Optical Properties and Amplified Spontaneous Emission of Novel MDMO-PPV/C500 Hybrid
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials
2.2. Characterization
3. Results and Discussion
3.1. Optical Properties of the MDMO-PPV
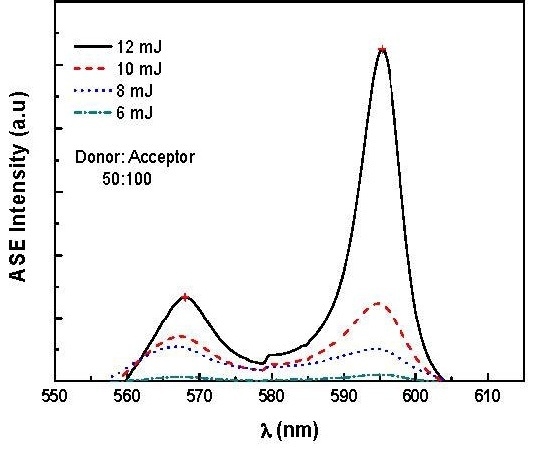
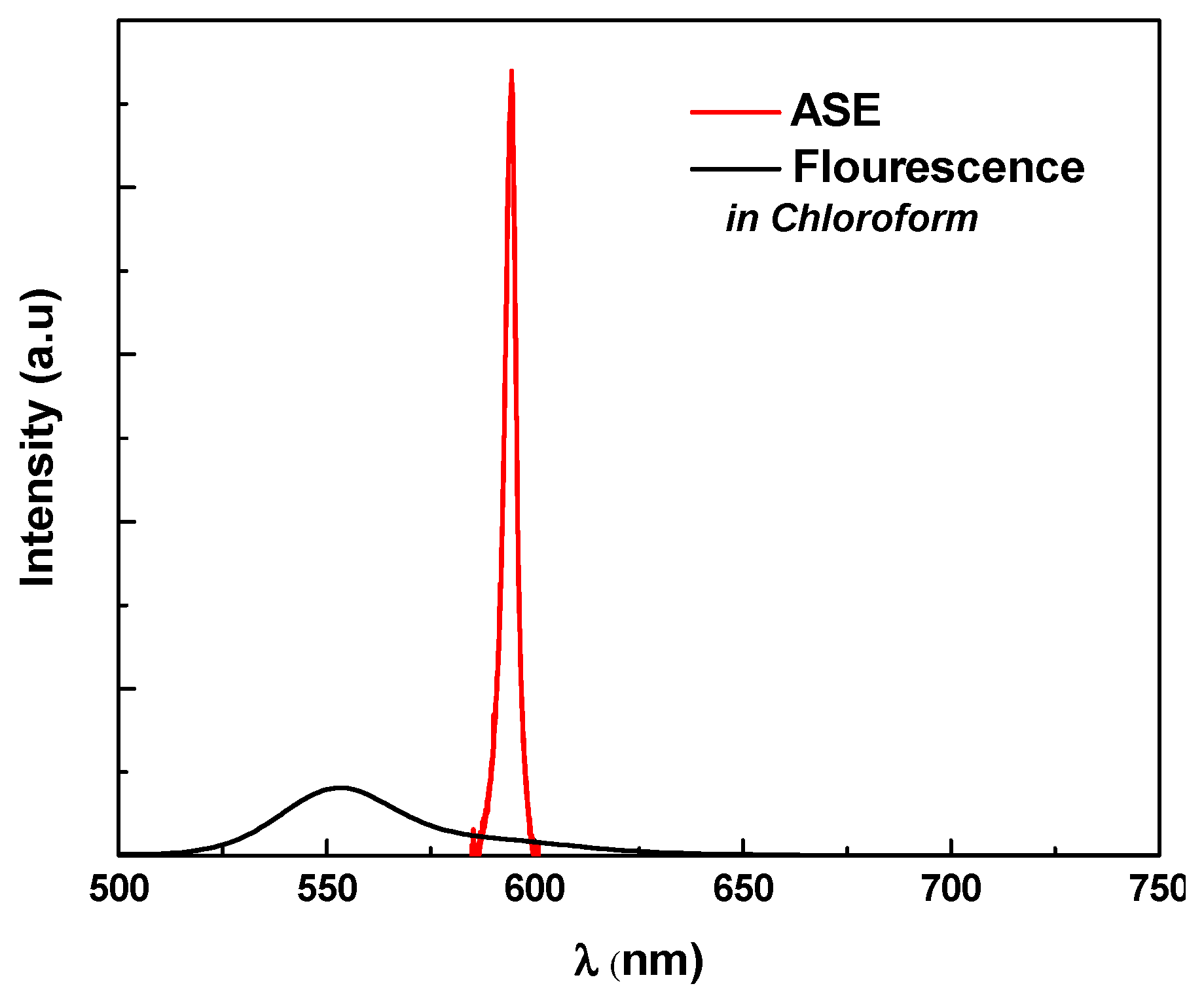
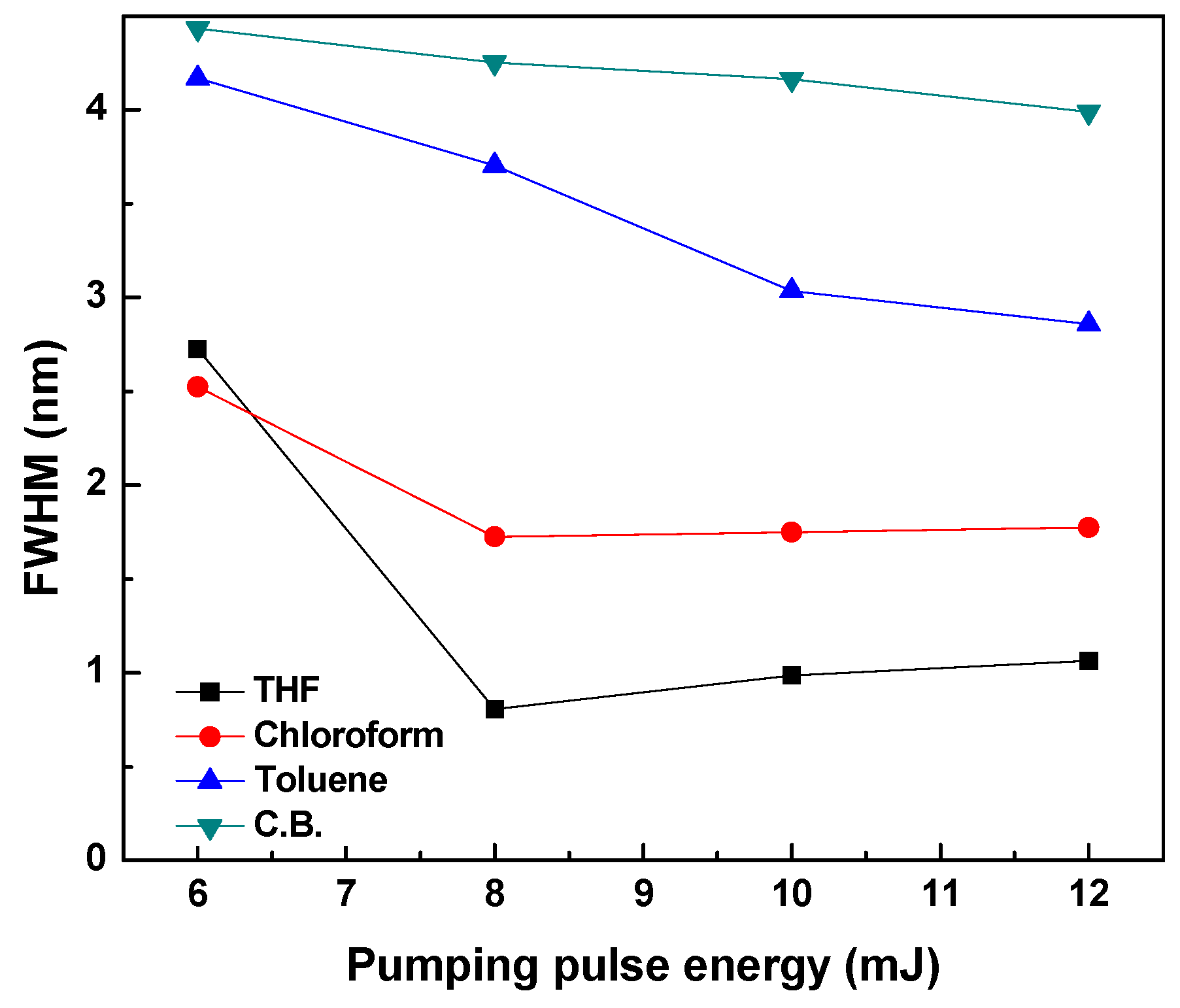
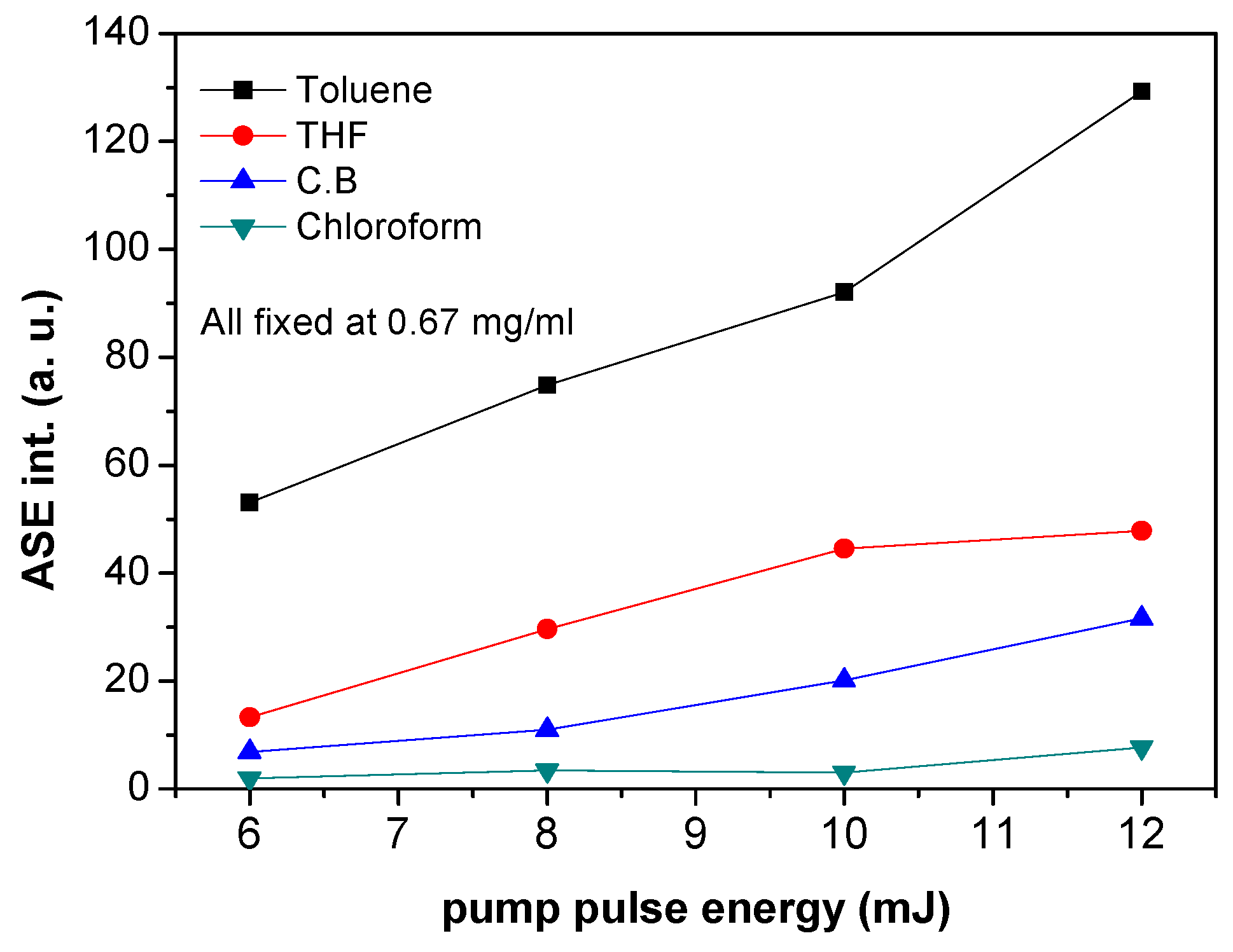
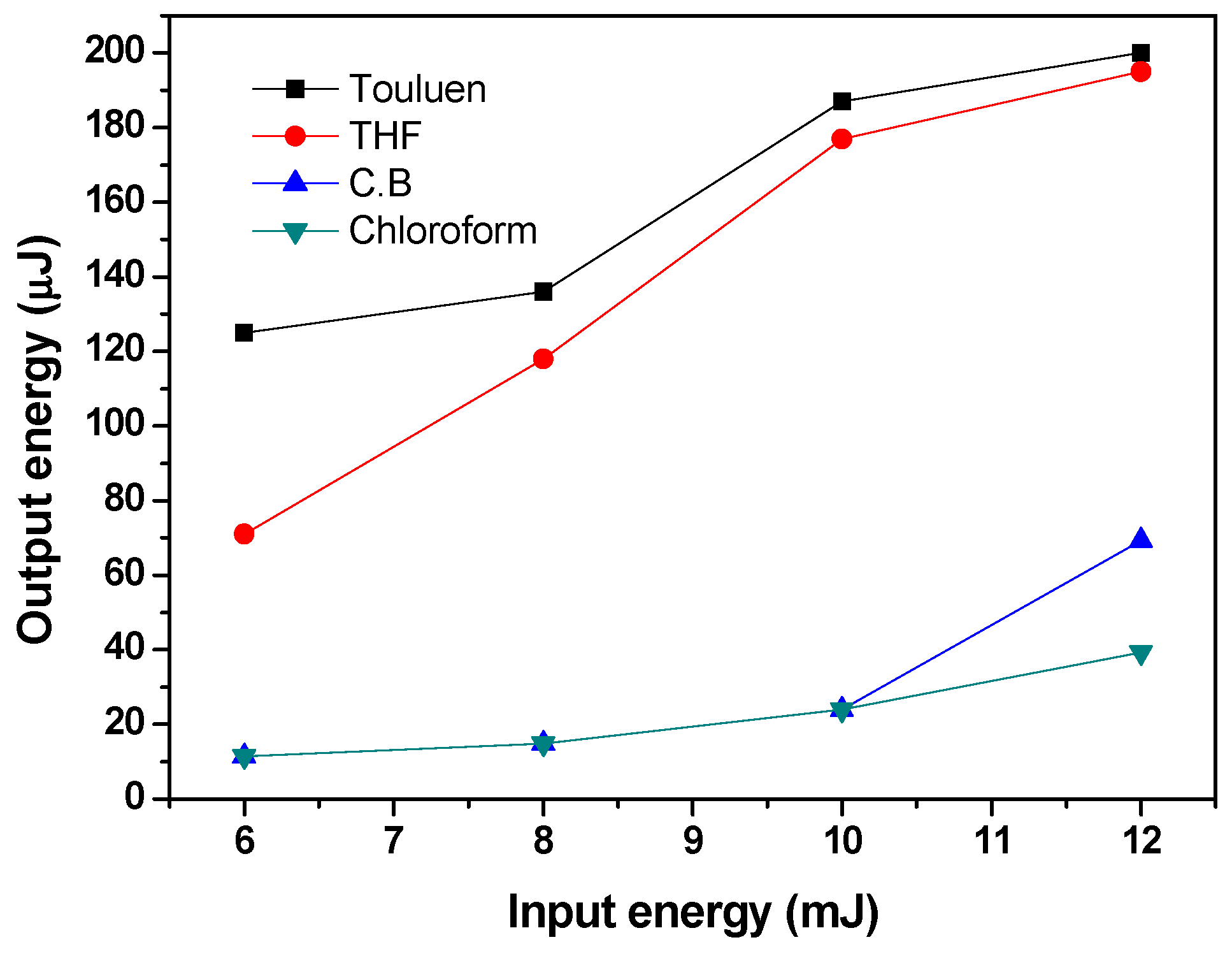
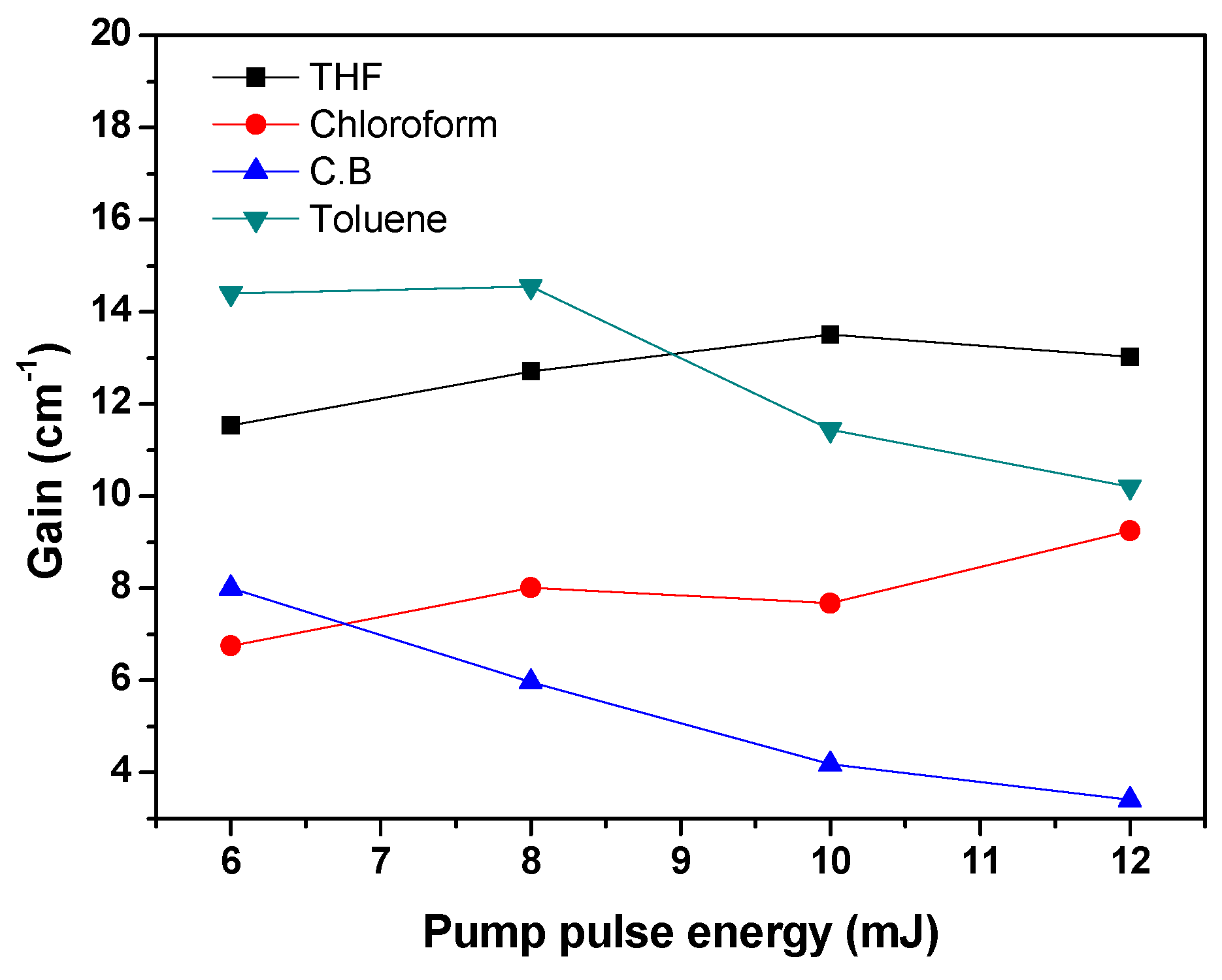
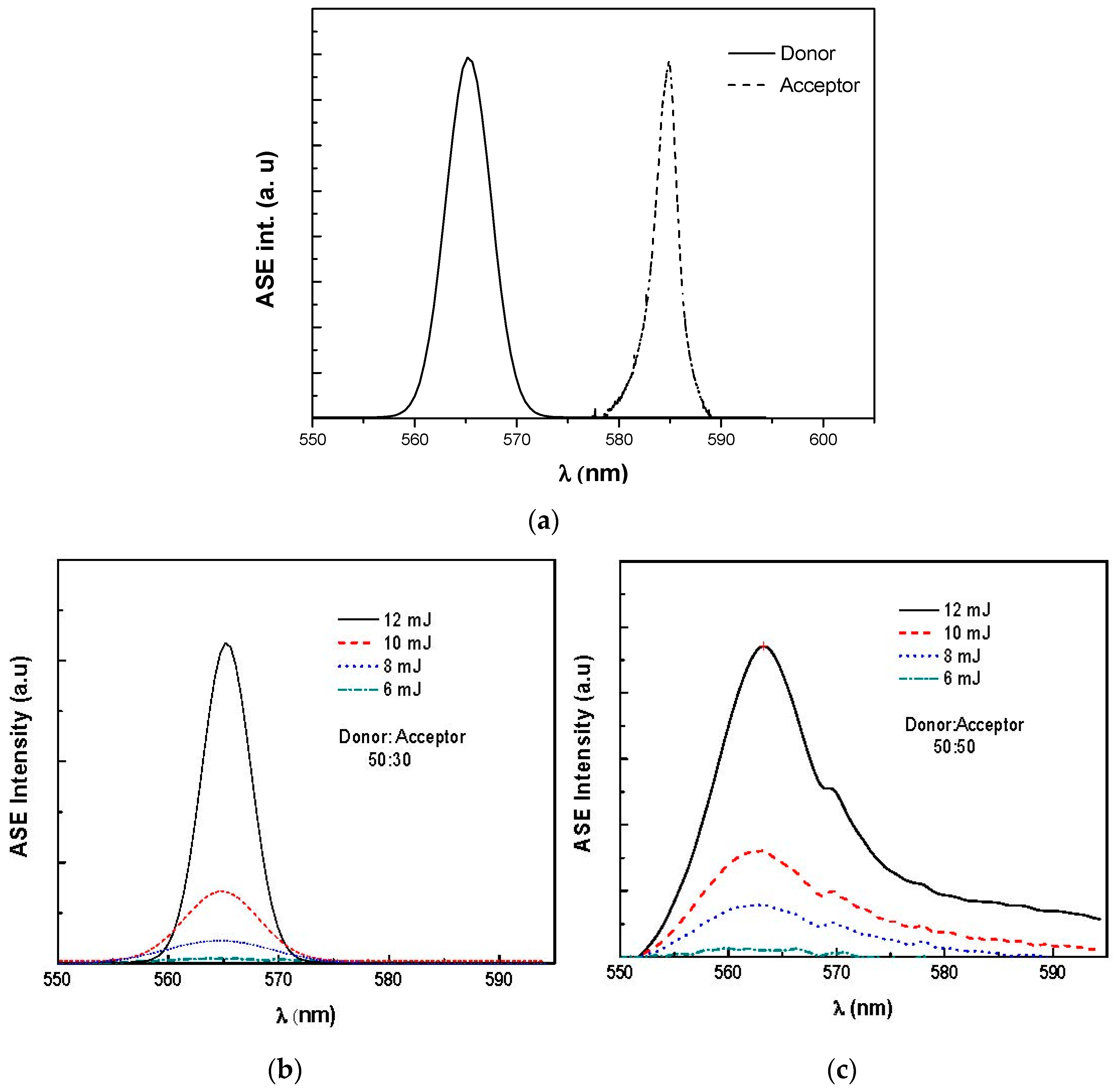
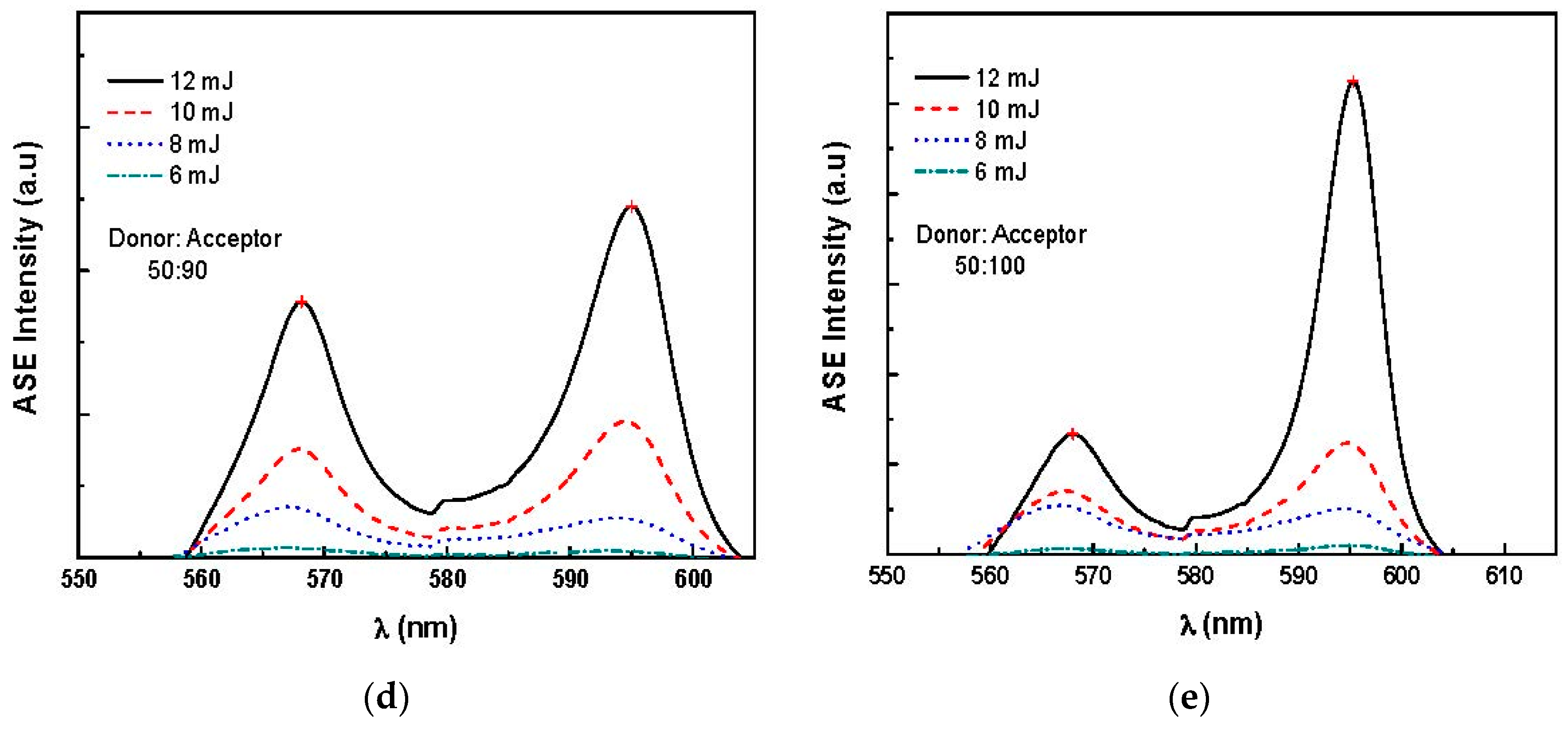
3.2. Amplified Spontaneous Emission and Energy Transfer
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hsiow, C.-Y.; Wang, H.-Y.; Lin, Y.-H.; Raja, R.; Rwei, S.-P.; Chiu, W.-Y.; Dai, C.-A.; Wang, L. Synthesis and characterization of two-dimensional conjugated polymers incorporating electron-deficient moieties for application in organic photovoltaics. Polymers 2016, 8, 382. [Google Scholar] [CrossRef]
- Yiğit, D.; Udum, Y.A.; Güllü, M.; Toppare, L. Electrochemical and optical properties of novel terthienyl based azobenzene, coumarine and fluorescein containing polymers: Multicolored electrochromic polymers. J. Electroanal. Chem. 2014, 712, 215–222. [Google Scholar] [CrossRef]
- Al-Asbahi, B.A.; Jumali, M.H.H. Enhancement of optical properties of poly (9,9′-di-n-octylfluorenyl-2,7-diyl) in conjugated polymer/TiO2 nanocomposites. Can. J. Phys. 2013, 92, 1021–1025. [Google Scholar] [CrossRef]
- Jumali, M.H.H.; Al-Asbahi, B.A.; Yap, C.C.; Salleh, M.M.; Alsalhi, M.S. Optoelectronic property enhancement of conjugated polymer in poly (9,9′-di-n-octylfluorenyl-2.7-diyl)/titania nanocomposites. Thin Solid Films 2012, 524, 257–262. [Google Scholar] [CrossRef]
- Braun, D.; Heeger, A.J. Visible light emission from semiconducting polymer diodes. Appl. Phys. Lett. 1991, 58, 1982–1984. [Google Scholar] [CrossRef]
- Al-Asbahi, B.A.; Jumali, M.H.H.; Yap, C.C.; Salleh, M.M. Influence of TiO2 nanoparticles on enhancement of optoelectronic properties of PFO-based light emitting diode. J. Nanomater. 2013, 2013, 130. [Google Scholar] [CrossRef]
- Chen, L.; McBranch, D.W.; Wang, H.-L.; Helgeson, R.; Wudl, F.; Whitten, D.G. Highly sensitive biological and chemical sensors based on reversible fluorescence quenching in a conjugated polymer. Proc. Natl. Acad. Sci. USA 1999, 96, 12287–12292. [Google Scholar] [CrossRef] [PubMed]
- Ryskulova, K.; Rao Gulur Srinivas, A.; Kerr-Phillips, T.; Peng, H.; Barker, D.; Travas-Sejdic, J.; Hoogenboom, R. Multiresponsive behavior of functional poly(p-phenylene vinylene)s in water. Polymers 2016, 8, 365. [Google Scholar] [CrossRef]
- Sariciftci, N.S. Polymeric photovoltaic materials. Curr. Opin. Solid State Mater. Sci. 1999, 4, 373–378. [Google Scholar] [CrossRef]
- Pei, Q.; Yu, G.; Zhang, C.; Yang, Y.; Heeger, A.J. Polymer light-emitting electrochemical cells. Science 1995, 269, 1086. [Google Scholar] [CrossRef] [PubMed]
- AlTal, F.; Gao, J. Charging and discharging of a planar polymer light-emitting electrochemical cell. Electrochim. Acta 2016, 220, 529–535. [Google Scholar] [CrossRef]
- Friend, R.; Gymer, R.; Holmes, A.; Burroughes, J.; Marks, R.; Taliani, C.; Bradley, D.; Dos Santos, D.; Bredas, J.; Lögdlund, M. Electroluminescence in conjugated polymers. Nature 1999, 397, 121–128. [Google Scholar] [CrossRef]
- Al-Asbahi, B.; Haji Jumali, M.; AlSalhi, M. Enhanced optoelectronic properties of PFO/Fluorol 7GA hybrid light emitting diodes via additions of TiO2 nanoparticles. Polymers 2016, 8, 334. [Google Scholar] [CrossRef]
- Holzer, W.; Penzkofer, A.; Pertsch, T.; Danz, N.; Bräuer, A.; Kley, E.; Tillmann, H.; Bader, C.; Hörhold, H.-H. Corrugated neat thin-film conjugated polymer distributed-feedback lasers. Appl. Phys. B 2002, 74, 333–342. [Google Scholar] [CrossRef]
- Mujamammi, W.; Prasad, S.; AlSalhi, M.; Masilamani, V. Relaxation oscillation with picosecond spikes in a conjugated polymer laser. Polymers 2016, 8, 364. [Google Scholar] [CrossRef]
- Nguyen, T.-Q.; Doan, V.S.; Benjamin, J. Conjugated polymer aggregates in solution: Control of interchain interactions. J. Chem. Phys. 1999, 8, 4068–4078. [Google Scholar]
- Stryer, L.; Haugland, R.P. Energy transfer: A spectroscopic ruler. Proc. Natl. Acad. Sci. USA 1967, 58, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, A.; Buckley, A.; Fox, A.; Bacher, A.; Bradley, D.; Samuel, I. Efficient energy transfer in organic thin films—Implications for organic lasers. J. Appl. Phys. 2002, 92, 6367–6371. [Google Scholar] [CrossRef]
- Kozlov, V.; Bulovic, V.; Burrows, P.; Baldo, M.; Khalfin, V.; Parthasarathy, G.; Forrest, S.; You, Y.; Thompson, M. Study of lasing action based on förster energy transfer in optically pumped organic semiconductor thin films. J. Appl. Phys. 1998, 84, 4096–4108. [Google Scholar] [CrossRef]
- Peter, J.; Vallabhan, C.P.G.; Radhakrishnan, P.; Nampoori, V.P.N.; Kailasnath, M. Ase and photostability measurements in dye doped step index, graded index and hollow polymer optical fiber. Opt. Laser Technol. 2014, 63, 34–38. [Google Scholar] [CrossRef]
- Chen, F.; Nunzi, J.-M. Distributed feedback laser action in reflection geometry from a dye-doped polymer film. Opt. Mater. 2012, 34, 1415–1418. [Google Scholar] [CrossRef]
- Nguyen, T.-Q.; Martini, I.B.; Liu, J.; Schwartz, B.J. Controlling interchain interactions in conjugated polymers: The effects of chain morphology on exciton-exciton annihilation and aggregation in MEH-PPV films. J. Phys. Chem. B 2000, 104, 237–255. [Google Scholar] [CrossRef]
- Quan, S.; Teng, F.; Xu, Z.; Qian, L.; Hou, Y.; Wang, Y.; Xu, X. Solvent and concentration effects on fluorescence emission in MEH-PPV solution. Eur. Polym. J 2006, 42, 228–233. [Google Scholar] [CrossRef]
- Reufer, M.; Feldmann, J.; Rudati, P.; Ruhl, A.; Müller, D.; Meerholz, K.; Karnutsch, C.; Gerken, M.; Lemmer, U. Amplified spontaneous emission in an organic semiconductor multilayer waveguide structure including a highly conductive transparent electrode. Appl. Phys. Lett. 2005, 86, 221102. [Google Scholar] [CrossRef]
- Sabry, M.; Ebeid, E.; Issa, Y.; El-Daly, S. Fluorescence and laser activity of some pyrazinyl schiff-base derivatives. J. Chim. Phys. 1989, 86, 2162–2172. [Google Scholar]
- Fangzhong, S.; Feng, H.; Dan, L.; Zengqi, X.; Weijie, X.; Yuguang, M.; Bin, H. Bright and colour stable white polymer light-emitting diodes. Semicond. Sci. Technol. 2006, 21, L16. [Google Scholar]
- Joseph, R.L. Principles of Fluorescence Spectroscopy; Springer: New York, NY, USA, 2006. [Google Scholar]
- Heliotis, G.; Bradley, D.D.; Turnbull, G.A.; Samuel, I.D. Light amplification and gain in polyfluorene waveguides. Appl. Phys. Lett. 2002, 81, 415–417. [Google Scholar] [CrossRef]
- Fakis, M.; Polyzos, I.; Tsigaridas, G.; Giannetas, V.; Persephonis, P.; Spiliopoulos, I.; Mikroyannidis, J. Laser action of two conjugated polymers in solution and in solid matrix: The effect of aggregates on spontaneous and stimulated emission. Phys. Rev. B 2002, 65, 195203. [Google Scholar] [CrossRef]
- Soukoulis, C.M. Photonic Crystals and Light Localization in the 21st Century; Springer Science & Business Media: Berlin, Germany, 2012; Volume 563. [Google Scholar]
- Camposeo, A.; Mele, E.; Persano, L.; Pisignano, D.; Cingolani, R. Role of doping concentration on the competition between amplified spontaneous emission and nonradiative energy transfer in blends of conjugated polymers. Phys. Rev. B 2006, 16, 165201. [Google Scholar] [CrossRef]
- Al-Asbahi, B.A.; Jumali, M.H.H.; Yap, C.C.; Salleh, M.M.; AlSalhi, M.S. Inhibition of dark quenching by TiO2 nanoparticles content in novel PFO/Fluorol 7GA hybrid: A new role to improve OLED performance. Chem. Phys. Lett. 2013, 570, 109–112. [Google Scholar] [CrossRef]
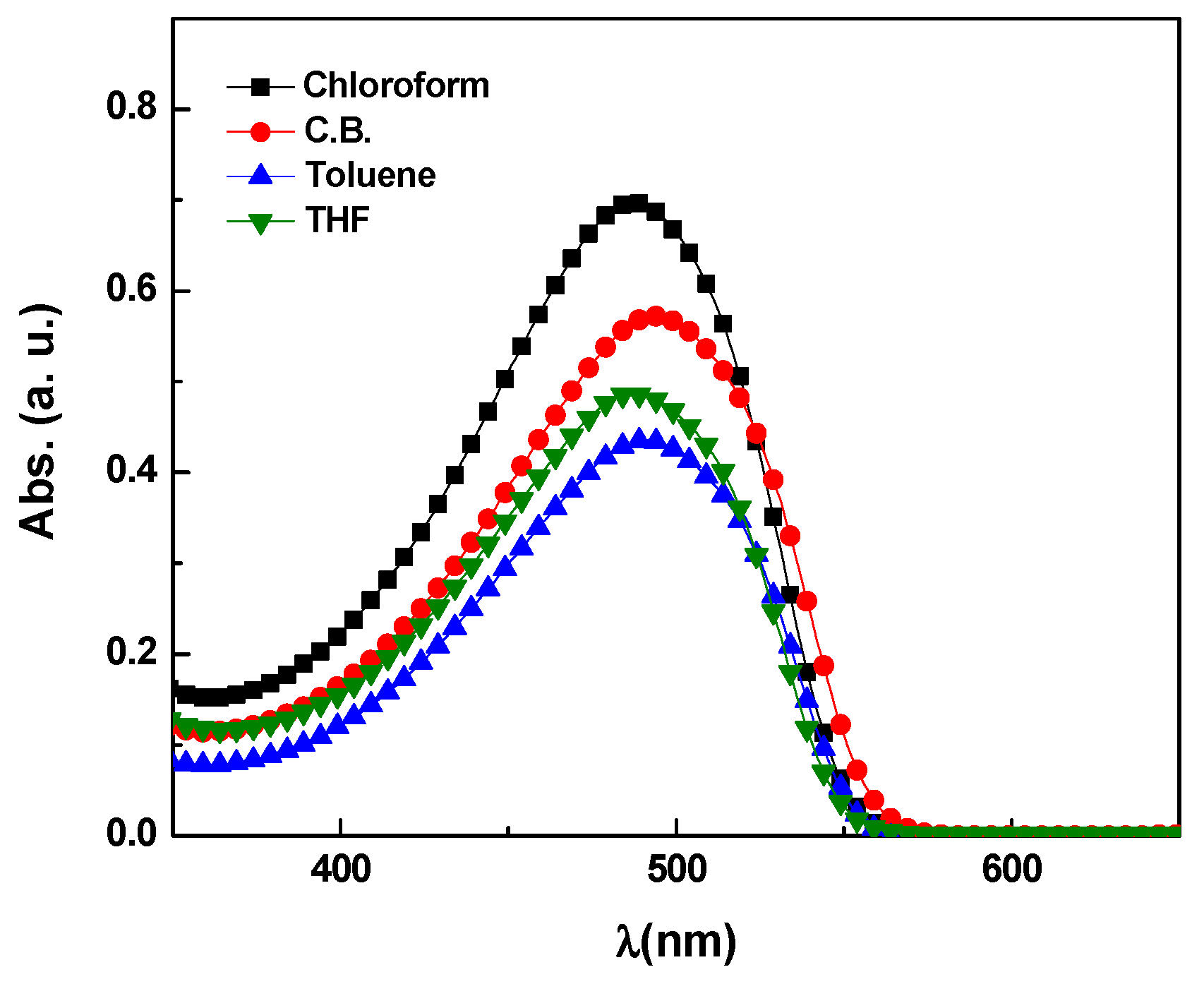


| Solvent | Quantum yield % | Polarity index | εmax × 107 (M·cm)−1 | σa × 10−14 (cm2) | σe × 10−16 (cm2) |
|---|---|---|---|---|---|
| Toluene | 40.36 | 2.3 | 1.36 | 0.20 | 1.83 |
| CB | 33.02 | 2.7 | 2.23 | 0.26 | 2.87 |
| THF | 33.63 | 4.2 | 2.96 | 0.22 | 2.64 |
| Chloroform | 25.55 | 4.4 | 3.96 | 0.32 | 4.23 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abumosa, R.A.; Al-Asbahi, B.A.; AlSalhi, M.S. Optical Properties and Amplified Spontaneous Emission of Novel MDMO-PPV/C500 Hybrid. Polymers 2017, 9, 71. https://doi.org/10.3390/polym9020071
Abumosa RA, Al-Asbahi BA, AlSalhi MS. Optical Properties and Amplified Spontaneous Emission of Novel MDMO-PPV/C500 Hybrid. Polymers. 2017; 9(2):71. https://doi.org/10.3390/polym9020071
Chicago/Turabian StyleAbumosa, Rasha A., Bandar Ali Al-Asbahi, and Mohamad Saleh AlSalhi. 2017. "Optical Properties and Amplified Spontaneous Emission of Novel MDMO-PPV/C500 Hybrid" Polymers 9, no. 2: 71. https://doi.org/10.3390/polym9020071