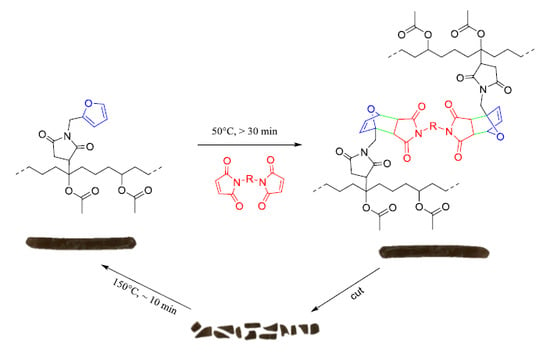
Thermoreversible Cross-Linking of Furan-Containing Ethylene/Vinyl Acetate Rubber with Bismaleimide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
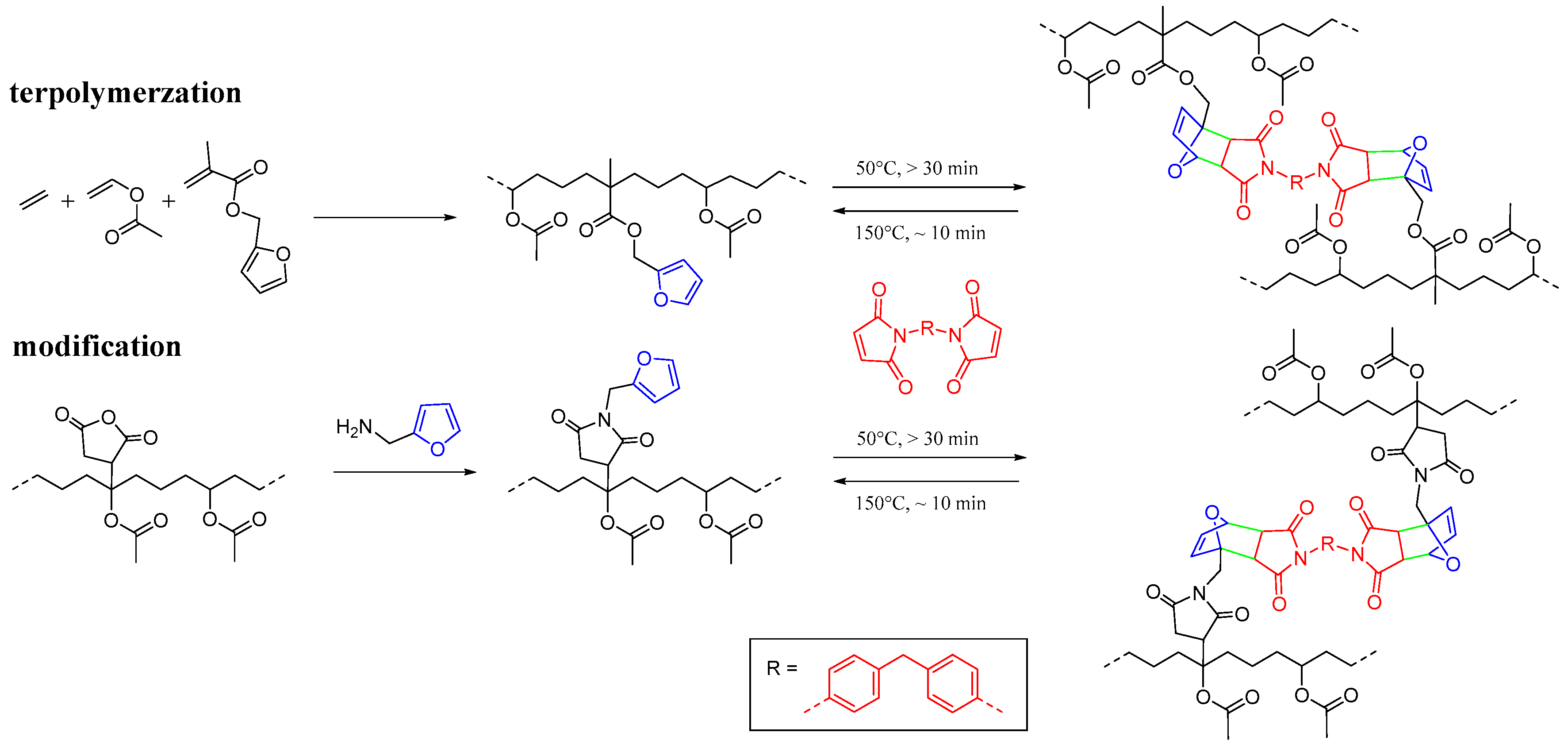
2.2.1. Terpolymerization of Ethylene, Vinyl Acetate, and Furfuryl Methacrylate
2.2.2. Furan Functionalization of EVA-g-MA
2.2.3. Diels–Alder Cross-Linking
2.2.4. Peroxide Curing of EVA
2.2.5. Compounding of EVA-g-Furan
2.3. Characterization
3. Results
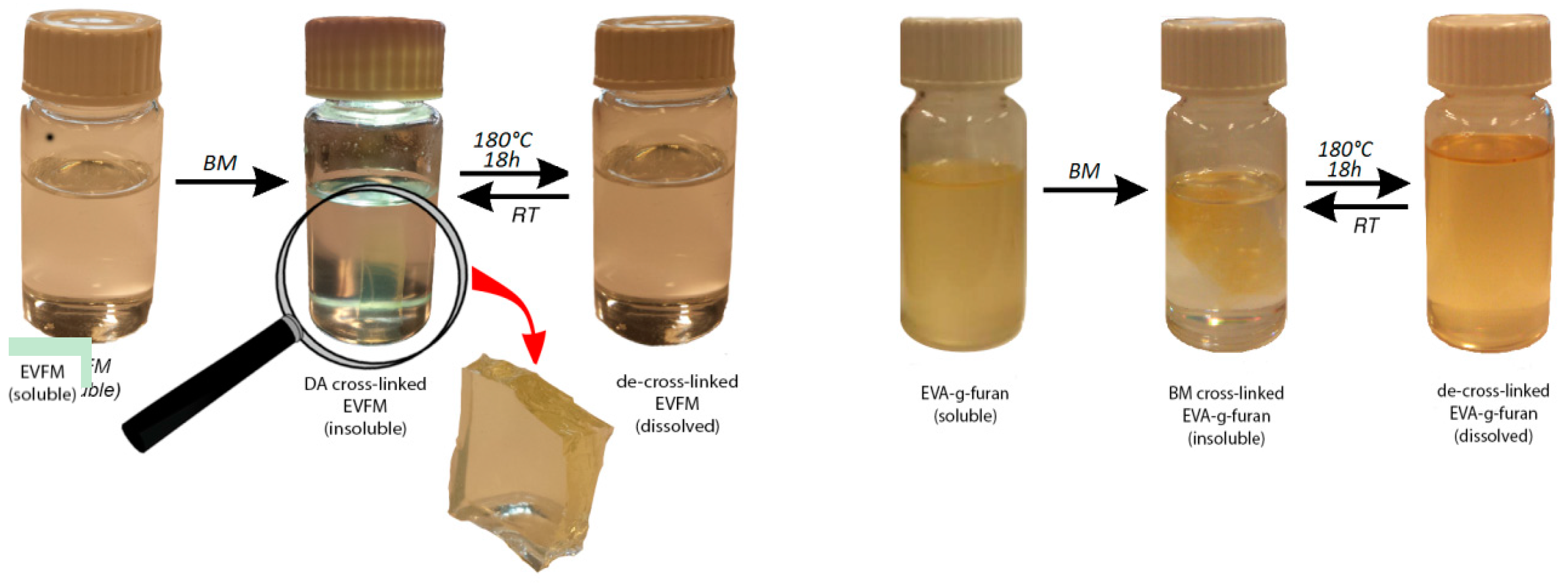
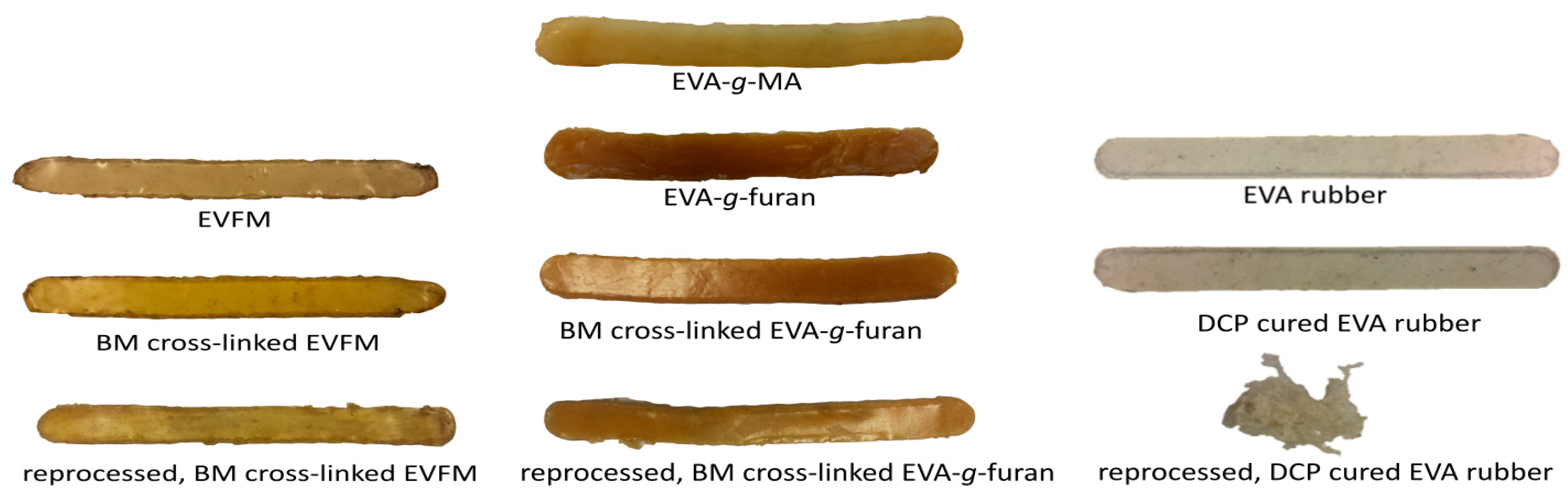
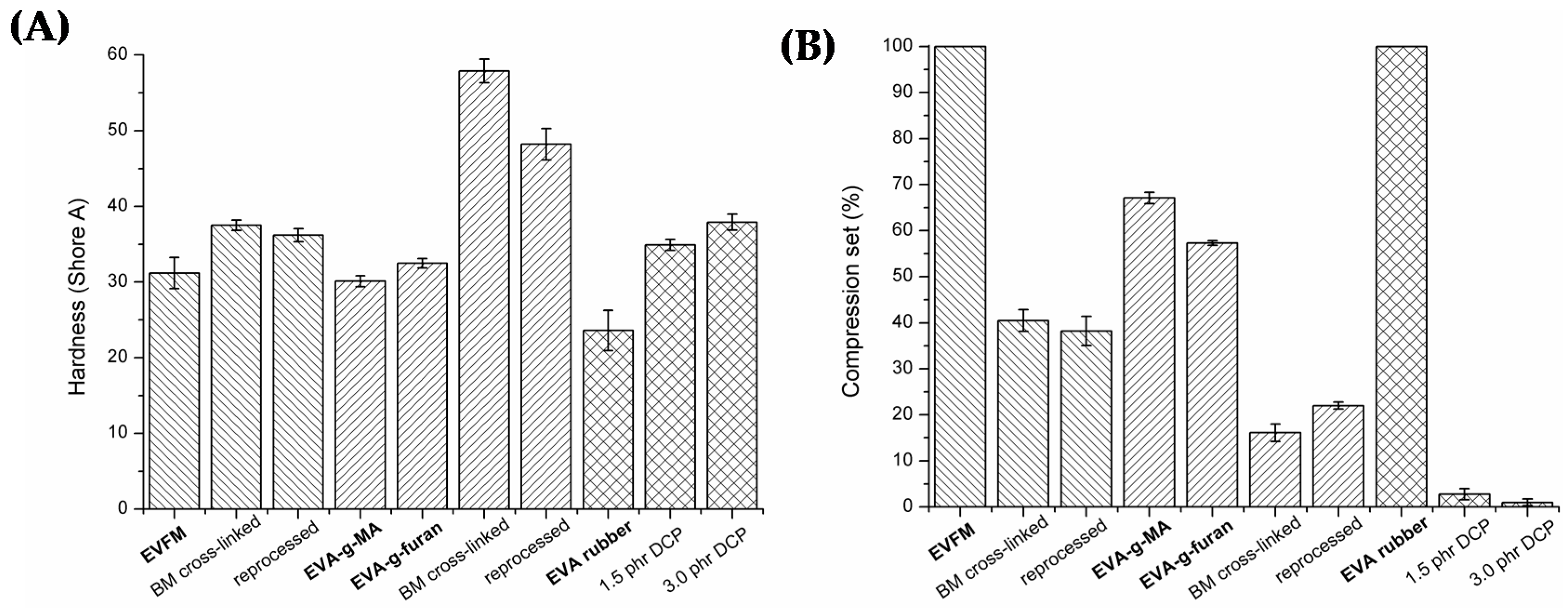
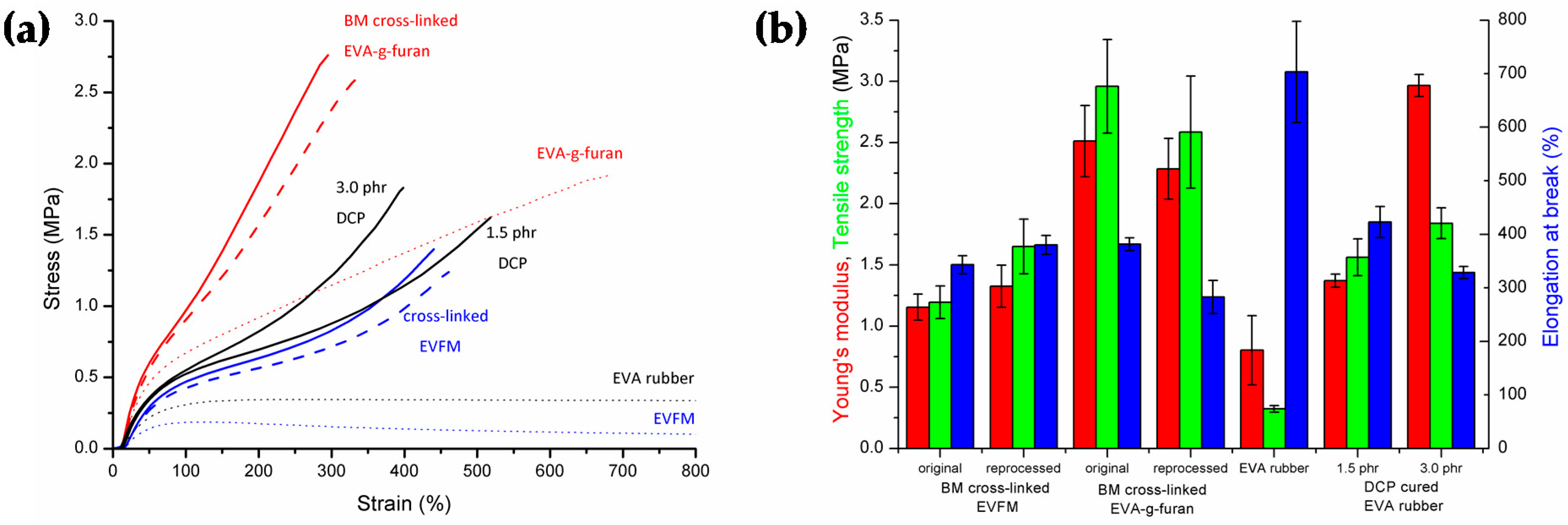
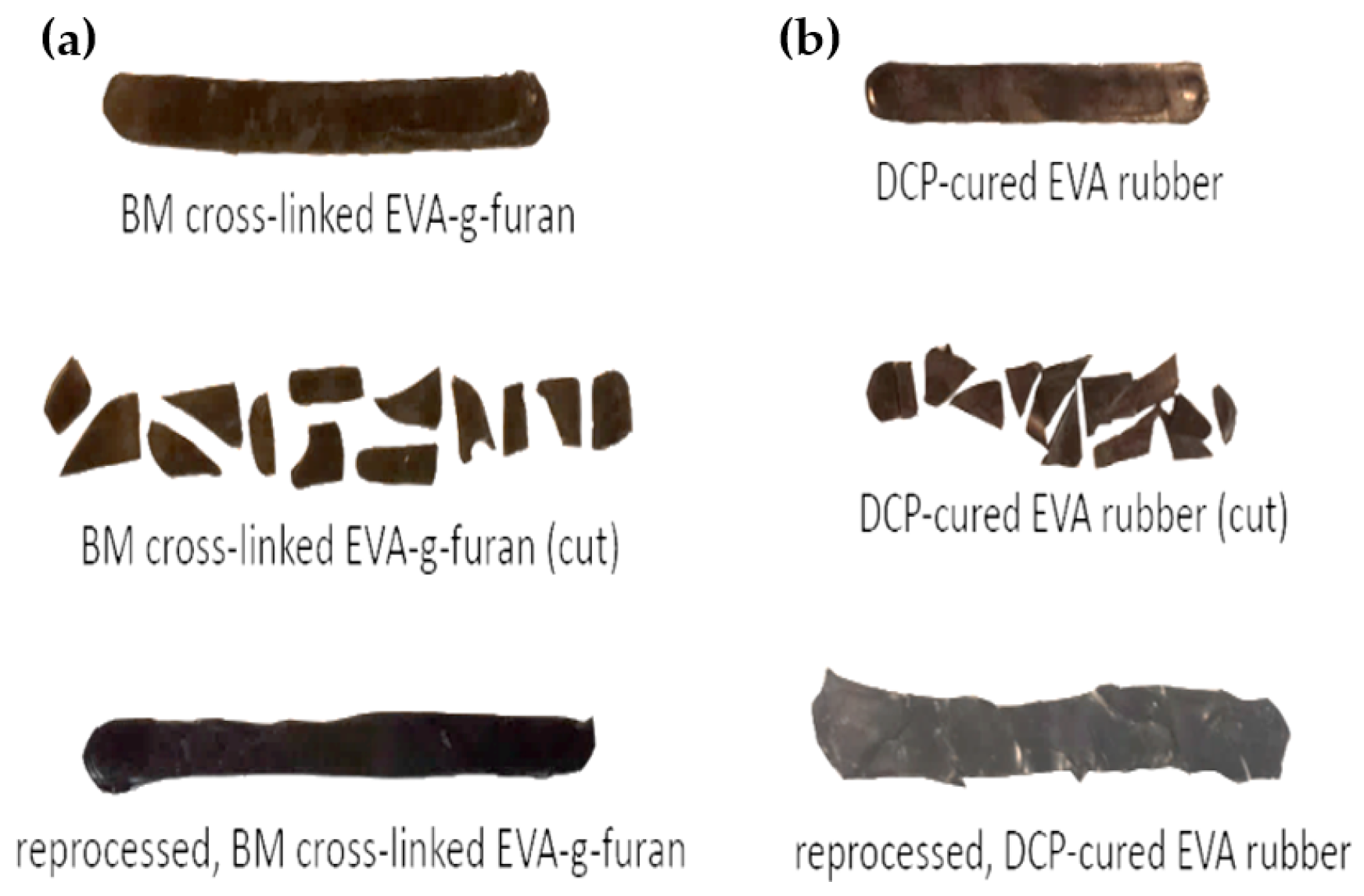
3.1. Characteristics of (Cross-Linked) EVA Gum Rubber
3.2. Properties of (Cross-Linked) EVA Gum Rubber
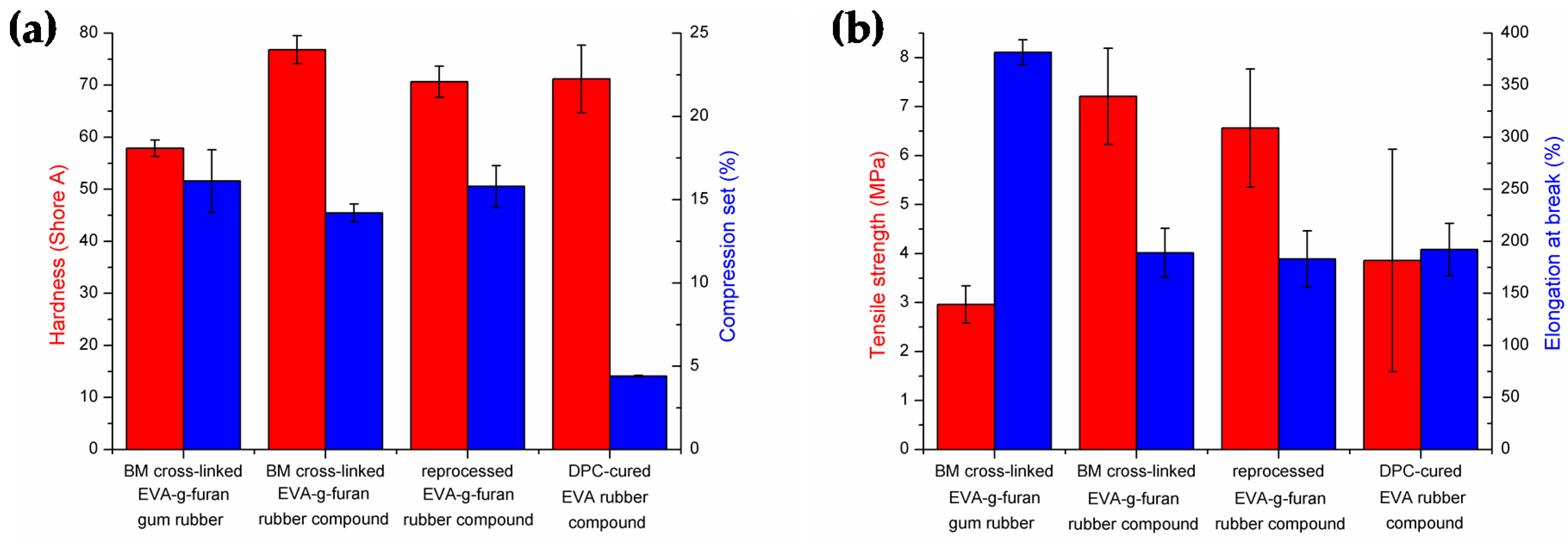
3.3. Cross-Linked EVA Rubber Compounds
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pazur, R.J. EVM copolymers: The forgotten rubber. Rubber World 2005, 231, 27–34. [Google Scholar]
- Andersen, B. Investigations on environmental stress cracking resistance of LDPE/EVA blends. Halle-Wittenberg 2004, 6, 28–30. [Google Scholar]
- Yao, D.; Qu, B.; Wu, Q. Photoinitiated crosslinking of ethylene-vinyl acetate copolymers and characterization of related properties. Polym. Eng. Sci. 2007, 47, 1761–1767. [Google Scholar] [CrossRef]
- Biron, M. Polyolefin and non-polyolefin copolymers. In Thermoplastics and Thermoplastic Composites, 2nd ed.; William, A., Ed.; Elsevier: Oxford, UK, 2007; Volume 4, pp. 281–293. [Google Scholar]
- Pern, F.J. Ethylene-vinyl acetate (EVA) encapsulants for photovoltaic modules: Degradation and discoloration mechanisms and formulation modifications for improved photostability. Angew. Makromol. Chem. 1997, 252, 195–216. [Google Scholar] [CrossRef]
- Lee, B.; Liu, J.Z.; Sun, B.; Shen, C.Y.; Dai, G.C. Thermally conductive and electrically insulating EVA composite encapsulants for solar photovoltaic (PV) cell. Express Polym. Lett. 2008, 2, 357–363. [Google Scholar] [CrossRef]
- Agroui, K.; Collins, G.; Farenc, J. Measurement of glass transition temperature of crosslinked EVA encapsulant by thermal analysis for photovoltaic application. Renew. Energy 2012, 43, 218–223. [Google Scholar] [CrossRef]
- Zhou, S. The influence of crosslinking agent on the properties of EVA resin. In Proceedings of the 2014 IEEE 40th Photovoltaic Specialist Conference (PVSC), Denver, CL, USA, 8 June 2014.
- Stelescu, M.D.; Manaila, E.; Craciun, G.; Zuga, N. Crosslinking and grafting ethylene vinyl acetate copolymer with accelerated electrons in the presence of polyfunctional monomers. Polym. Bull. 2012, 68, 263–285. [Google Scholar] [CrossRef]
- Sen, M.; Copuroglu, M. A comparative study of gamma irradiation of poly (ethylene-co-vinyl acetate) and poly(ethylene-co-vinyl acetate)/carbon black mixture. Mater. Chem. Phys. 2005, 93, 154–158. [Google Scholar] [CrossRef]
- Ivanov, V.S.; Migunova, I.I.; Kalinina, N.A.; Aleksandrov, G.N. Radiation processing of polymer insulators: A method for improving their properties and performance. Polym. Eng. Sci. 1996, 36, 1941–1949. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Rawlins, J.W.; Ray, P. Cable technology. In Polymer Grafting and Crosslinking; Sen, A., Ed.; John Wiley & Sons: Hoboken, New Jersey, USA, 2008; p. 227. [Google Scholar]
- Posadas, P.; Fernandez-Torres, A.; Chamorro, C.; Mora-Barrantes, I.; Rodriguez, A.; Gonzalez, L.; Valentin, J.L. Study on peroxide vulcanization thermodynamics of ethylenevinyl acetate copolymer rubber using 2,2,6,6,-tetramethylpiperidinyloxyl nitroxide. Polym. Int. 2013, 62, 909–918. [Google Scholar] [CrossRef]
- Wang, L.; Hong, Y.; Li, J.X. Durability of running shoes with ethylene vinyl acetate or polyurethane midsoles. J. Sports Sci. 2012, 30, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Loan, L.D. Mechanism of peroxide vulcanization of elastomers. Rubber Chem. Technol. 1967, 40, 149–176. [Google Scholar] [CrossRef]
- Huskic, M.; Sebenik, A. Characterization of cross-linked ethylene vinylacetate copolymers. Polym. Int. 1993, 31, 41–44. [Google Scholar] [CrossRef]
- Narkis, M.; Miltz, J. Peroxide crosslinking of ethylene-vinyl acetate copolymer. J. Appl. Polym. Sci. 1977, 21, 703–709. [Google Scholar] [CrossRef]
- Manaila, E.; Stelescu, M.D.; Craciun, G. Aspects regarding radiation crosslinking of elastomers. In Advanced Elastomers—Technology, Properties and Application, 1st ed.; Boczkowska, A., Ed.; InTech: Rijeka, Croatia, 2012; pp. 254–269. [Google Scholar]
- Odian, G. Peroxide and radiation crosslinking. In Principles of polymerization, 4th ed.; John Wiley & Sons: New York, NY, USA, 2004; pp. 742–744. [Google Scholar]
- Franc, G.; Kakkar, A.K. Diels-Alder “click” chemistry in designing dendritic macromolecules. Chem. Eur. J. 2009, 15, 48–69. [Google Scholar] [CrossRef] [PubMed]
- Gandini, A. The furan/maleimide Diels-Alder reaction: A versatile click-unclick tool in macromolecular synthesis. Prog. Polym. Sci. 2013, 38, 1–29. [Google Scholar] [CrossRef]
- Gheneim, R.; Perez-Berumen, C.; Gandini, A. Diels-Alder reactions with novel polymeric dienes and dienophiles: Synthesis of reversibly cross-linked elastomers. Macromolecules 2002, 35, 8–14. [Google Scholar] [CrossRef]
- Toncelli, C.; De Reus, D.C.; Picchioni, F.; Broekhuis, A.A. Properties of reversible Diels-Alder furan/maleimide polymer networks as function of crosslink density. Macromol. Chem. Phys. 2012, 213, 157–165. [Google Scholar] [CrossRef]
- Gandini, A.; Silvestre, A.J.D.; Coelho, D. Reversible click chemistry at the service of macromolecular materials. Polym. Chem. 2011, 2, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Gandini, A.; Coelho, D.; Silvestre, A.J.D. Reversible click chemistry at the service of macromolecular materials part 1: Kinetics of the Diels-Alder reaction applied to furan-maleimide model compounds and linear polymerizations. Eur. Polym. J. 2008, 44, 4029–4036. [Google Scholar] [CrossRef]
- Ax, J.; Wenz, G. Thermoreversible networks by Diels-Alder reaction of cellulose furoates with bismaleimides. Macromol. Chem. Phys. 2012, 213, 182–186. [Google Scholar] [CrossRef]
- Canary, S.A.; Stevens, M.P. Thermally reversible cross-linking of polystyrene via the furan-maleimide Diels-Alder reaction. J. Polym. Sci. Pol. Chem. 1992, 30, 1755–1760. [Google Scholar] [CrossRef]
- Polgar, L.M.; van Duin, M.; Broekhuis, A.A.; Picchioni, F. The use of Diels-Alder chemistry for thermo-reversible cross-linking of rubbers: The next step towards recycling of rubber products? Macromolecules 2015, 48, 7096–7105. [Google Scholar] [CrossRef]
- Polgar, L.M.; van Duin, M.; Pucci, A.; Broekhuis, A.A.; Picchioni, F. Chemical modification of hydrocarbon elastomers: A versatile toolbox for the design of novel functional elastomers. Prog. Polym. Sci. 2017. (under review). [Google Scholar]
- Burlett, D.J.; Lindt, J.T. Reactive processing of rubbers. Rubber Chem. Technol. 1993, 66, 411–434. [Google Scholar] [CrossRef]
- Saelao, J.; Phinyocheep, P. Influence of styrene on grafting efficiency of maleic anhydride onto natural rubber. J. Appl. Polym. Sci. 2005, 95, 28–38. [Google Scholar] [CrossRef]
- Van Duin, M.; Dikland, H.G. Effect of third monomer type and content on peroxide crosslinking efficiency of EPDM. Rubber Chem. Technol. 2003, 76, 132–144. [Google Scholar] [CrossRef]
- Barra, G.M.O.; Crespo, J.S.; Bertolino, J.R.; Soldi, V.; Pires, A.T.N. Maleic anhydride grafting on EPDM: Qualitative and quantitative determination. J. Braz. Chem. Soc. 1999, 10, 31–34. [Google Scholar] [CrossRef]
- Oostenbrink, A.J.; Gaymans, R.J. Maleic anhydride grafting on EPDM rubber in the melt. Polymer 1992, 33, 3086–3088. [Google Scholar] [CrossRef]
- Rzayev, Z.M.O. Graft copolymers of maleic anhydride and its isostructural analogues: High performance engineering materials. Int. Rev. Chem. Eng. 2011, 3, 1105–1129. [Google Scholar]
- Gancarz, I.; Laskawski, W. Peroxide-initiated crosslinking of maleic anhydride-modified low-molecular-weight polybutadiene 1: Mechanism and kinetics of the reaction. J. Polym. Sci. Pol. Chem. 1979, 17, 683–692. [Google Scholar] [CrossRef]
- Fink, J.K. Maleic anhydride. In Reactive Polymers Fundamentals and Applications: A Concise Guide to Industrial Polymers, 2nd ed.; Elsevier: Oxford, UK, 2013; p. 433. [Google Scholar]
- Laita, H.; Boufi, S.; Gandini, A. The application of the Diels-Alder reaction to polymers bearing furan moieties 1: Reactions with maleimides. Eur. Polym. J. 1997, 33, 1203–1211. [Google Scholar] [CrossRef]
- Zhang, Y.; Broekhuis, A.A.; Picchioni, F. Thermally self-healing polymeric materials: The next step to Recycling thermoset polymers? Macromolecules 2009, 42, 1906–1912. [Google Scholar] [CrossRef] [Green Version]
- Gousse, C.; Gandini, A.; Hodge, P. Application of the Diels-Alder reaction to polymers bearing furan moieties 2: Diels-Alder and retro-Diels-Alder reactions involving furan rings in some styrene copolymers. Macromolecules 1998, 31, 314–321. [Google Scholar] [CrossRef]
- Rigbi, Z. Reinforcement of rubber by carbon black. Properties of polymers. In Advances in Polymer Science, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1980; Volume 36, pp. 21–68. [Google Scholar]
- Frohlich, J.; Niedermeier, W.; Luginsland, H.D. The effect of filler-filler and filler-elastomer interaction on rubber reinforcement. Compos. Part A 2005, 36, 449–460. [Google Scholar] [CrossRef]
- Wolff, S. Chemical aspects of rubber reinforcement by fillers. Rubber Chem. Technol. 1996, 69, 325–346. [Google Scholar] [CrossRef]
- Ignatz-Hoover, F.; To, B.H.; Datta, R.N.; De Hoog, A.J.; Huntink, N.M.; Talma, A.G. Chemical additives migration in rubber. Rubber Chem. Technol. 2003, 76, 747–767. [Google Scholar] [CrossRef]
- Van der Mee, M.A.J.; I′Abee, R.M.A.; Portale, G.; Goossens, J.G.P.; van Duin, M. Synthesis, structure, and properties of ionic thermoplastic elastomers based on maleated ethylene/propylene copolymers. Macromolecules 2008, 41, 5493–5501. [Google Scholar] [CrossRef]
- Hirschl, C.; Biebl-Rydlo, M.; DeBiasio, M.; Muehleisen, W.; Neumaier, L.; Scherf, W.; Oreski, G.; Eder, G.; Chernev, B.; Schwab, W.; Kraft, M. Determining the degree of crosslinking of ethylene vinyl acetate photovoltaic module encapsulants—A comparative study. Sol. Energy Mat. Sol. C 2013, 116, 203–218. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J. Statistical theory of chain configuration and physical properties of high polymers. I. Rubberlike elasticity. II. Swelling. J. Chem. Phys. 1943, 11, 419–429. [Google Scholar]
- Ghosh, S.K. Self-Healing Mateirals: Fundamentals, Design Strategies and Applications. In Self-healing mateirals: Fundamentals, design strategies and applications, 1st ed.; Ghosh, S.K., Ed.; Wiley: Weinheim, Germany, 2009; pp. 1–28. [Google Scholar]
- Nielsen, L.E. Cross-linking-effect on physical properties of polymers. J. Macromol. Sci. 1969, 3, 69–74. [Google Scholar] [CrossRef]
- Zhao, F.; Bi, W.; Zhao, S. Influence of crosslink density on mechanical properties of natural rubber vulcanizates. J. Macromol. Sci. Phys. 2011, 50, 1460–1469. [Google Scholar] [CrossRef]
- Boye, W.M. Utilizing coagents in the electron beam cure of elastomers. Rubber World 2009, 241, 38–44. [Google Scholar]
- Machado, A.V.; Covas, J.A.; van Duin, M. Effect of polyolefin structure on maleic anhydride grafting. Polymer 2001, 42, 3649–3655. [Google Scholar] [CrossRef]
- Polgar, L.M.; Hagting, E.; van Duin, M.; Picchioni, F. The effect of polar clusters on thermoreversibly cross-linked elastomers. Polymer 2017. (submitted). [Google Scholar]
- Nunes, R.W.; Martin, J.R.; Johnson, J.F. Influence of molecular-weight and molecular-weight distribution on mechanical-properties of polymers. Polym. Eng. Sci. 1982, 22, 205–228. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, C. Stress-Strain behaviors and crosslinked networks studies of natural rubber-zinc dimethacrylate composites. J. Macromol. Sci. Phys. 2012, 51, 1384–1400. [Google Scholar] [CrossRef]
- Hofmann, W. Rubber Technology Handbook, 1st ed.; Hanser Publishers: Münich, Germany, 1989; pp. 246–258. [Google Scholar]
- Polgar, L.M.; Blom, R.; Keizer, J.C.; van Duin, M.; Picchioni, F. Recyclable EPM rubber compounds via thermoreversible DA chemistry and their interaction with carbon black. Rubber Chem. Technol. 2017. (submitted). [Google Scholar]
- Bull, S.D.; Davidson, M.G.; Van den Elsen, J.M.H.; Fossey, J.S.; Jenkins, A.T.A.; Jiang, Y.; Kubo, Y.; Marken, F.; Sakurai, K.; Zhao, J.; James, T.D. Exploiting the reversible covalent bonding of boronic acids: Recognition, sensing, and assembly. ACC Chem. Res. 2013, 46, 312–326. [Google Scholar] [CrossRef] [PubMed]
| Component | phr | Time of Addition (min) |
|---|---|---|
| EVA-g-furan | 100 | 0 |
| Carbon black (N550) | 55 | 2 |
| Lubricant (DOS) | 15 | 2 |
| Antioxidant | 0.1 | 2 |
| BM/DCP cross-linker | 2.6/1.6 | 5 |
| Mn (kg/mol) | PDI (-) | N, C, H → O Content (wt %) | #/Chain (-) | Tg (°C) | [XLD] (mol/mL) | |
|---|---|---|---|---|---|---|
| EVA rubber | 35 | 9.1 | <0.01, 64.41, 9.19 → 26.40 | – | −11.9 | - |
| EVFM | 46 | 2.7 | <0.01, 67.41, 9.87 → 22.72 | 5.8 | −21.7 | - |
| EVA-g-MA | 31 | 17 | <0.01, 65.40, 9.61 → 24.99 | 4.4 | −12.7 | - |
| EVA-g-furan | 31 | 17 | 0.20, 66.37, 9.52 → 23.91 | 4.3 | −11.0 | - |
| BM cross-linked EVFM | - | - | 0.35, 67.34, 9.62 → 22.69 | 5.5 | −22.3 | 8.62 × 10−5 ± 2.0 × 10−5 |
| BM cross-linked EVA-g-furan | - | - | 0.42, 65.16, 9.34 → 25.08 | 3.9 | −9.7 | 8.75 × 10−5 ± 3.0 × 10−6 |
| 1.5 phr DCP cured EVA | - | - | <0.01, 64.44, 9.04 → 26.52 | - | −12.3 | 4.8 × 10−5 ± 1.1 × 10−5 |
| 3.0 phr DCP cured EVA | - | - | <0.01, 62.47, 8.91 → 28.62 | - | −12.7 | 2.4 × 10−4 ± 2.3 × 10−5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polgar, L.M.; Hagting, E.; Koek, W.-J.; Picchioni, F.; Van Duin, M. Thermoreversible Cross-Linking of Furan-Containing Ethylene/Vinyl Acetate Rubber with Bismaleimide. Polymers 2017, 9, 81. https://doi.org/10.3390/polym9030081
Polgar LM, Hagting E, Koek W-J, Picchioni F, Van Duin M. Thermoreversible Cross-Linking of Furan-Containing Ethylene/Vinyl Acetate Rubber with Bismaleimide. Polymers. 2017; 9(3):81. https://doi.org/10.3390/polym9030081
Chicago/Turabian StylePolgar, Lorenzo Massimo, Erik Hagting, Wouter-Jan Koek, Francesco Picchioni, and Martin Van Duin. 2017. "Thermoreversible Cross-Linking of Furan-Containing Ethylene/Vinyl Acetate Rubber with Bismaleimide" Polymers 9, no. 3: 81. https://doi.org/10.3390/polym9030081