Shape Memory Investigation of α-Keratin Fibers as Multi-Coupled Stimuli of Responsive Smart Materials
Abstract
:1. Introduction
2. Materials and Methods
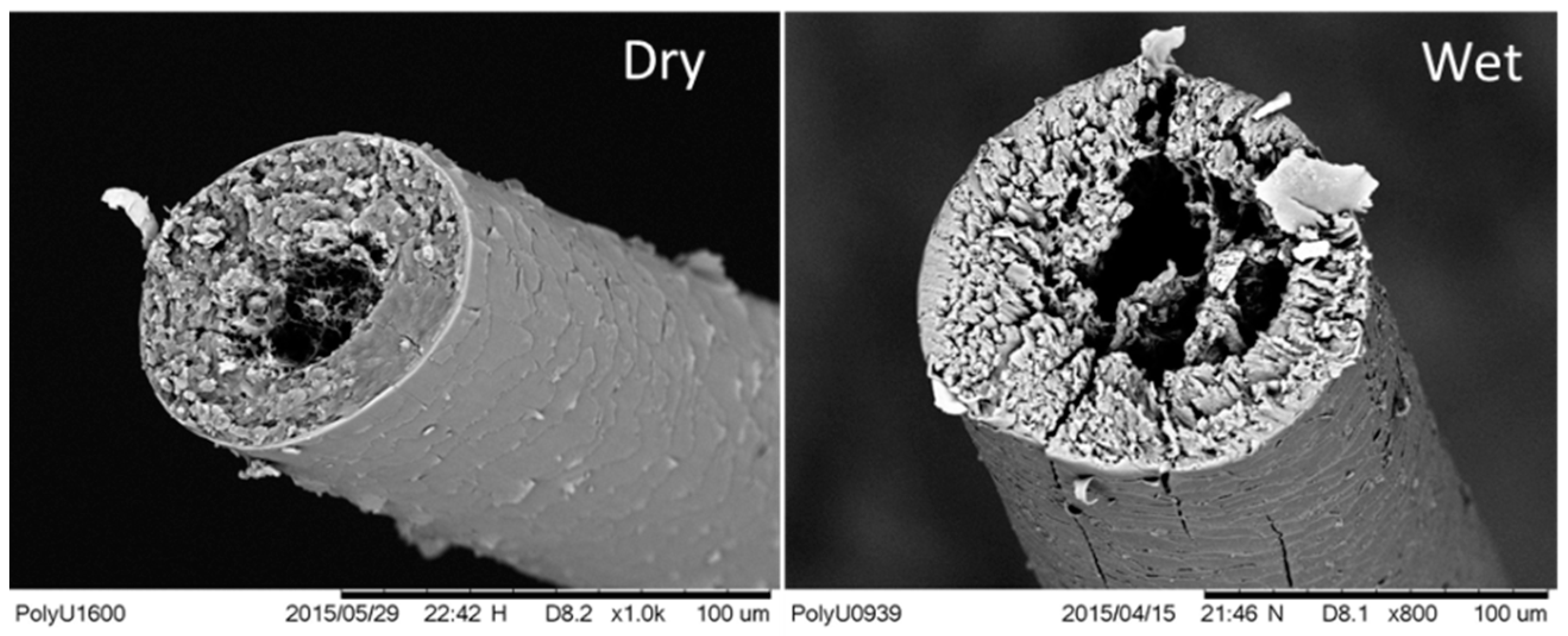
2.1. Preparation of -Keratin Hair Fibers
2.2. Characterization of Fiber Coupled Stimuli of SME
2.3. Quantitative Characterization of Hair Coupled Stimuli of SME
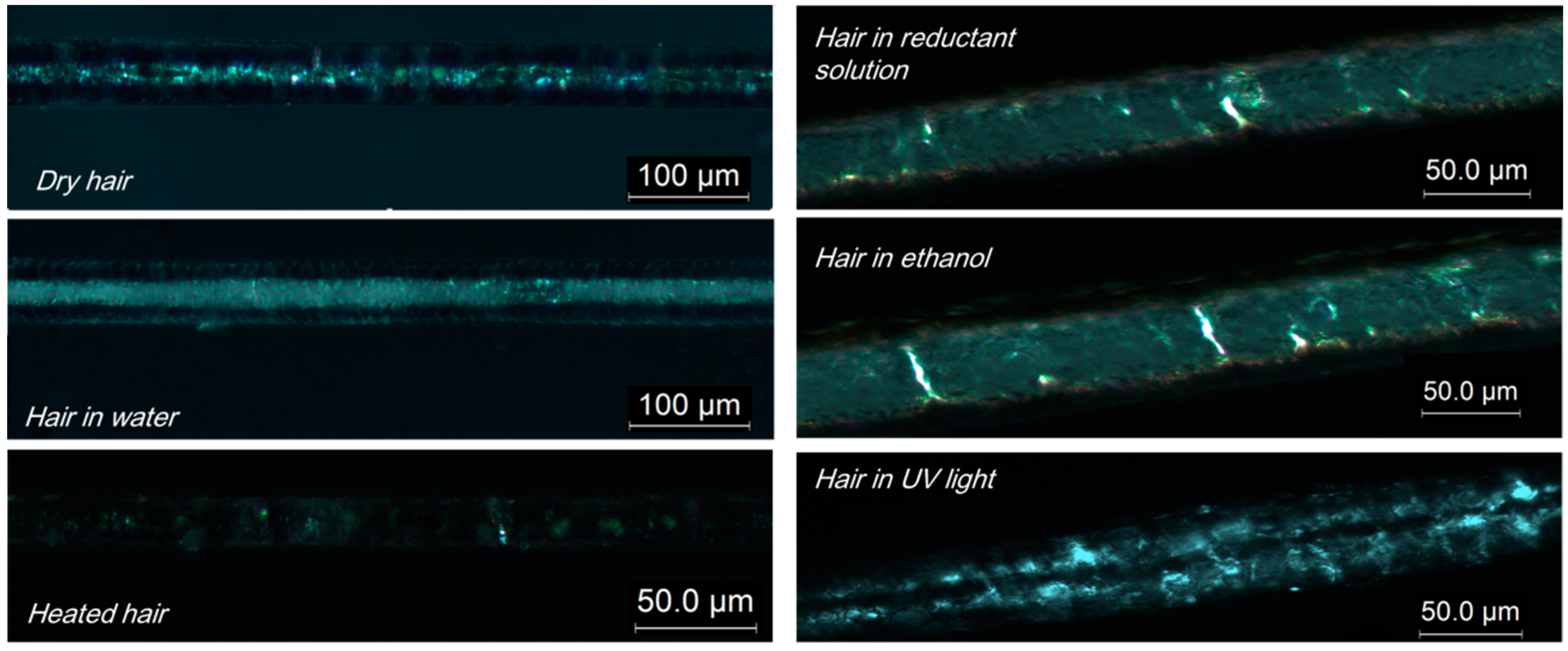
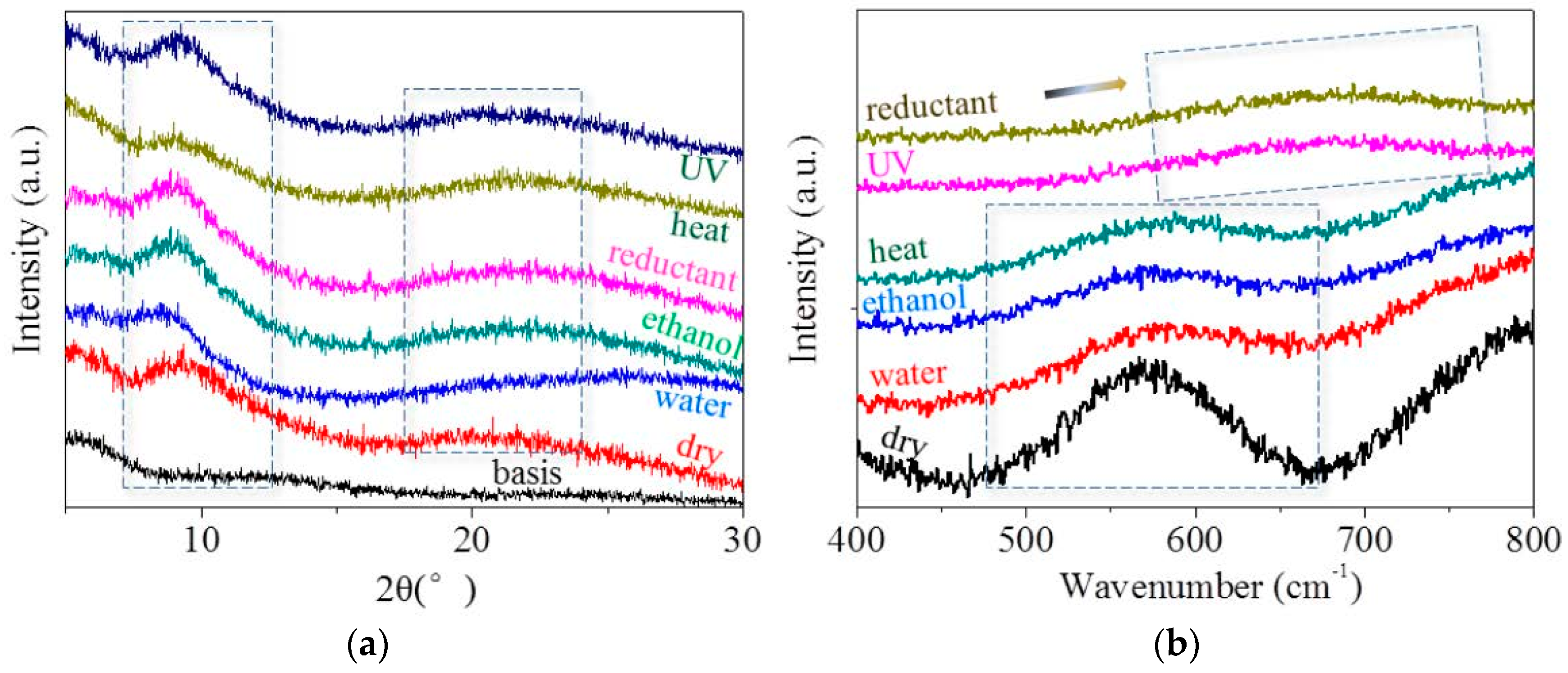
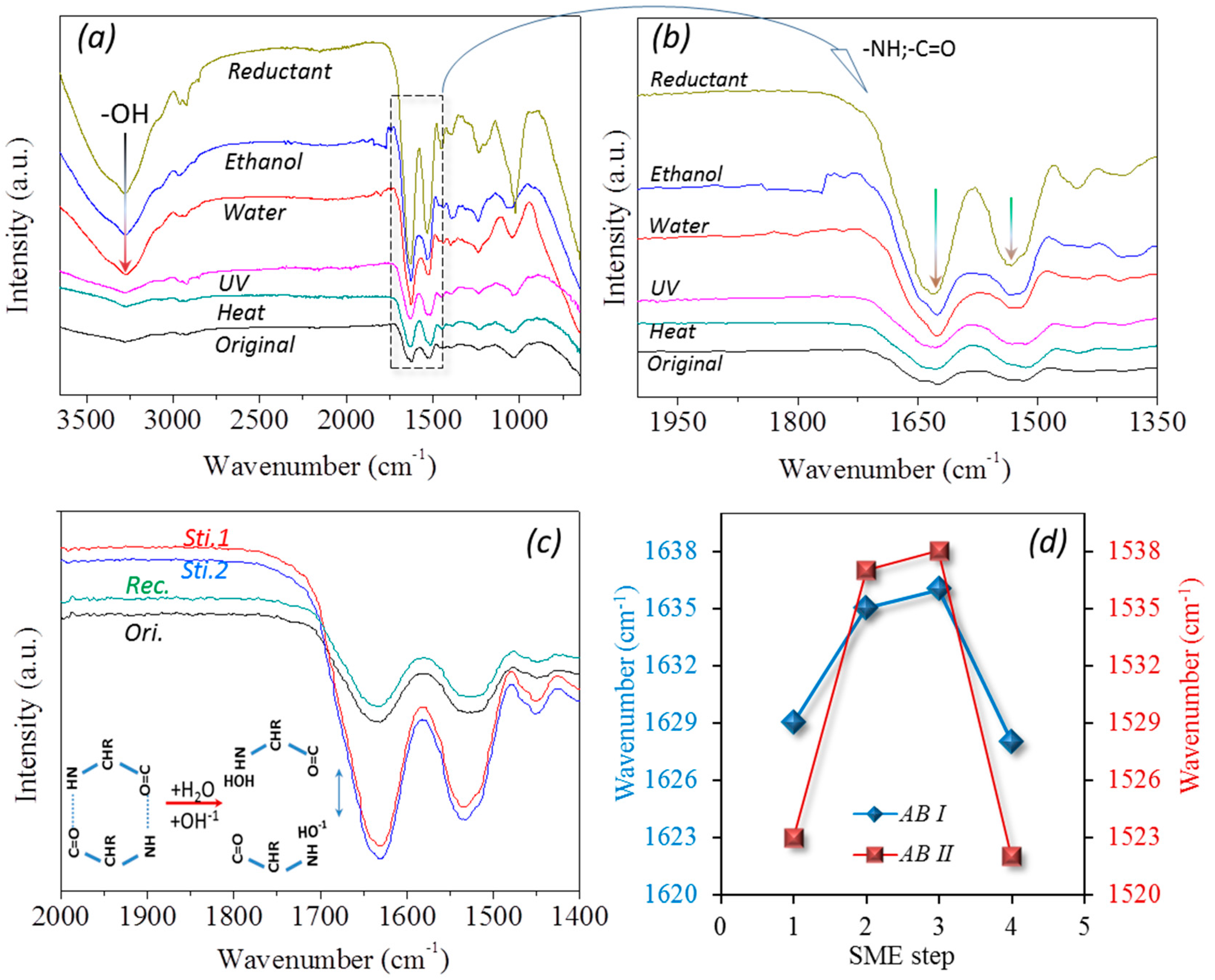
2.4. Molecule Network Characterization of Hair Fiber during SME
3. Results and Discussions
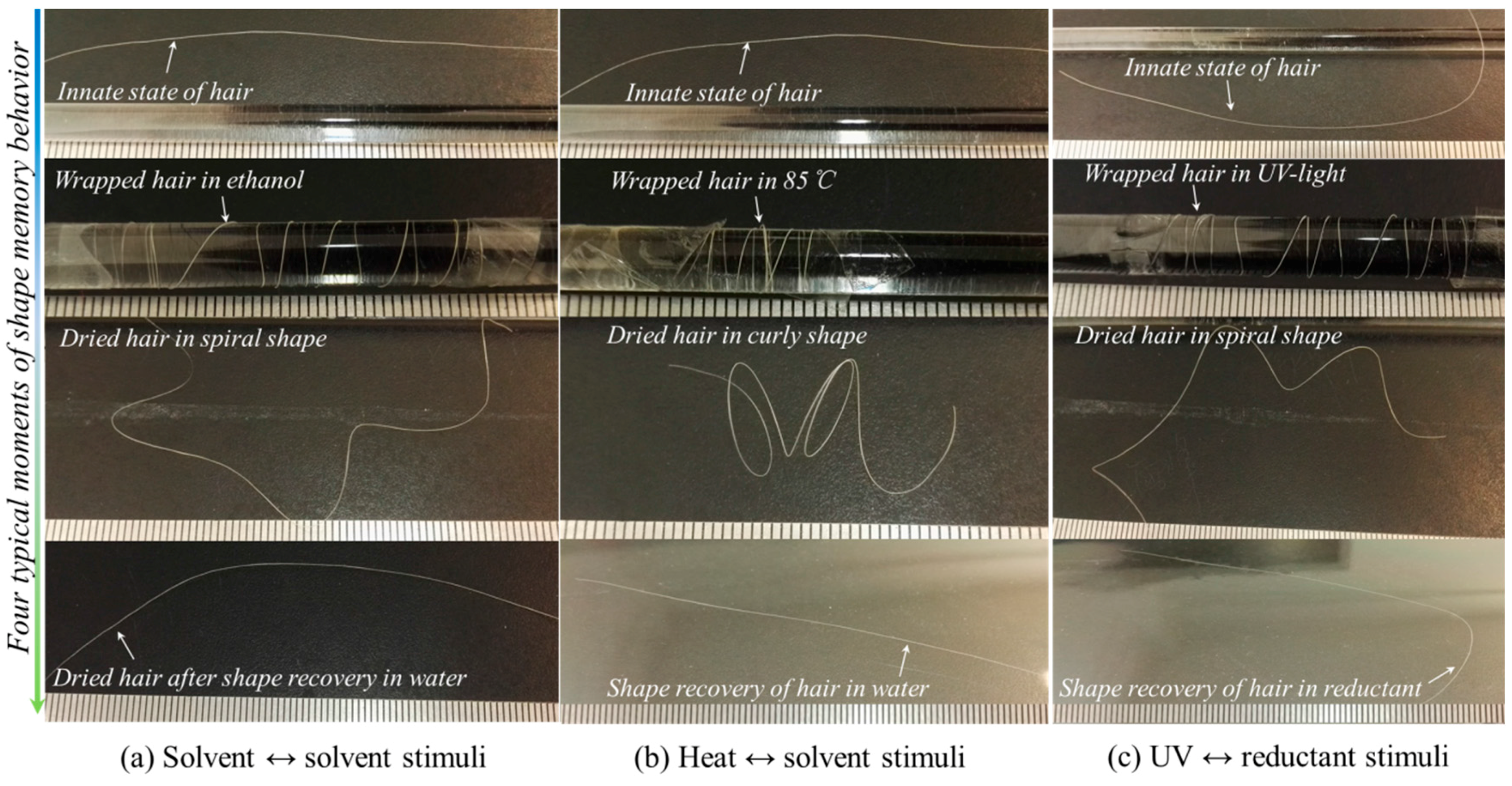
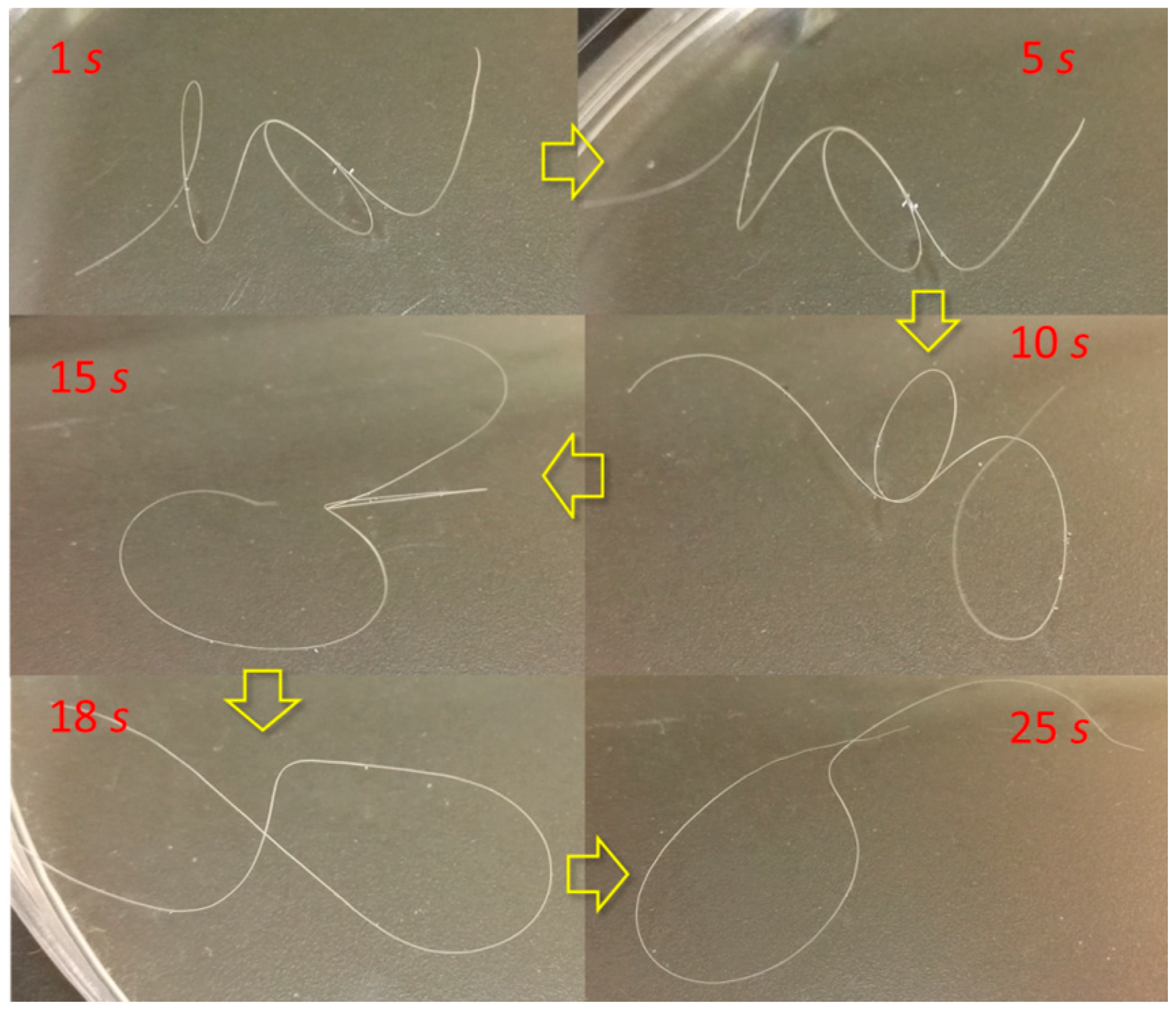
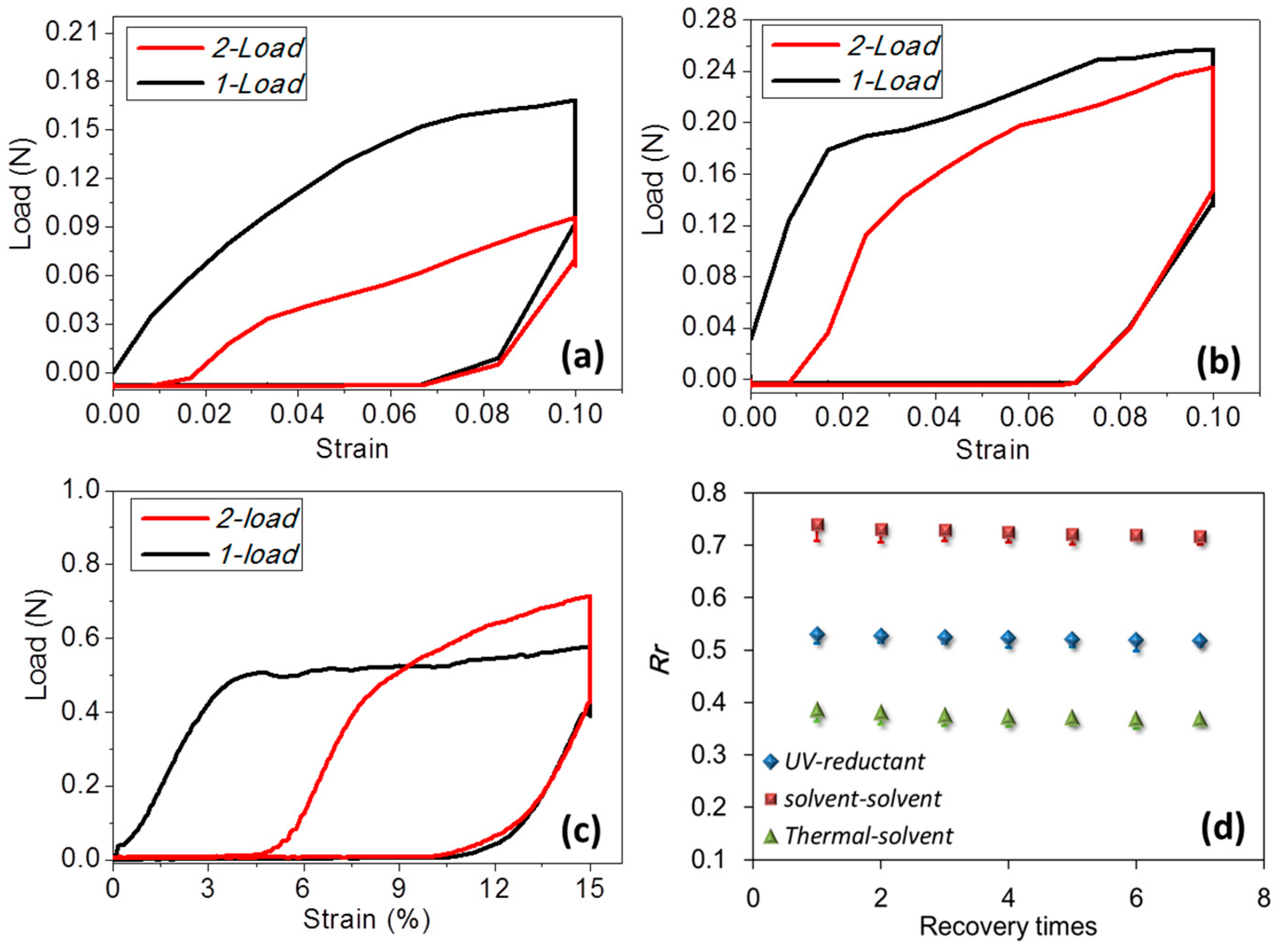
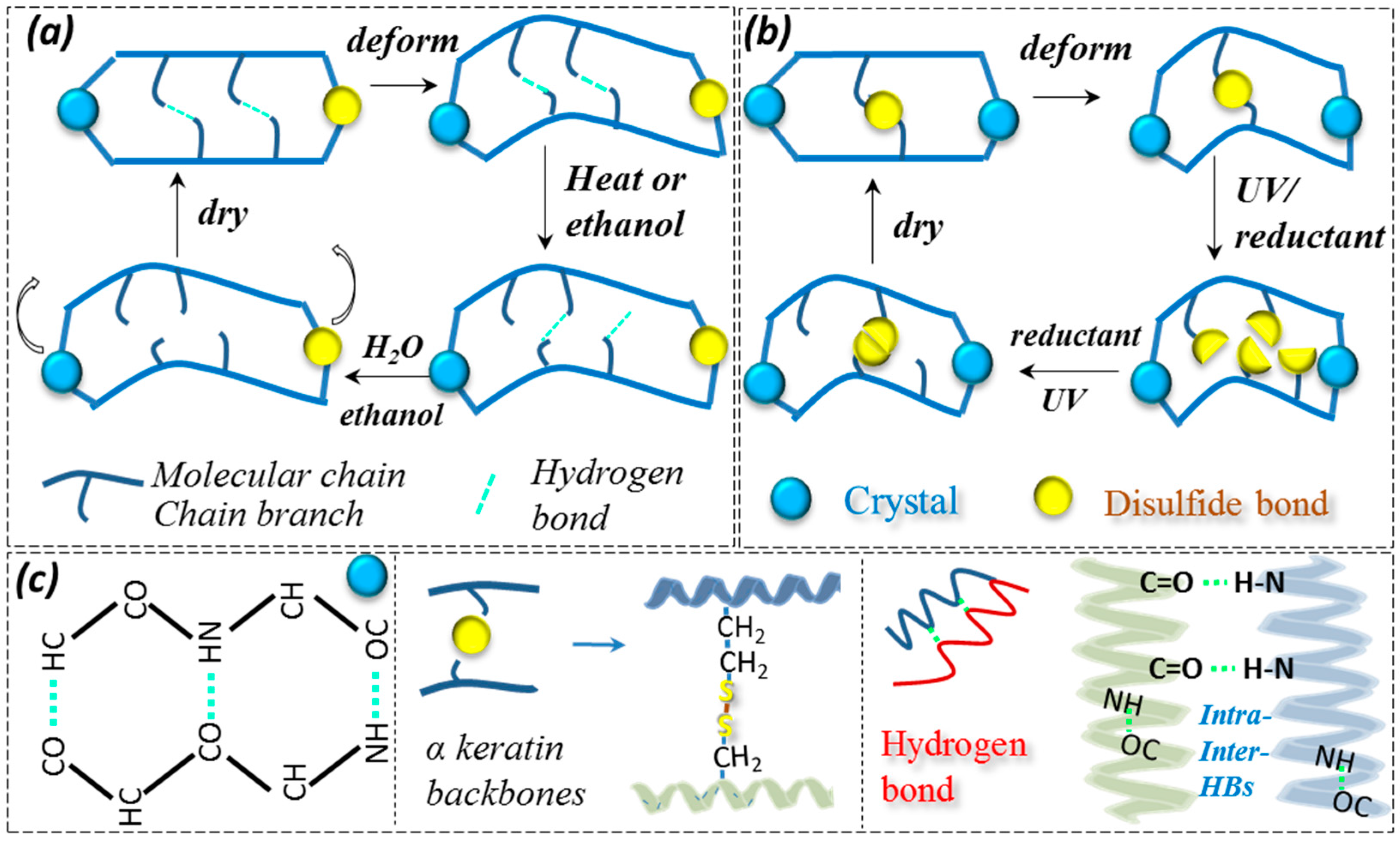
3.1. Coupled Stimuli of SM Behaviors of -Keratin Hair Fiber
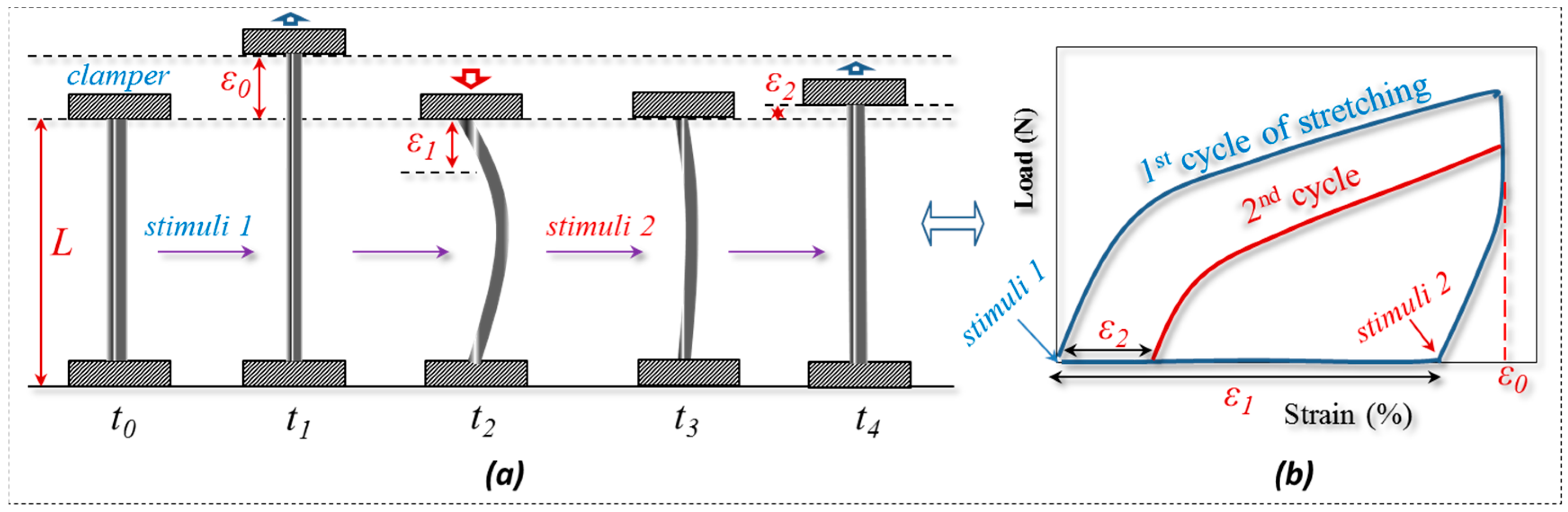
3.2. Quantification of Coupled Stimuli Sensitive SME Ability
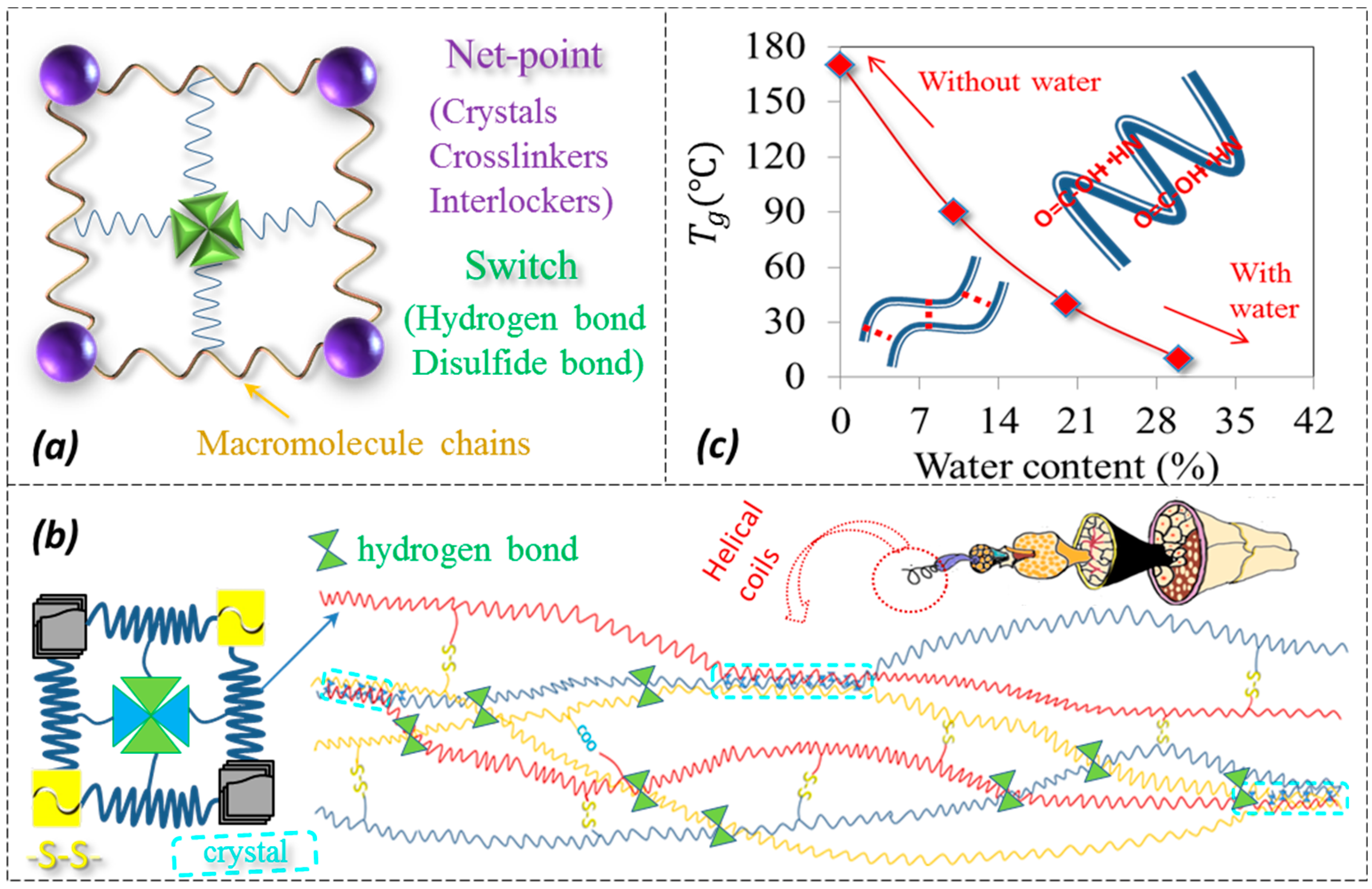
3.3. Characterization of Netpoint and Switch of SM Hairs
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tcharkhtchi, A.; Abdallah-Elhirtsi, S.; Ebrahimi, K.; Fitoussi, J.; Shirinbayan, M.; Farzaneh, S. Some new concepts of shape memory effect of polymers. Polymers 2014, 6, 1144–1163. [Google Scholar] [CrossRef] [Green Version]
- Leng, J.; Lan, X.; Liu, Y.; Du, S. Shape-memory polymers and their composites: Stimulus methods and applications. Prog. Mater. Sci. 2011, 56, 1077–1135. [Google Scholar] [CrossRef]
- Stuart, M.A.C.; Huck, W.T.; Genzer, J.; Multer, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, W.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhu, Y.; Huang, H.; Lv, J. Recent advances in shape-memory polymers: Structure, mechanism, functionality, modeling and applications. Prog. Polym. Sci. 2012, 37, 1720–1763. [Google Scholar] [CrossRef]
- Pretsch, T. Review on the functional determinants and durability of shape memory polymers. Polymers 2010, 2, 120–158. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, J.; Luo, H.; Young, R.J.; Deng, L.; Zhang, S.; Fan, Y.; Ye, G. Rapidly switchable water-sensitive shape-memory cellulose/elastomer nano-composites. Soft Matter 2012, 8, 2509–2517. [Google Scholar] [CrossRef]
- Li, J.; Su, Z.; Xu, H.; Ma, X.; Yin, J.; Jiang, X. Supramolecular networks of hyperbranched poly(ether amine) (hPEA) nanogel/chitosan (CS) for selective adsorption and separation of guest molecules. Macromolecules 2015, 48, 2022–2029. [Google Scholar] [CrossRef]
- Bai, Q.M.; Zhang, G.; Xu, B.; Feng, X.; Jiang, H.; Li, H. Thermal and water dual-responsive shape memory poly(vinyl alcohol)/Al2O3 nanocomposite. RSC Adv. 2015, 5, 91213–91217. [Google Scholar] [CrossRef]
- Meng, Q.; Hu, J. A temperature-regulating fiber made of PEG-based smart copolymer. Sol. Energy Mater. Sol. Cells 2008, 92, 1245–1252. [Google Scholar] [CrossRef]
- Jung, Y.C.; So, H.H.; Cho, J.W. Water-responsive shape memory polyurethane block copolymer modified with polyhedral oligomeric silsesquioxane. J. Macromol. Sci. B 2006, 45, 453–461. [Google Scholar] [CrossRef]
- Belmonte, A.; Guzman, D.; Fernandez-Francos, X.; De la Flor, S. Effect of the network structure and programming temperature on the shape-memory response of thiol-epoxy ‘click’ systems. Polymers 2015, 7, 2146–2164. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.M.; Yang, B.; An, L.; Li, C.; Chan, Y.S. Water-driven programmable polyurethane shape memory polymer: Demonstration and mechanism. Appl. Phys. Lett. 2005, 86, 114105–114110. [Google Scholar] [CrossRef]
- Xie, T. Recent advances in polymer shape memory. Polymer 2011, 52, 4985–5000. [Google Scholar] [CrossRef]
- Dagnon, K.L.; Way, A.E.; Carson, S.O.; Silva, J.; Maia, J.; Rowan, S.J. Controlling the rate of water-induced switching in mechanically dynamic cellulose nanocrystal composites. Macromolecules 2013, 46, 8203–8212. [Google Scholar] [CrossRef]
- Chen, S.; Hu, J.; Zhuo, H. Properties and mechanism of two-way shape memory polyurethane composites. Compos. Sci. Technol. 2010, 70, 1437–1443. [Google Scholar] [CrossRef]
- Huang, H.; Hu, J.; Zhu, Y. Shape-memory biopolymers based on β-sheet structures of polyalanine segments inspired by spider silks. Macromol. Biosci. 2013, 13, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, G.; Kato, R.; Tokuda, M.; Nishitani, Y. Intraoral temperature triggered shape-memory effect and sealing capability of a transpolyisoprene-based polymer. Polymers 2015, 7, 2259–2275. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, Y. Spider Silk: A smart biopolymer with water switchable shape memory effects—Unraveling the mystery of supercontraction. Res. J. Text. Appar. 2013, 17, 1–9. [Google Scholar] [CrossRef]
- Hu, J.; Dong, Z.E.; Liu, Y. The investigation about the shape memory behavior of wool. Adv. Sci. Technol. 2008, 60, 1–10. [Google Scholar] [CrossRef]
- Fratzl, P.; Barth, F.G. Biomaterial systems for mechano sensing and actuation. Nature 2009, 462, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Meyers, M.A.; McKittrick, J.; Chen, P.Y. Structural biological materials: Critical mechanics-materials connections. Science 2013, 339, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Elbaum, R.; Zaltzman, L.; Burgert, I.; Fratzl, P. The role of wheat awns in the seed dispersal unit. Science 2007, 316, 884–886. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Jiao, D.; Zhang, Z.F. Remarkable shape memory effect of a natural biopolymer in aqueous environment. Biomaterials 2015, 65, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.L.; Hu, J.L. Animal Hairs as Water-stimulated Shape Memory Materials: Mechanism and Structural Networks in Molecular Assemblies. Sci. Rep. 2016, 6, 26393–26405. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Hu, J.; Gui, X.; Lu, J.; Luo, H. Is biopolymer hair a multi-responsive smart material? Polym. Chem. 2017, 8, 283–294. [Google Scholar] [CrossRef]
- Xu, W.; Ke, G.; Wu, J.; Wang, X. Modification of wool fiber using steam explosion. Eur. Polym. J. 2006, 42, 2168–2173. [Google Scholar] [CrossRef]
- Barba, C.; Marti, M.; Manich, A.M.; Carilla, J.; Coderch, L. Water absorption/desorption of human hair and nails. Thermochim. Acta 2010, 503, 33–39. [Google Scholar] [CrossRef]
- Pauling, L. The Nature of the Chemical Bond, 2nd ed.; Pauling, L., Ed.; Cornel University Press: New York, NY, USA, 1948. [Google Scholar]
- Fracer, R.D.B.; MacRae, T.P.; Rogers, G.E. Keratins, Their Composition, Structure and Biosynthesis; Charles C Thomas Publishing: Springfield, IL, USA, 1972; pp. 9–17. [Google Scholar]
- Wortmann, F.J.; Rigby, B.J.; Phillips, D.G. Glass transition temperature of wool as a function of regain. Text. Res. J. 1984, 54, 6–8. [Google Scholar] [CrossRef]
- Xiao, X.L.; Hu, J.L.; Hui, D. Tensile-relaxation study of camel hair fiber at elastic stretching region: Analytical model and experiment. Compos. Part B Eng. 2016, 91, 559–568. [Google Scholar] [CrossRef]
- Meredith, R. The Mechanical Properties of Textile Fibers; Inter-Science Publishers: New York, NY, USA, 1956. [Google Scholar]
- Eaves, J.D.; Loparo, J.J.; Fecko, C.J.; Roberts, S.T.; Tokmakoff, A. HBs in liquid water are broken only fleetingly. Proc. Natl. Acad. Sci. USA 2005, 102, 13019–13022. [Google Scholar] [CrossRef] [PubMed]
- Kopke, V. The role of water in the setting of wool: A study of setting at temperatures above 100 °C: Part 1: Degree of set. J. Text. Inst. 1970, 61, 361–387. [Google Scholar] [CrossRef]
- Millington, K.R. Rapid permanent setting of wool fabrics by conductive heat transfer. Text. Res. J. 2012, 82, 1414–1421. [Google Scholar] [CrossRef]
- Le, C.V.; Jiang, X.H. The effects of steaming condition and wrapper type on the permanent setting of wool fabric. J. Text. Inst. 1997, 88, 53–63. [Google Scholar] [CrossRef]
- Nagia, F.A.; EL-Mohamedy, R.S.R. Dyeing of wool with natural anthraquinone dyes from Fusarium oxysporum. Dyes Pigments 2007, 75, 550–555. [Google Scholar] [CrossRef]
- Li, Y.T.; Lian, H.Q.; Hu, Y.N.; Chang, W.; Cui, X.G.; Liu, Y. Enhancement in mechanical and shape memory properties for liquid crystalline polyurethane strengthened by graphene oxide. Polymers 2016, 8, 236. [Google Scholar] [CrossRef]
- Richard-Lacroix, M.; Pellerin, C. Accurate new method for molecular orientation quantification using polarized Raman spectroscopy. Macromolecules 2013, 46, 5561–5569. [Google Scholar] [CrossRef]
- Gong, N.; He, X.; Zhou, M.; He, L.Q.; Fan, L.M.; Song, W.Z.; Sun, C.L.; Li, Z.W. Effects of fermi resonance of nu(1) and 2nu(2) on the Raman scattering of fundamental mode nu(2) from liquid carbon disulfide. Mater. Res. Bull. 2017, 85, 104–108. [Google Scholar] [CrossRef]
- Yao, J.; Liu, Y.; Yang, S.; Liu, J. Characterization of secondary structure transformation of stretched and slenderized wool fibers with FTIR spectra. J. Eng. Fabr. Fibers 2008, 3, 1–10. [Google Scholar]
- Ishida, Y.; Chabanne, L.; Antonietti, M.; Shalom, M. Morphology control and photocatalysis enhancement by the one-pot synthesis of carbon nitride from preorganized hydrogen bonded supra-molecular precursors. Langmuir 2014, 30, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, R.; Shi, Z.Q.; Huang, X.L.; Zhao, W.F.; Zhao, C.S. Multi-responsive, tough and reversible hydrogels with tunable swelling property. J. Hazard. Mater. 2017, 322, 499–507. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, X.; Hu, J.; Gui, X.; Qian, K. Shape Memory Investigation of α-Keratin Fibers as Multi-Coupled Stimuli of Responsive Smart Materials. Polymers 2017, 9, 87. https://doi.org/10.3390/polym9030087
Xiao X, Hu J, Gui X, Qian K. Shape Memory Investigation of α-Keratin Fibers as Multi-Coupled Stimuli of Responsive Smart Materials. Polymers. 2017; 9(3):87. https://doi.org/10.3390/polym9030087
Chicago/Turabian StyleXiao, Xueliang, Jinlian Hu, Xiaoting Gui, and Kun Qian. 2017. "Shape Memory Investigation of α-Keratin Fibers as Multi-Coupled Stimuli of Responsive Smart Materials" Polymers 9, no. 3: 87. https://doi.org/10.3390/polym9030087





