Monitoring Moisture Damage Propagation in GFRP Composites Using Carbon Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Fabrication
2.2. Electrical Conductivity of the Epoxy Nanocomposites
2.3. Moisture Absorption of GFRP Composites
2.4. Mechanical Characterization of the GFRP Composites
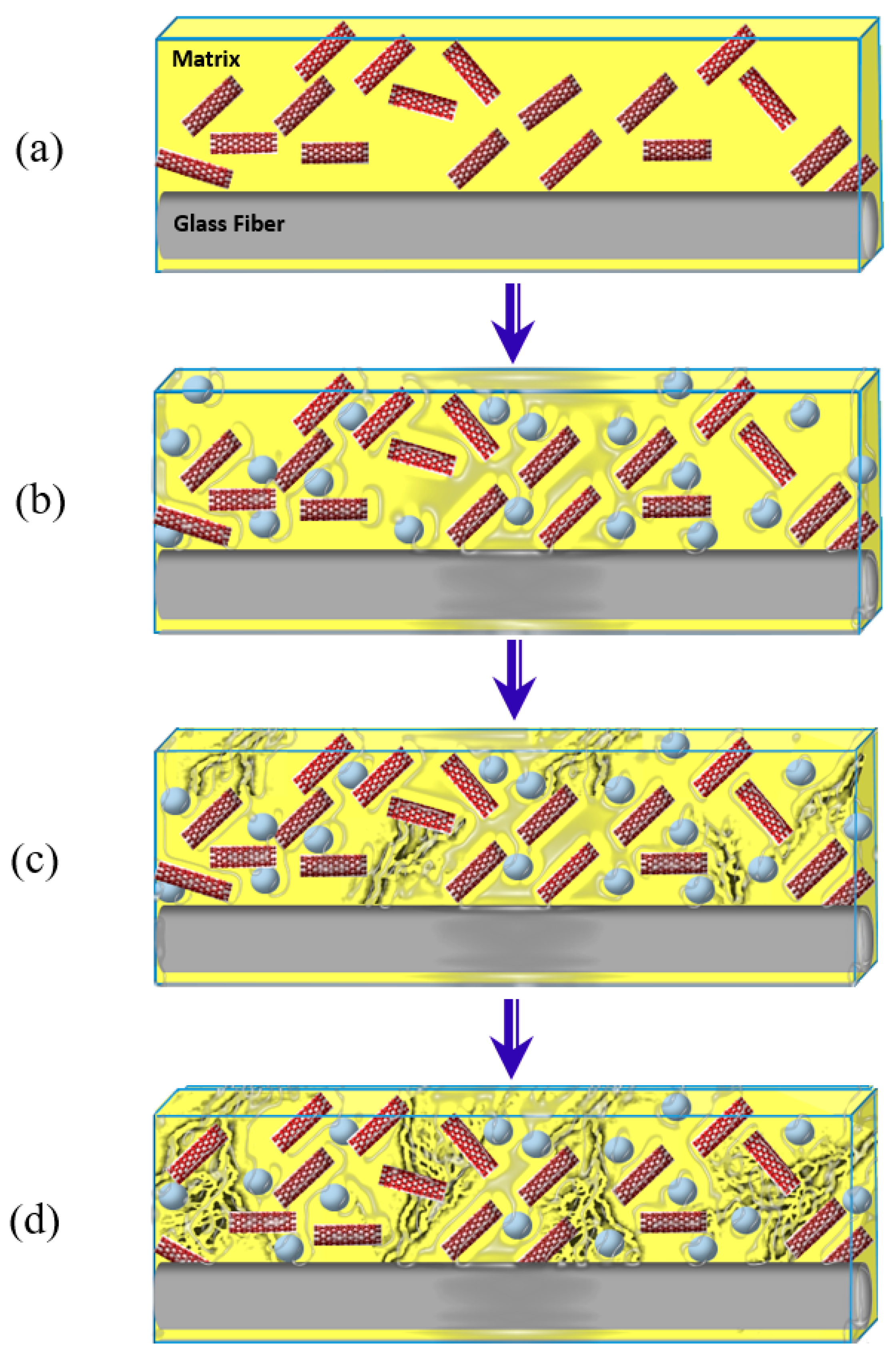
2.5. Microstructural Investigation of the GFRP Composites
3. Results
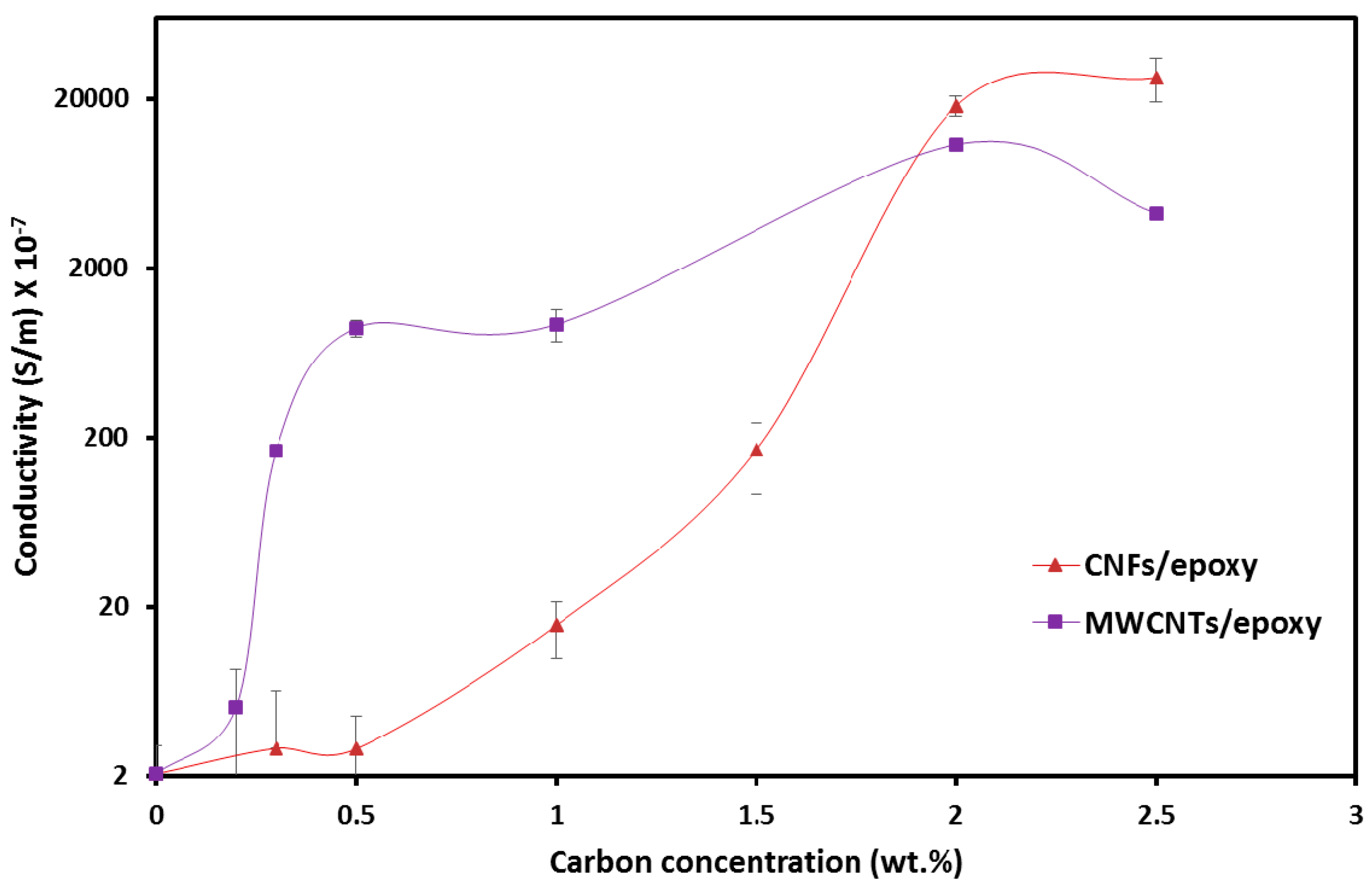
3.1. Electrical Properties of Epoxy Nanocomposites
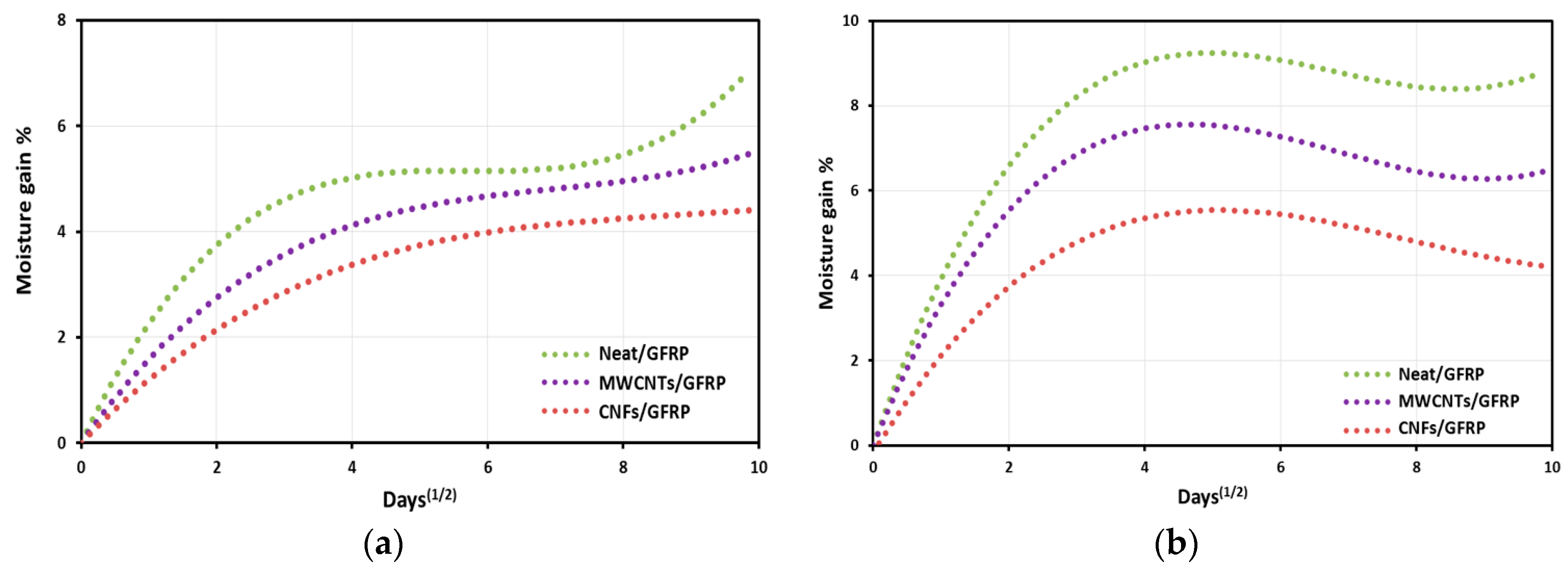
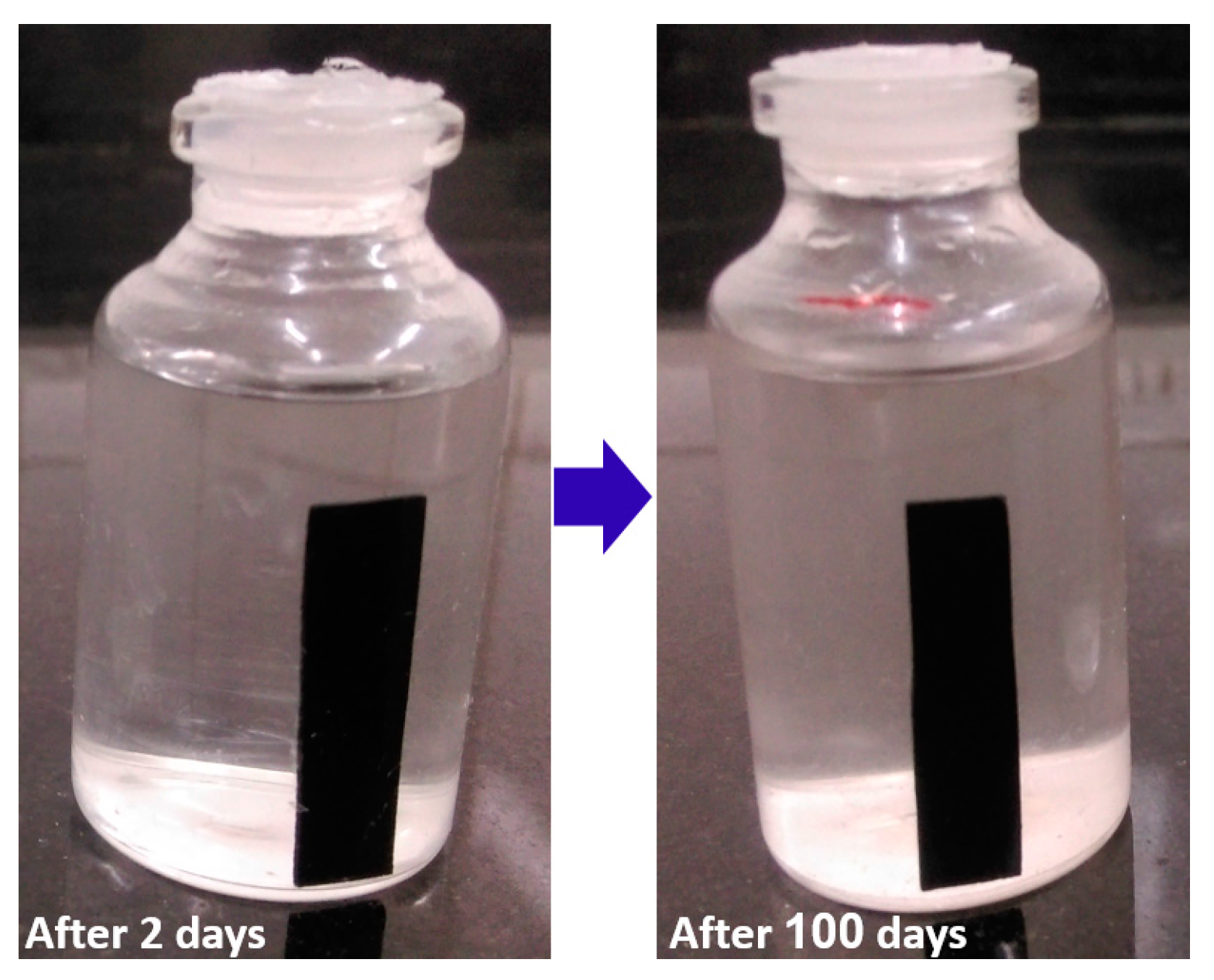
3.2. Moisture Absorption
3.3. Mechnical Properties of GFRP Composites
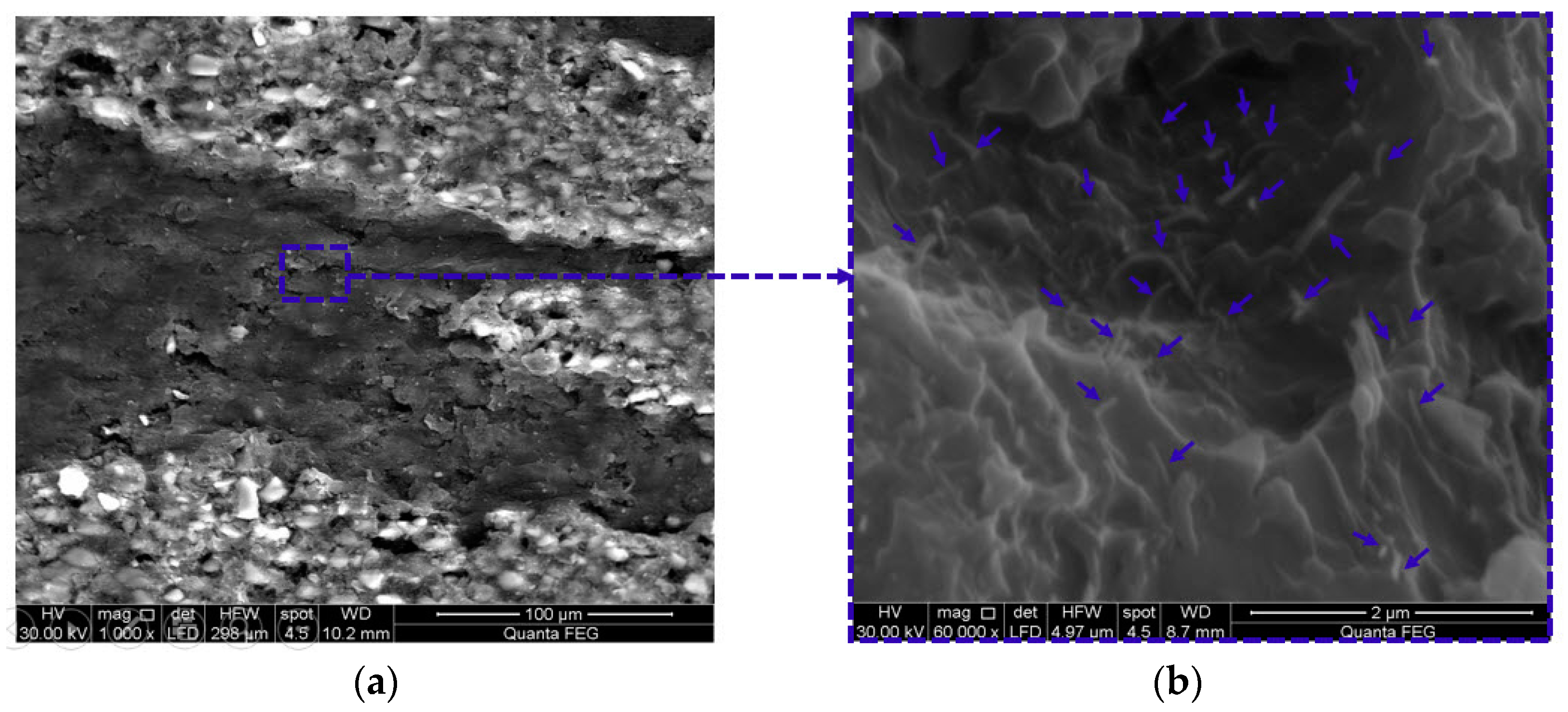
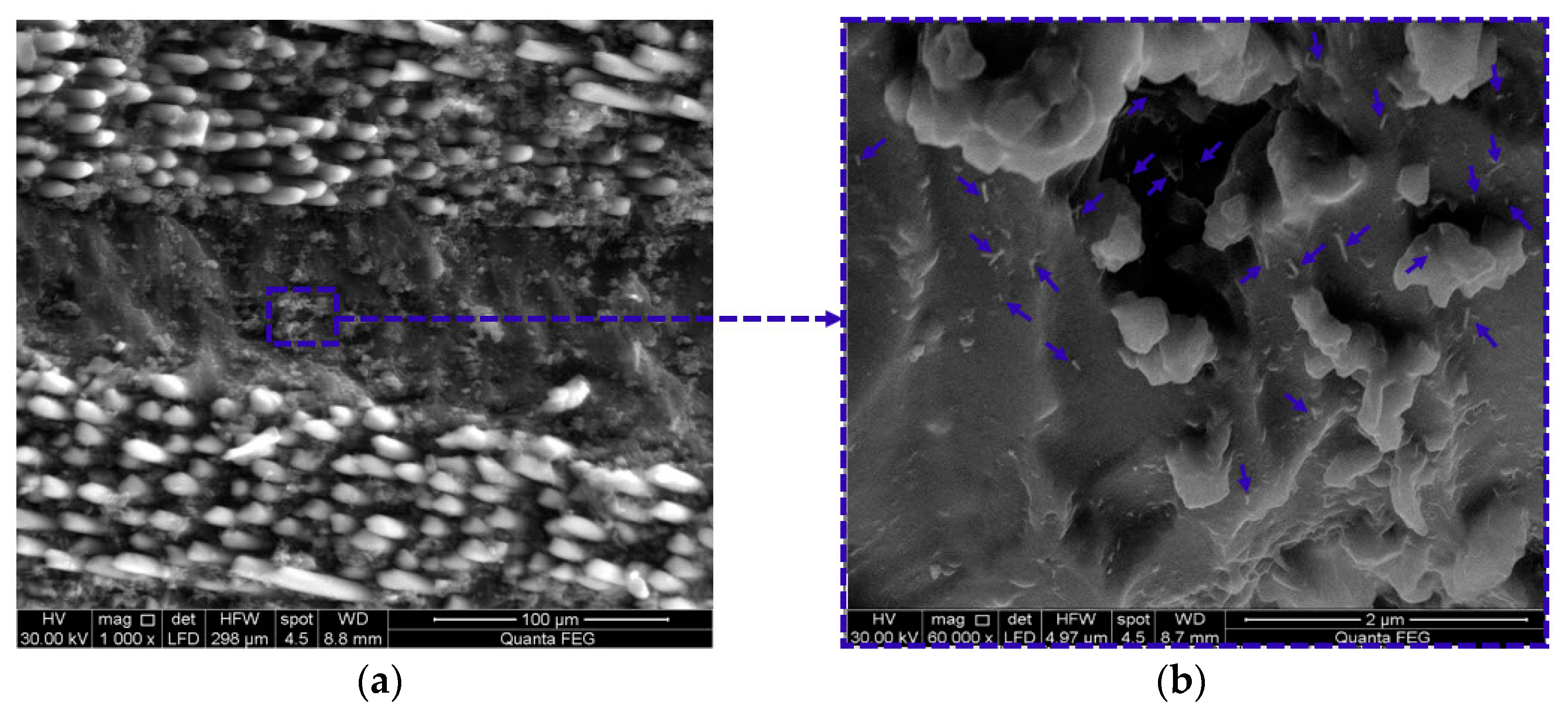
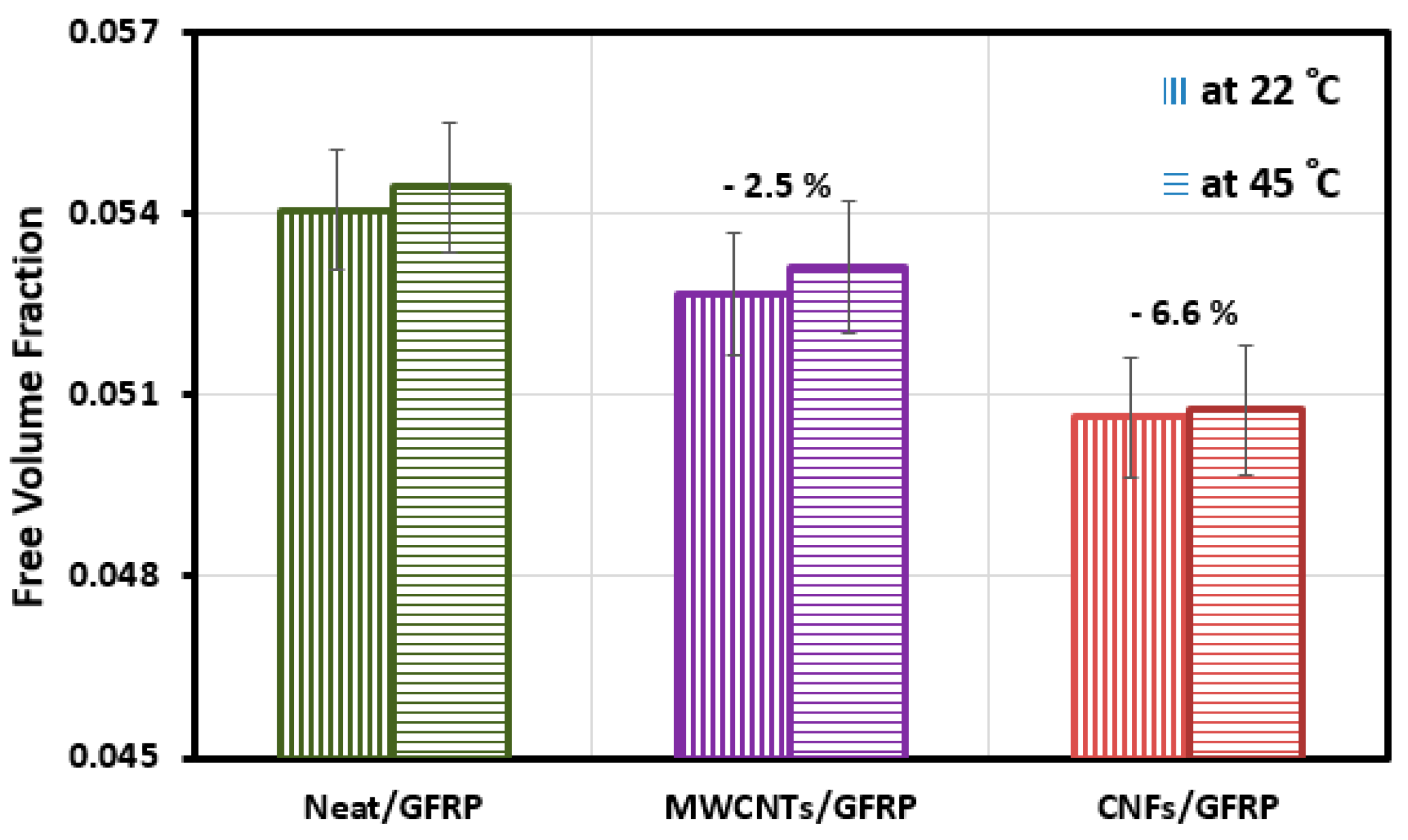
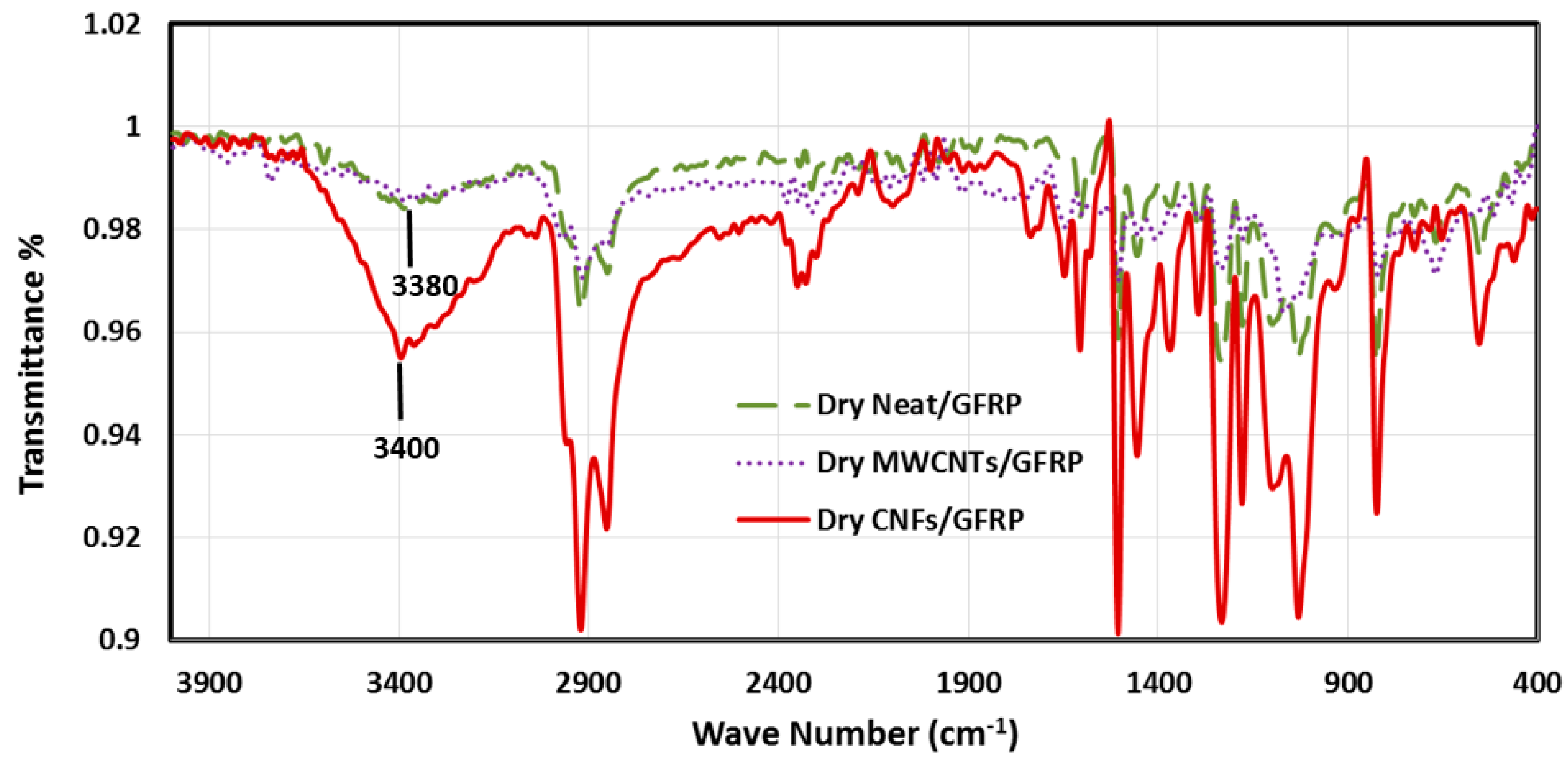
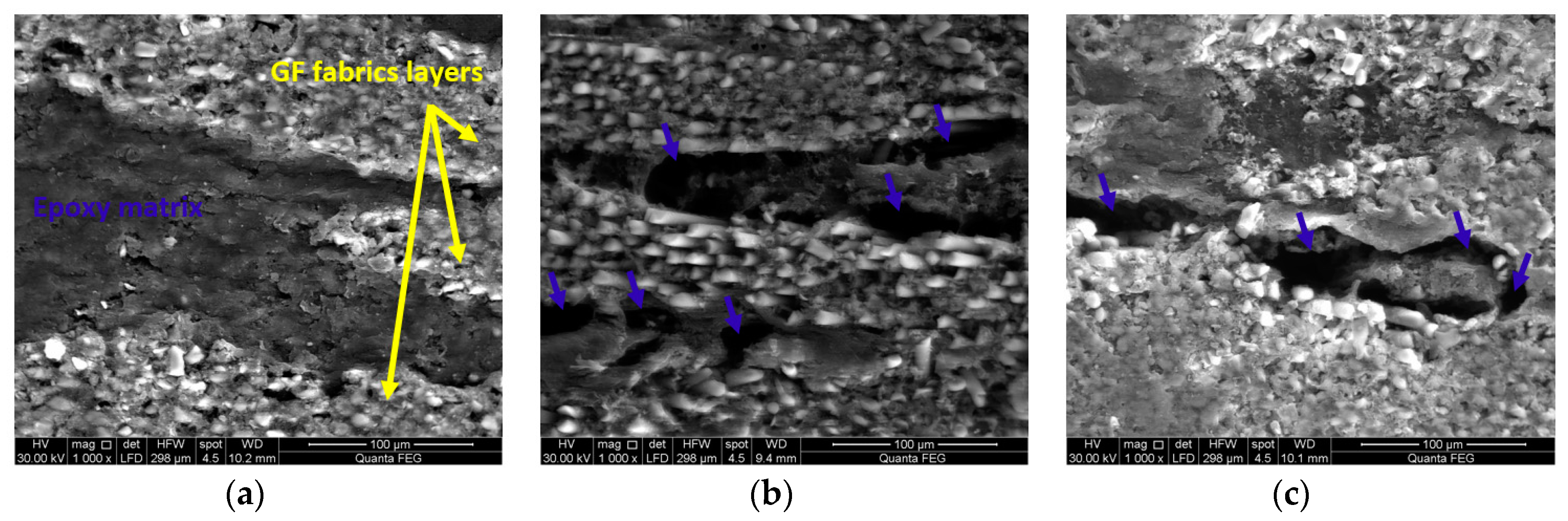
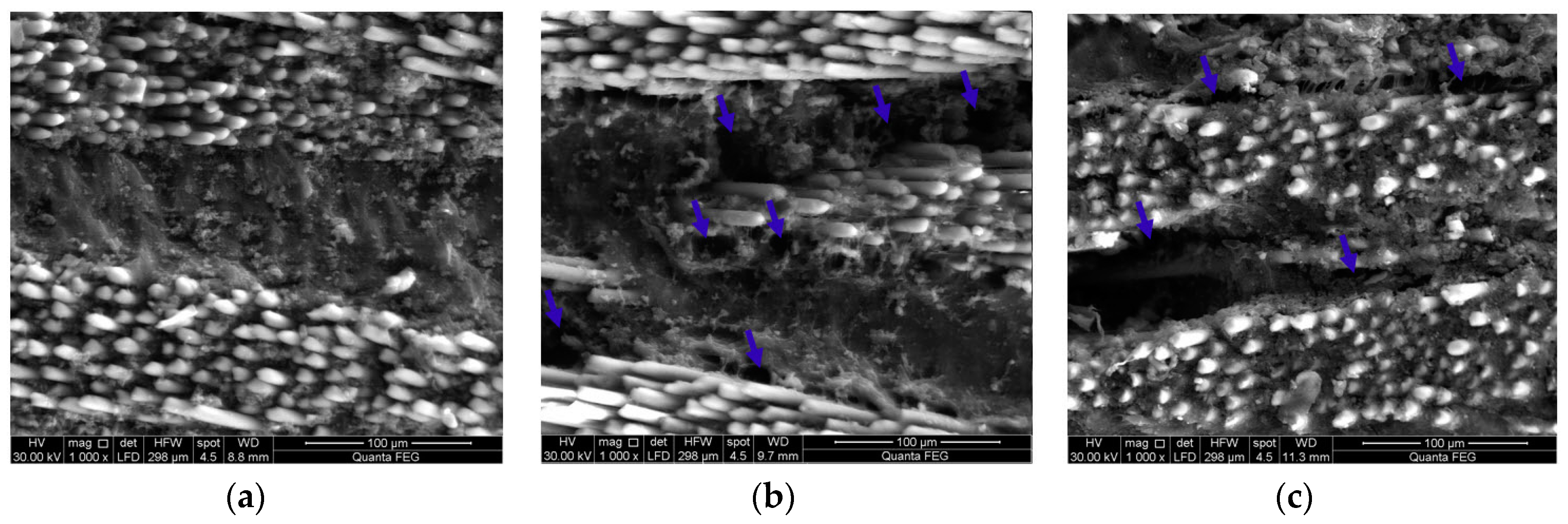
3.4. Microstructural Investigations of GFRP Composites
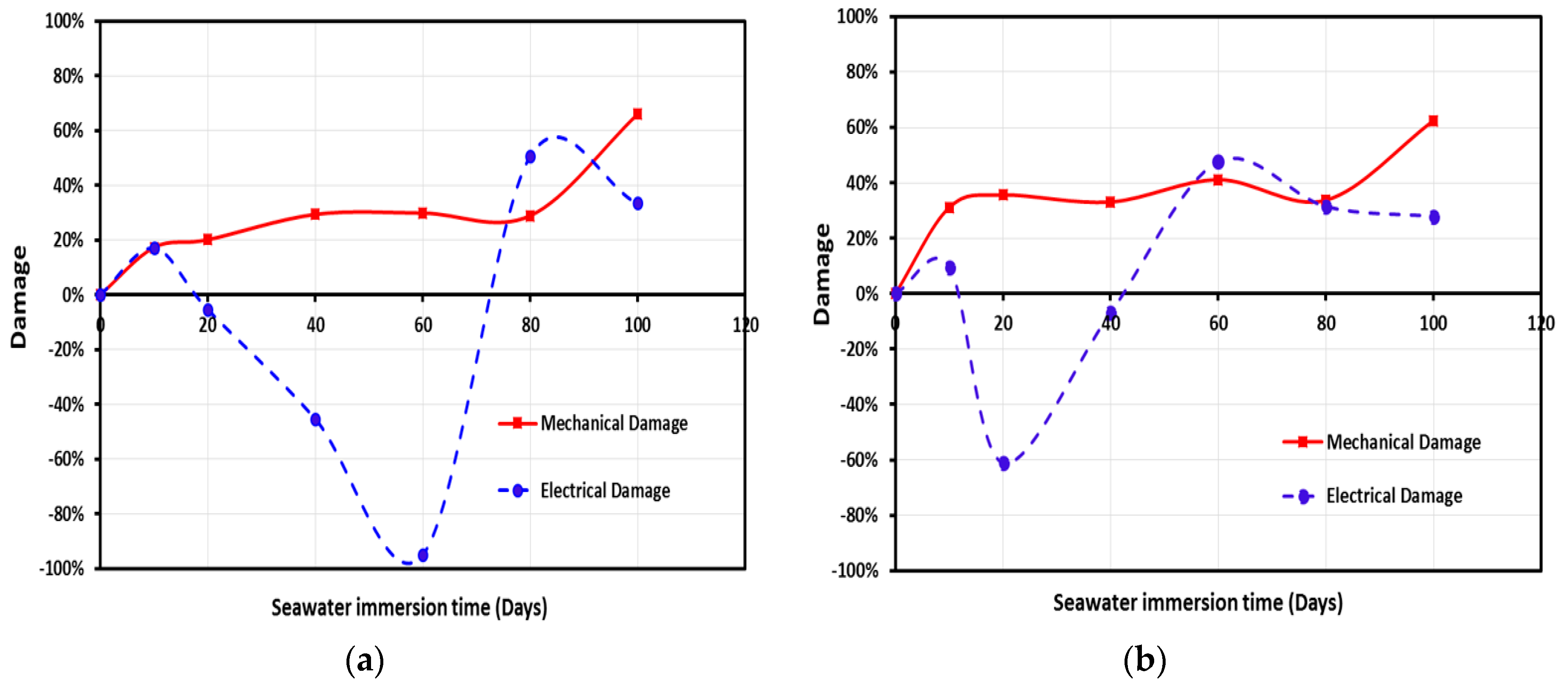
3.5. Monitoring Moisture Damage in GFRP Composites
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Einde, L.V.D.; Zhao, L.; Seible, F. Use of FRP composites in civil structural applications. Constr. Build. Mater. 2003, 17, 389–403. [Google Scholar] [CrossRef]
- Boisseau, A.; Davies, P.; Thiebaud, F. Sea water ageing of composites for ocean energy conversion systems: Influence of glass fibre type on static behavior. Appl. Compos. Mater. 2012, 19, 459–473. [Google Scholar] [CrossRef]
- Kafodya, I.; Xian, G.; Li, H. Durability study of pultruded CFRP plates immersed in water and seawater under sustained bending: Water uptake and effects on the mechanical properties. Compos. A Appl. Sci. 2015, 70, 138–148. [Google Scholar] [CrossRef]
- Bian, L.; Xiao, J.; Zeng, J.; Xing, S. Effects of seawater immersion on water absorption and mechanical properties of GFRP composites. J. Compos. Mater. 2012, 46, 3151–3162. [Google Scholar] [CrossRef]
- Srivastava, V.K. Influence of water immersion on mechanical properties of quasi-isotropic glass fibre reinforced epoxy vinylester resin composites. Mater. Sci. Eng. A. 1999, 263, 56–63. [Google Scholar] [CrossRef]
- Nakada, M.; Miyano, Y. Accelerated testing for long-term fatigue strength of various FRP laminates for marine use. Compos. Sci. Technol. 2009, 69, 805–813. [Google Scholar] [CrossRef]
- Robert, M.; Cousin, P.; Fam, A.; Benmokrane, B. Effect of the addition of modified mica on mechanical and durability properties of FRP composite materials for civil engineering. Polym. Compos. 2011, 32, 1202–1209. [Google Scholar] [CrossRef]
- Miyazaki, N.; Funakura, S. Solid particle erosion behavior of FRP degraded by hot water. J. Compos. Mater. 1998, 32, 1295–1305. [Google Scholar] [CrossRef]
- Karbhari, V.M.; Xian, G. Hygrothermal effects on high VF pultruded unidirectional carbon/epoxy composites: Moisture uptake. Compos. B Eng. 2009, 40, 41–49. [Google Scholar] [CrossRef]
- Gao, L.; Chou, T.; Thostenson, E.T.; Zhang, Z.; Coulaud, M. In situ sensing of impact damage in epoxy/glass fiber composites using percolating carbon nanotube networks. Carbon 2011, 49, 3371–3391. [Google Scholar] [CrossRef]
- Nanni, F.; Ruscito, G.; Puglia, D.; Terenzi, A.; Kenny, J.M.; Gusmano, G. Effect of carbon black nanoparticle intrinsic properties on the self-monitoring performance of glass fibre reinforced composite rods. Compos. Sci. Technol. 2011, 71, 1–8. [Google Scholar] [CrossRef]
- Nofar, M.; Hoa, S.V.; Pugh, M.D. Failure detection and monitoring in polymer matrix composites subjected to static and dynamic loads using carbon nanotube networks. Compos. Sci. Technol. 2009, 69, 1599–1606. [Google Scholar] [CrossRef]
- Kupke, M.; Schulte, K.; Schulte, R. Non-destructive testing of FRP by D.C. and A.C. electrical methods. Compos. Sci. Technol. 2001, 61, 837–847. [Google Scholar] [CrossRef]
- Fiedler, B.; Gojny, F.H.; Wichmann, M.H.G.; Bauhofer, W.; Schulte, K. Can carbon nanotubes be used to sense damage in composites? Ann. Chim. Sci. Mater. 2004, 29, 81–94. [Google Scholar] [CrossRef]
- Wang, S.; Kowalik, D.P.; Chung, D.D.L. Effects of the temperature, humidity, and stress on the interlaminar interface of carbon fiber polymer-matrix composites, studied by contact electrical resistivity measurement. J. Adhes. 2002, 78, 189–200. [Google Scholar] [CrossRef]
- Wang, S.; Chung, D.D.L. Effect of moisture on the interlaminar interface of a carbon fiber polymer–matrix composite, studied by contact electrical resistivity measurement. Compos. Interfaces 2002, 9, 453–458. [Google Scholar] [CrossRef]
- Belani, J.G.; Broutman, L.J. Moisture induced resistivity changes in graphite-reinforced plastics. Composites 1978, 9, 273–277. [Google Scholar] [CrossRef]
- Zhai, Z.; Feng, L.; Liu, Z.; Li, G. Water absorption test for carbon fiber epoxy resin composite based on electrical resistance. Polym. Test. 2016, 56, 394–397. [Google Scholar] [CrossRef]
- Kotrotsos, A.; Vavouliotis, A.; Tsantzalis, S.; Kostopoulos, V. Effect of CNT modified matrix of epoxy CFRPs on hydrothermal behaviour of material. Evaluation of water uptake using electrical resistance measurements. Plast. Rubber Compos. 2014, 43, 122–129. [Google Scholar] [CrossRef]
- Barkoula, N.M.; Paipetis, A.; Matikas, T.; Vavouliotis, A.; Karapappas, P.; Kostopoulos, V. Environmental degradation of carbon nanotube modified composite laminates: A study of electrical resistivity. Mech. Compos. Mater. 2009, 45, 21–32. [Google Scholar] [CrossRef]
- Al-Sabagh, A.; Taha, E.; Kandil, U.; Nasr, G.; Taha, M.R. Monitoring damage propagation in glass fiber composites using carbon nanofibers. Nanomaterials 2016, 6, 169. [Google Scholar] [CrossRef]
- Borowski, E.; Soliman, E.; Kandil, U.F.; Taha, M.R. Interlaminar fracture toughness of CFRP laminates incorporating multi-walled carbon nanotubes. Polymers 2015, 7, 1020–1045. [Google Scholar] [CrossRef]
- Standard Guide for Preparation of Flat Composite Panels with Processing Guidelines for Specimen Preparation; ASTM D5687/D5687M-07; ASTM International Standards: West Conshohocken, PA, USA, 2005.
- Strong, A.B. Fundamentals of Composites Manufacturing: Materials, Methods and Applications, 2nd ed.; Society of Manufacturing Engineers: Dearborn, MI, USA, 2008. [Google Scholar]
- Genedy, M.; Daghash, S.; Soliman, E.; Taha, M.R. Improving fatigue performance of GFRP composite using carbon nanotubes. Fibers 2015, 3, 13–29. [Google Scholar] [CrossRef]
- Kumar, D.S.; Shukla, M.J.; Mahato, K.K.; Rathore, D.K.; Prusty, R.K.; Ray, B.C. Effect of post-curing on thermal and mechanical behavior of GFRP composites. IOP Conf. Ser. Mater. Sci. Eng. 2015, 75, 012012. [Google Scholar] [CrossRef]
- Krishna, R.; Revathi, A.; Srihari, S.; Rao, R. Post-curing effects on hygrothermal behavior of RT-cured glass/epoxy composites. J. Reinf. Plast. Compos. 2012, 29, 325–330. [Google Scholar] [CrossRef]
- Cain, J.J.; Post, N.I.; Lin, Y.-N.; Hess, P.E. Post-curing effects on marine VARTM FRP composite material properties for test and implementation. J. Eng. Mater. Technol. 2006, 128, 34–39. [Google Scholar] [CrossRef]
- Standard Test Methods for Constituent Content of Composite Materials; ASTM D3171-06; ASTM International Standards: West Conshohocken, PA, USA, 2005.
- Standard Test Methods for DC Resistance or Conductance of Insulating Materials; ASTM D257-99; ASTM International Standards: West Conshohocken, PA, USA, 2005.
- Standard Test Method for Water Absorption of Plastics; ASTM D 570-98; ASTM International Standards: West Conshohocken, PA, USA, 2005.
- Standard Practice for the Preparation of Substitute Ocean Water; ASTM D 1141-98; ASTM International Standards: West Conshohocken, PA, USA, 2005.
- Shen, C.-H.; Springer, G.S. Moisture absorption and desorption of composite materials. J. Compos. Mater. 1976, 10, 2–20. [Google Scholar] [CrossRef]
- Gellert, E.P.; Turley, D.M. Seawater immersion ageing of glass-fibre reinforced polymer laminates for marine applications. Compos. A Appl. Sci. 1999, 30, 1259–1265. [Google Scholar] [CrossRef]
- Bauhofer, W.; Kovacs, J.Z. A review and analysis of electrical percolation in carbon nanotube polymer Composites. Compos. Sci. Technol. 2009, 69, 1486–1498. [Google Scholar] [CrossRef]
- Khurram, A.A.; Rakha, S.A.; Zhou, P.; Shafi, M.; Munir, A. Correlation of electrical conductivity, dielectric properties, microwave absorption, and matrix properties of composites filled with graphene nanoplatelets and carbon nanotubes. J. Appl. Phys. 2015, 118, 044105. [Google Scholar] [CrossRef]
- Ma, P.; Siddiqui, N.A.; Marom, G.; Kim, J. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. A Appl. Sci. 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Gu, H. Behaviours of glass fibre/unsaturated polyester composites under seawater environment. Mater. Des. 2009, 30, 1337–1340. [Google Scholar] [CrossRef]
- Wei, B.; Cao, H.; Song, S. Degradation of basalt fibre and glass fibre/epoxy resin composites in seawater. Corros. Sci. 2011, 53, 426–431. [Google Scholar] [CrossRef]
- Tsotsis, T.K. A simple graphical method for determining diffusion parameters for two-stage sorption in composites. J. Mater. Sci. Lett. 1994, 13, 1635–1636. [Google Scholar] [CrossRef]
- Apicella, A.; Nicolais, L.; Cataldis, C. Characterization of the morphological fine structure of commercial thermosetting resins through hygrothermal experiments. Adv. Polym. Sci. 1985, 66, 189–207. [Google Scholar]
- Apicella, A.; Nicolais, L. Effect of water on the properties of epoxy matrix and composite. Adv. Polym. Sci. 1986, 72, 69–77. [Google Scholar]
- Adams, D.F.; Miller, A.K. Hygrothermal microstresses in a unidirectional composite exhibiting inelastic behavior. J. Compos. Mater. 1977, 11, 285–299. [Google Scholar] [CrossRef]
- Hahn, H.T. Hygrothermal Damage in graphite/epoxy laminates. J. Eng. Mater. Technol. 1987, 109, 3–11. [Google Scholar] [CrossRef]
- Illinger, J.L.; Schneider, N.S. Water in Polymers; ACS Symposium Series 127; ACS Publications: Washington, DC, USA, 1980. [Google Scholar]
- Ishida, H.; Koenig, J.L. A fourier-transform infrared spectroscopic study of the hydrolytic stability of silane coupling agents on E-glass fibers. J. Polym. Sci. Part B Polym. Phys. 1980, 18, 1931–1943. [Google Scholar] [CrossRef]
- Antoon, M.K.; Koenig, J.L. Irreversible effects of moisture on the epoxy matrix in glass-reinforced composites. J. Polym. Sci. Part B Polym. Phys. 1981, 19, 197–212. [Google Scholar] [CrossRef]
- Antoon, M.K.; Koenig, J.L. Fourier-transform infrared study of the reversible interaction of water and a crosslinked epoxy matrix. J. Polym. Sci. Part B Polym. Phys. 1981, 19, 1567–1575. [Google Scholar] [CrossRef]
- Ehrenstein, G.W.; Spaude, R. A study of the corrosion resistance of glass fiber reinforced polymers. Compos. Struct. 1984, 2, 191–200. [Google Scholar] [CrossRef]
- Cohen, M.H.; Turnull, D. Molecular transport in liquids and glasses. J. Chem. Phys. 1959, 31, 1164–1169. [Google Scholar] [CrossRef]
- Tsenoglou, C.J.; Pavlidou, S.; Papaspyrides, C.D. Evaluation of interfacial relaxation due to water absorption in fiber–polymer composites. Compos. Sci. Technol. 2006, 66, 2855–2864. [Google Scholar] [CrossRef]
- Tan, H.; Jiang, L.Y.; Huang, Y.; Liu, B.; Hwang, K.C. The effect of van der Waals-based interface cohesive law on carbon nanotube-reinforced composite materials. Compos. Sci. Technol. 2007, 67, 2941–2946. [Google Scholar] [CrossRef]
- Sheng, Y.; Yang, H.; Li, J.; Sun, M. Predicting glass transition temperature of polyethylene/graphene nanocomposites by molecular dynamic simulation. Chem. Res. Chin. Univ. 2013, 29, 788–792. [Google Scholar] [CrossRef]
- Nielsen, L.E. Cross-linking: Effect on physical properties of polymers. J. Macromol. Sci. C Polym. Rev. 1969, 3, 69–103. [Google Scholar] [CrossRef]
- Hill, L.W. Calculation of crosslink density in short chain networks. Prog. Org. Coat. 1997, 31, 235–243. [Google Scholar] [CrossRef]
- Golru, S.S.; Attar, M.M.; Ramezanzadeh, B. Studying the influence of nano-Al2O3 particles on the corrosion performance and hydrolytic degradation resistance of an epoxy/polyamide coating on AA-1050. Prog. Org. Coat. 2014, 77, 1391–1399. [Google Scholar] [CrossRef]
- Mikhaylova, Y.; Adam, G.; Haussler, L.; Eichhorn, K.J.; Voit, B. Temperature-dependent FTIR spectroscopic and thermoanalytic studies of hydrogen bonding of hydroxyl (phenolic group) terminated hyperbranched aromatic polyesters. J. Mol. Struct. 2006, 788, 80–88. [Google Scholar] [CrossRef]
- Larson, R.A.; Weber, E.J. Reaction Mechanisms in Environmental Organic Chemistry; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Nabavizadehrafsanjani, S. The Effect of Moisture on Electrical Resistivity of MWCNT Reinforced Epoxy Nanocomposites. Master’s Thesis, Concordia University, Montreal, QC, Canada, 2015. [Google Scholar]







| Immersion Time (Days) | CNFs/GFRP | MWCNTs/GFRP | Neat/GFRP | |||
|---|---|---|---|---|---|---|
| Tg (°C) | E′ (GPa) | Tg (°C) | E′ (GPa) | Tg (°C) | E′ (GPa) | |
| Dry | 51.7 | 5.14 | 53.9 | 4.78 | 56.4 | 3.81 |
| 20 | 43.3 | 4.10 | 50.6 | 4.44 | 50.2 | 4.53 |
| 40 | 44.5 | 3.61 | 44 | 2.99 | 50.2 | 4.21 |
| 60 | 43.6 | 3.60 | 44 | 3.19 | 54.7 | 4.11 |
| 80 | 47.3 | 3.65 | 46.7 | 2.57 | 54.0 | 2.37 |
| 100 | 44.8 | 1.74 | 44.2 | 1.68 | 53.4 | 2.09 |
| Immersion Time (Days) | CNFs/GFRP | MWCNTs/GFRP | Neat/GFRP | |||
|---|---|---|---|---|---|---|
| Tg (°C) | E′ (GPa) | Tg (°C) | E′ (GPa) | Tg (°C) | E′ (GPa) | |
| Dry | 51.7 | 5.14 | 53.9 | 4.78 | 56.4 | 3.81 |
| 20 | 42.1 | 3.30 | 44.9 | 4.88 | 50.4 | 4.04 |
| 40 | 39.3 | 3.41 | 48 | 4.81 | 47.6 | 3.53 |
| 60 | 41.8 | 3.01 | 43.3 | 4.43 | 46.4 | 3.71 |
| 80 | 46.3 | 3.39 | 48.3 | 3.32 | 47.3 | 2.06 |
| 100 | 44.5 | 1.92 | 43.9 | 1.30 | 46.9 | 2.10 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Sabagh, A.; Taha, E.; Kandil, U.; Awadallah, A.; Nasr, G.-a.M.; Reda Taha, M. Monitoring Moisture Damage Propagation in GFRP Composites Using Carbon Nanoparticles. Polymers 2017, 9, 94. https://doi.org/10.3390/polym9030094
Al-Sabagh A, Taha E, Kandil U, Awadallah A, Nasr G-aM, Reda Taha M. Monitoring Moisture Damage Propagation in GFRP Composites Using Carbon Nanoparticles. Polymers. 2017; 9(3):94. https://doi.org/10.3390/polym9030094
Chicago/Turabian StyleAl-Sabagh, Ahmed, Eman Taha, Usama Kandil, Ahmed Awadallah, Gamal-abdelnaser M. Nasr, and Mahmoud Reda Taha. 2017. "Monitoring Moisture Damage Propagation in GFRP Composites Using Carbon Nanoparticles" Polymers 9, no. 3: 94. https://doi.org/10.3390/polym9030094







