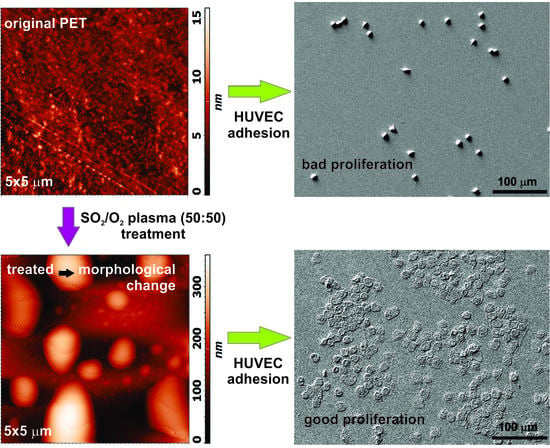
Cell Proliferation on Polyethylene Terephthalate Treated in Plasma Created in SO2/O2 Mixtures
Abstract
:1. Introduction
2. Materials and Methods
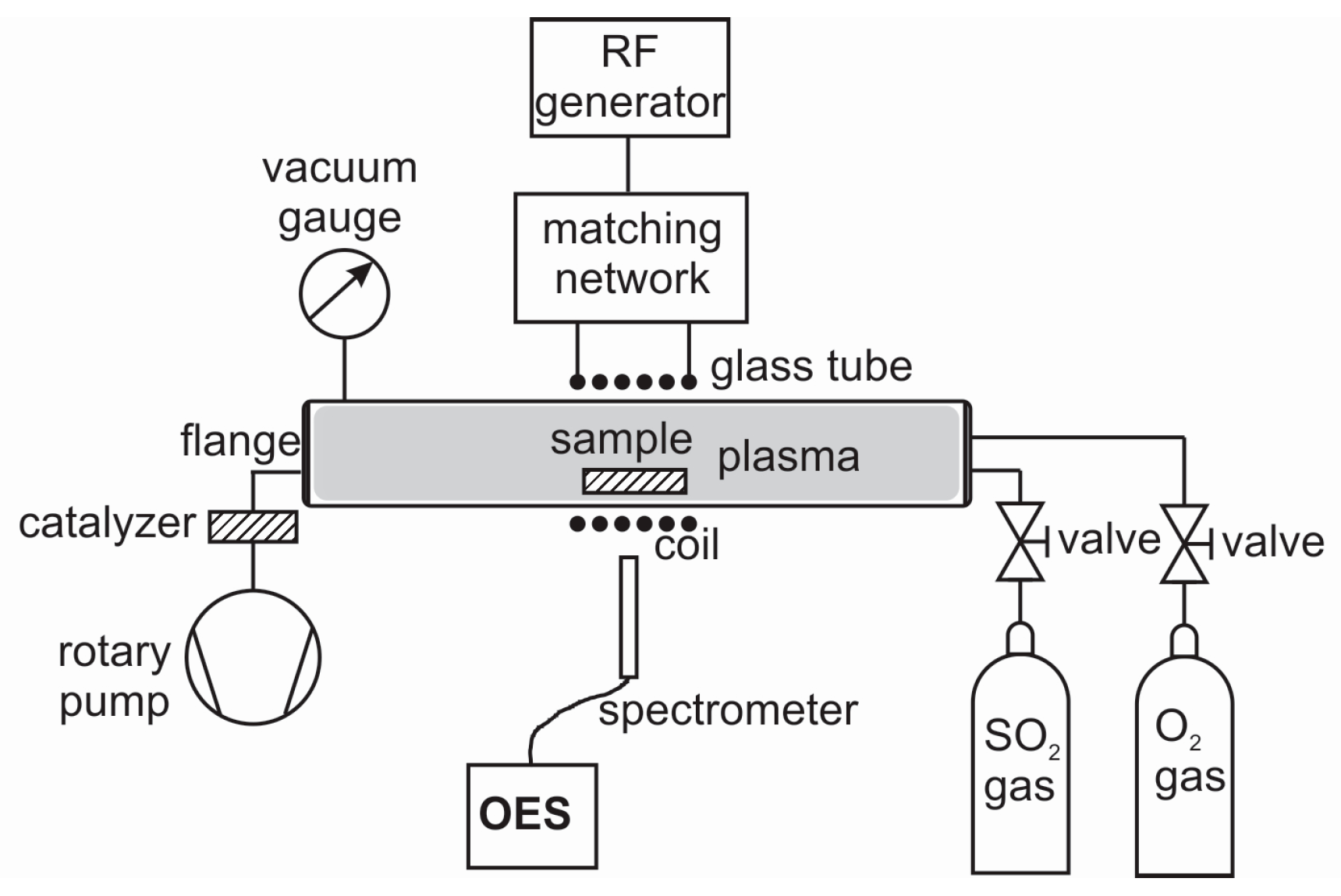
2.1. Plasma Treatment
2.2. Plasma Characterisation
2.3. Atomic Force Microscopy (AFM) Measurements
2.4. Characterisation by X-Ray Photoelectron Spectroscopy (XPS)
2.5. Contact Angle Measurements
2.6. Cell Adhesion and Morphology Studies
2.6.1. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) Assay
2.6.2. In Vitro Toxicology Assay (TOX)
2.6.3. Statistical Analysis
3. Results and Discussion
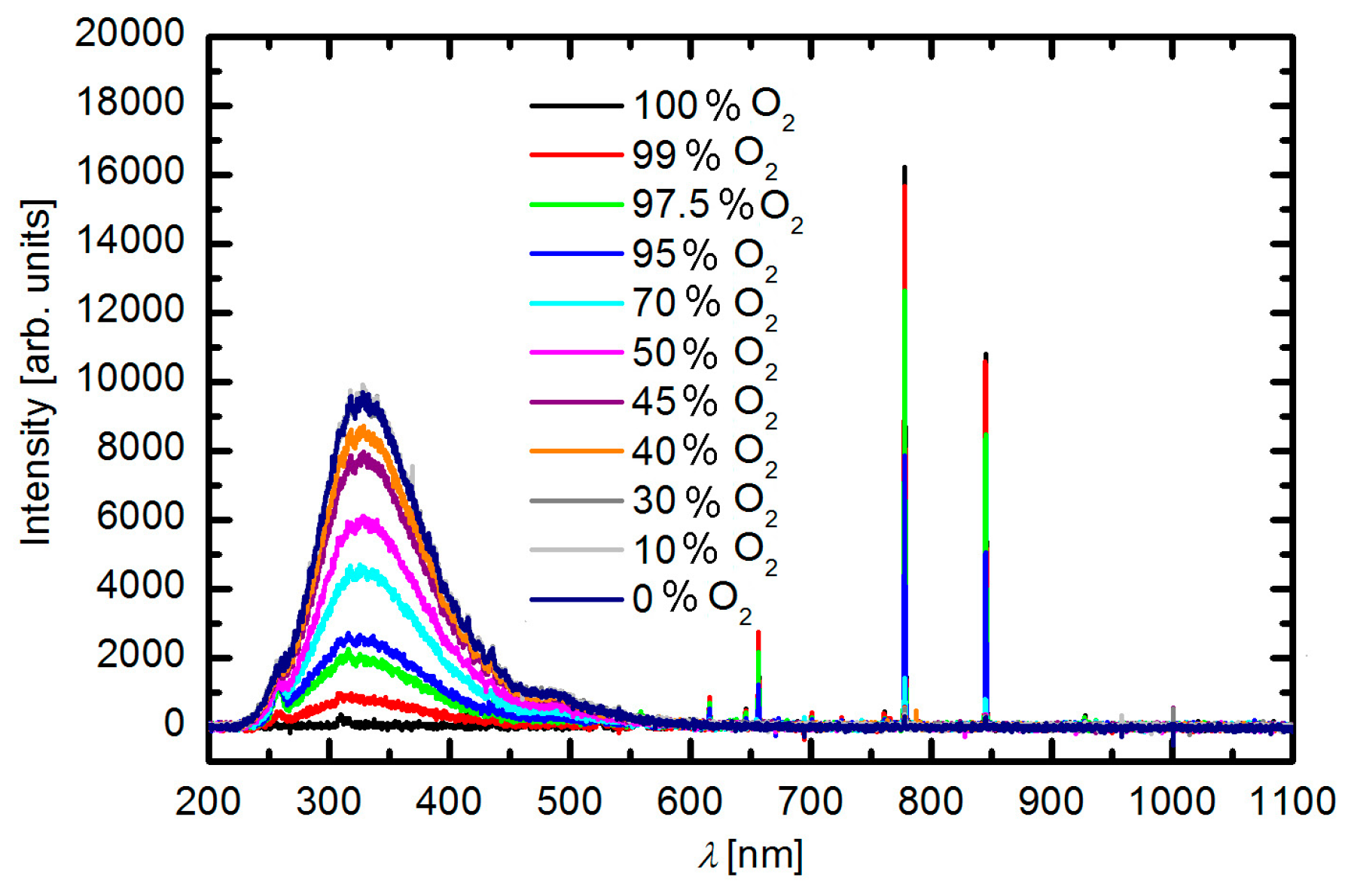
3.1. Plasma Characterisation
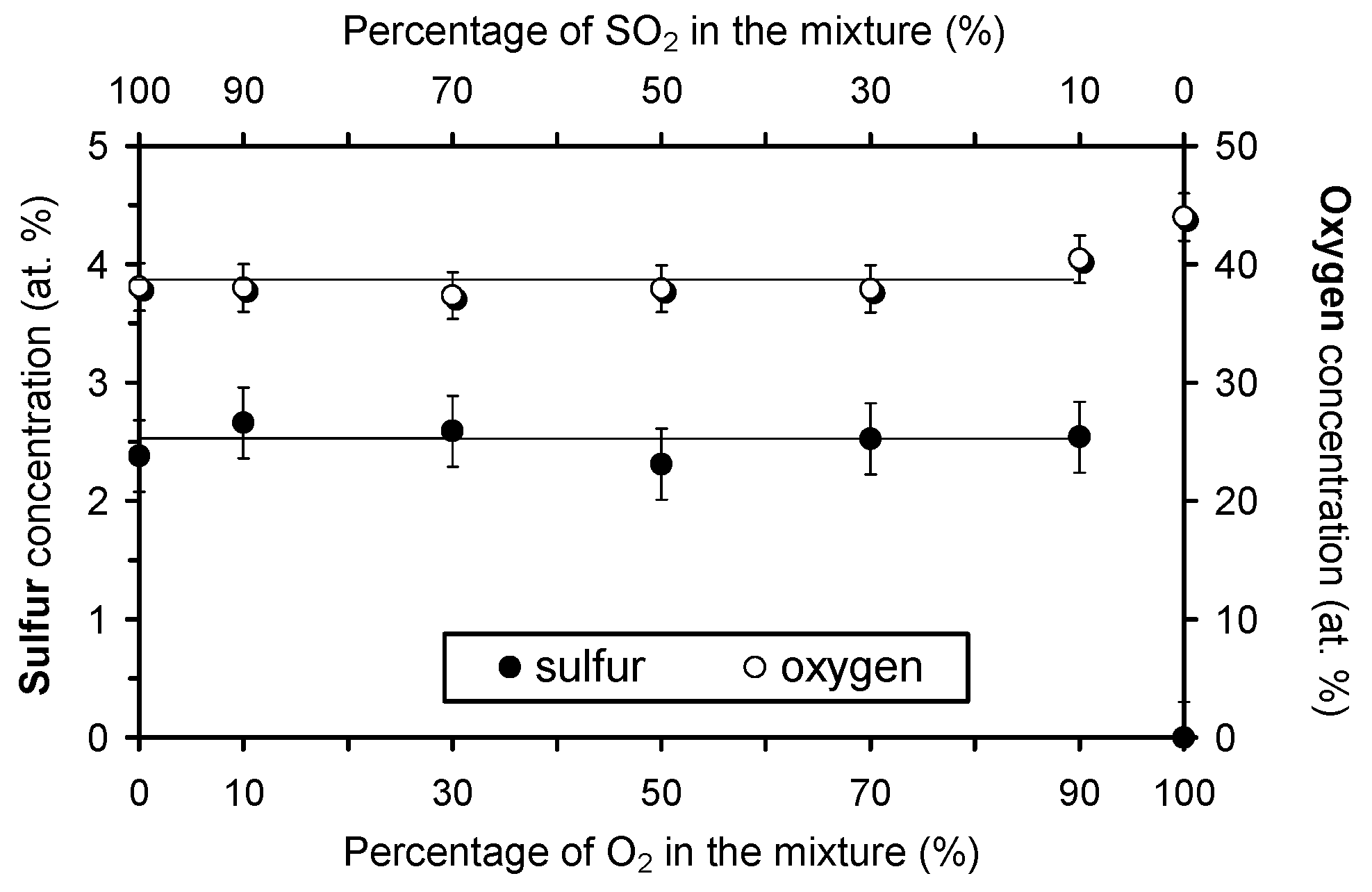
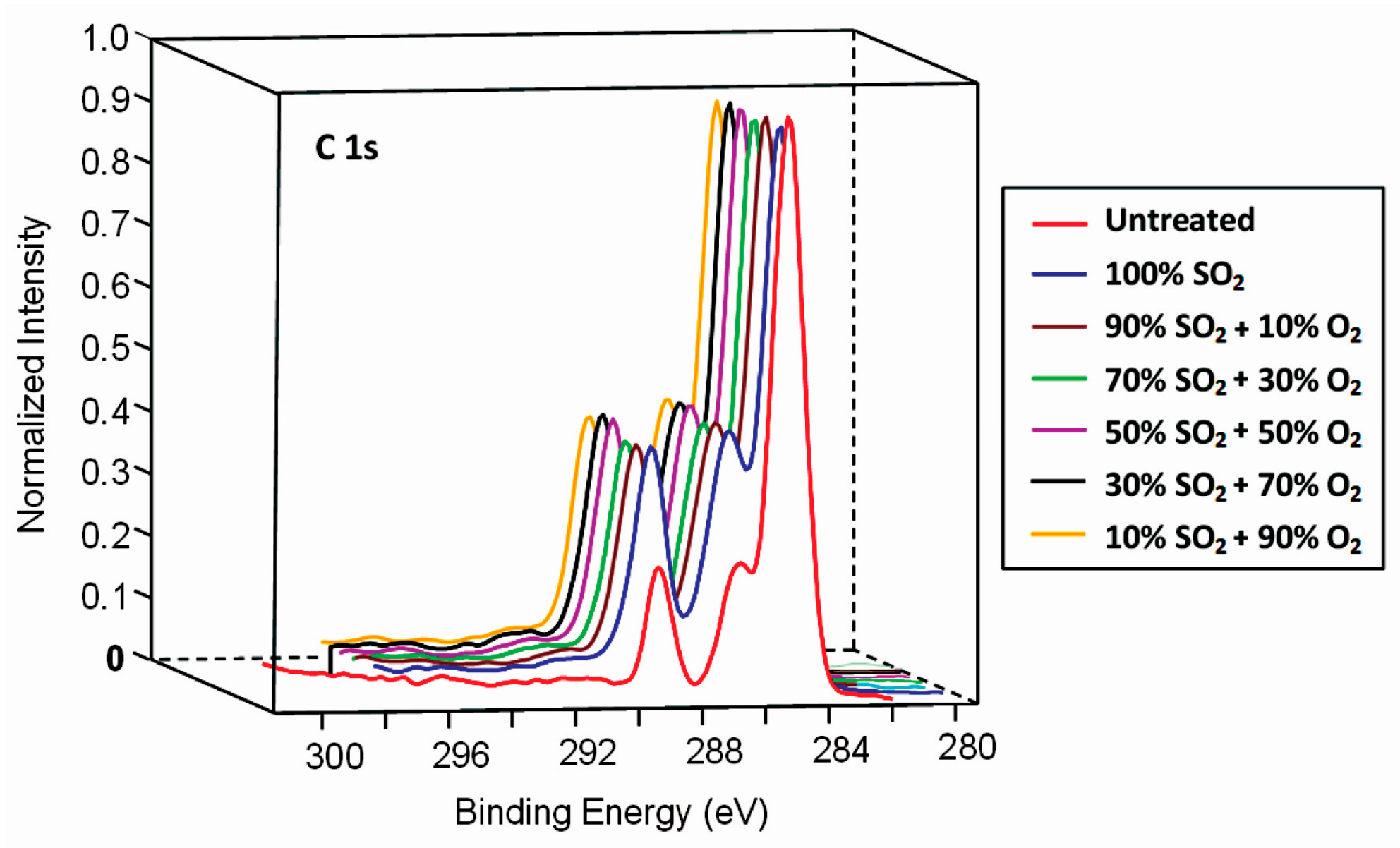
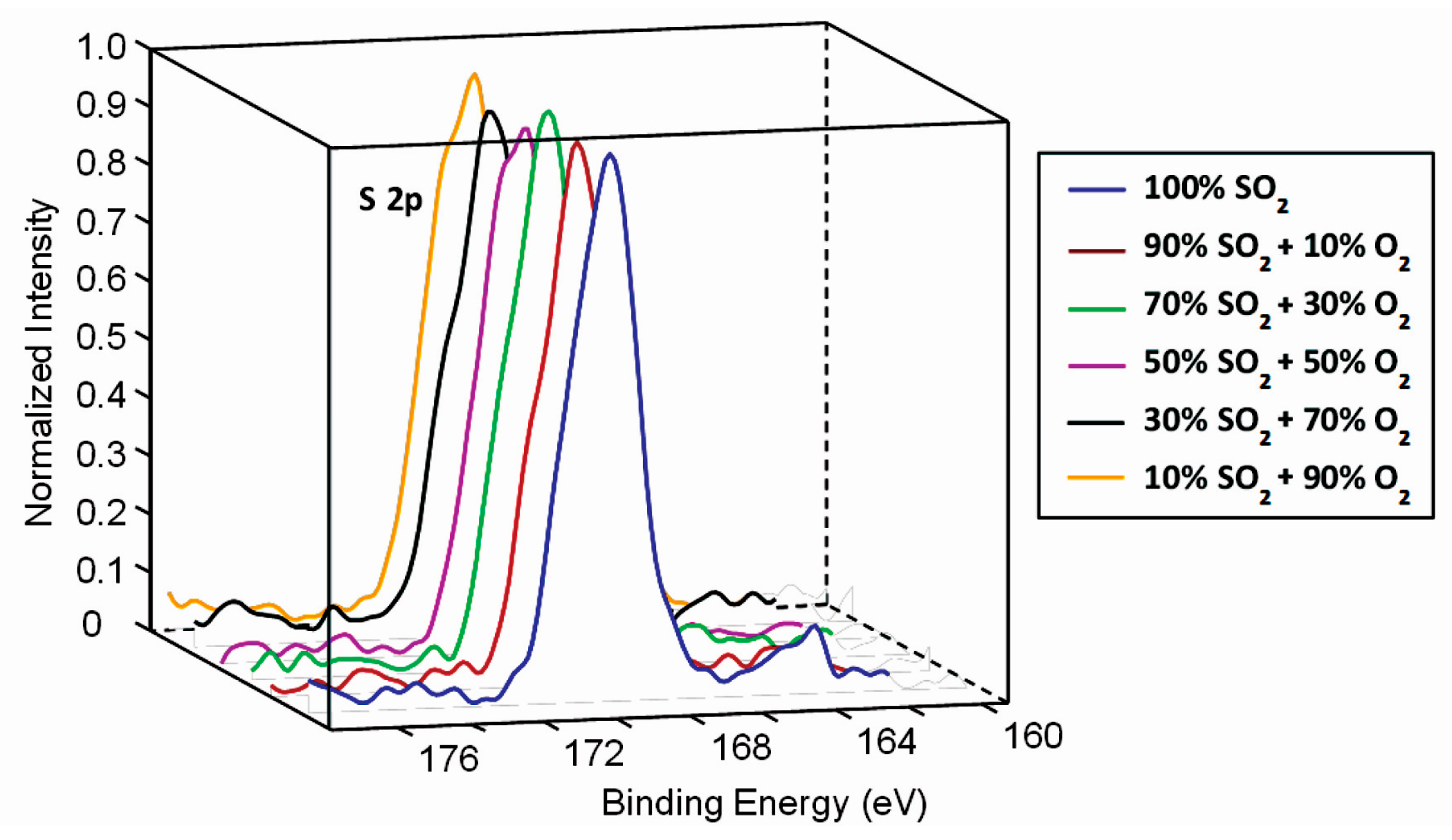
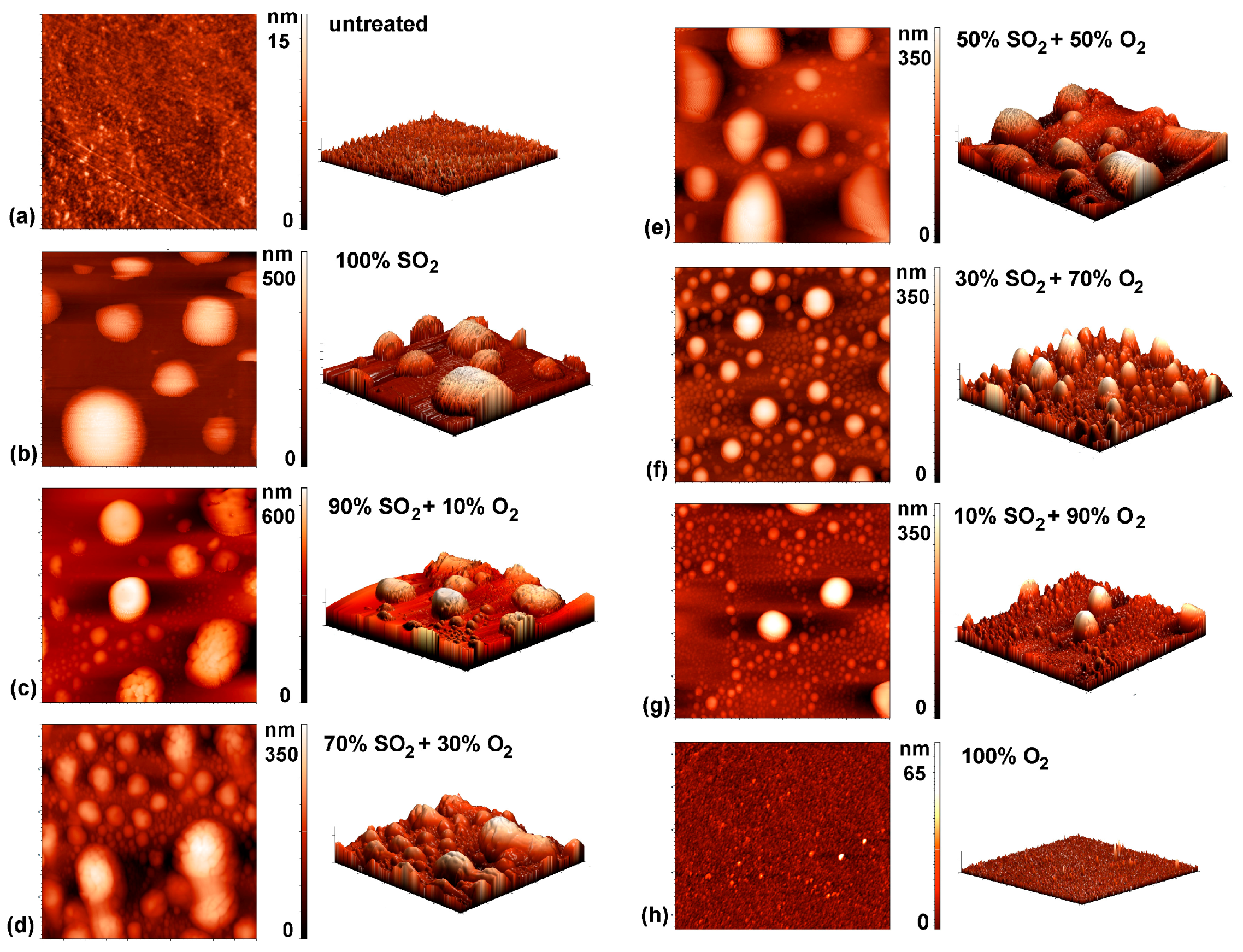
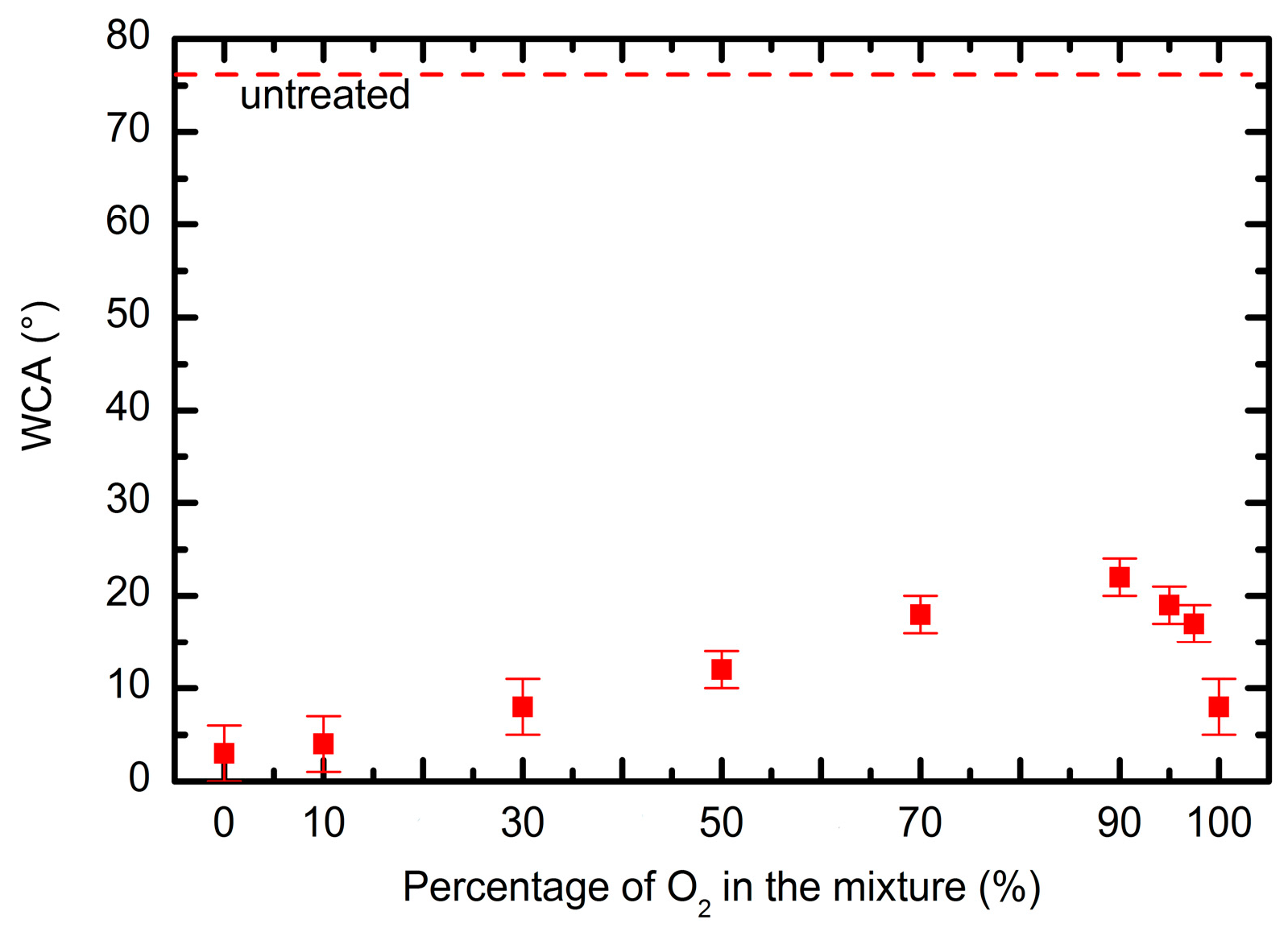
3.2. Surface Characterisation of Plasma-Treated Samples
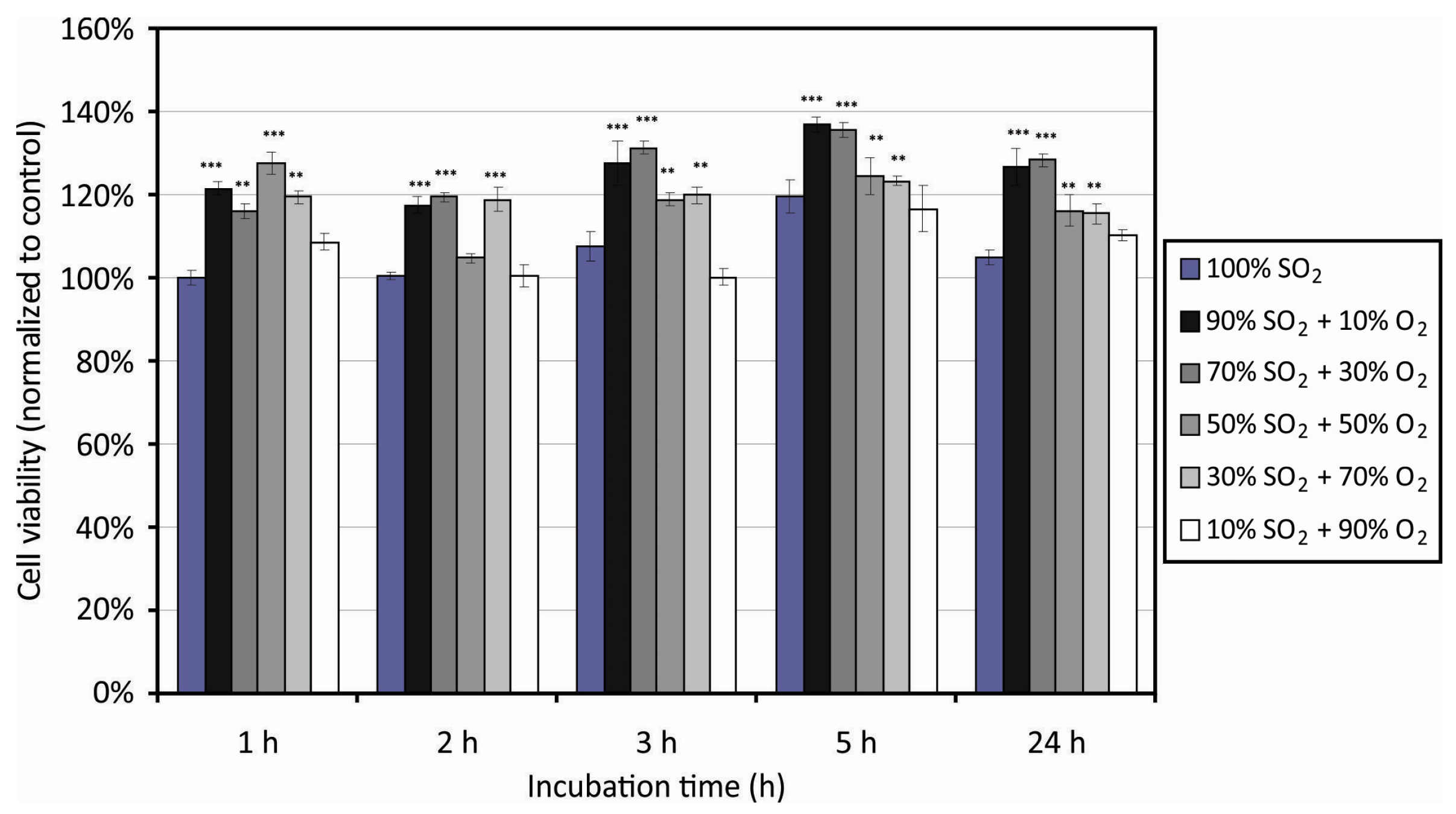
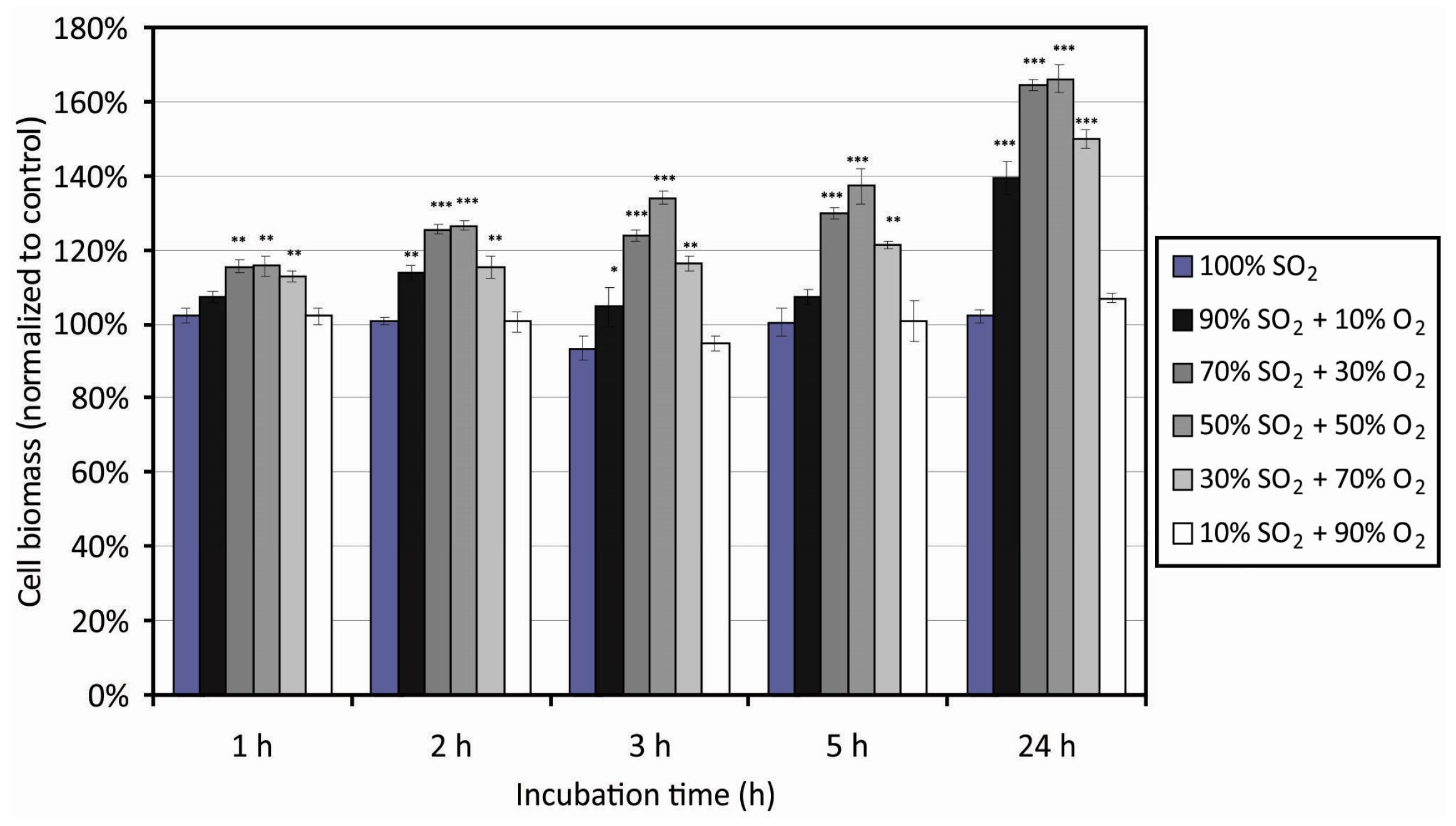
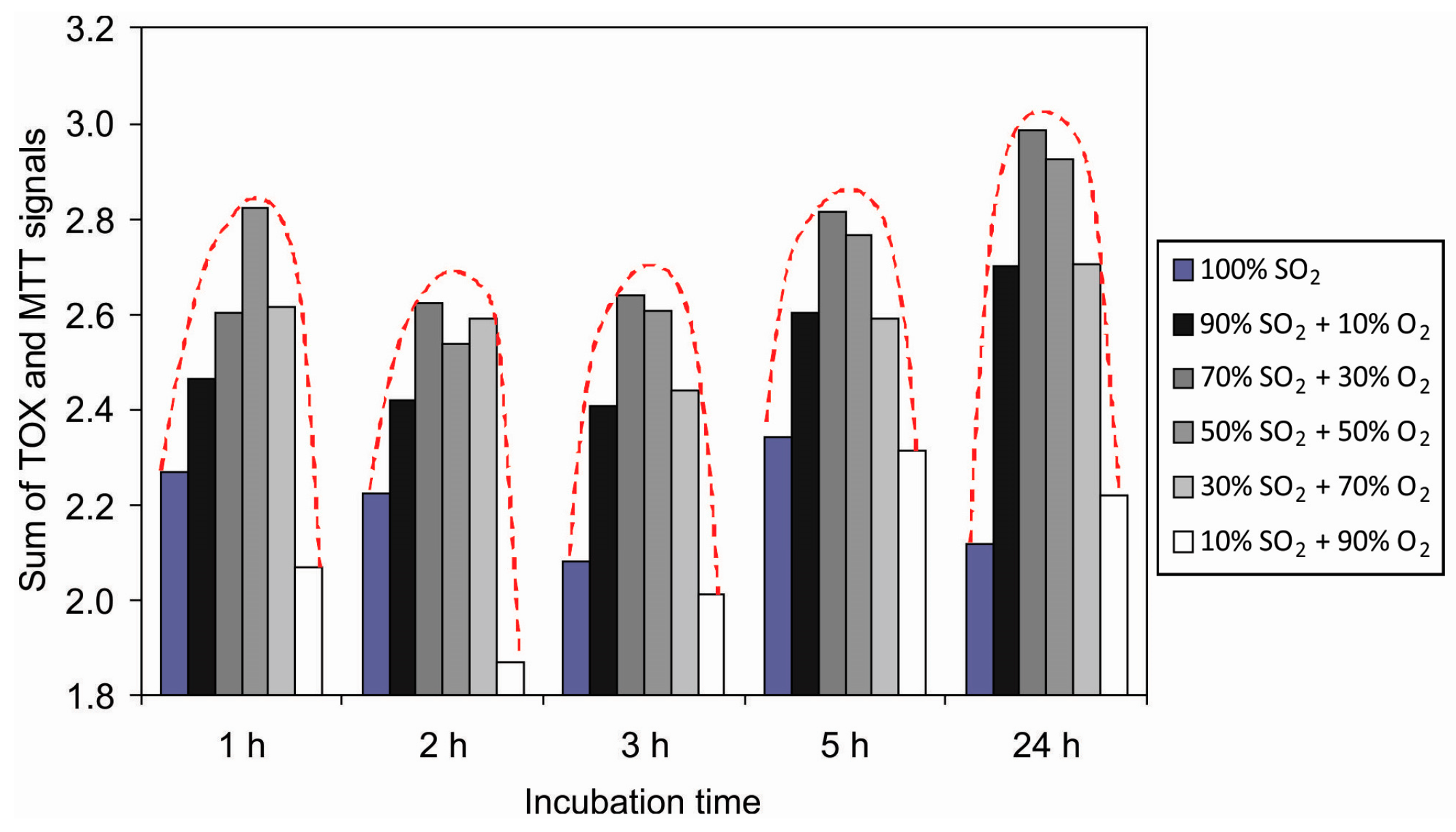
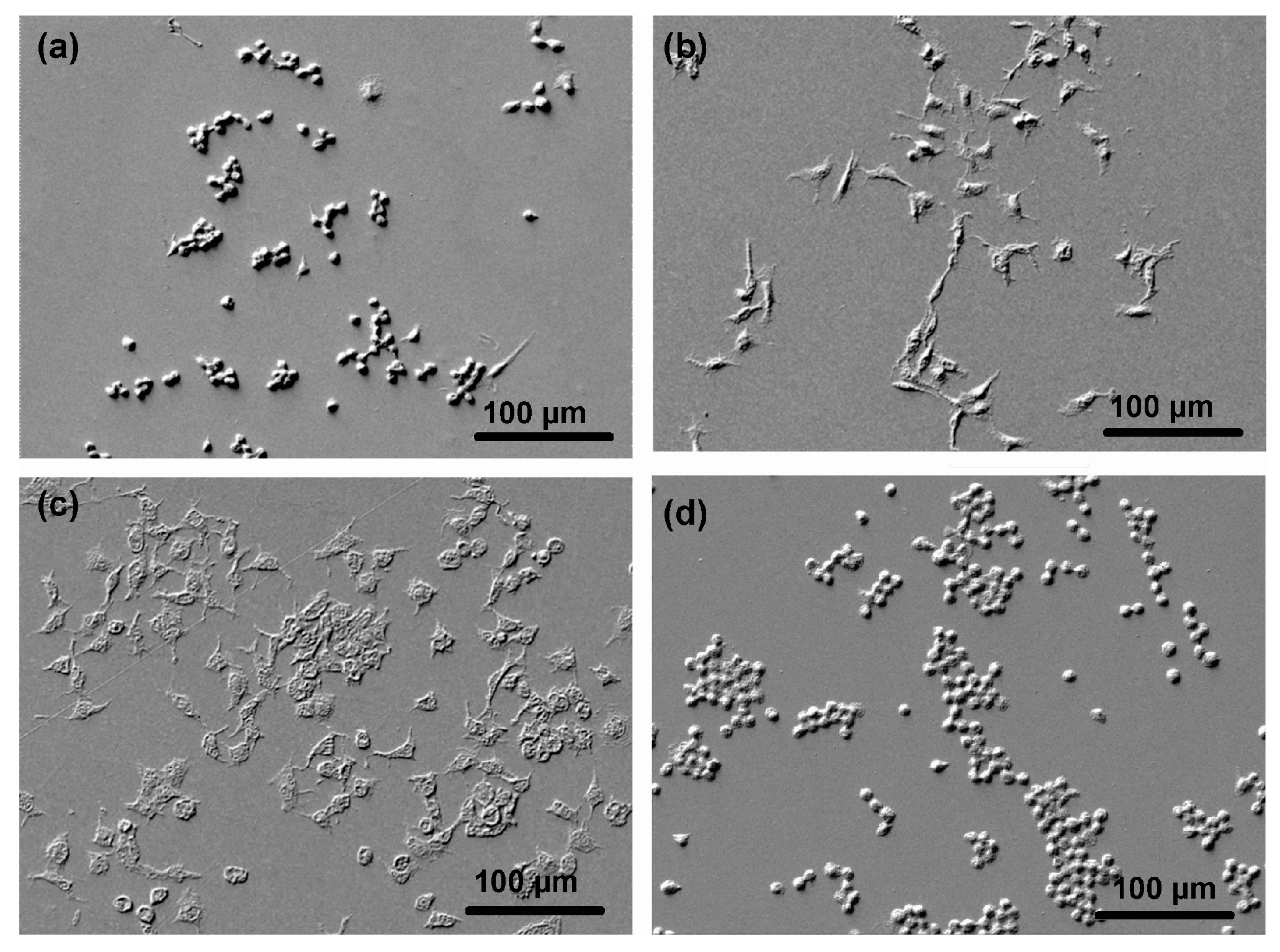
3.3. Cell Adhesion on Plasma-Treated Surfaces
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mozetic, M.; Primc, G.; Vesel, A.; Zaplotnik, R.; Modic, M.; Junkar, I.; Recek, N.; Klanjsek-Gunde, M.; Guhy, L.; Sunkara, M.K.; et al. Application of extremely non-equilibrium plasmas in the processing of nano and biomedical materials. Plasma Sources Sci. Technol. 2015, 24, 015026. [Google Scholar] [CrossRef]
- Labay, C.; Canal, J.M.; Modic, M.; Cvelbar, U.; Quiles, M.; Armengol, M.; Arbos, M.A.; Gil, F.J.; Canal, C. Antibiotic-loaded polypropylene surgical meshes with suitable biological behaviour by plasma functionalization and polymerization. Biomaterials 2015, 71, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Puac, N.; Petrovic, Z.L.; Radetic, M.; Djordjevic, A. Low pressure RF capacitively coupled plasma reactor for modification of seeds, polymers and textile fabrics. Mater. Sci. Forum 2005, 494, 291–296. [Google Scholar] [CrossRef]
- Lopez-Garcia, J.; Lehocky, M.; Humpolicek, P.; Novak, I. On the correlation of surface charge and energy in non-thermal plasma-treated polyethylene. Surf. Interface Anal. 2014, 46, 625–629. [Google Scholar] [CrossRef]
- Vandrovcova, M.; Grinevich, A.; Drabik, M.; Kylian, O.; Hanus, J.; Stankova, L.; Lisa, V.; Choukourov, A.; Slavinska, D.; Biederman, H.; Bacakova, L. Effect of various concentrations of Ti in hydrocarbon plasma polymer films on the adhesion, proliferation and differentiation of human osteoblast-like MG-63 cells. Appl. Surf. Sci. 2015, 357, 459–472. [Google Scholar] [CrossRef]
- Labay, C.; Canal, C.; Garcia-Celma, M.J. Influence of corona plasma treatment on polypropylene and polyamide 6.6 on the release of a model drug. Plasma Chem. Plasma Process. 2010, 30, 885–896. [Google Scholar] [CrossRef]
- Rodriguez-Emmenegger, C.; Kylian, O.; Houska, M.; Brynda, E.; Artemenko, A.; Kousal, J.; Alles, A.B.; Biederman, H. Substrate-independent approach for the generation of functional protein resistant surfaces. Biomacromolecules 2011, 12, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, Z.L.; Puac, N.; Malovic, G.; Lazovic, S.; Maletic, D.; Miletic, M.; Mojsilovic, S.; Milenkovic, P.; Bugarski, D. Application of non-equilibrium plasmas in medicine. J. Serb. Chem. Soc. 2012, 77, 1689–1699. [Google Scholar] [CrossRef]
- Popelka, A.; Novak, I.; Lehocky, M.; Bilek, F.; Kleinova, A.; Mozetic, M.; Spirkova, M.; Chodak, I. Antibacterial treatment of LDPE with halogen derivatives via cold plasma. Express. Polym. Lett. 2015, 9, 402–411. [Google Scholar] [CrossRef]
- Labay, C.; Canal, J.M.; Canal, C. Relevance of surface modification of polyamide 6.6 fibers by air plasma treatment on the release of caffeine. Plasma Process. Polym. 2012, 9, 165–173. [Google Scholar] [CrossRef]
- Kuzminova, A.; Shelemin, A.; Kylian, O.; Choukourov, A.; Valentova, H.; Krakovsky, I.; Nedbal, J.; Slavinska, D.; Biederman, H. Study of the effect of atmospheric pressure air dielectric barrier discharge on Nylon 6,6 foils. Polym. Degrad. Stabil. 2014, 110, 378–388. [Google Scholar] [CrossRef]
- Kregar, Z.; Biscan, M.; Milosevic, S.; Mozetic, M.; Vesel, A. Interaction of argon, hydrogen and oxygen plasma early afterglow with polyvinyl chloride (PVC) materials. Plasma Process. Polym. 2012, 9, 1020–1027. [Google Scholar] [CrossRef]
- Novak, I.; Popelka, A.; Valentin, M.; Chodak, I.; Spirkova, M.; Toth, A.; Kleinova, A.; Sedliacik, J.; Lehocky, M.; Maronek, M. Surface behavior of polyamide 6 modified by barrier plasma in oxygen and nitrogen. Int. J. Polym. Anal. Ch. 2014, 19, 31–38. [Google Scholar] [CrossRef]
- Labay, C.; Canal, J.M.; Navarro, A.; Canal, C. Corona plasma modification of polyamide 66 for the design of textile delivery systems for cosmetic therapy. Appl. Surf. Sci. 2014, 316, 251–258. [Google Scholar] [CrossRef]
- Lopez-Garcia, J.; Primc, G.; Junkar, I.; Lehocky, M.; Mozetic, M. On the hydrophilicity and water resistance effect of styrene-acrylonitrile copolymer treated by CF4 and O2 plasmas. Plasma Process. Polym. 2015, 12, 1075–1084. [Google Scholar] [CrossRef]
- Modic, M.; Junkar, I.; Vesel, A.; Mozetic, M. Aging of plasma treated surfaces and their effects on platelet adhesion and activation. Surf. Coat. Technol. 2012, 213, 98–104. [Google Scholar] [CrossRef]
- Recek, N.; Resnik, M.; Motaln, H.; Lah-Turnsek, T.; Augustine, R.; Kalarikkal, N.; Thomas, S.; Mozetic, M. Cell adhesion on polycaprolactone modified by plasma treatment. Int. J. Polym. Sci. 2016, 2016, 354–366. [Google Scholar] [CrossRef]
- Kuzminova, A.; Vandrovcova, M.; Shelemin, A.; Kylian, O.; Choukourov, A.; Hanus, J.; Bacakova, L.; Slavinska, D.; Biederman, H. Treatment of poly(ethylene terephthalate) foils by atmospheric pressure air dielectric barrier discharge and its influence on cell growth. Appl. Surf. Sci. 2015, 357, 689–695. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- Klement, P.; Du, Y. J.; Berry, L.; Andrew, M.; Chan, A.K.C. Blood-compatible biomaterials by surface coating with a novel antithrombin-heparin covalent complex. Biomaterials 2002, 23, 527–535. [Google Scholar] [CrossRef]
- Grasel, T.G.; Cooper, S.L. Properties and biological inteactions of polyurethane anionomers: Effect of sulfonate incorporation. J. Biomed. Mater. Res. 1989, 23, 311–338. [Google Scholar] [CrossRef] [PubMed]
- Okkema, A.Z.; Visser, S.A.; Cooper, S.I. Physical and blood-contacting properties of polyurethanes based on a sulfonic acid-containing diol chain extender. J. Biomed. Mater. Res. 1991, 25, 1371–1395. [Google Scholar] [CrossRef] [PubMed]
- Vesel, A.; Mozetic, M.; Strnad, S. Improvement of adhesion of fucoidan on polyethylene terephthalate surface using gas plasma treatments. Vacuum 2011, 85, 1083–1086. [Google Scholar] [CrossRef]
- Ozaltin, K.; Lehocky, M.; Humpolicek, P.; Pelkova, J.; Saha, P. A new route of fucoidan immobilization on low density polyethylene and its blood compatibility and anticoagulation activity. Int. J. Mol. Sci. 2016, 17, 908–911. [Google Scholar] [CrossRef] [PubMed]
- Popelka, A.; Novak, I.; Lehocky, M.; Junkar, I.; Mozetic, M.; Kleinova, A.; Janigova, I.; Slouf, M.; Bilek, F.; Chodak, I. A new route for chitosan immobilization onto polyethylene surface. Carbohyd. Polym. 2012, 90, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Jui-Che, L.; Cooper, S.L. Surface characterization and ex vivo blood compatibility study of plasmamodified small diameter tubing: effect of sulphur dioxide and hexamethyl-disiloxane plasmas. Biomaterials 1995, 16, 1017–1023. [Google Scholar] [CrossRef]
- Tze-Man, K.; Jui-Che, L.; Cooper, S.L. Surface characterization and platelet adhesion studies of plasma-sulphonated polyethylene. Biomaterials 1993, 14, 657–664. [Google Scholar] [CrossRef]
- Inagaki, N.; Tasaka, S.; Miyazaki, H. Sulfonic acid group-containing thin films prepared by plasma polymerization. J. Appl. Polym. Sci. 1989, 38, 1829–1838. [Google Scholar] [CrossRef]
- Klee, D.; Villari, R.V.; Höcker, H.; Dekker, B.; Mittermayer, C. Surface modification of a new flexible polymer with improved cell adhesion. J. Mater. Sci. Mater. Med. 1994, 5, 592–595. [Google Scholar] [CrossRef]
- Shin, Y.N.; Kim, B.S.; Ahn, H.H.; Lee, J.H.; Kim, K.S.; Lee, J.Y.; Kim, M.S.; Khang, G.; Lee, H.B. Adhesion comparison of human bone marrow stem cells on a gradient wettable surface prepared by corona treatment. Appl. Surf. Sci. 2008, 255, 293–296. [Google Scholar] [CrossRef]
- Wang, L.C.; Chen, J.W.; Liu, H.I.; Chen, Z.Q.; Zhang, Y.; Wang, C.Y.; Feng, Z.G. Synthesis and evaluation of biodegradable segmented multiblock poly(ether ester) copolymers for biomaterial applications. Polym. Int. 2004, 53, 2145–2154. [Google Scholar] [CrossRef]
- Gugala, Z.; Gogolewski, S. Attachment, growth, and activity of rat osteoblasts on polylactide membranes treated with various lowtemperature radiofrequency plasmas. J. Biomed. Mater. Res. A 2006, 76A, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Holländer, A.; Kröpke, S. Polymer surface treatment with SO2-containing plasmas. Plasma Process. Polym. 2010, 7, 390–402. [Google Scholar] [CrossRef]
- Hiratsuka, A.; Fukui, H.; Suzuki, Y.; Muguruma, H.; Sakairi, K.; Matsushima, T.; Maruo, Y.; Yokoyama, K. Sulphur dioxide plasma modification on poly(methyl methacrylate) for fluidic devices. Curr. Appl. Phys. 2008, 8, 198–205. [Google Scholar] [CrossRef]
- Caro, J.C.; Lappan, U.; Simon, F.; Pleul, D.; Lunkwitz, K. On the low-pressure plasma treatment of PTFE (polytetrafluoroethylene) with SO2 as process gas. Eur. Polym. J. 1999, 35, 1149–1152. [Google Scholar] [CrossRef]
- Coen, M.C.; Keller, B.; Groening, P.; Schlapbach, L. Functionalization of graphite, glassy carbon, and polymer surfaces with highly oxidized sulfur species by plasma treatments. J. Appl. Phys. 2002, 92, 5077–5083. [Google Scholar] [CrossRef]
- Siow, K.S.; Britcher, L.; Kumar, S.; Griesser, H.J. Sulfonated surfaces by sulfur dioxide plasma surface treatment of plasma polymer films. Plasma Process. Polym. 2009, 6, 583–592. [Google Scholar] [CrossRef]
- Fatyeyeva, K.; Poncin-Epaillard, F. Sulfur dioxide plasma treatment of the clay (laponite) particles. Plasma Chem. Plasma Process. 2011, 31, 449–464. [Google Scholar] [CrossRef]
- Kregar, Z.; Biscan, M.; Milosevic, S.; Elersic, K.; Zaplotnik, R.; Primc, G.; Cvelbar, U. Optical emission characterization of extremely reactive oxygen plasma during treatment of graphite samples. Mater. Tehnol. 2012, 46, 25–30. [Google Scholar]
- Kregar, Z.; Biscan, M.; Milosevic, S.; Vesel, A. Monitoring oxygen plasma treatment of polypropylene with optical emission spectroscopy. IEEE Trans. Plasma Sci. 2011, 39, 1239–1246. [Google Scholar] [CrossRef]
- Krstulovic, N.; Labazan, I.; Milosevic, S.; Cvelbar, U.; Vesel, A.; Mozetic, M. Optical emission spectroscopy characterization of oxygen plasma during treatment of a PET foil. J. Phys. D Appl. Phys. 2006, 39, 3799–3804. [Google Scholar] [CrossRef]
- Xie, C.; Hu, X.; Zhou, L.; Xie, D.; Guo, H. Ab initio determination of potential energy surfaces for the first two UV absorption bands of SO2. J. Chem. Phys. 2013, 139, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Zaplotnik, R.; Vesel, A.; Mozetič, M. Characteristics of gaseous plasma created in SO2 by inductively coupled RF discharge in E and H modes. J. Appl. Phys. 2016, 120, 163–169. [Google Scholar] [CrossRef]
- Vesel, A.; Zaplotnik, R.; Modic, M.; Mozetic, M. Hemocompatibility properties of a polymer surface treated in plasma containing sulfur. Surf. Interface Anal. 2016, 48, 601–605. [Google Scholar] [CrossRef]
- Cvelbar, U.; Mozetič, M.; Junkar, I.; Vesel, A.; Kovač, J.; Drenik, A.; Vrlinič, T.; Hauptman, N.; Klanjšek-Gunde, M.; Markoli, B.; Krstulović, N.; Milošević, S.; Gaboriau, F.; Belmonte, T. Oxygen plasma functionalization of poly(p-phenilene sulphide). Appl. Surf. Sci. 2007, 253, 8669–8673. [Google Scholar] [CrossRef]
- Tsougeni, K.; Vourdas, N.; Tserepi, A.; Gogolides, E.; Cardinaud, C. Mechanisms of oxygen plasma nanotexturing of organic polymer surfaces: from stable super hydrophilic to super hydrophobic surfaces. Langmuir 2009, 25, 11748–11759. [Google Scholar] [CrossRef] [PubMed]
- Wohlfart, E.; Fernández-Blázquez, J.P.; Knoche, E.; Bello, A.; Pérez, E.; Arzt, E.; del Campo, A. Nanofibrillar Patterns by Plasma Etching: The influence of polymer crystallinity and orientation in surface morphology. Macromolecules 2010, 43, 9908–9917. [Google Scholar] [CrossRef]
- Slepicka, P.; Kasalkova, N.S.; Stranska, E.; Bacakova, L.; Svorcik, V. Surface characterization of plasma treated polymers for applications as biocompatible carriers. Express Polym. Lett. 2013, 7, 535–545. [Google Scholar] [CrossRef]
- Kontziampasis, D.; Constantoudis, V.; Gogolides, E. Plasma Directed organization of nanodots on polymers: Effects of polymer type and etching time on morphology and order. Plasma Process. Polym. 2012, 9, 866–872. [Google Scholar] [CrossRef]
- Li, Y.P.; Shi, W.; Li, S.Y.; Lei, M.K. Transition of water adhesion on superhydrophobic surface during aging of polypropylene modified by oxygen capacitively coupled radio frequency plasma. Surf. Coat. Technol. 2012, 213, 139–144. [Google Scholar] [CrossRef]
- Li, Y.P.; Li, S.Y.; Shi, W.; Lei, M.K. Hydrophobic over-recovery during aging of polyethylene modified by oxygen capacitively coupled radio frequency plasma: A new approach for stable superhydrophobic surface with high water adhesion. Surf. Coat. Technol. 2012, 206, 4952–4958. [Google Scholar] [CrossRef]
- Cortese, B.; Morgan, H. Controlling the wettability of hierarchically structured thermoplastics. Langmuir 2012, 28, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Blázquez, J.P.; Fell, D.; Bonaccurso, E.; Campo, A.D. Superhydrophilic and superhydrophobic nanostructured surfaces via plasma treatment. J. Colloid Interface Sci. 2011, 357, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, F.; Di Mundo, R.; Cappelluti, D.; d′Agostino, R. Superhydrophobic and superhydrophilic polycarbonate by tailoring chemistry and nano-texture with plasma processing. Plasma Process. Polym. 2011, 8, 118–126. [Google Scholar] [CrossRef]
- Doliska, A.; Vesel, A.; Kolar, M.; Stana-Kleinschek, K.; Mozetic, M. Interaction between model poly(ethylene terephthalate) thin films and weakly ionised oxygen plasma. Surf. Interface Anal. 2012, 44, 56–61. [Google Scholar] [CrossRef]
- Kim, J.; Ahn, D.U.; Sancaktar, E. The effects of excimer laser irradiation on surface morphology development in stretched poly(ethylene therephthalate), poly(butylene therephthalate) and polystyrene films. In Polymer Surface Modification, Relevance to Adhesion, 1st ed.; Mittal, K.L., Ed.; CRC Press: Boca Raton, FL, USA, 2007; Volume 4, pp. 33–86. [Google Scholar]
- Kostov, K.G.; Nishime, T.M.C.; Hein, L.R.O.; Toth, A. Study of polypropylene surface modification by air dielectric barrier discharge operated at two different frequencies. Surf. Coat. Technol. 2013, 234, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Cismaru, C.; Shohet, J.L. Plasma vacuum ultraviolet emission in an electron cyclotron resonance etcher. Appl. Phys. Lett. 1999, 74, 2599–2601. [Google Scholar] [CrossRef]
- Vesel, A.; Junkar, I.; Cvelbar, U.; Kovac, J.; Mozetic, M. Surface modification of polyester by oxygen- and nitrogen-plasma treatment. Surf. Interface Anal. 2008, 40, 1444–1453. [Google Scholar] [CrossRef]
- Izdebska, J.; Sabu, T. Printing on polymers: Fundamentals and applications, 1st ed.; Elsevier Science Publishing: Waltham, USA, 2016; pp. 116–118. [Google Scholar]
- Ramiasa-MacGregor, M.; Mierczynska, A.; Sedev, R.; Vasilev, K. Tuning and predicting the wetting of nanoengineered material surface. Nanoscale 2016, 8, 4635–4642. [Google Scholar] [CrossRef] [PubMed]
- Bico, J.; Tordeux, C.; Quere, D. Rough wetting. Europhys. Lett. 2001, 55, 214–220. [Google Scholar] [CrossRef]
- Encinas, N.; Pantoja, M.; Abenojar, J.; Martínez, M.A. Control of wettability of polymers by surface roughness modification. J. Adhes. Sci. Technol. 2010, 24, 1869–1883. [Google Scholar] [CrossRef]
- Flemming, R.G.; Murphy, C.J.; Abrams, G.A.; Goodman, S.L.; Nealey, P.F. Effects of synthetic micro- and nano-structured surfaces on cell behavior. Biomaterials 1999, 20, 573–588. [Google Scholar] [CrossRef]
- Anselme, K.; Davidson, P.; Popa, A.M.; Giazzon, M.; Liley, M.; Ploux, L. The interaction of cells and bacteria with surfaces structured at the nanometre scale. Acta Biomater. 2010, 6, 3824–3846. [Google Scholar] [CrossRef] [PubMed]
- Martínez, E.; Engel, E.; Planell, J.A.; Samitier, J. Effects of artificial micro- and nano-structured surfaces on cell behaviour. Ann. Anat. 2009, 191, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Lord, M.S.; Foss, M.; Besenbacher, F. Influence of nanoscale surface topography on protein adsorption and cellular response. Nano Today 2010, 5, 66–78. [Google Scholar] [CrossRef]
- Mendes, P.M. Cellular nanotechnology: Making biological interfaces smarter. Chem. Soc. Rev. 2013, 42, 9207–9218. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Recek, N.; Resnik, M.; Zaplotnik, R.; Mozetic, M.; Motaln, H.; Lah-Turnsek, T.; Vesel, A. Cell Proliferation on Polyethylene Terephthalate Treated in Plasma Created in SO2/O2 Mixtures. Polymers 2017, 9, 82. https://doi.org/10.3390/polym9030082
Recek N, Resnik M, Zaplotnik R, Mozetic M, Motaln H, Lah-Turnsek T, Vesel A. Cell Proliferation on Polyethylene Terephthalate Treated in Plasma Created in SO2/O2 Mixtures. Polymers. 2017; 9(3):82. https://doi.org/10.3390/polym9030082
Chicago/Turabian StyleRecek, Nina, Matic Resnik, Rok Zaplotnik, Miran Mozetic, Helena Motaln, Tamara Lah-Turnsek, and Alenka Vesel. 2017. "Cell Proliferation on Polyethylene Terephthalate Treated in Plasma Created in SO2/O2 Mixtures" Polymers 9, no. 3: 82. https://doi.org/10.3390/polym9030082