Characterization of Comb-Shaped Copolymers by Multidetection SEC, DLS and SANS
Abstract
:1. Introduction
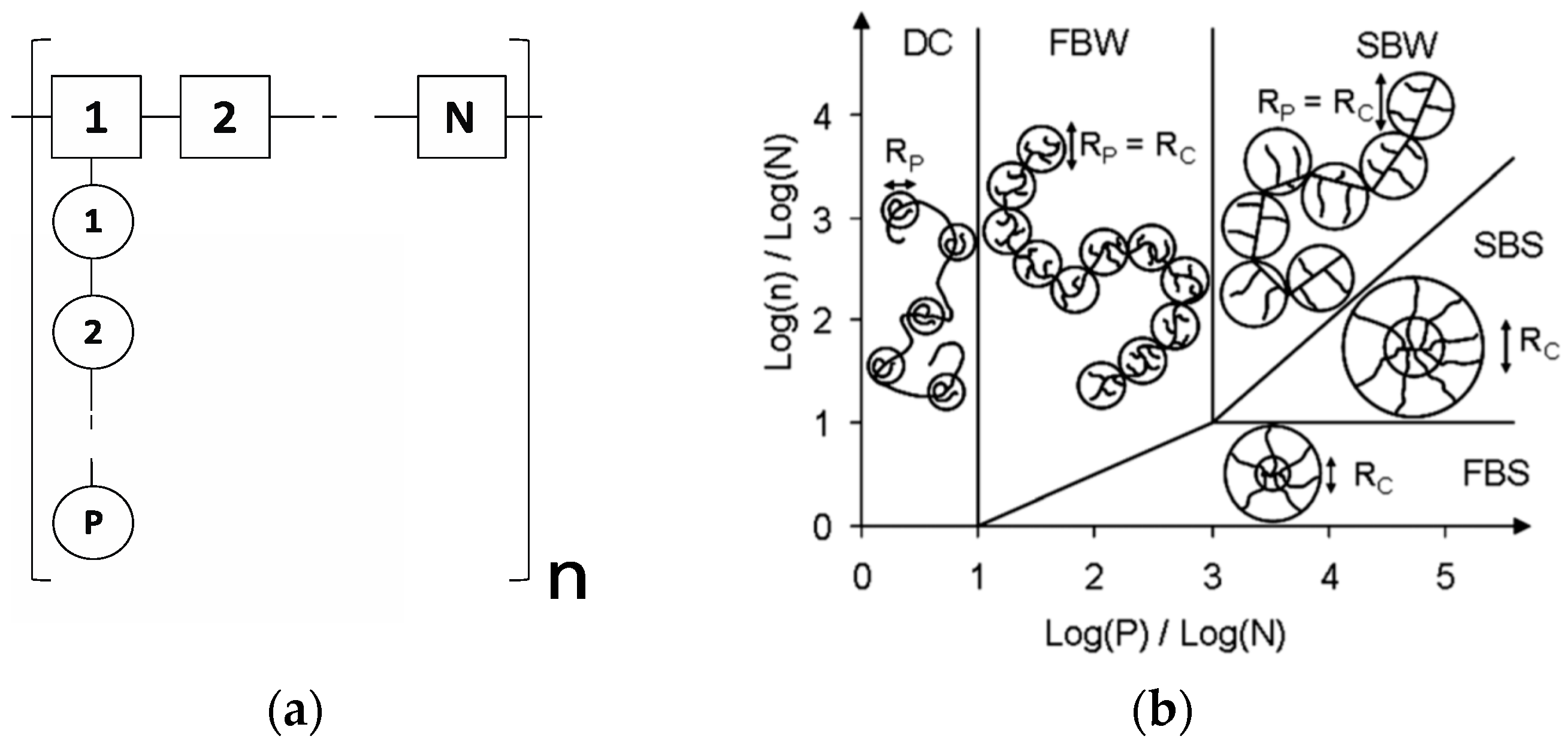
Conformation in Solution: A Theoretical Approach
2. Materials and Methods
2.1. Materials
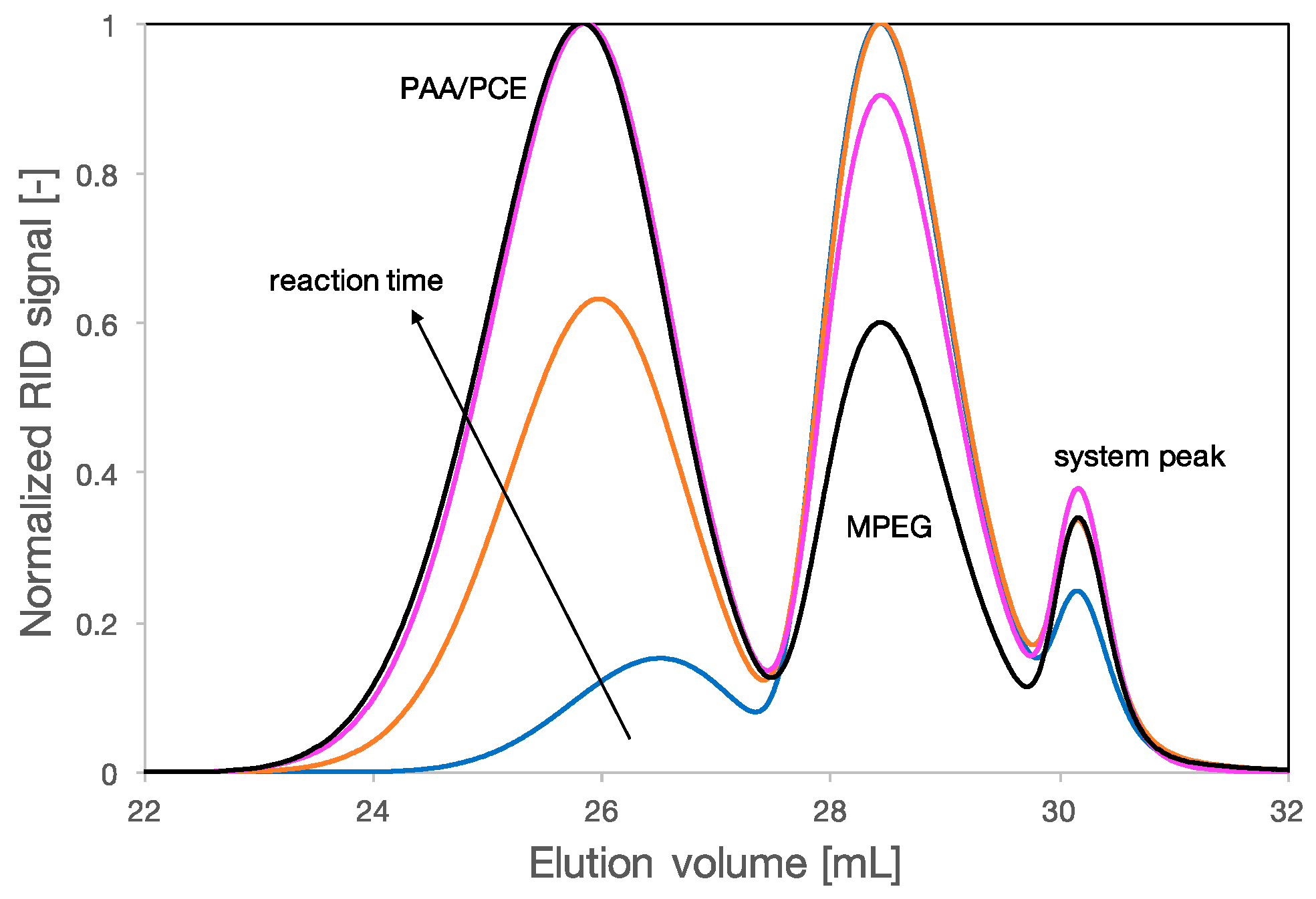
2.2. Synthesis of PCEs
2.3. Proton Nuclear Magnetic Resonance (1H-NMR)
2.4. Multidetection Size Exclusion Chromatography (SEC)
2.5. Dynamic Light Scattering (DLS)
2.6. Small-Angle Neutron Scattering (SANS)
3. Results
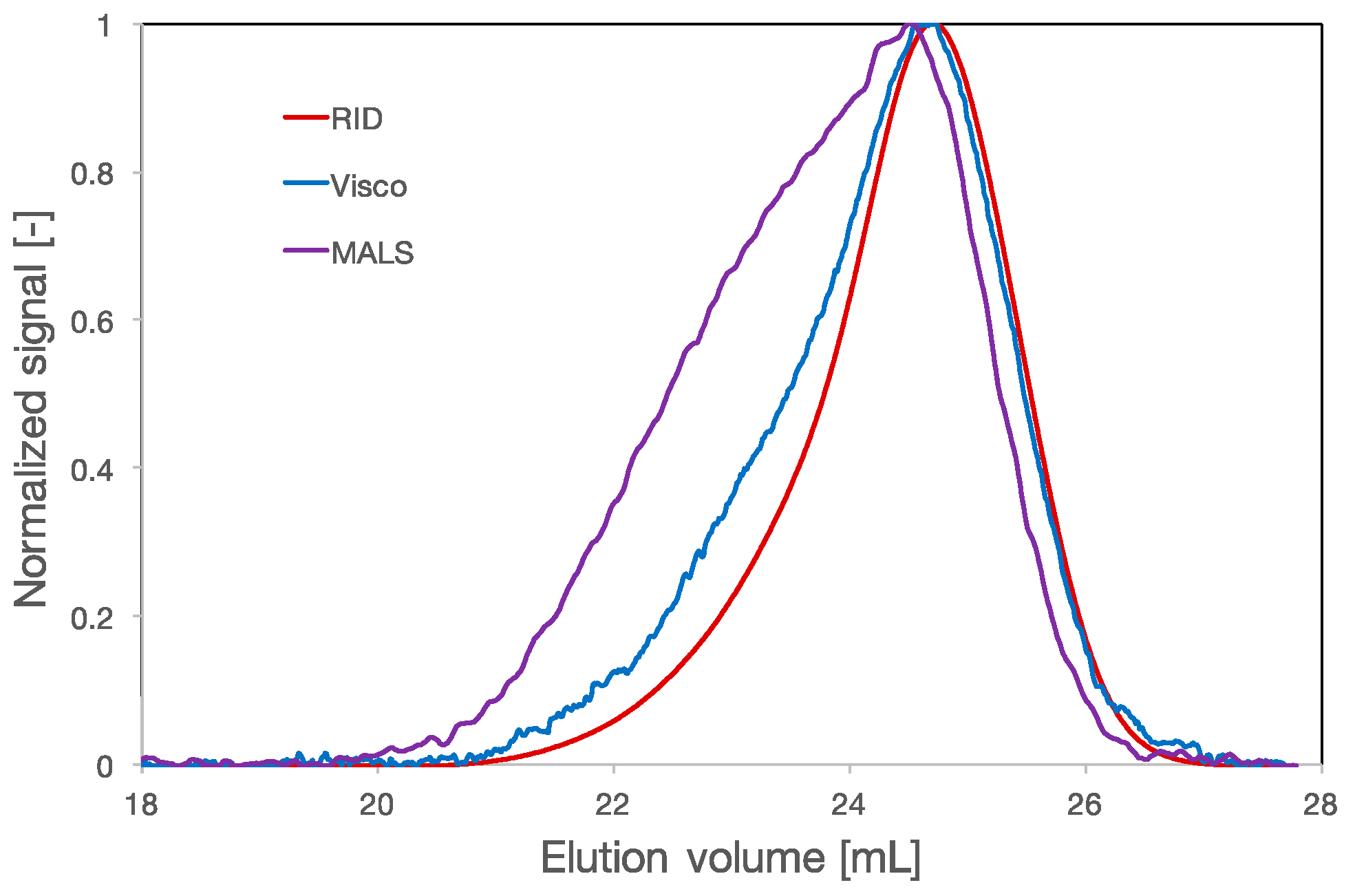
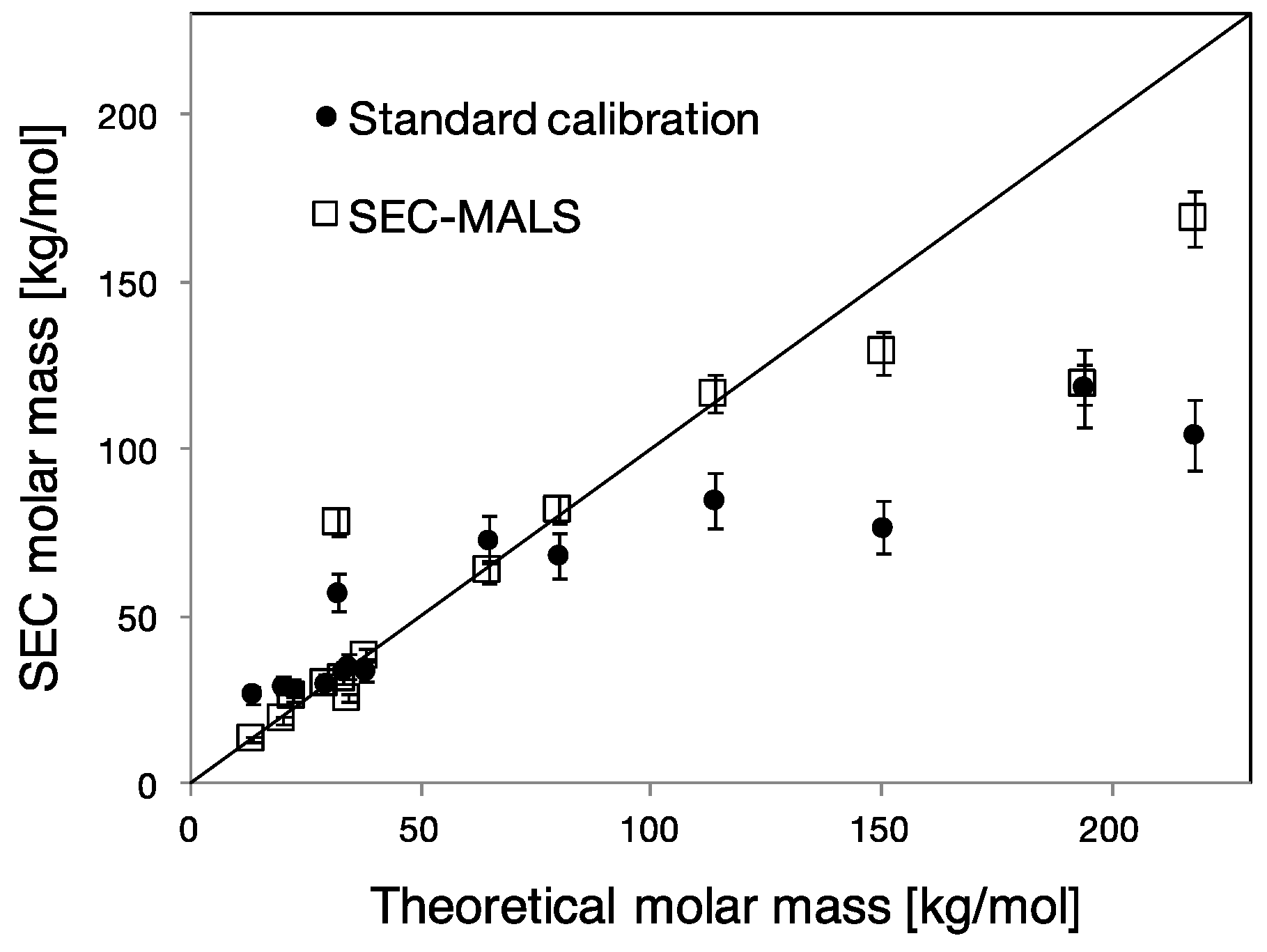
3.1. Multidetection Size Exclusion Chromatography
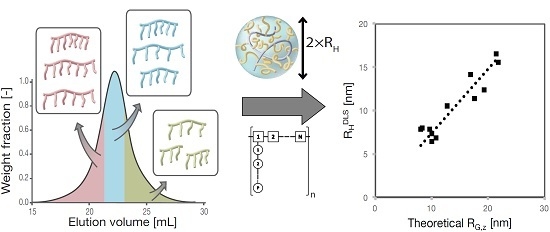
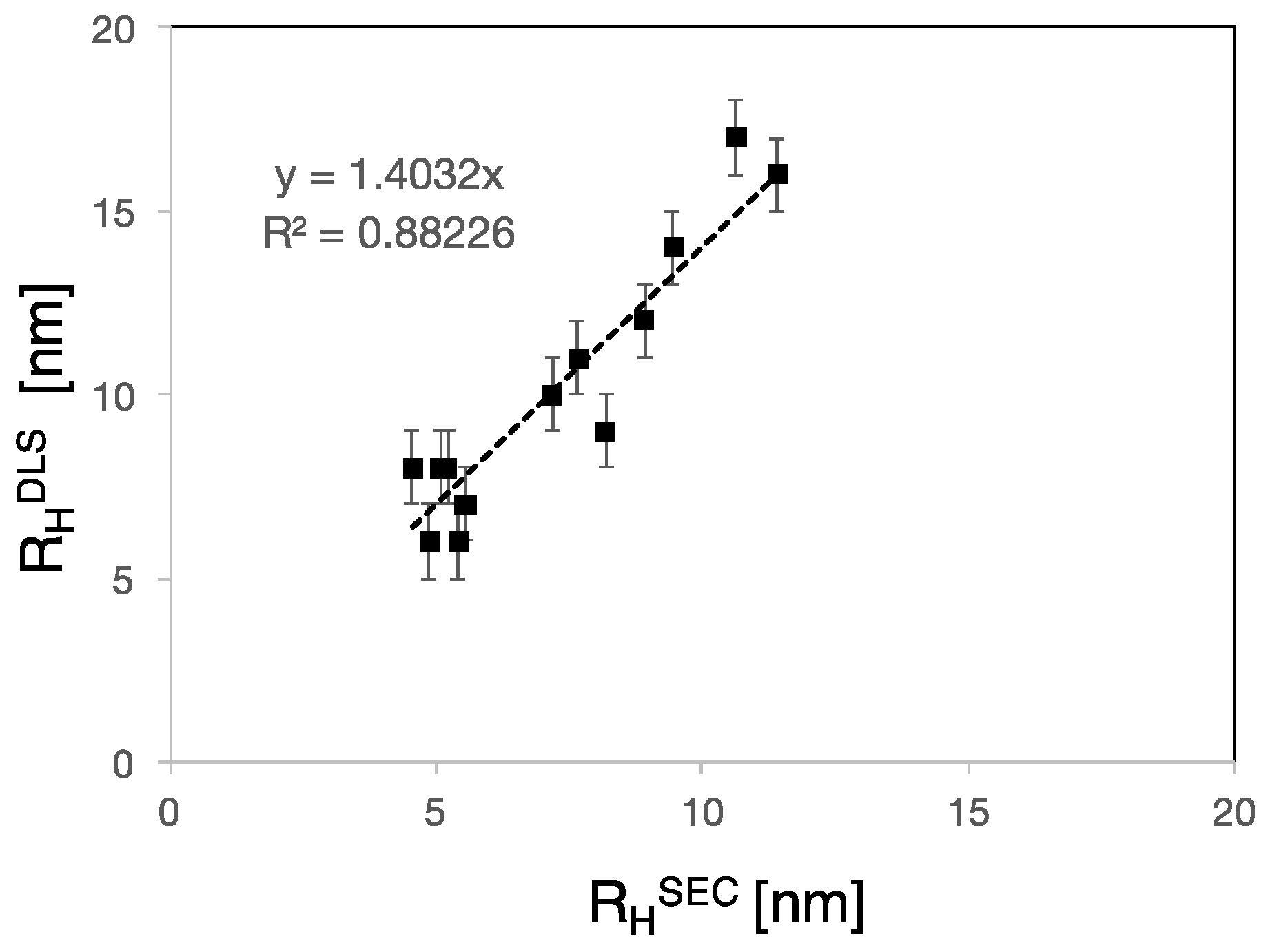
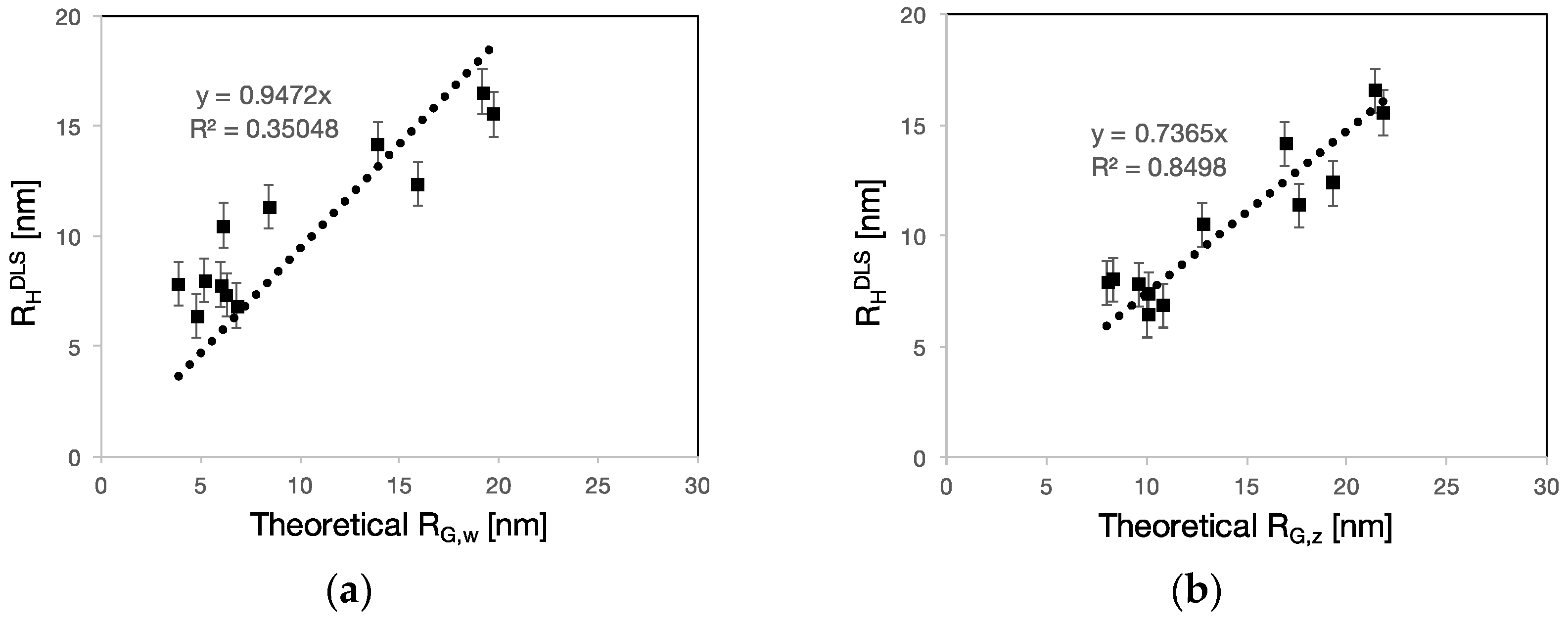
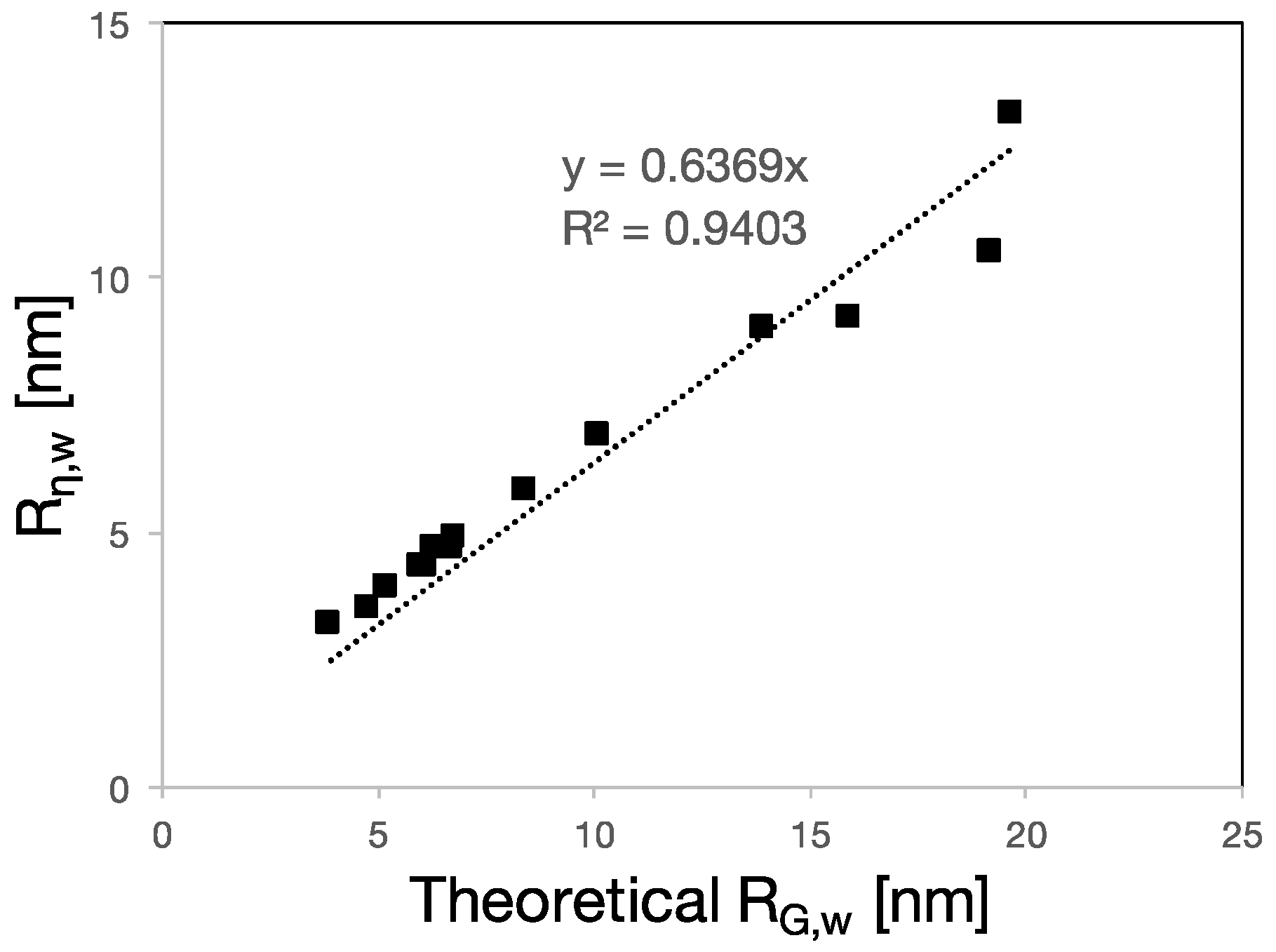
3.2. RH, RG and Rη of Polycarboxylate Ethers
4. Discussion
4.1. Molar Mass Determination of PCEs
4.2. PCE Conformation in Solution
4.3. Impact of Grafting Density on PCE Dispersity
4.4. Proposal for a Better Use of SEC in Characterizing PCEs
4.5. Implications for the Structure–Property Characterization of PCEs
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kirby, G.H.; Lewis, J.A. Comb Polymer Architecture Effects on the Rheological Property Evolution of Concentrated Cement Suspensions. J. Am. Ceram. Soc. 2004, 87, 1643–1652. [Google Scholar] [CrossRef]
- Houst, Y.F.; Bowen, P.; Perche, F.; Kauppi, A.; Borget, P.; Galmiche, L.; Le Meins, J.-F.; Lafuma, F.; Flatt, R.J.; Schober, I.; et al. Design and function of novel superplasticizers for more durable high performance concrete (superplast project). Cem. Concr. Res. 2008, 38, 1197–1209. [Google Scholar] [CrossRef]
- Sakai, E.; Kawakami, A.; Daimon, M. Dispersion mechanisms of comb-type superplasticizers containing grafted poly(ethylene oxide) chains. Macromol. Symp. 2001, 175, 367–376. [Google Scholar] [CrossRef]
- Whitby, C.P.; Scales, P.J.; Grieser, F.; Healy, T.W.; Kirby, G.; Lewis, J.A.; Zukoski, C.F. PAA/PEO comb polymer effects on rheological properties and interparticle forces in aqueous silica suspensions. J. Colloid Interface Sci. 2003, 262, 274–281. [Google Scholar] [CrossRef]
- Peng, J.; Qu, J.; Zhang, J.; Chen, M.; Wan, T. Adsorption characteristics of water-reducing agents on gypsum surface and its effect on the rheology of gypsum plaster. Cem. Concr. Res. 2005, 35, 527–531. [Google Scholar] [CrossRef]
- Kirby, G.H.; Harris, D.J.; Li, Q.; Lewis, J.A. Poly(acrylic acid)–Poly(ethylene oxide) Comb Polymer Effects on BaTiO3 Nanoparticle Suspension Stability. J. Am. Ceram. Soc. 2004, 87, 181–186. [Google Scholar] [CrossRef]
- Kjeldsen, A.M.; Flatt, R.J.; Bergström, L. Relating the molecular structure of comb-type superplasticizers to the compression rheology of MgO suspensions. Cem. Concr. Res. 2006, 36, 1231–1239. [Google Scholar] [CrossRef]
- Spiratos, N.; Pagé, M.; Mailvaganam, N.P.; Malhotra, V.M.; Jolicoeur, C. Superplasticizers for Concrete: Fundamentals, Technology and Practice; Supplementary Cementing Materials for Sustainable Development Inc.: Ottawa, ON, Canada, 2003. [Google Scholar]
- Gelardi, G.; Flatt, R.J. Working mechanisms of water reducers and superplasticizers. In Science and Technology of Concrete Admixtures; Woodhead Publishing: Sawston, UK, 2016; pp. 257–278. [Google Scholar]
- Aïtcin, P.-C. The importance of the water–cement and water–binder ratios. In Science and Technology of Concrete Admixtures; Woodhead Publishing: Sawston, UK, 2016; pp. 3–13. [Google Scholar]
- Flatt, R.J.; Roussel, N.; Cheeseman, C.R. Concrete: An eco material that needs to be improved. J. Eur. Ceram. Soc. 2012, 32, 2787–2798. [Google Scholar] [CrossRef]
- Gelardi, G.; Mantellato, S.; Marchon, D.; Palacios, M.; Eberhardt, A.B.; Flatt, R.J. Chemistry of chemical admixtures. In Science and Technology of Concrete Admixtures; Woodhead Publishing: Sawston, UK, 2016; pp. 149–218. [Google Scholar]
- Habbaba, A.; Lange, A.; Plank, J. Synthesis and performance of a modified polycarboxylate dispersant for concrete possessing enhanced cement compatibility. J. Appl. Polym. Sci. 2013, 129, 346–353. [Google Scholar] [CrossRef]
- Liu, M.; Lei, J.; Du, X.; Huang, B.; Chen, L. Synthesis and properties of methacrylate-based and allylether-based polycarboxylate superplasticizer in cementitious system. J. Sustain. Cem. Based Mater. 2013, 2, 218–226. [Google Scholar] [CrossRef]
- Lei, L.; Plank, J. Synthesis and Properties of a Vinyl Ether-Based Polycarboxylate Superplasticizer for Concrete Possessing Clay Tolerance. Ind. Eng. Chem. Res. 2014, 53, 1048–1055. [Google Scholar] [CrossRef]
- Marchon, D.; Sulser, U.; Eberhardt, A.; Flatt, R.J. Molecular design of comb-shaped polycarboxylate dispersants for environmentally friendly concrete. Soft Matter 2013, 9, 10719. [Google Scholar] [CrossRef]
- Fan, W.; Stoffelbach, F.; Rieger, J.; Regnaud, L.; Vichot, A.; Bresson, B.; Lequeux, N. A new class of organosilane-modified polycarboxylate superplasticizers with low sulfate sensitivity. Cem. Concr. Res. 2012, 42, 166–172. [Google Scholar] [CrossRef]
- Marchon, D. Controlling Cement Hydration through the Molecular Structure of Comb Copolymer Superplasticizers; ETH Zurich: Zurich, Switzerland, 2016. [Google Scholar]
- Marchon, D.; Juilland, P.; Gallucci, E.; Frunz, L.; Flatt, R.J. Molecular and sub-molecular scale effects of comb-copolymers on tri-calcium silicate reactivity: Towards molecular design. J. Am. Ceram. Soc. 2016. [Google Scholar] [CrossRef]
- Striegel, A.; Yau, W.W.; Kirkland, J.J.; Bly, D.D. Modern Size-Exclusion Liquid Chromatography: Practice of Gel Permeation and Gel Filtration Chromatography; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Adler, M.; Rittig, F.; Becker, S.; Pasch, H. Multidimensional Chromatographic and Hyphenated Techniques for Hydrophilic Copolymers, 1. Macromol. Chem. Phys. 2005, 206, 2269–2277. [Google Scholar] [CrossRef]
- Palacios, M.; Puertas, F.; Bowen, P.; Houst, Y.F. Effect of PCs superplasticizers on the rheological properties and hydration process of slag-blended cement pastes. J. Mater. Sci. 2009, 44, 2714–2723. [Google Scholar] [CrossRef]
- Santos, H.; Puertas, F.; Martínez-Ramírez, S.; Palacios, M. Polycarboxylate superplasticiser admixtures: effect on hydration, microstructure and rheological behaviour in cement pastes. Adv. Cem. Res. 2005, 17, 77–89. [Google Scholar]
- Shin, J.-Y.; Hong, J.-S.; Suh, J.-K.; Lee, Y.-S. Effects of polycarboxylate-type superplasticizer on fluidity and hydration behavior of cement paste. Korean J. Chem. Eng. 2008, 25, 1553–1561. [Google Scholar] [CrossRef]
- Yamada, K.; Takahashi, T.; Hanehara, S.; Matsuhisa, M. Effects of the chemical structure on the properties of polycarboxylate-type superplasticizer. Cem. Concr. Res. 2000, 30, 197–207. [Google Scholar] [CrossRef]
- Lacík, I.; Stach, M.; Kasák, P.; Semak, V.; Uhelská, L.; Chovancová, A.; Reinhold, G.; Kilz, P.; Delaittre, G.; Charleux, B.; et al. SEC analysis of poly(acrylic acid) and poly(methacrylic acid). Macromol. Chem. Phys. 2015, 216, 23–37. [Google Scholar] [CrossRef]
- Gay, C.; Raphaël, E. Comb-like polymers inside nanoscale pores. Adv. Colloid Interface Sci. 2001, 94, 229–236. [Google Scholar] [CrossRef]
- Flatt, R.J.; Schober, I.; Raphael, E.; Plassard, C.; Lesniewska, E. Conformation of Adsorbed Comb Copolymer Dispersants. Langmuir 2009, 25, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Flory, P.J. Principles of Polymer Chemistry; The George Fisher Baker Non-Resident Lectureship in Chemistry at Cornell University; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]
- Plank, J.; Sakai, E.; Miao, C.W.; Yu, C.; Hong, J.X. Chemical admixtures—Chemistry, applications and their impact on concrete microstructure and durability. Cem. Concr. Res. A 2015, 78, 81–99. [Google Scholar] [CrossRef]
- Pourchet, S.; Liautaud, S.; Rinaldi, D.; Pochard, I. Effect of the repartition of the PEG side chains on the adsorption and dispersion behaviors of PCP in presence of sulfate. Cem. Concr. Res. 2012, 42, 431–439. [Google Scholar] [CrossRef]
- Strunz, P.; Mortensen, K.; Janssen, S. SANS-II at SINQ: Installation of the former Risø-SANS facility. Phys. B Condens. Matter 2004, 350, E783–E786. [Google Scholar] [CrossRef]
- Breßler, I.; Kohlbrecher, J.; Thünemann, A.F. SASfit: A tool for small-angle scattering data analysis using a library of analytical expressions. J. Appl. Crystallogr. 2015, 48, 1587–1598. [Google Scholar] [CrossRef] [PubMed]
- Giraudeau, C.; D’Espinose De Lacaillerie, J.-B.; Souguir, Z.; Nonat, A.; Flatt, R.J. Surface and Intercalation Chemistry of Polycarboxylate Copolymers in Cementitious Systems. J. Am. Ceram. Soc. 2009, 92, 2471–2488. [Google Scholar] [CrossRef]
- Devanand, K.; Selser, J.C. Asymptotic behavior and long-range interactions in aqueous solutions of poly(ethylene oxide). Macromolecules 1991, 24, 5943–5947. [Google Scholar] [CrossRef]
- Sanson, N.; Bouyer, F.; Destarac, M.; In, M.; Gérardin, C. Hybrid Polyion Complex Micelles Formed from Double Hydrophilic Block Copolymers and Multivalent Metal Ions: Size Control and Nanostructure. Langmuir 2012, 28, 3773–3782. [Google Scholar] [CrossRef] [PubMed]
- Hirzinger, B.; Helmstedt, M.; Stejskal, J. Light scattering studies on core–shell systems: Determination of size parameters of sterically stabilized poly(methylmethacrylate) dispersions. Polymer 2000, 41, 2883–2891. [Google Scholar] [CrossRef]
- Plank, J.; Pöllmann, K.; Zouaoui, N.; Andres, P.R.; Schaefer, C. Synthesis and performance of methacrylic ester based polycarboxylate superplasticizers possessing hydroxy terminated poly(ethylene glycol) side chains. Cem. Concr. Res. 2008, 38, 1210–1216. [Google Scholar] [CrossRef]
- Flatt, R.; Schober, I. Superplasticizers and the rheology of concrete. In Understanding the Rheology of Concrete; Roussel, N., Ed.; Woodhead Publishing Series in Civil and Structural Engineering; Woodhead Publishing: Sawston, UK, 2012; pp. 144–208. [Google Scholar]


| PCE | BB type | MwBB (g/mol) | ĐBB | ĐPCE | nw | N | P | MMtheor. (g/mol) | RG,w (nm) | RG,z (nm) |
|---|---|---|---|---|---|---|---|---|---|---|
| A1 | PAA | 23,000 | 1.8 | 1.6 | 64 | 5.0 | 45 | 150,700 | 15.9 | 19.3 |
| A2 | PAA | 23,000 | 1.8 | 1.5 | 46 | 7.0 | 45 | 114,200 | 13.9 | 16.9 |
| A3 | PAA | 26,000 | 1.3 | 1.5 | 96 | 3.7 | 45 | 218,000 | 19.2 | 21.4 |
| A4 | PAA | 63,000 | 1.3 | 1.3 | 66 | 13.3 | 45 | 194,000 | 19.7 | 21.8 |
| B1 | PMA | 5300 | 9.2 | 1.5 | 15 | 4.2 | 23 | 20,100 | 4.8 | 10.0 |
| B2 | PMA | 9000 | not available | 1.5 | 26 | 4.1 | 23 | 34,500 | 6.7 | - |
| B3 | PMA | 9000 | not available | 1.9 | 24 | 4.4 | 68 | 80,300 | 10.1 | - |
| B4 | PMA | 5300 | 9.2 | 2.0 | 20 | 3.1 | 68 | 65,200 | 8.4 | 17.6 |
| B5 | PMA | 5300 | 9.2 | 1.6 | 8 | 7.3 | 23 | 13,800 | 3.9 | 8.0 |
| B6 | PMA | 5300 | 9.2 | 2.4 | 27 | 2.3 | 23 | 32,200 | 6.1 | 12.8 |
| C1 | PAA | 4600 | 4.0 | 1.4 | 9 | 7.3 | 45 | 22,200 | 5.2 | 8.3 |
| C2 | PAA | 4600 | 4.0 | 1.4 | 12 | 5.2 | 45 | 29,300 | 6.0 | 9.6 |
| C3 | PAA | 4600 | 4.0 | 1.4 | 14 | 4.5 | 45 | 33,100 | 6.3 | 10.1 |
| C4 | PAA | 4600 | 4.0 | 1.5 | 17 | 3.8 | 45 | 38,400 | 6.8 | 10.8 |
| PCE | RHDLS (nm) | RGSANS (nm) | RηSEC (nm) | RGSANS/RHDLS |
|---|---|---|---|---|
| A1 | 12 | 4.8 | 9.2 | 0.40 |
| A2 | 14 | 5.9 | 9.0 | 0.42 |
| A3 | 17 | 6.1 | 10.5 | 0.36 |
| A4 | 16 | 5.6 | 13.2 | 0.35 |
| B1 | 6 | 3.0 | 3.5 | 0.49 |
| B2 | 6 | 2.5 | 4.7 | 0.41 |
| B3 | 9 | 4.7 | 6.9 | 0.52 |
| B4 | 11 | 5.4 | 5.8 | 0.49 |
| B5 | 8 | 3.3 | 3.2 | 0.41 |
| B6 | 10 | 3.1 | 4.3 | 0.31 |
| C1 | 8 | 3.1 | 3.9 | 0.39 |
| C2 | 8 | 3.6 | 4.3 | 0.45 |
| C3 | 7 | 3.6 | 4.7 | 0.52 |
| C4 | 7 | 3.4 | 4.9 | 0.48 |
| PCE structure parameters | Characterization method | Esterification | Copolymeriazation |
|---|---|---|---|
| P | SEC, 1H-NMR | ✔ | ✔ |
| N | 1H-NMR, HPLC, titration | ✔ | ✔ |
| n | SEC | ✔ * | ✗ ** |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gelardi, G.; Sanson, N.; Nagy, G.; Flatt, R.J. Characterization of Comb-Shaped Copolymers by Multidetection SEC, DLS and SANS. Polymers 2017, 9, 61. https://doi.org/10.3390/polym9020061
Gelardi G, Sanson N, Nagy G, Flatt RJ. Characterization of Comb-Shaped Copolymers by Multidetection SEC, DLS and SANS. Polymers. 2017; 9(2):61. https://doi.org/10.3390/polym9020061
Chicago/Turabian StyleGelardi, Giulia, Nicolas Sanson, Gergely Nagy, and Robert J. Flatt. 2017. "Characterization of Comb-Shaped Copolymers by Multidetection SEC, DLS and SANS" Polymers 9, no. 2: 61. https://doi.org/10.3390/polym9020061