Visible Light-Induced Metal Free Surface Initiated Atom Transfer Radical Polymerization of Methyl Methacrylate on SBA-15
Abstract
:1. Introduction
2. Materials and Characterization
2.1. Materials
2.2. Synthesis of SBA-15
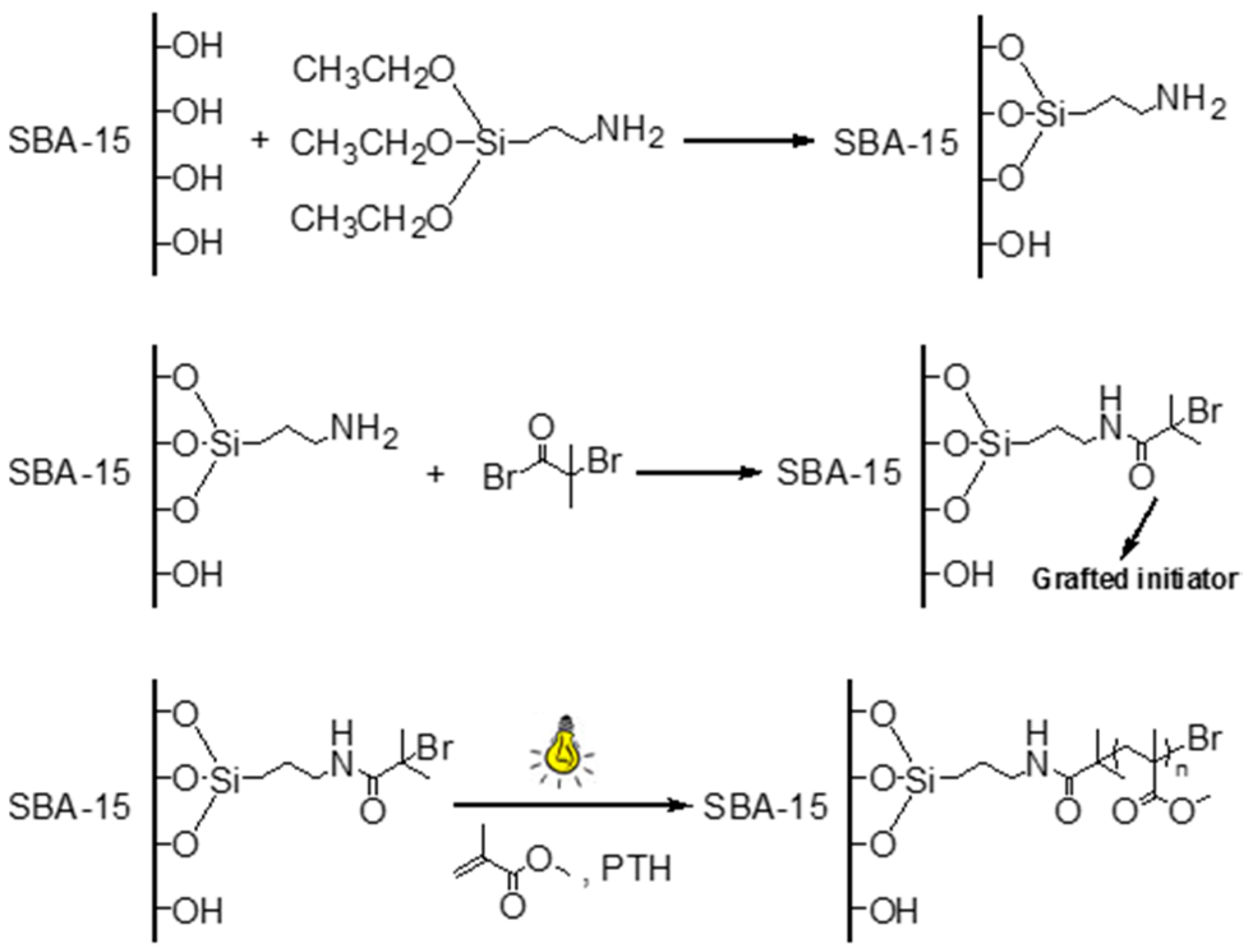
2.3. Synthesis of SBA-APTES
2.4. Synthesis of SBA-Br
2.5. Synthesis of SBA-PMMA
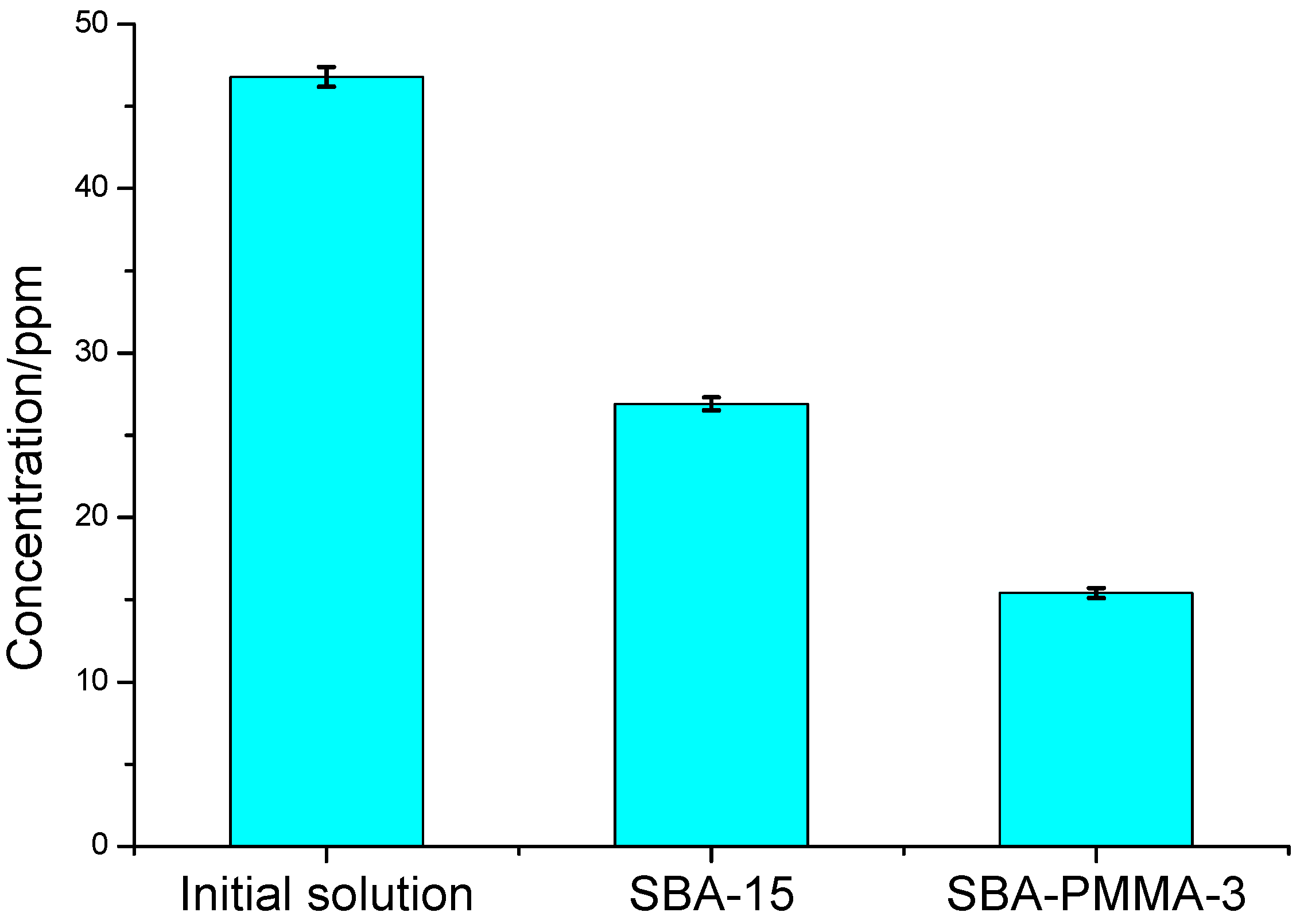
2.6. Batch Adsorption
2.7. Characterization
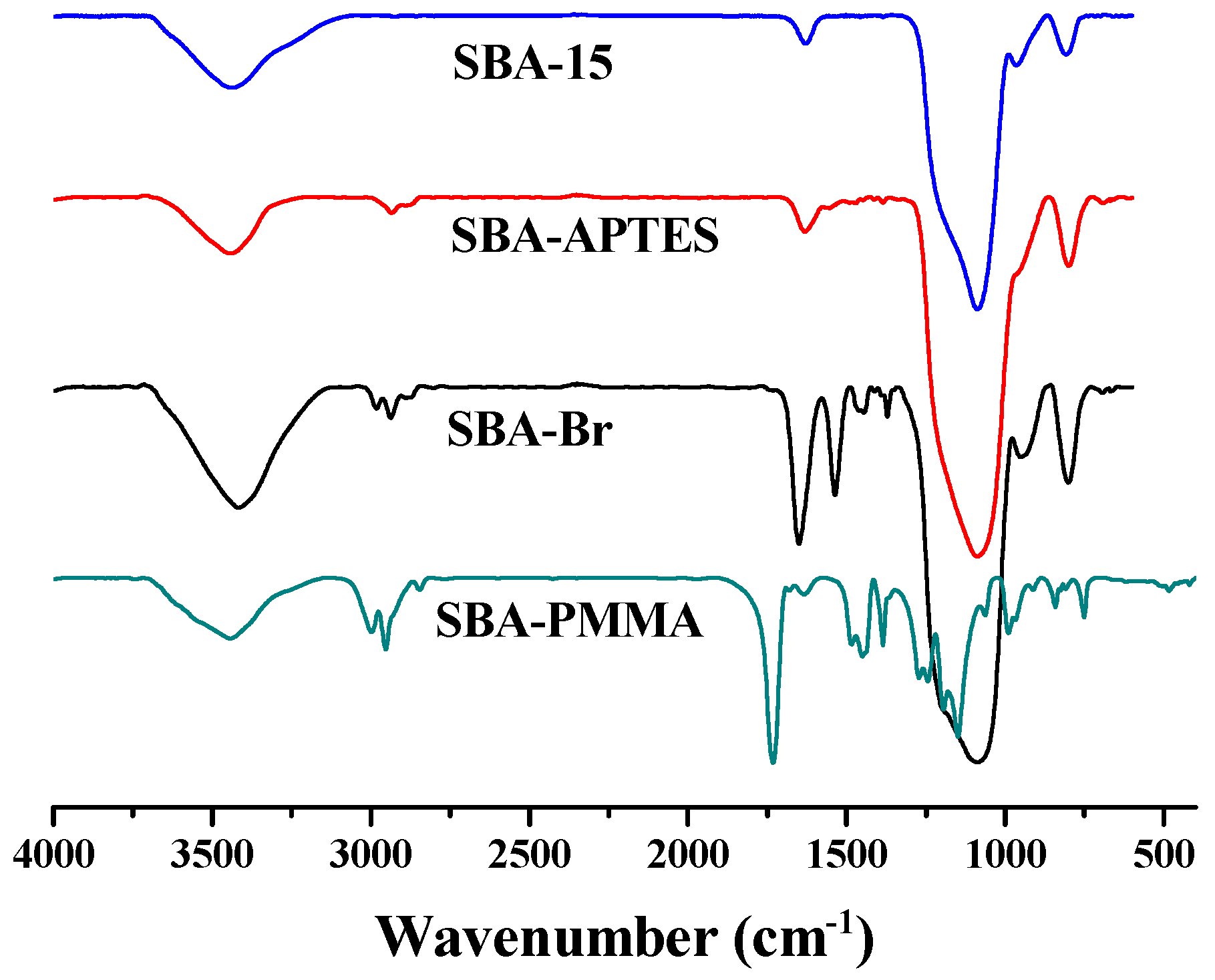
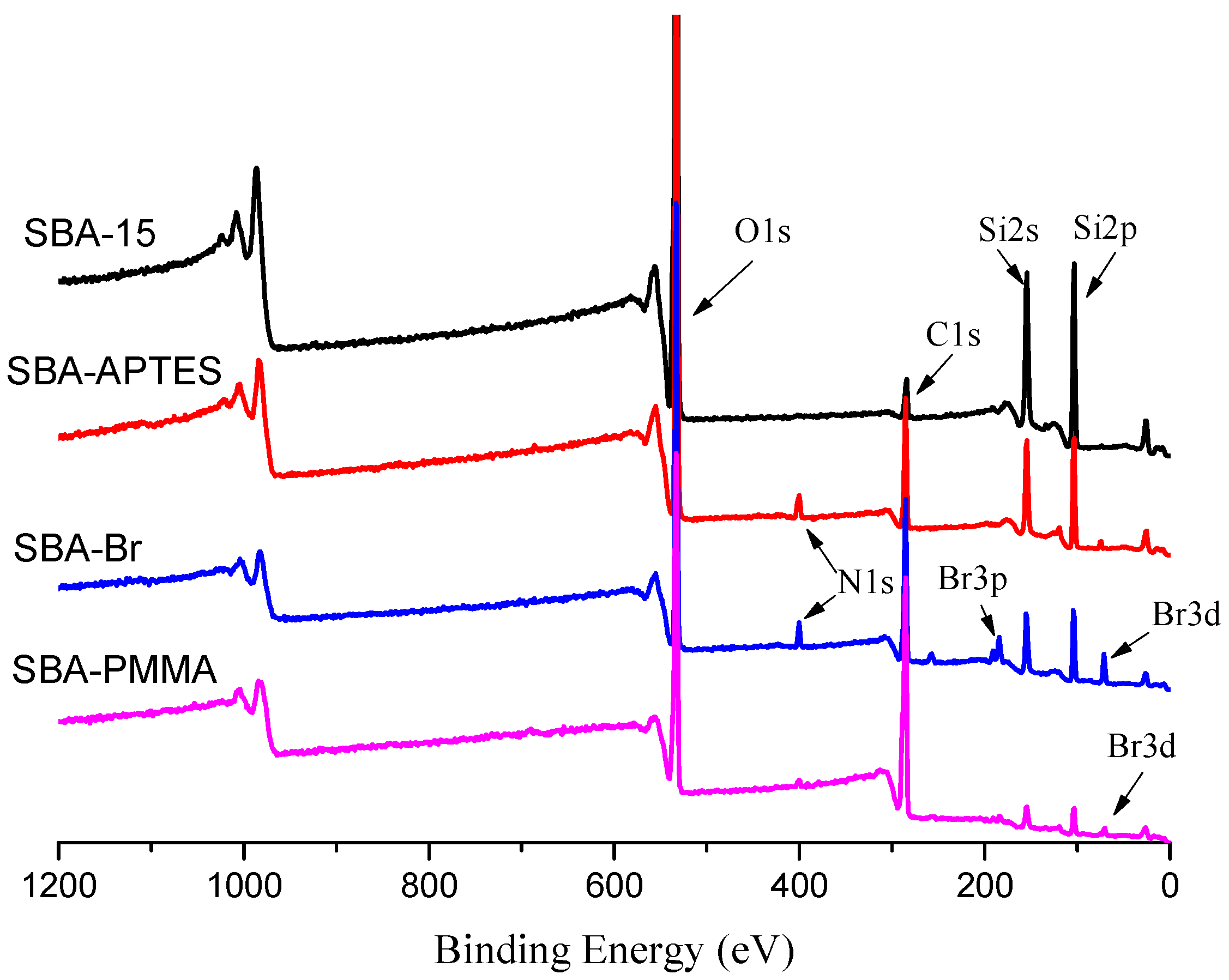
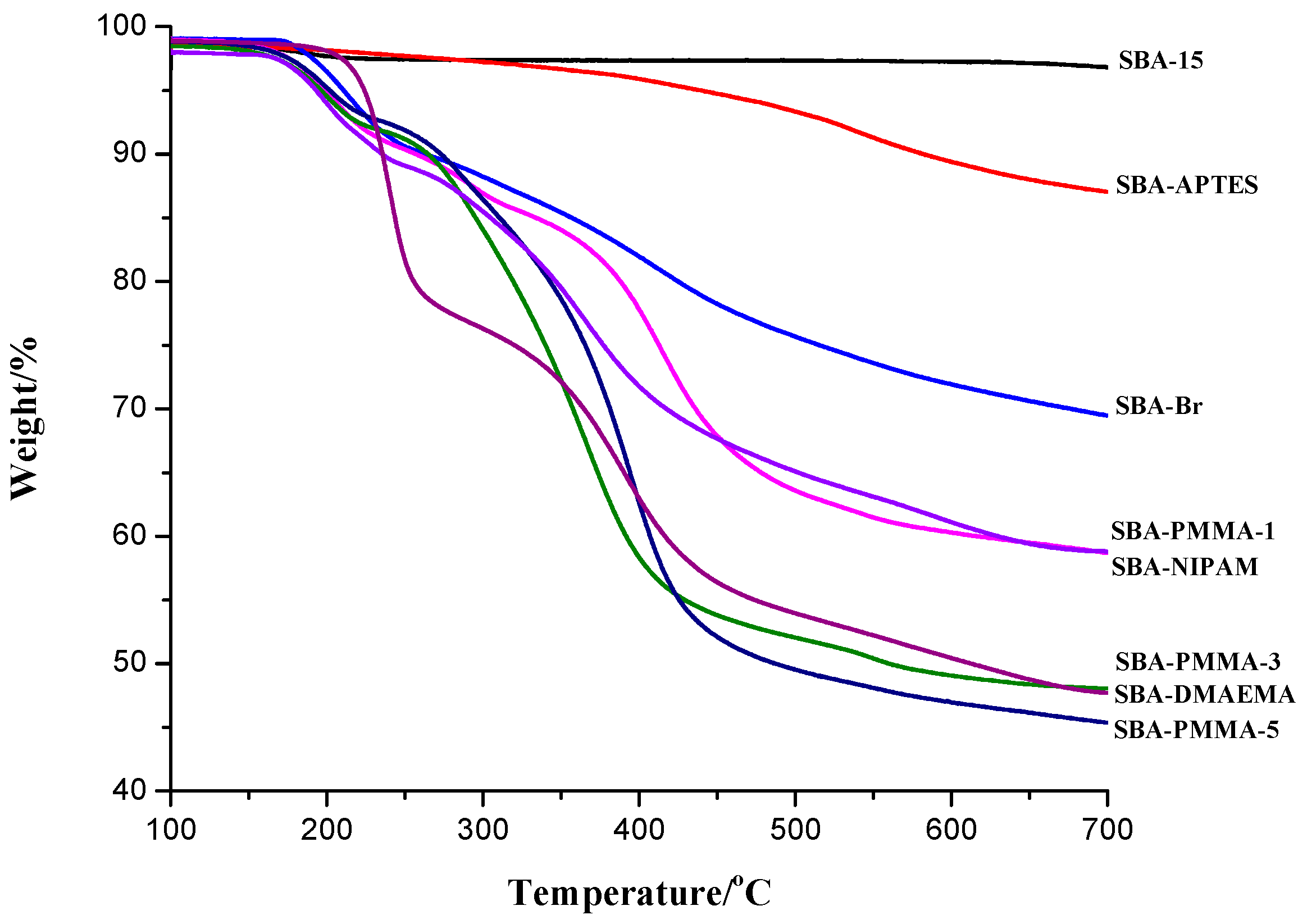
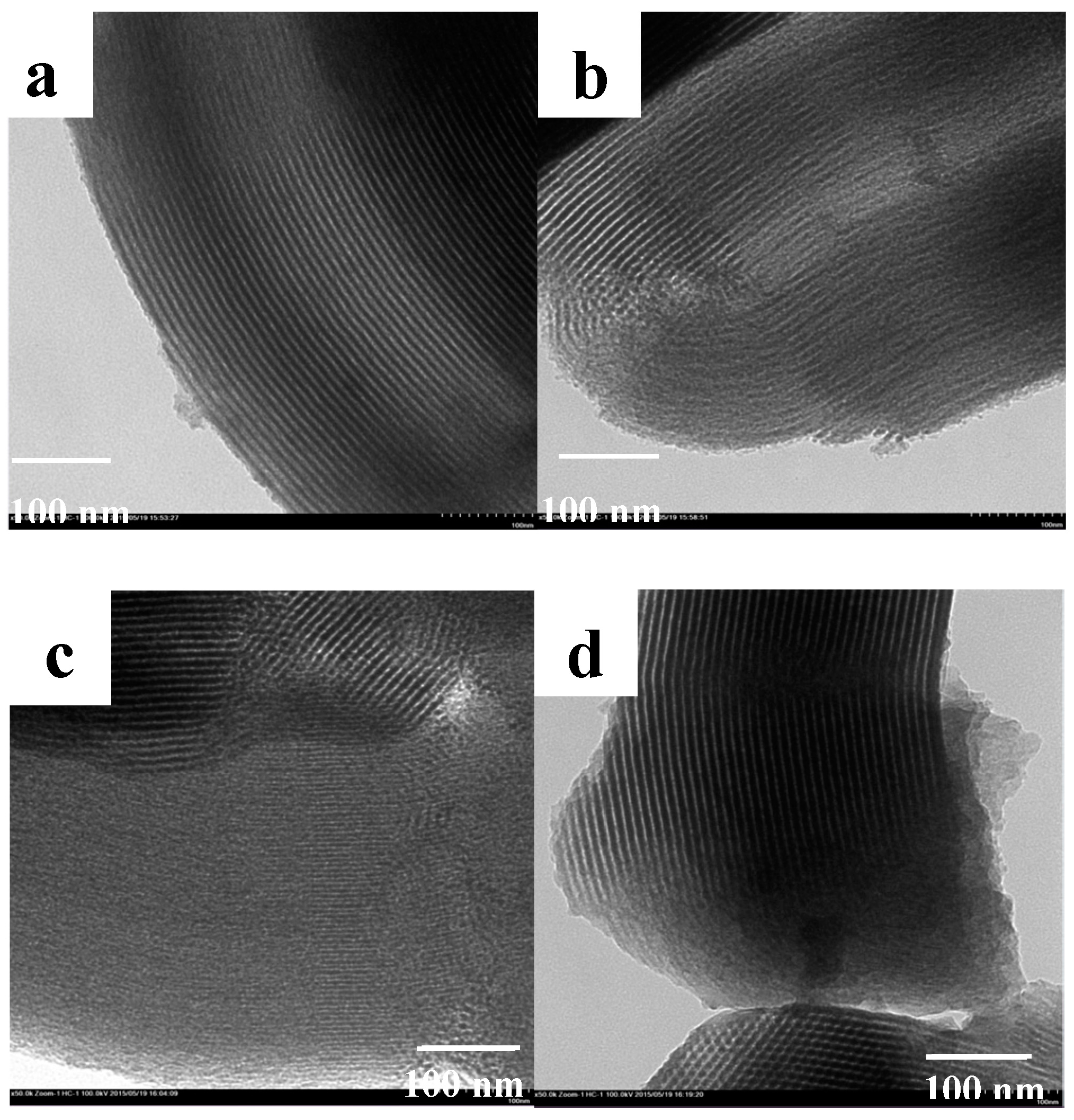
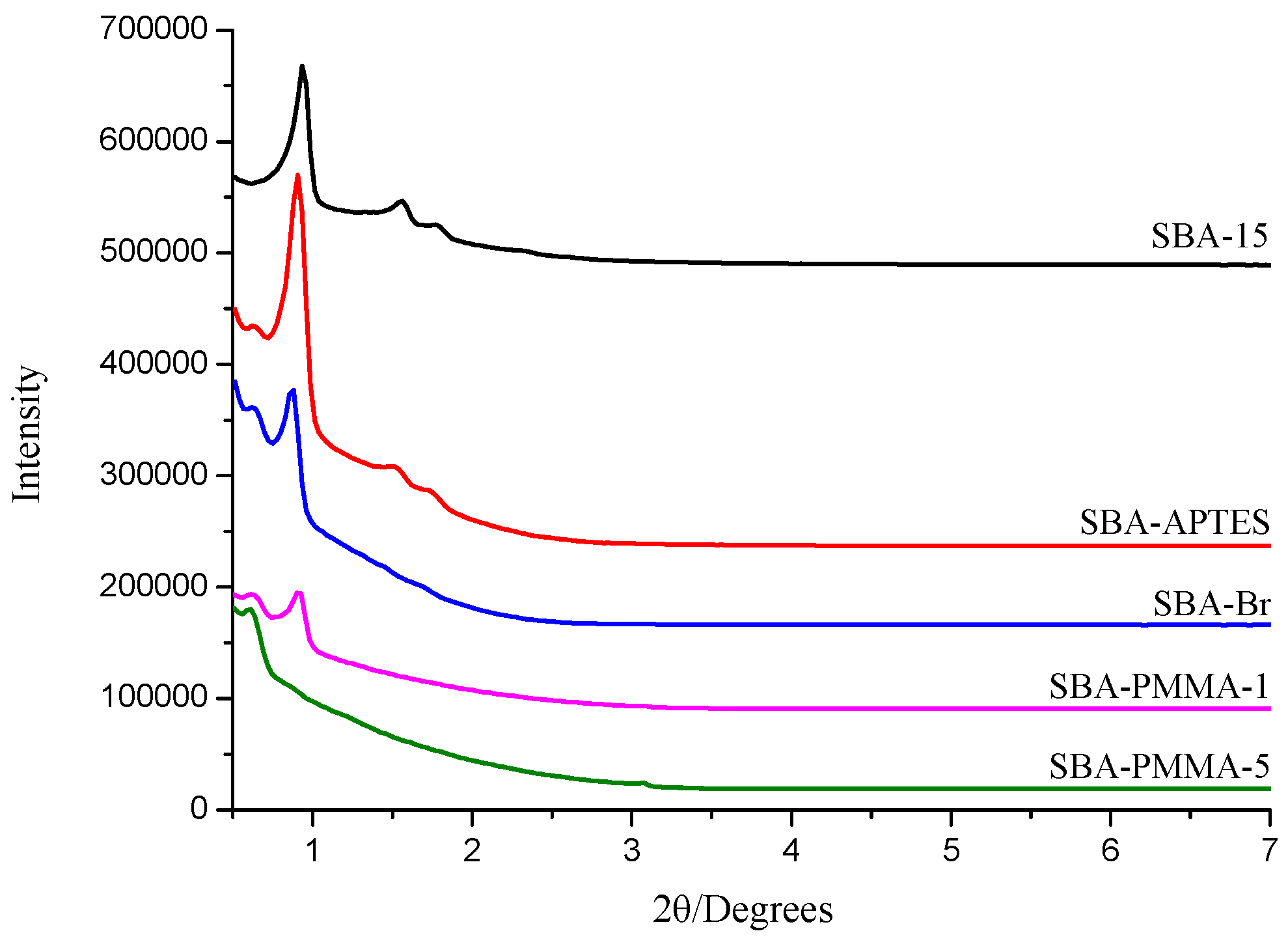
3. Results
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhu, Y.; Li, H.; Zheng, Q.; Xu, J.; Li, X. Amine-Functionalized SBA-15 with Uniform Morphology and Well-Defined Mesostructure for Highly Sensitive Chemosensors To Detect Formaldehyde Vapor. Langmuir 2012, 28, 7843–7850. [Google Scholar] [CrossRef] [PubMed]
- Barbey, R.; Lavanant, L.; Paripovic, D.; Schüwer, N.; Sugnaux, C.; Tugulu, S.; Klok, H.-A. Polymer Brushes via Surface-Initiated Controlled Radical Polymerization: Synthesis, Characterization, Properties, and Applications. Chem. Rev. 2009, 109, 5437–5527. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.; Sherrington, D. Well-Defined Mesostructured Organic-Inorganic Hybrid Materials via Atom Transfer Radical Grafting of Oligomethacrylates onto SBA-15 Pore Surfaces. Chem. Mater. 2008, 20, 4468–4474. [Google Scholar] [CrossRef]
- Wight, A.; Davis, M. Design and Preparation of Organic-Inorganic Hybrid Catalysts. Chem. Rev. 2002, 102, 3589–3614. [Google Scholar] [CrossRef] [PubMed]
- Moller, K.; Bein, T.; Fischer, R.X. Synthesis of Ordered Mesoporous Methacrylate Hybrid Systems: Hosts for Molecular Polymer Composites. Chem. Mater. 1999, 11, 665–673. [Google Scholar] [CrossRef]
- Wan, Y.; Zhao, D. On the Controllable Soft-Templating Approach to Mesoporous Silicates. Chem. Rev. 2007, 107, 2821–2860. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Shi, Y.; Meng, Y.; Chen, D.; Cheng, S.; Chen, P.; Yang, H.; Wan, Y.; Zhao, D. Highly ordered mesoporous silicon carbide ceramics with large surface areas and high stability. Adv. Funct. Mater. 2006, 16, 561–567. [Google Scholar] [CrossRef]
- Liu, T.; Jia, S.; Kowalewski, T.; Matyjaszewski, K.; Casado-Portilla, R.; Belmont, J. Grafting poly (N-butyl acrylate) from a functionalized carbon black surface by atom transfer radical polymerization. Langmuir 2003, 19, 6342–6345. [Google Scholar] [CrossRef]
- Liu, Y.; Miao, X.; Zhu, J.; Zhang, Z.; Cheng, Z.; Zhu, X. Polymer-Grafted Modification of Activated Carbon by Surface-Initiated AGET ATRP. Macromol. Chem. Phys. 2012, 213, 868–877. [Google Scholar] [CrossRef]
- Husseman, M.; Malmström, E.E.; McNamara, M.; Mate, M.; Mecerreyes, D.; Benoit, D.G.; Hedrick, J.L.; Mansky, P.; Huang, E.; Russell, T.P. Controlled synthesis of polymer brushes by “living” free radical polymerization techniques. Macromolecules 1999, 32, 1424–1431. [Google Scholar] [CrossRef]
- Devonport, W.; Michalak, L.; Malmström, E.; Mate, M.; Kurdi, B.; Hawker, C.J.; Barclay, G.G.; Sinta, R. “Living” free-radical polymerizations in the absence of initiators: Controlled autopolymerization. Macromolecules 1997, 30, 1929–1934. [Google Scholar] [CrossRef]
- Baum, M.; Brittain, W.J. Synthesis of polymer brushes on silicate substrates via reversible addition fragmentation chain transfer technique. Macromolecules 2002, 35, 610–615. [Google Scholar] [CrossRef]
- Pyun, J.; Jia, S.; Kowalewski, T.; Patterson, G.D.; Matyjaszewski, K. Synthesis and characterization of organic/inorganic hybrid nanoparticles: Kinetics of surface-initiated atom transfer radical polymerization and morphology of hybrid nanoparticle ultrathin films. Macromolecules 2003, 36, 5094–5104. [Google Scholar] [CrossRef]
- Hui, C.M.; Pietrasik, J.; Schmitt, M.; Mahoney, C.; Choi, J.; Bockstaller, M.R.; Matyjaszewski, K. Surface-initiated polymerization as an enabling tool for multifunctional (nano-) engineered hybrid materials. Chem. Mater. 2013, 26, 745–762. [Google Scholar] [CrossRef]
- Khabibullin, A.; Mastan, E.; Matyjaszewski, K.; Zhu, S. Surface-initiated atom transfer radical polymerization. In Controlled Radical Polymerization at and from Solid Surfaces; Springer: Berlin, Germany, 2015; pp. 29–76. [Google Scholar]
- Tsujii, Y.; Ohno, K.; Yamamoto, S.; Goto, A.; Fukuda, T. Structure and properties of high-density polymer brushes prepared by surface-initiated living radical polymerization. In Surface-Initiated Polymerization I; Springer: Berlin, Germany, 2006; pp. 1–45. [Google Scholar]
- Fristrup, C.J.; Jankova, K.; Hvilsted, S. Surface-initiated atom transfer radical polymerization—A technique to develop biofunctional coatings. Soft Matter 2009, 5, 4623. [Google Scholar] [CrossRef]
- Dalsin, J.L.; Hu, B.-H.; Lee, B.P.; Messersmith, P.B. Mussel adhesive protein mimetic polymers for the preparation of nonfouling surfaces. J. Am. Chem. Soc. 2003, 125, 4253–4258. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, M.; Hakobyan, S.; Ramstedt, M.; Gautrot, J.E. Surface-initiated polymer brushes in the biomedical field: Applications in membrane science, biosensing, cell culture, regenerative medicine and antibacterial coatings. Chem. Rev. 2014, 114, 10976–11026. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.A.C.; Huck, W.T.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Baker, G.L.; Bruening, M.L. Polymer brush membranes for pervaporation of organic solvents from water. Macromolecules 2005, 38, 2307–2314. [Google Scholar] [CrossRef]
- Wang, J.-S.; Matyjaszewski, K. Controlled/“living” radical polymerization. Atom transfer radical polymerization in the presence of transition-metal complexes. J. Am. Chem. Soc. 1995, 117, 5614–5615. [Google Scholar] [CrossRef]
- Kato, M.; Kamigaito, M.; Sawamoto, M.; Higashimura, T. Polymerization of methyl methacrylate with the carbon tetrachloride/dichlorotris-(triphenylphosphine) ruthenium (II)/methylaluminum bis (2, 6-di-tert-butylphenoxide) initiating system: Possibility of living radical polymerization. Macromolecules 1995, 28, 1721–1723. [Google Scholar] [CrossRef]
- Patten, T.E.; Xia, J.; Abernathy, T.; Matyjaszewski, K. Polymers with very low polydispersities from atom transfer radical polymerization. Science 1996, 272, 866–869. [Google Scholar] [CrossRef] [PubMed]
- Matyjaszewski, K.; Xia, J. Atom transfer radical polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef] [PubMed]
- Matyjaszewski, K.; Tsarevsky, N.V. Macromolecular engineering by atom transfer radical polymerization. J. Am. Chem. Soc. 2014, 136, 6513–6533. [Google Scholar] [CrossRef] [PubMed]
- Pintauer, T.; Matyjaszewski, K. Atom transfer radical addition and polymerization reactions catalyzed by ppm amounts of copper complexes. Chem. Soc. Rev. 2008, 37, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H. The persistent radical effect: A principle for selective radical reactions and living radical polymerizations. Chem. Rev. 2001, 101, 3581–3610. [Google Scholar] [CrossRef] [PubMed]
- Treat, N.J.; Sprafke, H.; Kramer, J.W.; Clark, P.G.; Barton, B.E.; Read de Alaniz, J.; Fors, B.P.; Hawker, C.J. Metal-free atom transfer radical polymerization. J. Am. Chem. Soc. 2014, 136, 16096–16101. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Lamson, M.; Yan, J.; Matyjaszewski, K. Photoinduced Metal-Free Atom Transfer Radical Polymerization of Acrylonitrile. ACS Macro Lett. 2015, 4, 192–196. [Google Scholar] [CrossRef]
- Magenau, A.J.; Strandwitz, N.C.; Gennaro, A.; Matyjaszewski, K. Electrochemically mediated atom transfer radical polymerization. Science 2011, 332, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Theriot, J.C.; Lim, C.-H.; Yang, H.; Ryan, M.D.; Musgrave, C.B.; Miyake, G.M. Organocatalyzed atom transfer radical polymerization driven by visible light. Science 2016, 352, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Discekici, E.H.; Pester, C.W.; Treat, N.J.; Lawrence, J.; Mattson, K.M.; Narupai, B.; Toumayan, E.P.; Luo, Y.; McGrath, A.J.; Clark, P.G.; et al. Simple Benchtop Approach to Polymer Brush Nanostructures Using Visible-Light-Mediated Metal-Free Atom Transfer Radical Polymerization. ACS Macro Lett. 2016, 5, 258–262. [Google Scholar] [CrossRef]
- Yan, J.; Pan, X.; Schmitt, M.; Wang, Z.; Bockstaller, M.R.; Matyjaszewski, K. Organocatalyzed atom transfer radical polymerization driven by visible light. ACS Macro Lett. 2016, 5, 661–665. [Google Scholar] [CrossRef]
- Kar, M.; Malvi, B.; Das, A.; Panneri, S.; Gupta, S.S. Synthesis and characterization of poly-l-lysine grafted SBA-15 using NCA polymerization and click chemistry. J. Mater. Chem. 2011, 21, 6690. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, L.; Zhang, Z.; Zhou, N.; Cheng, Z.; Zhu, X. Air-tolerantly surface-initiated AGET ATRP mediated by iron catalyst from silica nanoparticles. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 2006–2015. [Google Scholar] [CrossRef]
| Entry | Label | Time (h) | Conv. (%) | Mn,GPC d,e (g/mol) | Ð d,e | Grafting Ratio f (%) |
|---|---|---|---|---|---|---|
| 1 | SBA-PMMA-C1 b | 72.0 | -- | -- | -- | -- |
| 2 | SBA-PMMA-C2 c | 72.0 | -- | -- | -- | -- |
| 3 | SBA-PMMA-1 | 15.0 | 10.8 | 12,800 | 1.24 | 12.5 |
| 4 | SBA-PMMA-2 | 24.0 | 9.4 | 11,100 | 1.29 | 21.6 |
| 5 | SBA-PMMA-3 | 36.0 | 20.0 | 13,300 | 1.28 | 24.6 |
| 6 | SBA-PMMA-4 | 48.0 | 20.7 | 12,800 | 1.24 | 25.0 |
| 7 | SBA-PMMA-5 | 72.0 | 57.6 | 16,700 | 1.27 | 27.6 |
| 8 | SBA-DMAEMA e | 20.0 | 83.4 | 21,100 | 1.83 | 23.8 |
| 9 | SBA-NIPAM e | 18.0 | 85.0 | 13,400 | 2.25 | 14.4 |
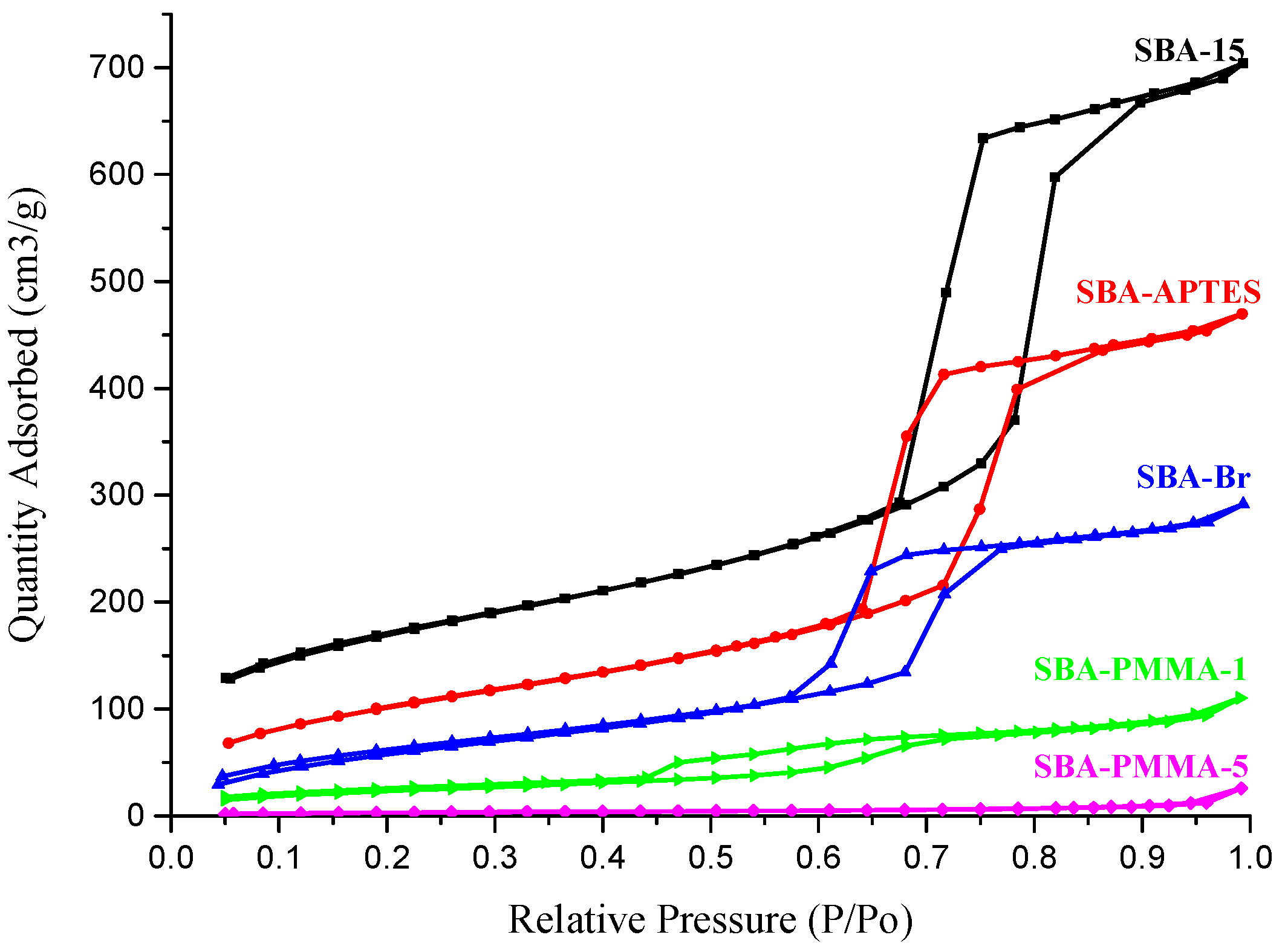
| Entry | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) | Grafting Ratio (%) |
|---|---|---|---|---|
| SBA-15 | 594.4 | 1.09 | 7.33 | - |
| SBA-APTES | 378.3 | 0.73 | 7.68 | - |
| SBA-Br | 239.3 | 0.45 | 7.54 | - |
| SBA-PMMA-1 | 86.8 | 0.17 | 7.86 | 12.5 |
| SBA-PMMA-5 | 11.4 | 0.04 | 14.0 | 27.6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Li, N.; Zhu, J.; Chen, X. Visible Light-Induced Metal Free Surface Initiated Atom Transfer Radical Polymerization of Methyl Methacrylate on SBA-15. Polymers 2017, 9, 58. https://doi.org/10.3390/polym9020058
Ma L, Li N, Zhu J, Chen X. Visible Light-Induced Metal Free Surface Initiated Atom Transfer Radical Polymerization of Methyl Methacrylate on SBA-15. Polymers. 2017; 9(2):58. https://doi.org/10.3390/polym9020058
Chicago/Turabian StyleMa, Liang, Na Li, Jian Zhu, and Xiaodong Chen. 2017. "Visible Light-Induced Metal Free Surface Initiated Atom Transfer Radical Polymerization of Methyl Methacrylate on SBA-15" Polymers 9, no. 2: 58. https://doi.org/10.3390/polym9020058






