1. Introduction
Biodegradable and biocompatible hydrogels used in drug delivery are obtained by both physical and chemical crosslinking strategies. By optimizing synthesis conditions, the physical and chemical properties of hydrogels can be controlled. By their relative deformability they can conform to the shape of the surface to which they are applied, and their muco- or bioadhesive properties are advantageous when used with intent to immobilize them at the site of application [
1]. Before use in disease therapy, the obtained hydrogels are loaded with drugs. Both quantity and homogeneity of drug loading depend on the morphology of hydrogels, swelling behaviour, and also on drug solubility. All these characteristics finally determine drug release. Therefore, the properties of each hydrogel should be tailored according to the desired drug delivery application.
According to O’Daly [
2] and Irvington et al. [
3] “psoriasis is a chronic inflammatory skin disease that affects 1% to 3% of the world population, with equal gender distribution” and it is appreciated that the incidence rates vary from 50 to 140 cases per 100,000 people per year [
4]. Severe psoriasis is associated with a decrease in the life quality of and risk of mortality [
5].
Active treatment for psoriasis using topical agents [
6] is typically sufficient to manage mild or moderate psoriasis [
7] when it affects less than 10% of the body surface area [
8]. With increasing severity of disease, other therapies are necessary, which sometimes involve non-biological systemic agents such as methotrexate, cyclosporine and orally administrated retinoids [
9].
Methotrexate (amethopterin, 4-amino-N10-methyl petroyl glutamic acid, MTX) is clinically used in formulations for therapy of several diseases such as acute lymphoblastic anaemia, choriocarcinoma, psoriasis, sarcoidosis, and trophoblastic tumours, etc. MTX inhibits epidermal cell proliferation [
10] and has anti-inflammatory action at low doses [
11]. However, the systemic administration of MTX leads to a large number of adverse effects (such as liver toxicity, and gastrointestinal side-effects, including nausea, vomiting, diarrhoea and stomatitis) which restrict its use. To overcome these limitations, its loading in matrices resulting from the combination of natural and synthetic polymers was developed as an important strategy, and also various formulations containing methotrexate have been developed. As methotrexate possesses two aromatic –NH
2 and two aliphatic –COOH groups, Riebeseel et al. esterified carboxylic groups with hydroxyl group of polyethylene glycol (PEG) and developed drug delivery systems based on methotrexate PEG esters [
12]. Pinto et al. [
13] reported nanostructured lipid carriers loaded with MTX with good results for the in vitro skin permeation studies. Goodarzi et al. [
14] reviewed the role of polysaccharides in the improvement of the cytotoxic drug delivery by increasing their water solubility and their safe use through a prolonged, targeted release [
15].
Among natural and semi-synthetic polysaccharides, hyaluronic acid, chitosan and dextran are preferred in bioconjugation studies [
16] as the therapeutic properties of the drugs can be combined with the biological properties of these polymers.
Hyaluronic acid (HA) is one of the most important topical carriers for the localized delivery of drugs to the skin and also as a drug delivery agent for ophthalmic, nasal, pulmonary, parenteral and topical routes of administration [
17]. HA acts as mucoadhesive, retaining the drug at specific site of action/absorption. It also can modify the in vivo release/absorption rate of the therapeutic agent and it is able, when applied topically, to localize delivery of drug to the epidermis [
18].
Poly(vinyl alcohol) (PVA) is a water soluble biodegradable synthetic polymer with good biocompatibility, and it can be physically cross-linked by the freeze-thaw method [
19] to form hydrogels useful in pharmaceutical formulations [
20].
HA–PVA hydrogels were prepared by Kim et al. [
21] and Ramires and Milella [
22] in the presence of a chemical crosslinker (glutaraldehyde) and 1-ethyl-(3-3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) as a coupling agent. They obtained pH- and electro-responsive hydrogels with different compositions. The presence of glutaraldehyde residuals unremoved by washing procedures imparts toxicity to the materials.
Freeze-thawed PVA [
23] and PVA/HA cryogels exhibited nontoxic behaviour and higher cell viability [
24].
In a previous paper [
25] the freeze-thawed PVA/HA hydrogels were prepared and tested as carriers for melatonin.
Loading of MTX within a hyaluronic acid-based matrix was done previously by using a peptide linker by Homma et al. [
26], which was designed to be used in the treatment of osteoarthritis, localizing the drug at the synovial membrane.
Methotrexate-loaded freeze-thawed HA/PVA hydrogels were not still found to be reported.
The aim of the present study is to prepare MTX-loaded freeze-thaw HA/PVA hydrogels and to characterize them in respect with the previous results on unloaded hydrogels and to determine their physical–chemical properties which assure a controlled release of the drug. The major outcome of the study is to obtain good protection of the drug and to reduce the reported side effects of MTX while it is carried and delivered to the affected site. This was done by analysing the acute toxicity and biocompatibility and by establishing the dependence of swelling and release behaviour on pH.
2. Materials and Methods
2.1. Materials
Hyaluronic acid sodium salt, from Streptococcus equi, was acquired from Sigma-Aldrich. Polyvinyl alcohol with 99% hydrolysis degree and a Mw of 89,000–98,000 as well as methotrexate, were purchased from Sigma-Aldrich (Buchs, Switzerland). All other chemicals and reagents used in this study were of analytical grade or higher, obtained commercially.
Hydrogels of PVA and HA prepared by method elaborated by Fahmy et al. [
27] which was a modified as described in the procedure given in previous paper [
25]. MTX was dissolved in the solution of HA with a concentration of 0.5% (
w/
w) in respect to polymeric matrix, and homogenized with vortex for 5 min and then added to the PVA solution drop-wisely. The volume ratio between the two solutions was PVA/HA 5:1 (
v/
v). The freeze-thaw procedure consists of freezing at −20 °C for 20 h and thawing for 4 h at room temperature. The formed gels were frozen and freeze-dried right after the freeze-thaw process. Applying the same procedure of consecutive freeze-thaw cycles resulted in MTX-loaded samples. Increasing or decreasing the freeze and thaw action time has a significant influence of the mechanical properties of the final gels. A list of samples and assigned codes are summarized in
Table 1.
2.3. Investigation Methods
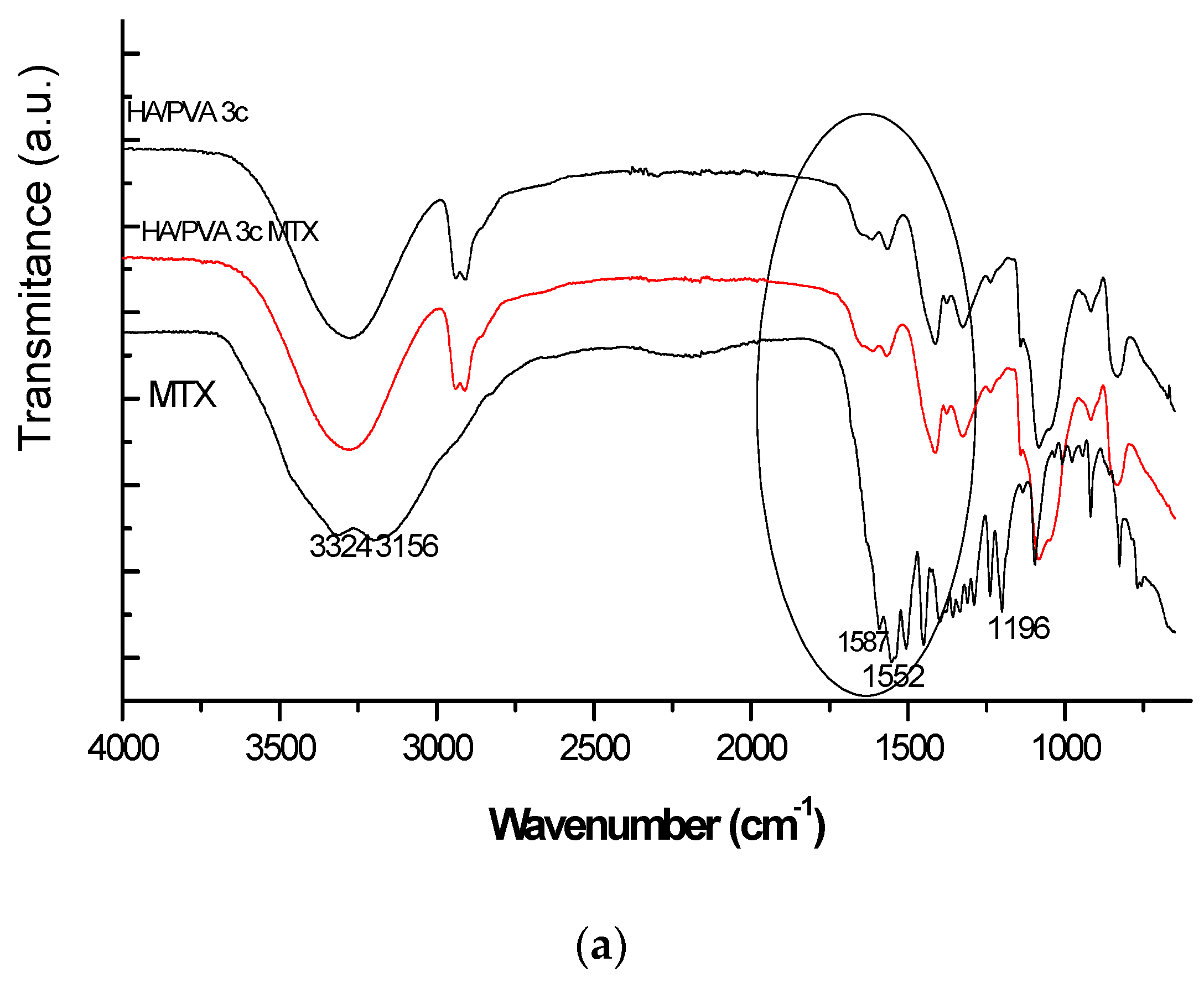
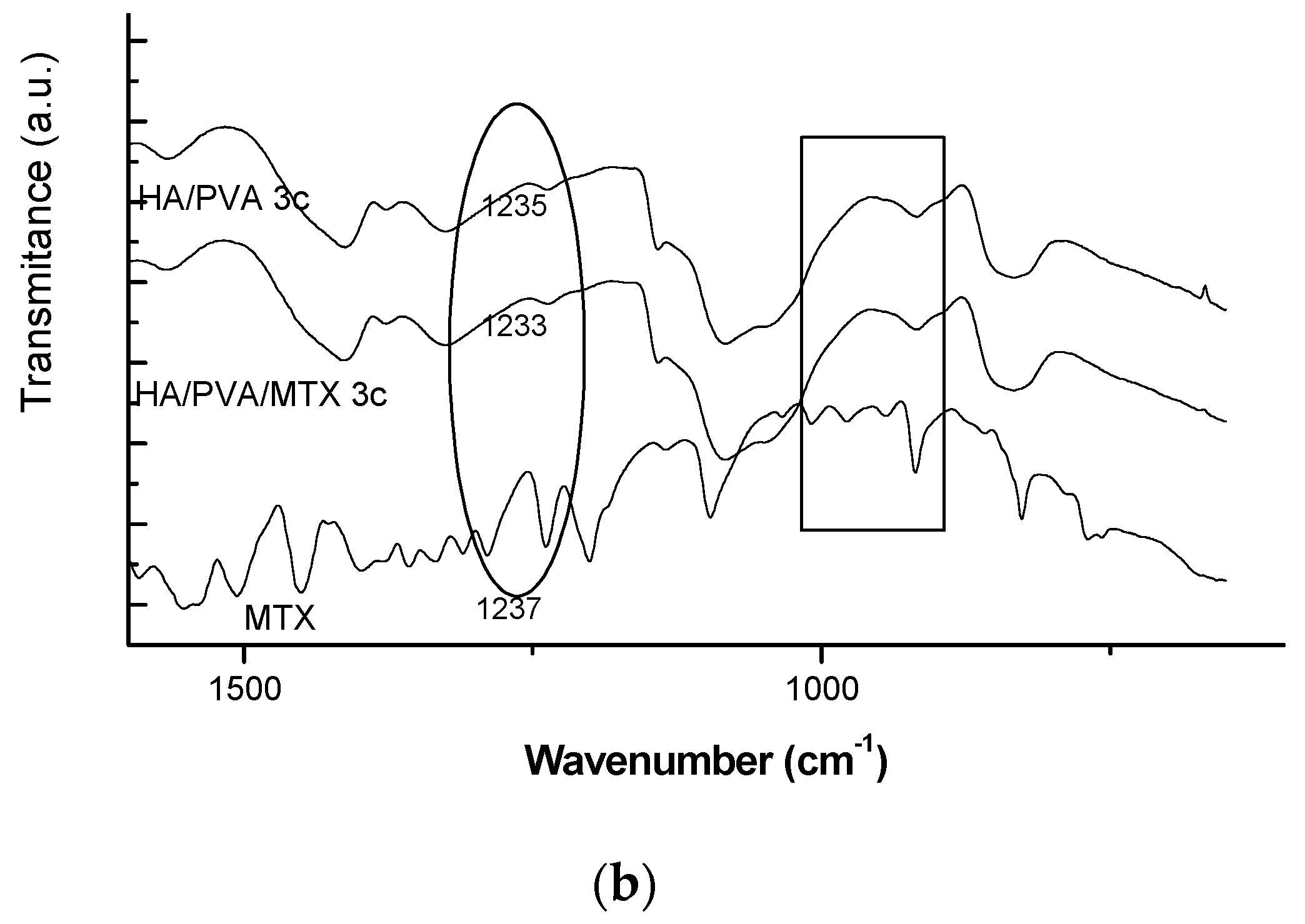
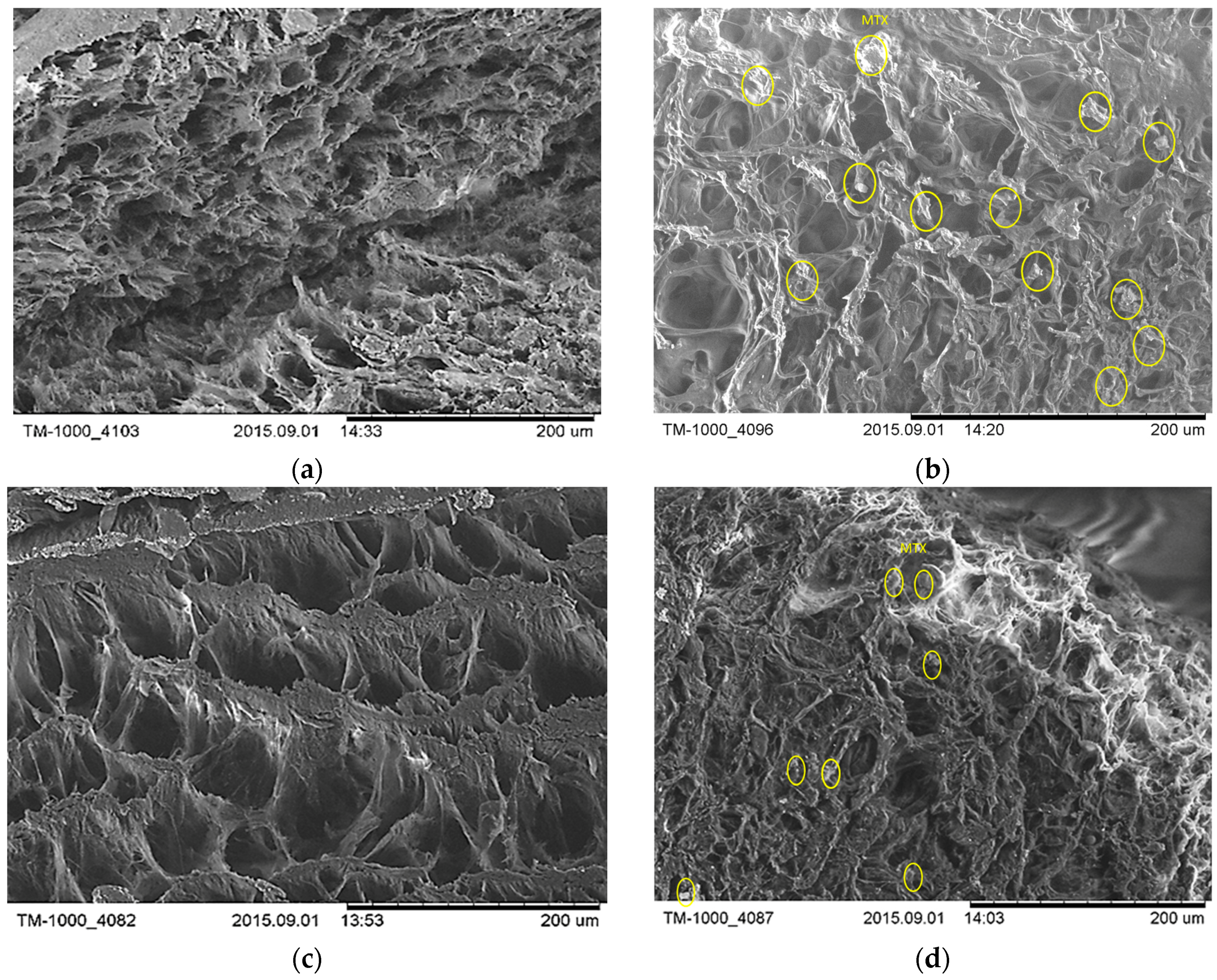
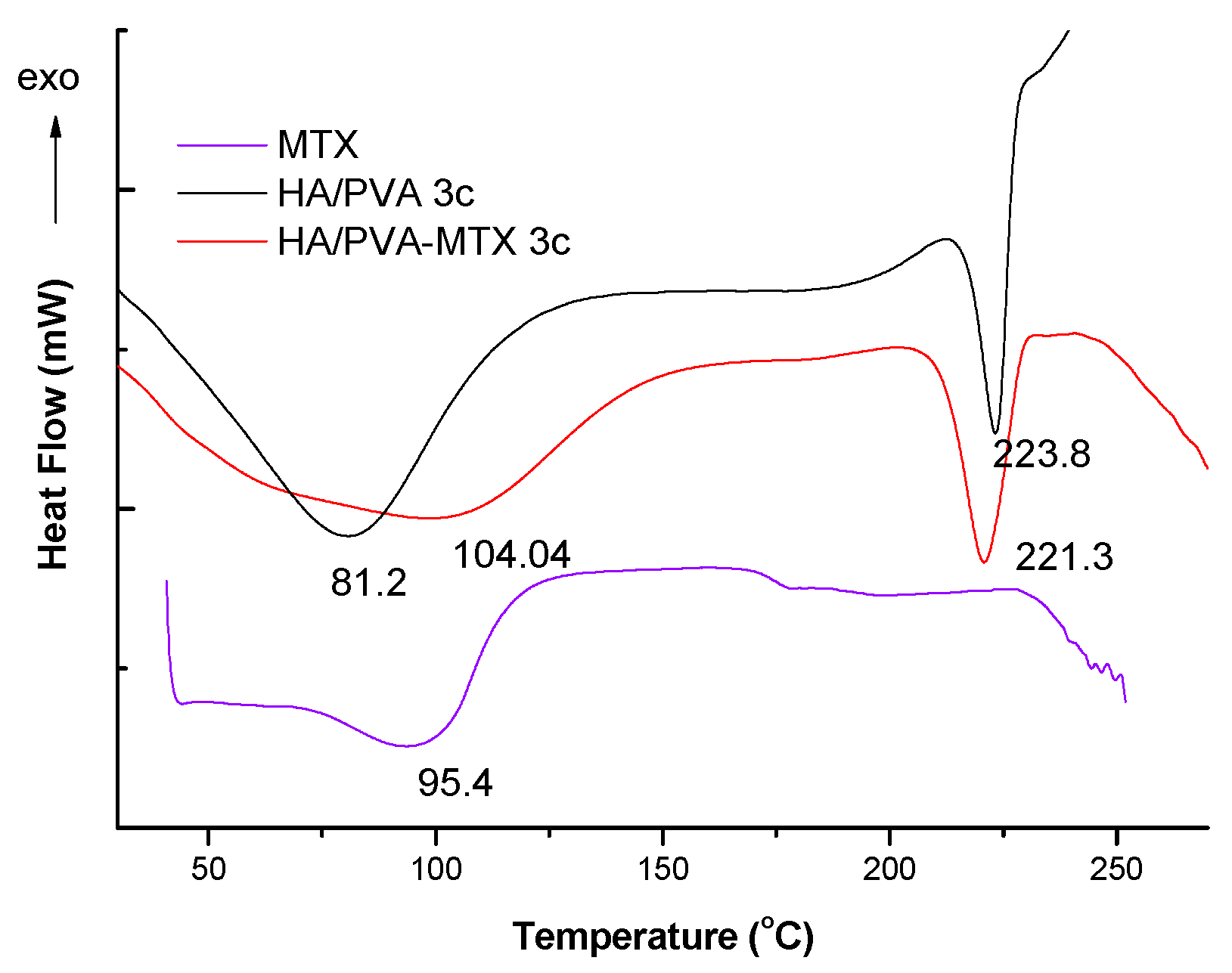
In this paper the freeze-thawed MTX loaded PVA/HA hydrogels underwent physical–chemical characterization and were analysed with respect to with toxicity and biocompatibility. Physical–chemical characterization of the PVA/HA hydrogels occurred using the following methods: FT-IR spectroscopy (ATR-FT-IR spectra were recorded using a Perkin Elmer Spectrum 100 Spectrometer (Shelton, CT, USA), through reflexion on a diamond crystal with an angle of 45 degrees, resolution 4 cm
−1), differential scanning calorimetry (DSC) (Perkin Elmer DSC-8000, Shelton, CT, USA within a temperature range of 30 and 220 °C at a heat rate of 10 °C/min under 20 mL/min nitrogen flow), and dynamic mechanical–thermal analysis (DMTA) (Anton Paar MCR301 Rheometer, Physica MCR, Berlin, Germany); polymer membranes under half-wet state with rectangular shape of 40 mm × 12 mm dimensions at a constant frequency of 1 Hz from −50 to 220 °C temperature range) was performed according to the procedures and experimental details described in previous paper [
25]. Here, only particular behaviour of MTX-loaded hydrogels is presented but sometimes reference to the previous results on unloaded hydrogels is made.
2.4. Drug Loading Evaluation
The loaded amount of methotrexate was evaluated by using HPLC method. An AGILENT 1200 Series, USA HPLC System (Agilent, New York, NY, USA) with vacuum Degasser, UV detector and ACE 5
® equipped with a column of Agilent Eclipse XDB-C18 with a length of 150 mm and a 4.6-mm internal diameter, particle size 5 µm was used. A degassed solution containing 90% (
V/
V) phosphate buffer pH 5.5 and 10% (
v/
v) acetonitrile was used as the mobile phase. The flow rate was set 1 mL/min and the UV detection was set at 302 nm, the characteristic wavelength of methotrexate. A modified HPLC method was used to determine amount of loaded methotrexate [
28]. The method was validated in terms of specificity, stability of solution, accuracy and precision. The linearity range was 0.24–10 µg/mL and the correlation coefficient (
R) was 0.989. The compounds were identified by comparing the retention times of the unknown peaks with the peaks of the reference standards. The retention time considered for methotrexate was found at 4.4 min. Weighed amounts of lyophilized hydrogels (0.027 g HA/PVA 2c-MTX and 0.03 g HA/PVA 3c-MTX) were placed in a vial and 10 mL of phosphate buffer pH 5.5 adjusted with NaOH 0.2 M were added. The systems were stirred overnight to ensure a good extraction of the drug from the polymeric matrix. Volumes of 1 mL were sampled for the HPLC characterization. The loading percent was calculated taking into account the initial amount of MTX loaded at the beginning of the experiment (0.4 mg) and it was found to be 66%.
2.5. Toxicity and Biocompatibility Tests
Ethics Statement for Experiments with Animals
Experimental protocols within the toxicity and biocompatibility studies were implemented according to the institutional recommendations of the “Gr. T. Popa” University of Medicine and Pharmacy of Iasi, Romania (official paper No. 15559/21.09.2010) Committee for Research and Ethical Issues, in rigorous accordance with international ethical regulations on laboratory animal work [
29,
30].
The laboratory animals were euthanized by exposure to ether vapour overdose in closed containers, as ether is one of the inhalant anaesthetics considered acceptable conditionally. The administration path for the in vivo studies was selected based on the characteristics related to each medium.
Prior to the biocompatibility tests, PVA/HA hydrogels were mashed and then suspended in saline solution with Tween 80.
For the in vivo experiments white Swiss mice were used (25–30 g). The mice were housed under standard laboratory conditions (relative humidity 55–65%, room temperature 23.0 ± 2.0 °C and 12 h light/dark cycle), with food and water ad libitum.
In order to determine the acute toxicity, the Lethal Dose LD50 was calculated, after intraperitoneal single dose administration in mice, using Karber arithmetic method [
31]. Both the minimal dose that kills all the animals (LD100) and the maximal dose that fails to kill any animal were determined. Between these two limits, different doses were chosen, and administered in groups of four animals [
32]. During the subsequent 14 days after immediate dosing, the animals were carefully observed for any behavioural changes, mortality and any change correlated with the manifestations of toxicity (lack of appetite, depression, immobility, respiratory distress). Mortality rates in each group were counted, and the LD50 was calculated, according to the Karber method by using Equation (1):
where
a—the difference between two successive doses of the substance administered,
b—the average of the number of death animals in two successive groups, and
n—the number of animals in a group.
The level of substance toxicity was appreciated according to the toxicity scale proposed by Voicu in 1997 [
33]. As already mentioned, at the end of the observation period the animals were euthanized with an overdose of ethyl ether.
The biocompatibility properties of doses representing 1/20 from LD50 of the substances were studied in two situations. Firstly, the effects on the blood and biochemical constants were assessed, 14 days after intraperitoneal unique administration of the substances. The experiment was carried out on mice distributed in four groups of six animals as listed within
Table 2, column 2. Each animal was treated intra-peritoneally, on the first day of the experiment.
The effect on the blood and biochemical parameters were determined after unique topical or intra-peritoneal administration for 7 or 14 days, respectively, of the loaded and unloaded gel formulations. The experiment was carried out on mice distributed in another four groups of six animals each treated topically with two drops of the formulations behind the left ear of the animal, on the first day of the experiment. The last column of the
Table 2 summarizes the group samples for the topical administration of the gel formulations.
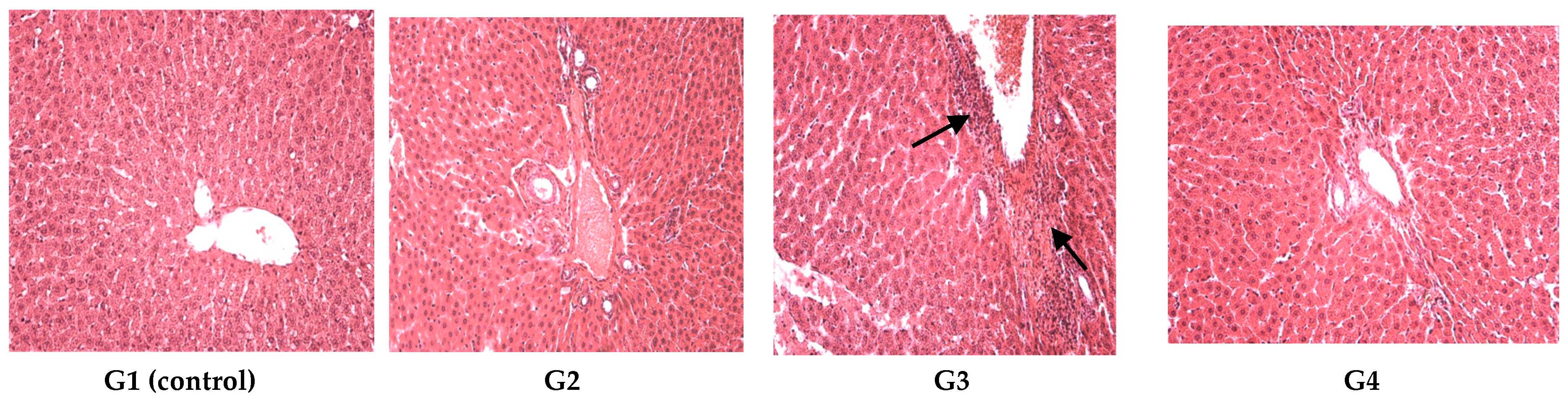
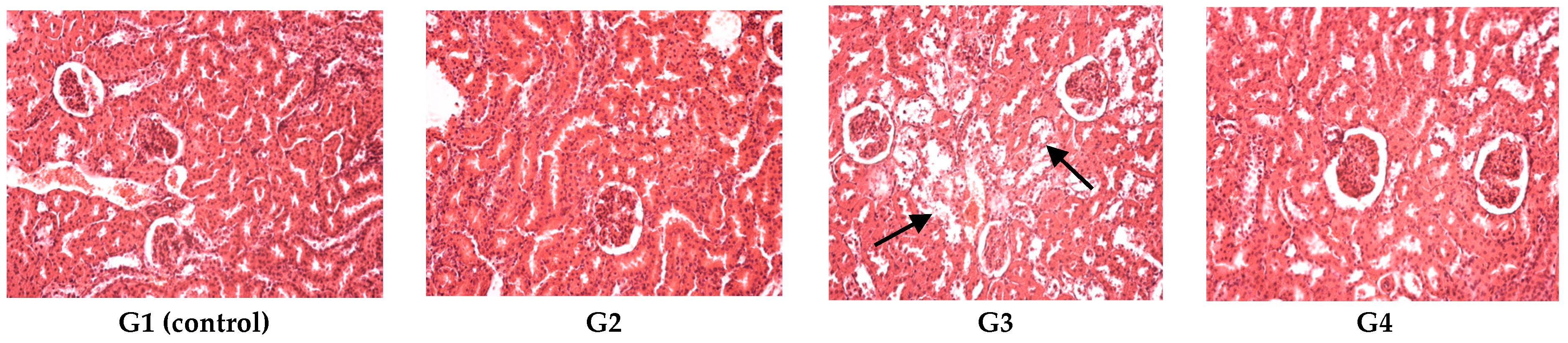
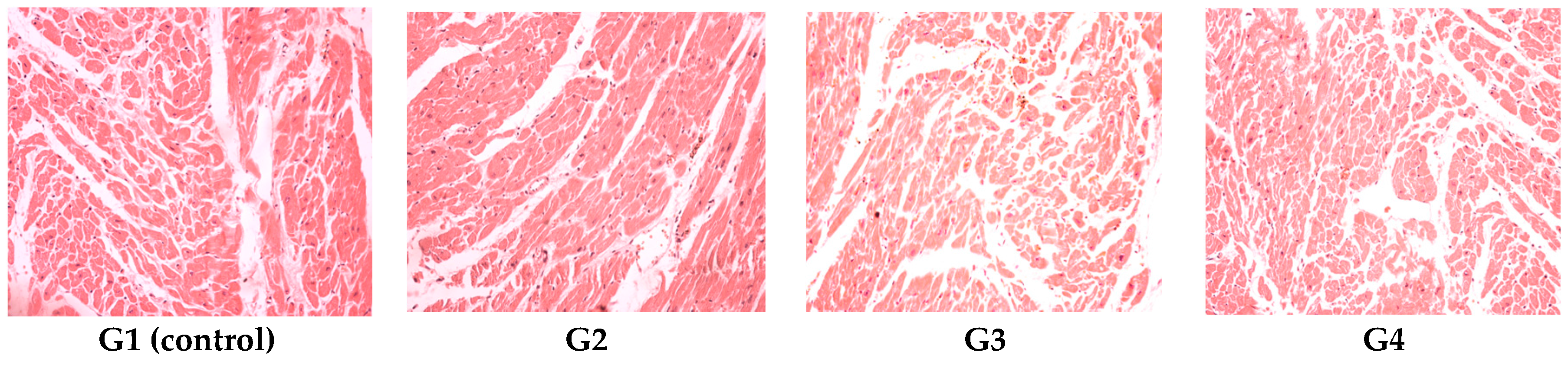
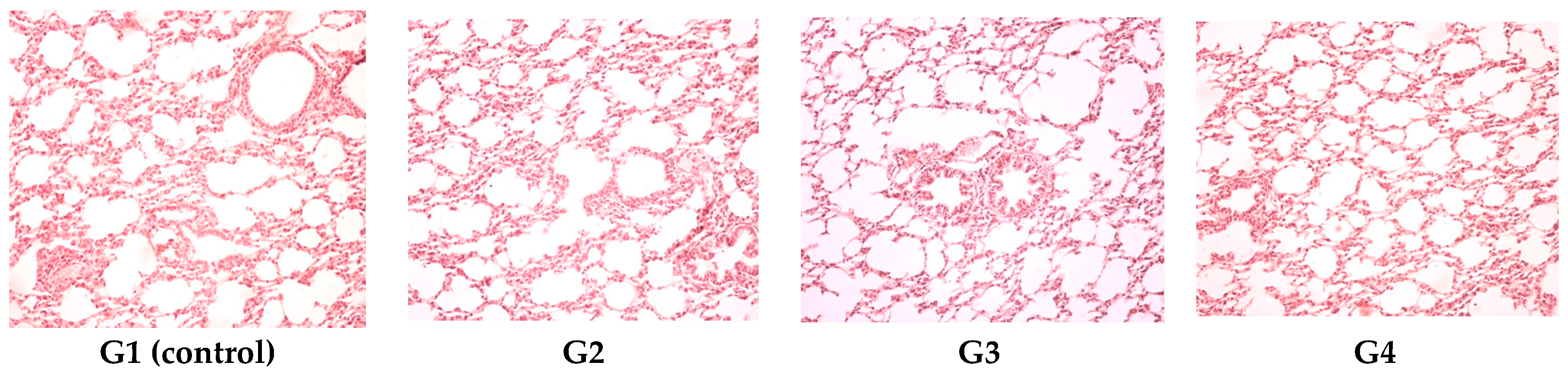
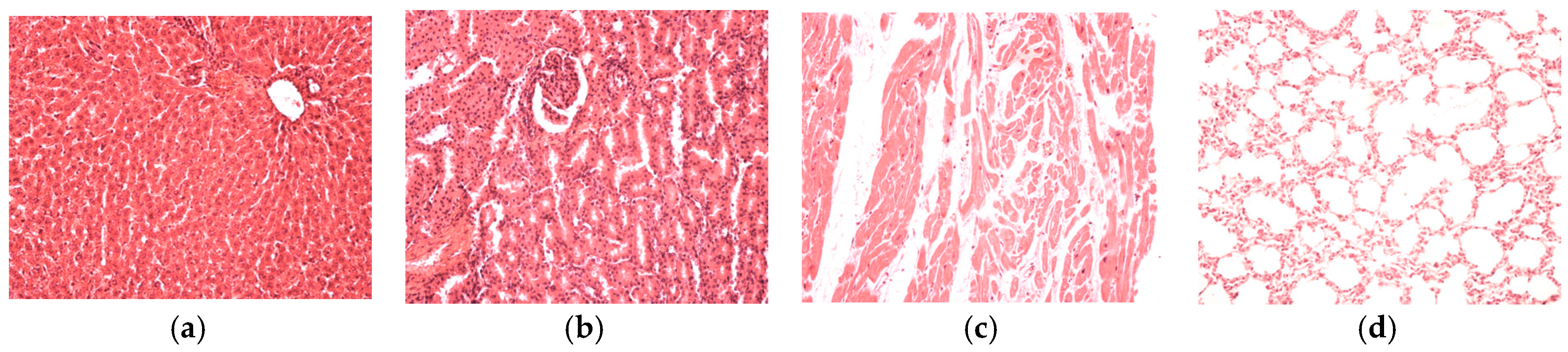
On the last day of both of the experiments, the blood was collected and with the automated haematological analyser (SySmex XT 1800 from SYSMEX Europe GmbH, Norderstedt, Germany) the following parameters were determined: white blood cell count and leukocyte formula, red blood cell count, platelet count, packed cell volume and haemoglobin levels. With the automated biochemical analyser RX Imola (from RANDOX laboratories, Crumlin, UK) the following parameters were determined in serum: aspartate aminotransferase, alanine aminotransferase, urea, creatinine, uric acid, total cholesterol and total proteins. After the experiments all animals were euthanized. After animal euthanasia, tissue fragments were collected for histological examination. The organ fragments were fixed in 10% formalin solution and prepared for light microscopic examination and stained with hematoxylin–eosin (HE).
2.6. Swelling Tests
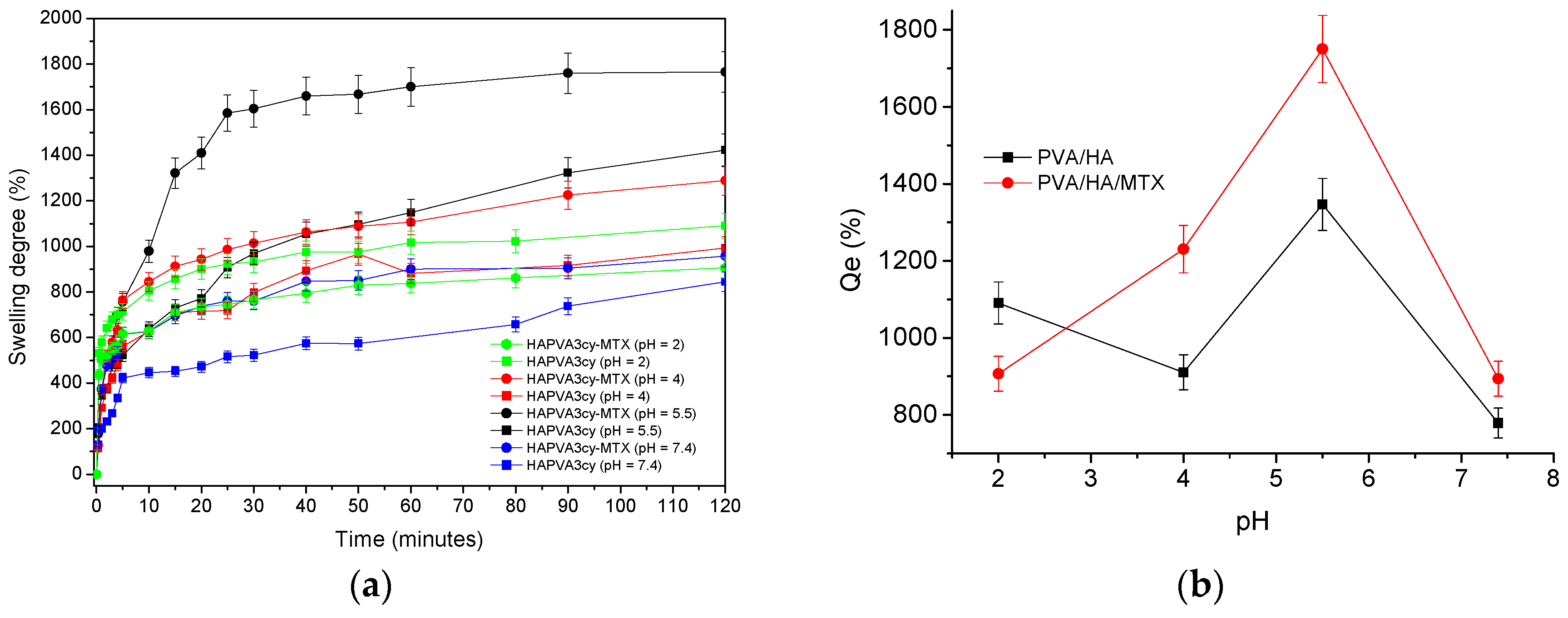
Swelling ability of the matrices was investigated by means of swelling tests by direct immersion in buffered solutions (PBS) of various pHs as 2, 4, 5.5 and 7.4 at a temperature of 37 ± 0.5 °C, simulating physiological conditions. The analysed hydrogels were removed from the solution at pre-determined intervals, the excess solution on the surface was removed with a soft tissue, weighed and then carefully placed back into the vessel with swelling solution as soon as possible for the next determination.
The following equation was used to evaluate the swelling degree (
Q):
where
Wt is the weight of the swollen samples at time
t and
Wd is the weight of the dry sample.
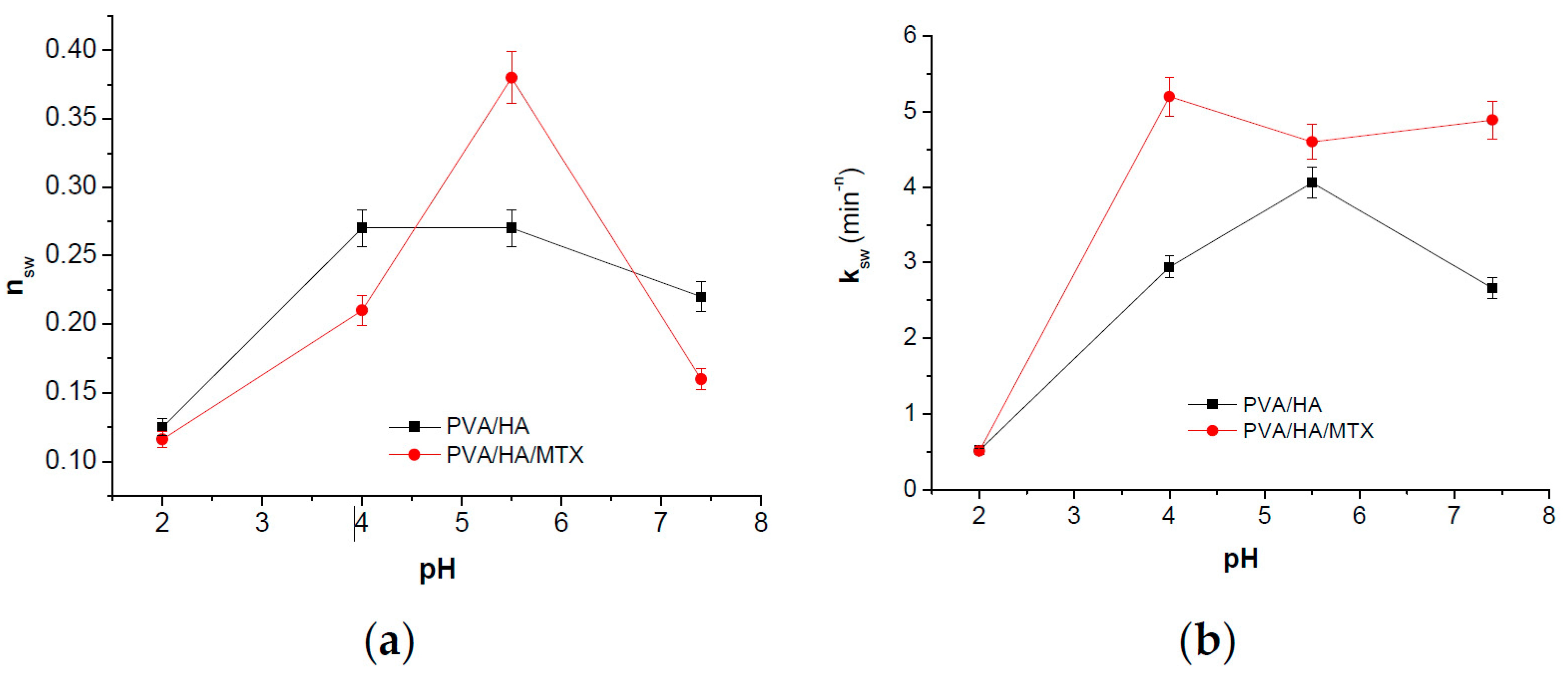
The swelling kinetic parameters have been evaluated by means of equations from Peppas et al. [
34,
35,
36]:
where
Wt and
Weq are the amounts of solution absorbed by polymeric hydrogels at time
t and at equilibrium, respectively;
ksw is the swelling specific rate constant of the studied sample and
nsw is the exponent in diffusion law expression whose values define the mechanism of the solvent transport. Equation (3) is valid for a swelling degree less than 60% where the plot of ln
Wt/
Weq versus ln
t gives a straight line.
2.7. In Vitro Release of MTX from Cryogels
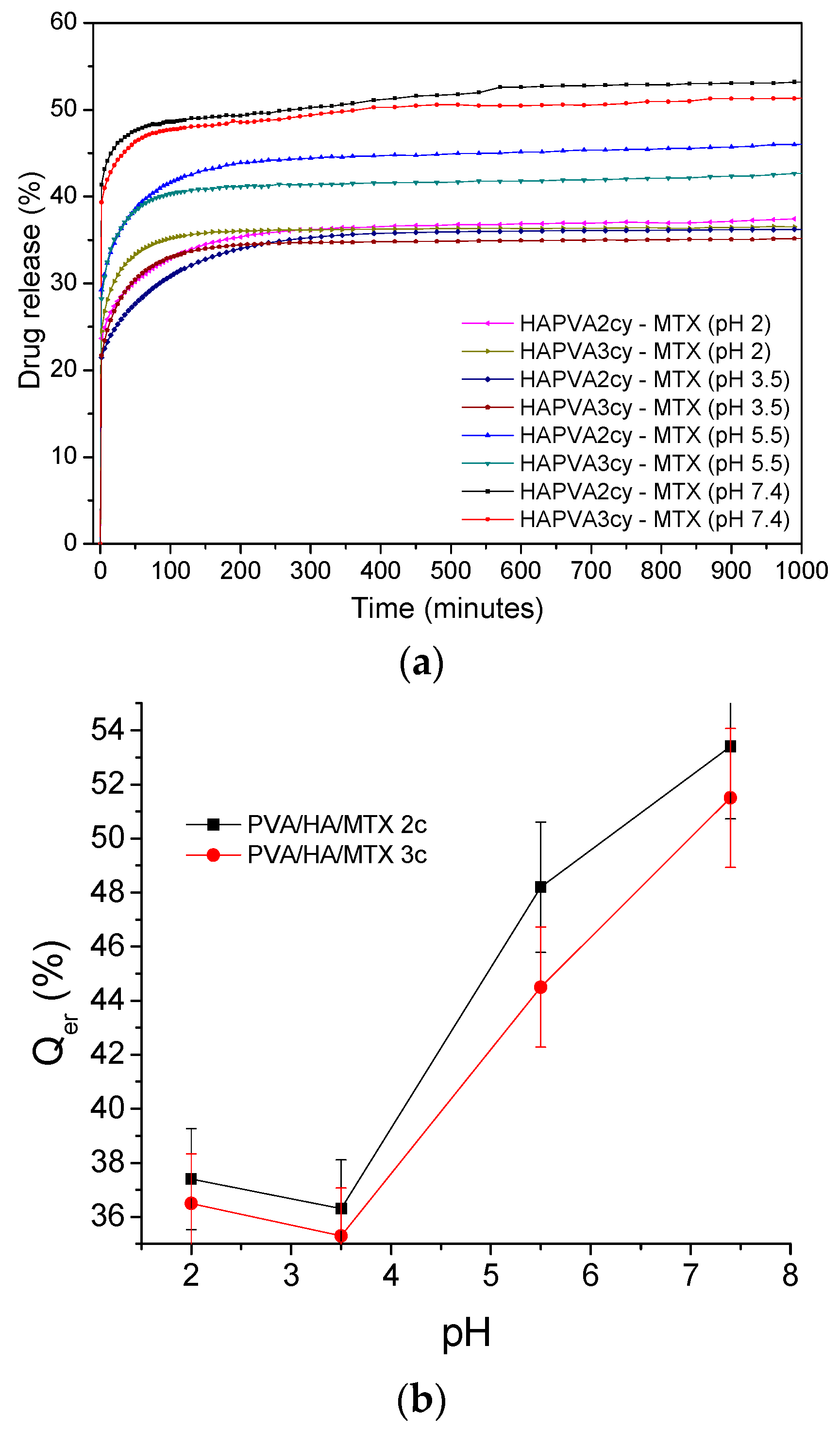
The in vitro release of the methotrexate at various pH values of 2, 4, 5.5 and 7.4 were performed by using a standard dissolution test [
37] carried out in conditions which mimic the physiological environment at a temperature of 37 ± 0.5 °C. The concentration of the drug was evaluated using a predetermined calibration curve for MTX recorded using an HP 8450A UV-visible spectrophotometer (Agilent, San Diego, CA, USA)—specific maximum absorption wavelength of 303 nm.
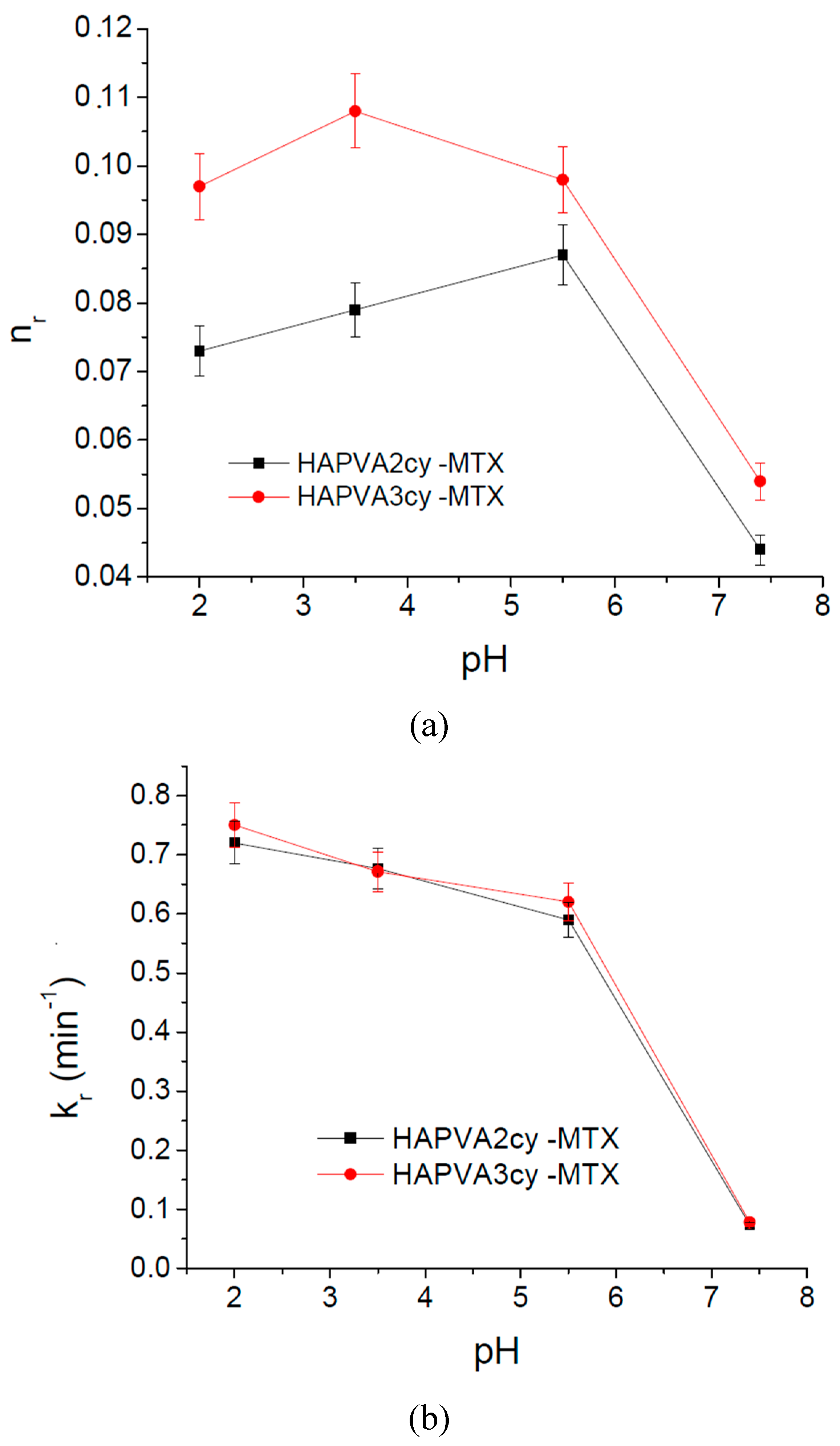
As in the case of swelling kinetics for the drug release kinetics, Korsmeyer–Peppas semi-empirical Equation (4) was applied for the initial release stages (~60% fractional release) [
36]:
where
is the fractional drug released,
Mt and
are the cumulative drug released amounts at time
t and at equilibrium, respectively (or experimental maximum released amount taken at the plateau of the release curves),
kr is rate constant dependent on the characteristics of the drug loaded system and
nr is the diffusional exponent which defines the type of the release mechanism. For example, a value of
nr ~ 0.5 is characteristic of the Fickian diffusion mechanism of the drug from the cryogel; the values in interval 0.5 <
nr < 1 are specific to an anomalous or non-Fickian behaviour. A case II of transport mechanism occurs when
nr = 1, which means zero-order kinetics, while a special case II of transport mechanism is indicated by values
nr > 1 [
35]. The MTX release profiles are plotted as the cumulative percentage of drug released versus time.
2.8. Statistical Analysis
The triplicate tests were performed and the obtained results are expressed as means ± SD (standard deviation) and significance was analysed using T-student test in Microsoft Excel for Windows. The StatView statistical software package (Apple Macintosh, BrainPower Inc., Cary, CA, USA) was used for data analysis. ANOVA and Fisher’s post hoc test consisting of 3 (groups) × 3 (time sample points) repeated measures of experimental results were analysed. The criterion for significance was p < 0.05.