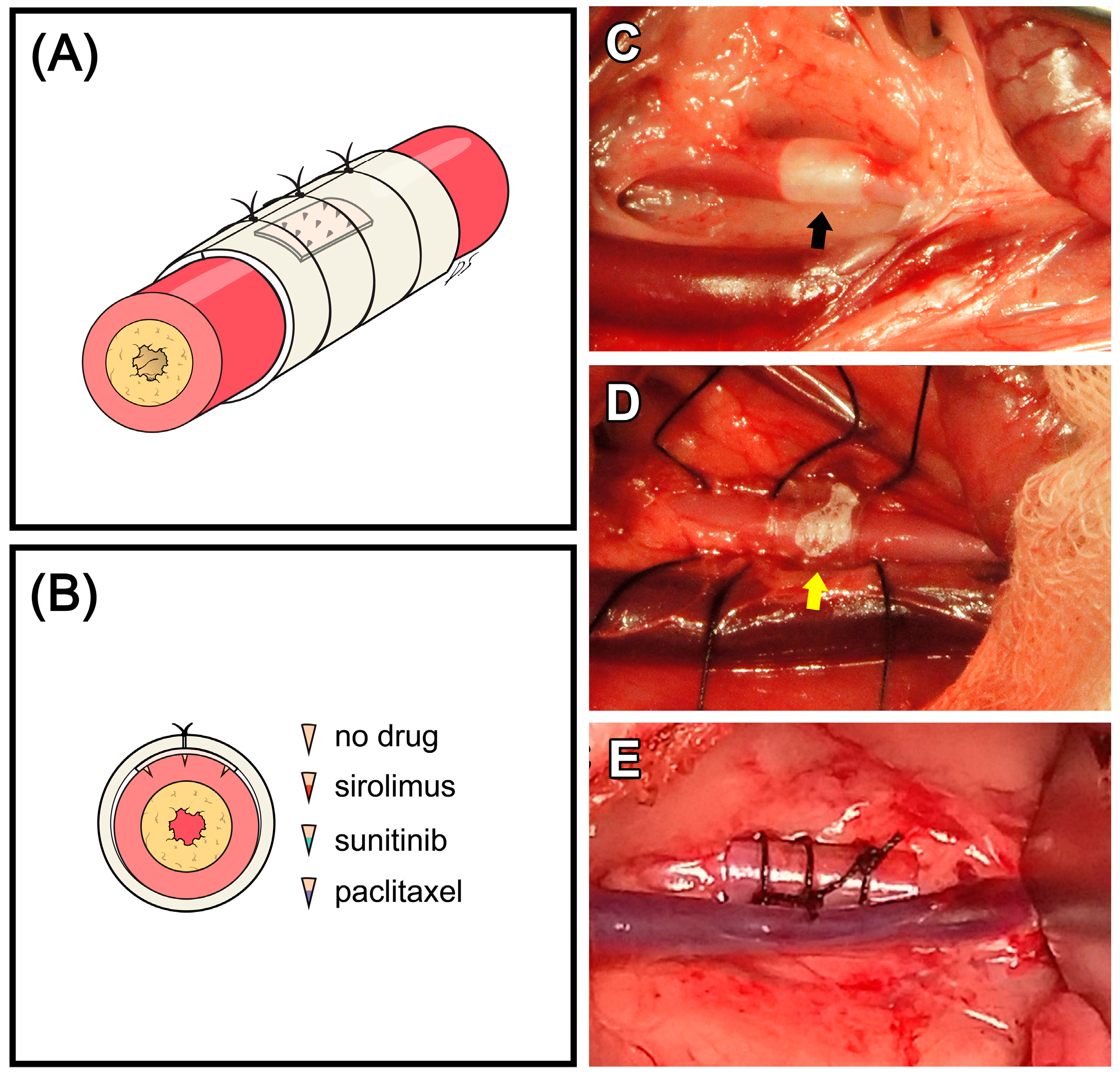
2.2. Animal Experiment
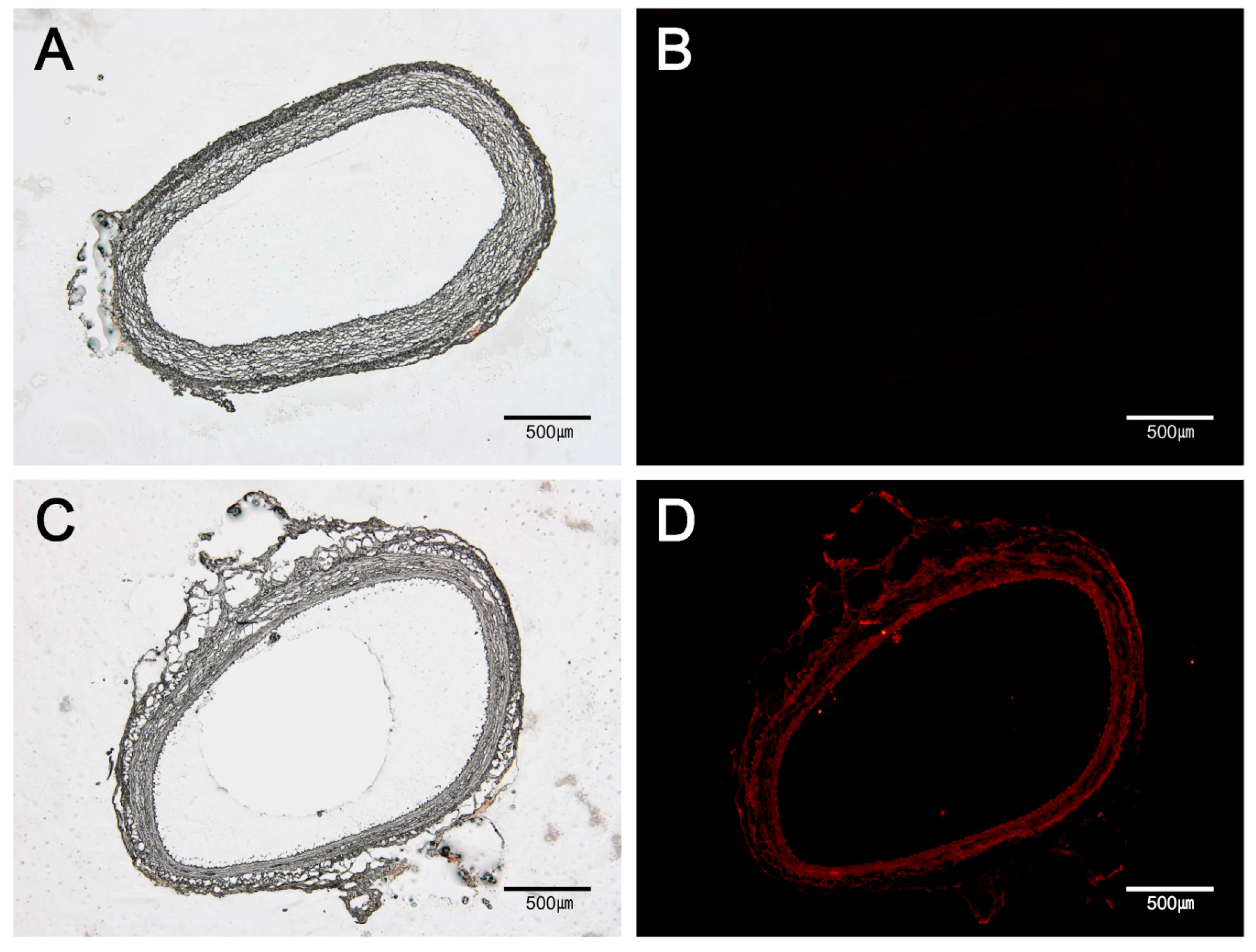
2.3. Fluorescent Microscopic Analysis
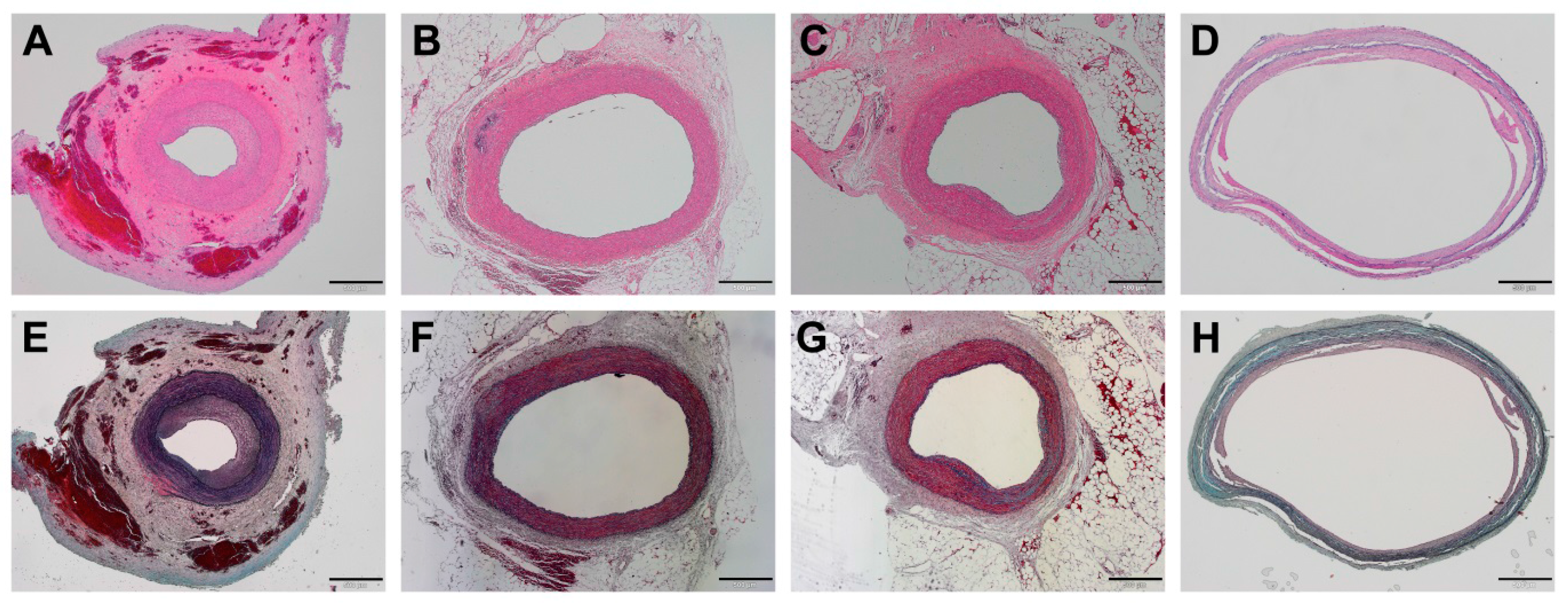
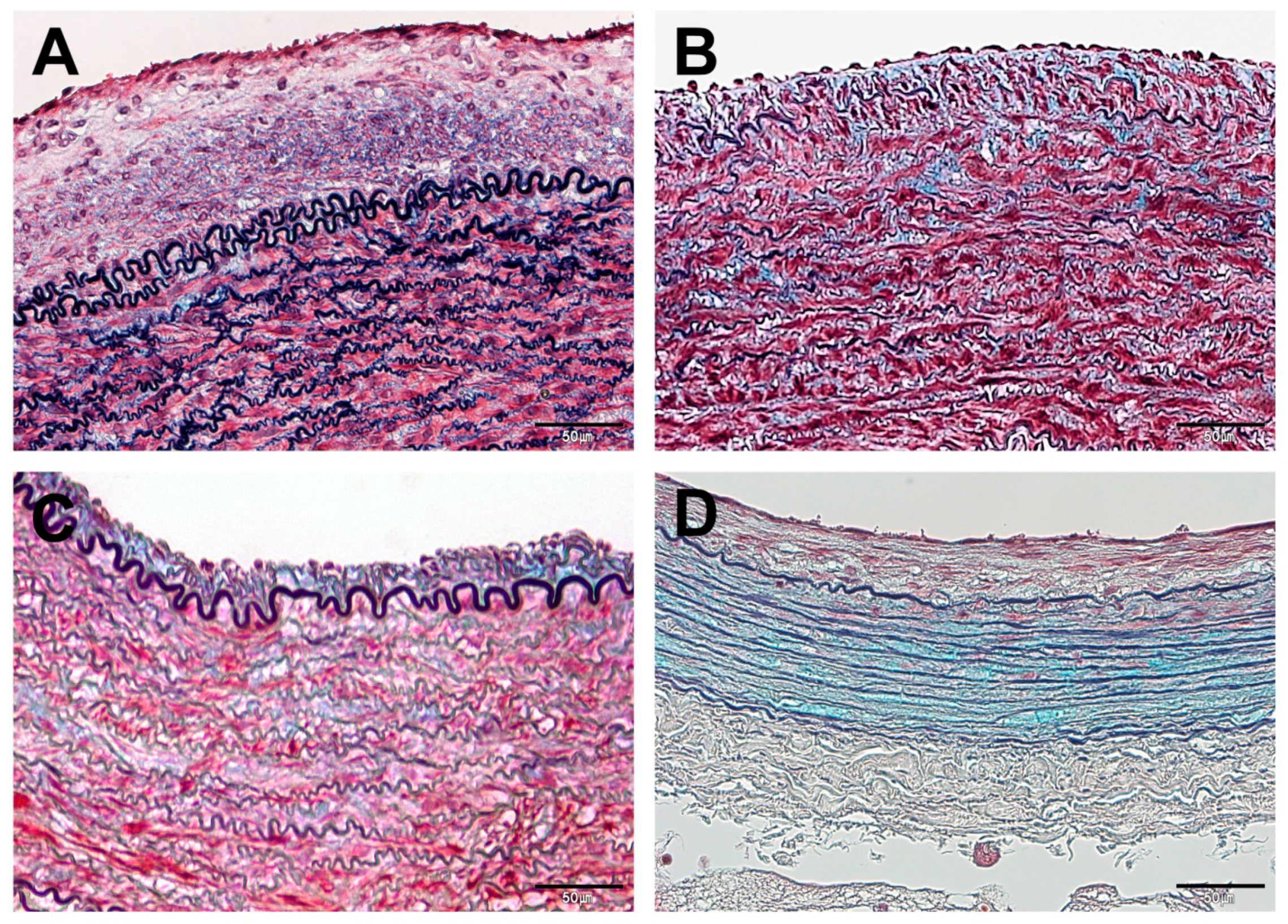
2.4. Histopathological Examinations
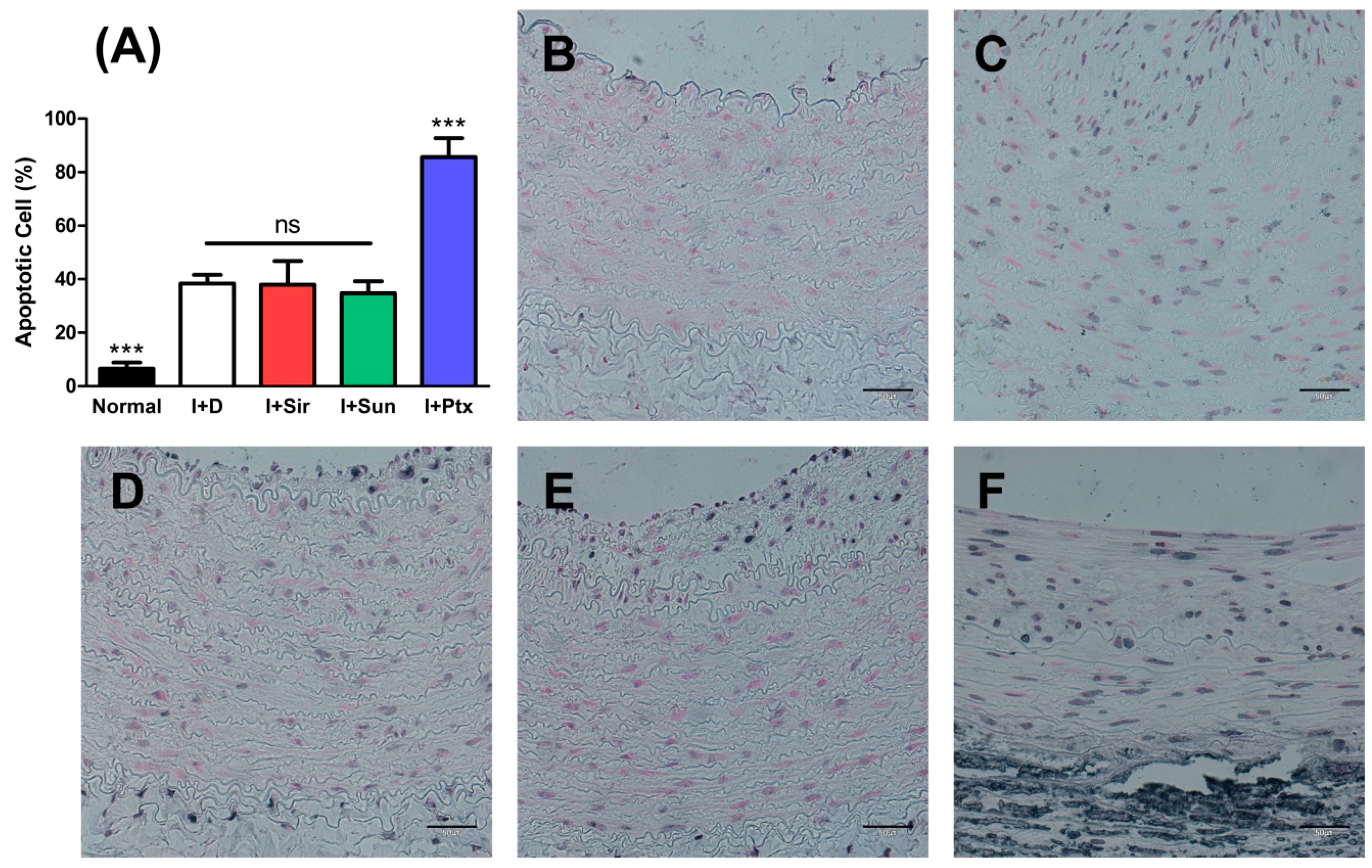
2.5. TUNEL (Terminal Deoxynucleotidyl Transferase dUTP Nick end Labeling) Assay
2.6. Statistical Analysis
3. Results
3.1. Drug Distribution in Vascular Tissue by MN Device
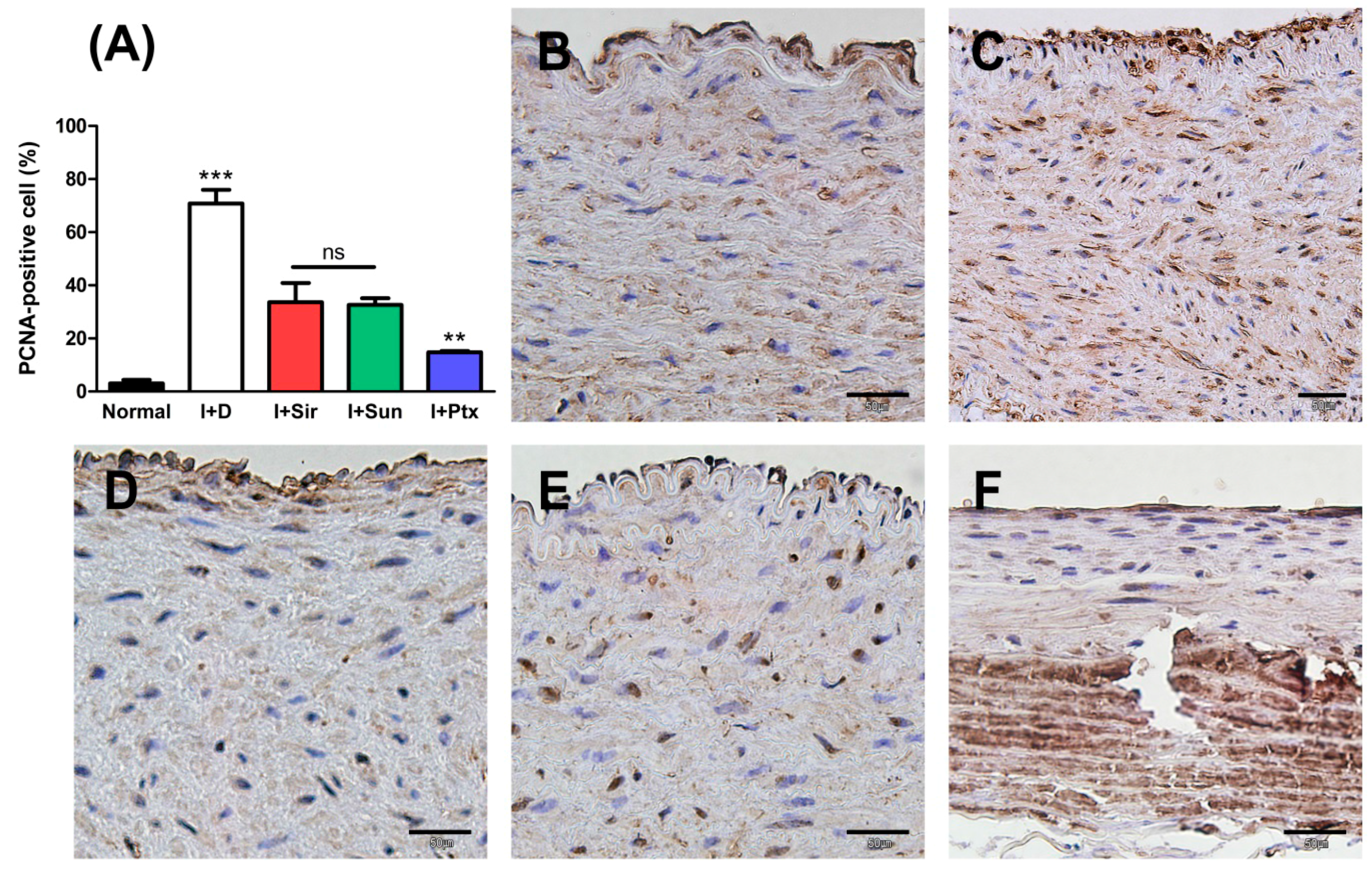
3.2. Quantitative Analysis
3.3. Histopathological Findings
3.4. Apoptosis of Vascular Smooth Muscle Cells
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brannigan, R.P.; Dove, A.P. Synthesis, properties and biomedical applications of hydrolytically degradable materials based on aliphatic polyesters and polycarbonates. Biomater. Sci. 2016, 5, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Daimon, Y.; Kamei, N.; Kawakami, K.; Takeda-Morishita, M.; Izawa, H.; Takechi-Haraya, Y.; Saito, H.; Sakai, H.; Abe, M.; Ariga, K. Dependence of Intestinal Absorption Profile of Insulin on Carrier Morphology Composed of β-Cyclodextrin-Grafted Chitosan. Mol. Pharm. 2016, 13, 4034–4042. [Google Scholar] [CrossRef] [PubMed]
- Tolstik, E.; Osminkina, L.A.; Akimov, D.; Gongalsky, M.B.; Kudryavtsev, A.A.; Timoshenko, V.Y.; Heintzmann, R.; Sivakov, V.; Popp, J. Linear and Non-Linear Optical Imaging of Cancer Cells with Silicon Nanoparticles. Int. J. Mol. Sci. 2016, 17, 1536. [Google Scholar] [CrossRef] [PubMed]
- Takanari, K.; Hashizume, R.; Hong, Y.; Amoroso, N.J.; Yoshizumi, T.; Gharaibeh, B.; Yoshida, O.; Nonaka, K.; Sato, H.; Huard, J.; et al. Skeletal muscle derived stem cells microintegrated into a biodegradable elastomer for reconstruction of the abdominal wall. Biomaterials 2017, 113, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, M.A.; Guo, L.W.; Shi, X.; Chen, G.; Gong, S.; Liu, B.; Kent, K.C. Periadventitial drug delivery for the prevention of intimal hyperplasia following open surgery. J. Control. Release 2016, 233, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Takayama, T.; Goel, S.A.; Shi, X.; Zhou, Y.; Kent, K.C.; Murphy, W.L.; Guo, L.W. A rapamycin-releasing perivascular polymeric sheath produces highly effective inhibition of intimal hyperplasia. J. Control. Release 2014, 191, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Skalský, I.; Szárszoi, O.; Filová, E.; Pařízek, M.; Lytvynets, A.; Malušková, J.; Lodererová, A.; Brynda, E.; Lisá, V.; Burdíková, Z.; et al. A perivascular system releasing sirolimus prevented intimal hyperplasia in a rabbit model in a medium-term study. Int. J. Pharm. 2012, 427, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Kanjickal, D.; Lopina, S.; Evancho-Chapman, M.M.; Schmidt, S.; Donovan, D. Sustained local drug delivery from a novel polymeric ring to inhibit intimal hyperplasia. J. Biomed. Mater. Res. A. 2010, 93, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Rajathurai, T.; Rizvi, S.I.; Lin, H.; Angelini, G.D.; Newby, A.C.; Murphy, G.J. Periadventitial rapamycin-eluting microbeads promote vein graft disease in long-term pig vein-into-artery interposition grafts. Circ. Cardiovasc. Interv. 2010, 3, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Chen, G.; Guo, L.W.; Si, Y.; Zhu, M.; Pilla, S.; Liu, B.; Gong, S.; Kent, K.C. Periadventitial application of rapamycin-loaded nanoparticles produces sustained inhibition of vascular restenosis. PLoS ONE 2014, 9, e89227. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, P.; Yao, Q.; Chen, C. Development of small-diameter vascular grafts. World J. Surg. 2007, 31, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Seedial, S.M.; Ghosh, S.; Saunders, R.S.; Suwanabol, P.A.; Shi, X.; Liu, B.; Kent, K.C. Local drug delivery to prevent restenosis. J. Vasc. Surg. 2013, 57, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Riede, F.N.; Pfisterer, M.; Jeger, R. Long-term safety of drug-eluting stents. Expert. Rev. Cardiovasc. Ther. 2013, 11, 1359–1378. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Roy-Chaudhury, P. Advances and new frontiers in the pathophysiology of venous neointimal hyperplasia and dialysis access stenosis. Adv. Chronic Kidney Dis. 2009, 16, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.S.; Chronos, N.A.; Virmani, R. Preclinical restenosis models and drug-eluting stents: Still important, still much to learn. J. Am. Coll. Cardiol. 2004, 44, 1373–1385. [Google Scholar] [CrossRef] [PubMed]
- Finn, A.V.; Kolodgie, F.D.; Harnek, J.; Guerrero, L.J.; Acampado, E.; Tefera, K.; Skorija, K.; Weber, D.K.; Gold, H.K.; Virmani, R. Differential response of delayed healing and persistent inflammation at sites of overlapping sirolimus- or paclitaxel-eluting stents. Circulation 2005, 112, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, G.; Finn, A.V.; John, M.C.; Kolodgie, F.D.; Virmani, R. The significance of preclinical evaluation of sirolimus-, paclitaxel-, and zotarolimus-eluting stents. Am. J. Cardiol. 2007, 100, 36M–44M. [Google Scholar] [CrossRef] [PubMed]
- Buechel, R.; Stirnimann, A.; Zimmer, R.; Keo, H.; Groechenig, E. Drug-eluting stents and drug-coated balloons in peripheral artery disease. Vasa 2012, 41, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, S.; Boey, F. Release profiles in drug-eluting stents: Issues and uncertainties. J. Control. Release 2007, 120, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Park, S.H.; Lee, J.Y.; Joo, H.C.; Jang, E.H.; Youn, Y.N.; Ryu, W. Perivascular biodegradable microneedle cuff for reduction of neointima formation after vascular injury. J. Control. Release 2014, 192, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Le, V.; Johnson, C.G.; Lee, J.D.; Baker, A.B. Murine model of femoral artery wire injury with implantation of a perivascular drug delivery patch. J. Vis. Exp. 2015, 96, e52403. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.K.; Kim, J.B.; Jang, E.H.; Youn, Y.N.; Ryu, W.H. Curved biodegradable microneedles for vascular drug delivery. Small 2012, 8, 2483–2488. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.K.; Lee, K.J.; Youn, Y.N.; Jang, E.H.; Kim, W.; Min, B.K.; Ryu, W. Spatially discrete thermal drawing of biodegradable microneedles for vascular drug delivery. Eur. J. Pharm. Biopharm. 2013, 83, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Signore, P.E.; Machan, L.S.; Jackson, J.K.; Burt, H.; Bromley, P.; Wilson, J.E.; McManus, B.M. Complete inhibition of intimal hyperplasia by perivascular delivery of paclitaxel in balloon-injured rat carotid arteries. J. Vasc. Interv. Radiol. 2001, 12, 79–88. [Google Scholar] [CrossRef]
- Kohler, T.R.; Toleikis, P.M.; Gravett, D.M.; Avelar, R.L. Inhibition of neointimal hyperplasia in a sheep model of dialysis access failure with the bioabsorbable vascular wrap paclitaxel-eluting mesh. J. Vasc. Surg. 2007, 45, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Ikeno, F.; Lyons, J.; Kaneda, H.; Baluom, M.; Benet, L.Z.; Rezaee, M. Novel percutaneous adventitial drug delivery system for regional vascular treatment. Catheter. Cardiovasc. Interv. 2004, 63, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Parry, T.J.; Brosius, R.; Thyagarajan, R.; Carter, D.; Argentieri, D.; Falotico, R.; Siekierka, J. Drug-eluting stents: Sirolimus and paclitaxel differentially affect cultured cells and injured arteries. Eur. J. Pharmacol. 2005, 524, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Wessely, R.; Blaich, B.; Belaiba, R.S.; Merl, S.; Görlach, A.; Kastrati, A.; Schömig, A. Comparative characterization of cellular and molecular anti-restenotic profiles of paclitaxel and sirolimus. Implications for local drug delivery. Thromb. Haemost. 2007, 97, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Pires, N.M.; Eefting, D.; de Vries, M.R.; Quax, P.H.; Jukema, J.W. Sirolimus and paclitaxel provoke different vascular pathological responses after local delivery in a murine model for restenosis on underlying atherosclerotic arteries. Heart 2007, 93, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.V.; Fernandes, M.R.; Madonna, R.; Clubb, F.; Oliveira, E.; Jimenez-Quevedo, P.; Branco, R.; Lopez, J.; Angeli, F.S.; Sanz-Ruiz, R.; et al. Comparative healing response after sirolimus- and paclitaxel-eluting stent implantation in a pig model of restenosis. Catheter. Cardiovasc. Interv. 2009, 73, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Majesky, M.W.; Reidy, M.A.; Bowen-Pope, D.F.; Hart, C.E.; Wilcox, J.N.; Schwartz, S.M. PDGF ligand and receptor gene expression during repair of arterial injury. J. Cell Biol. 1990, 111, 2149–2158. [Google Scholar] [CrossRef] [PubMed]
- Ferns, G.A.; Raines, E.W.; Sprugel, K.H.; Motani, A.S.; Reidy, M.A.; Ross, R. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science 1991, 253, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Makiyama, Y.; Toba, K.; Kato, K.; Hirono, S.; Ozawa, T.; Saigawa, T.; Minagawa, S.; Isoda, M.; Asami, F.; Ikarashi, N.; et al. Imatinib mesilate inhibits neointimal hyperplasia via growth inhibition of vascular smooth muscle cells in a rat model of balloon injury. Tohoku J. Exp. Med. 2008, 215, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Vamvakopoulos, J.E.; Petrov, L.; Aavik, S.; Lehti, S.; Aavik, E.; Hayry, P. Synergistic suppression of rat neointimal hyperplasia by rapamycin and imatinib mesylate: Implications for the prevention of accelerated arteriosclerosis. J. Vasc. Res. 2006, 43, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Sanders, W.G.; Hogrebe, P.C.; Grainger, D.W.; Cheung, A.K.; Terry, C.M. A biodegradable perivascular wrap for controlled, local and directed drug delivery. J Control. Release 2012, 161, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Okamoto, Y.; Katsumata, H.; Egawa, S.; Yamanaka, D.; Fukushima, M.; Minami, S. Sunitinib, a small-molecule receptor tyrosine kinase inhibitor, suppresses neointimal hyperplasia in balloon-injured rat carotid artery. J. Cardiovasc. Pharmacol. Ther. 2013, 18, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Roque, M.; Cordon-Cardo, C.; Fuster, V.; Reis, E.D.; Drobnjak, M.; Badimon, J.J. Modulation of apoptosis, proliferation, and p27 expression in a porcine coronary angioplasty model. Atherosclerosis 2000, 153, 315–322. [Google Scholar] [CrossRef]
- Angelini, A.; Visonà, A.; Calabrese, F.; Pettenazzo, E.; Yacoub, A.; Valente, M.; Bonandini, E.M.; Jori, G.; Pagnan, A.; Thiene, G. Time Course of Apoptosis and Proliferation in vascular Smooth Muscle Cells after Balloon Angioplasty. Basic Appl. Myol. 2002, 12, 33–42. [Google Scholar]
- Markos, F.; Ruane O’Hora, T.; Noble, M.I. What is the mechanism of flow-mediated arterial dilatation. Clin. Exp. Pharmacol. Physiol. 2013, 40, 489–494. [Google Scholar] [CrossRef] [PubMed]
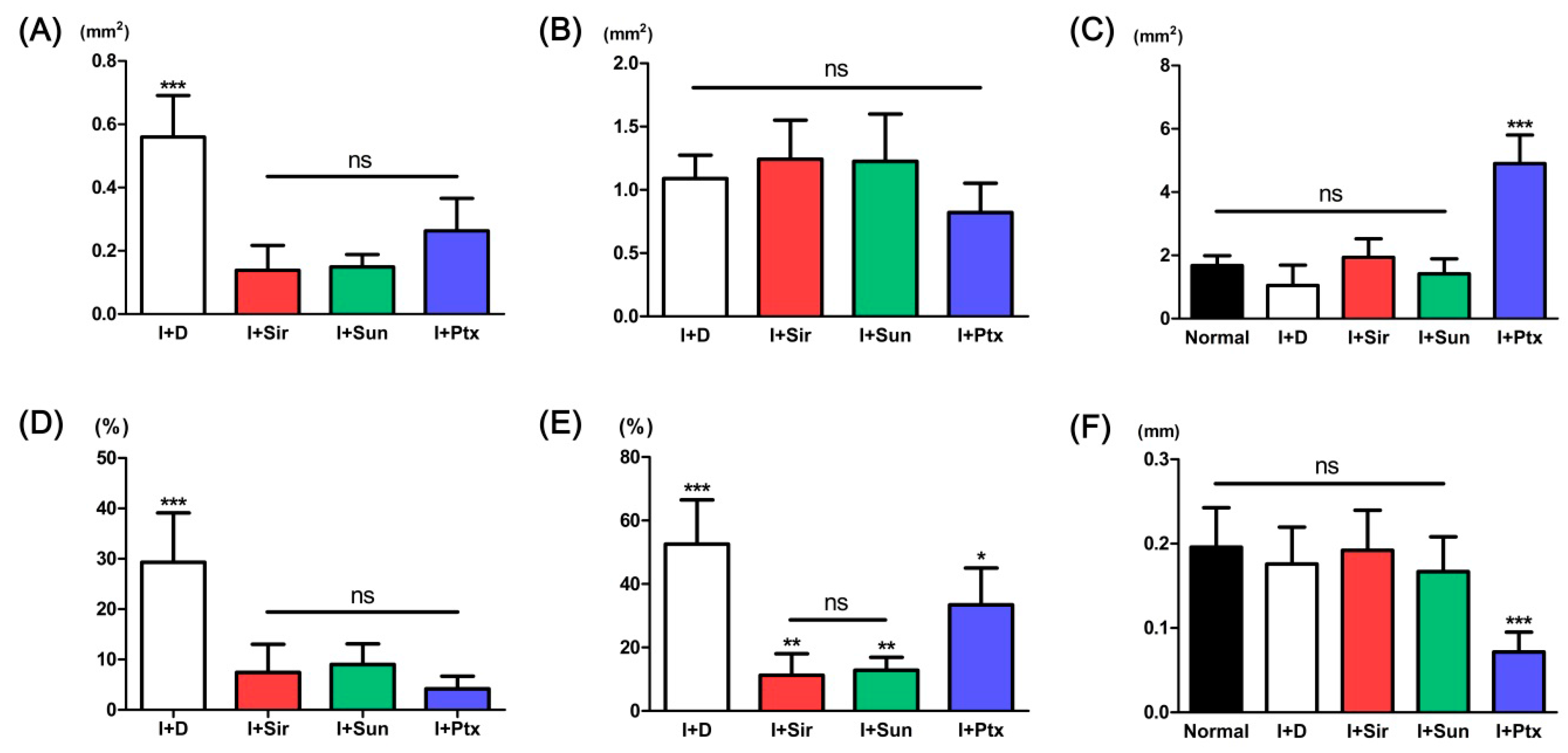
| Parameters | Normal artery | I + D | I + Sir | I + Sun | I + Ptx |
|---|---|---|---|---|---|
| Neointimal area (mm2) | - | 0.56 ± 0.13 *** | 0.14 ± 0.08 | 0.15 ± 0.04 | 0.26 ± 0.10 |
| Tunica media area (mm2) | - | 1.09 ± 0.18 | 1.24 ± 0.31 | 1.23 ± 0.37 | 0.82 ± 0.23 |
| Luminal area (mm2) | 1.67 ± 0.31 | 1.05 ± 0.64 | 1.94 ± 0.59 | 1.42 ± 0.47 | 4.90 ± 0.90 *** |
| Neointimal formation (%) | - | 33.59 ± 19.85 *** | 7.42 ± 5.59 | 9.02 ± 4.08 | 4.70 ± 2.31 |
| Neointimal area/tunica media area (%) | - | 52.57 ± 13.92 *** | 11.26 ± 6.80 ** | 12.86 ± 4.07 ** | 33.43 ± 11.62 * |
| Tunica media thickness (mm) | 0.20 ± 0.05 | 0.18 ± 0.04 | 0.19 ± 0.05 | 0.17 ± 0.04 | 0.07 ± 0.02 *** |
| PCNA-positive cells (%) | 3.03 ± 1.37 | 70.82 ± 5.11 *** | 33.59 ± 7.26 | 32.59 ± 2.49 | 14.82 ± 0.46 †† |
| TUNEL-positive cells (%) | 6.58 ± 2.27 *** | 38.33 ± 3.30 | 37.99 ± 8.78 | 34.71 ± 4.48 | 85.60 ± 7.14 *** |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-H.; Jang, E.H.; Lee, K.J.; Lee, J.Y.; Park, S.H.; Seo, I.H.; Lee, K.W.; Lee, S.H.; Ryu, W.; Youn, Y.-N. A Biodegradable Microneedle Cuff for Comparison of Drug Effects through Perivascular Delivery to Balloon-Injured Arteries. Polymers 2017, 9, 56. https://doi.org/10.3390/polym9020056
Kim D-H, Jang EH, Lee KJ, Lee JY, Park SH, Seo IH, Lee KW, Lee SH, Ryu W, Youn Y-N. A Biodegradable Microneedle Cuff for Comparison of Drug Effects through Perivascular Delivery to Balloon-Injured Arteries. Polymers. 2017; 9(2):56. https://doi.org/10.3390/polym9020056
Chicago/Turabian StyleKim, Dae-Hyun, Eui Hwa Jang, Kang Ju Lee, Ji Yong Lee, Seung Hyun Park, Il Ho Seo, Kang Woog Lee, Seung Hyun Lee, WonHyoung Ryu, and Young-Nam Youn. 2017. "A Biodegradable Microneedle Cuff for Comparison of Drug Effects through Perivascular Delivery to Balloon-Injured Arteries" Polymers 9, no. 2: 56. https://doi.org/10.3390/polym9020056






