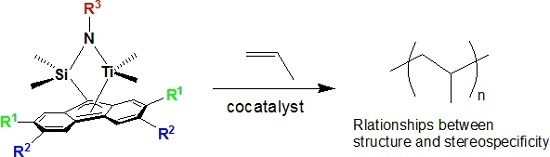
Living Polymerization of Propylene with ansa-Dimethylsilylene(fluorenyl)(cumylamido) Titanium Complexes
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
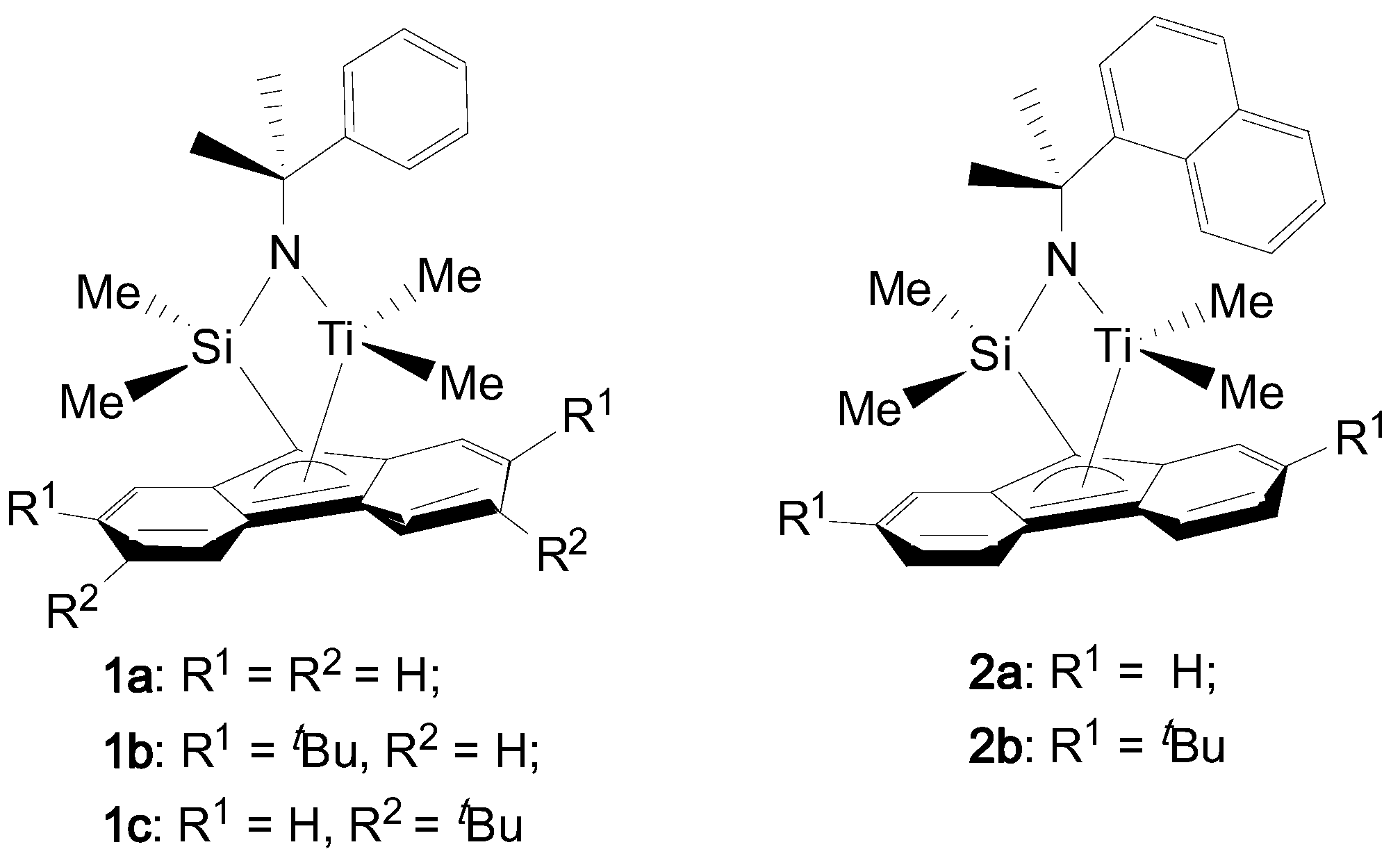
2.2. Synthesis of Complexes
2.2.1. Synthesis of (2,7-di-tBu)fluorenyl-cumylamido Titanium Complex (1b)
2.2.2. Synthesis of (3,6-di-tBu)fluorenyl-cumylamido Titanium Complex (1c)
2.2.3. Synthesis of Fluorenyl-(1-methyl-(1-naphthyl)ethyl)amido Titanium Complex (2a)
2.2.4. Synthesis of (2,7-di-tBu)fluorenyl-(1-methyl-(1-naphthyl)ethyl)amido Titanium Complex (2b)
2.3. Polymerization Procedure
2.4. Analytical Procedure
3. Results and Discussion
3.1. Molecular Structure of Complexes
3.2. Propylene Polymerization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stevent, J.C.; Timmers, F.J.; Wilson, D.R.; Schmidt, G.F.; Nickias, P.N.; Rosen, R.K.; Knight, G.W.; Lai, S.Y. Constrain Geometry Addition Polymerization Catalysts, Processer for Their Preparation, Precursors Therefor, Methods of use, and Novel Polymers Formed Therewith. Patent EP0416815, 1991. [Google Scholar]
- Canich, J.A.M. Monocyclopentadienyl Titanium Metal Compounds for Ethylene-α-Olefin-Copolymer Production Catalysts. U.S. Patent 5264405, 15 October 1993. [Google Scholar]
- Mcknight, A.L.; Waymouth, R.M. Group 4 ansa-cyclopentadienyl-amido catalysts for olefin polymerization. Chem. Rev. 1998, 98, 2587–2598. [Google Scholar] [CrossRef] [PubMed]
- Braunschweig, H.; Breitling, F.M. Constrained geometry complexes-synthesis and applications. Coord. Chem. Rev. 2006, 250, 2691–2720. [Google Scholar] [CrossRef]
- Nakayama, Y.; Sogo, Y.; Cai, Z. Copolymrization of ethylene with 1,1-disubstituted olefins catalyzed by ansa-(fluorenyl)(cyclododecylamido) dimethyltitanium complexes. J. Polym. Sci. Part A 2013, 51, 1223–1229. [Google Scholar] [CrossRef]
- Xu, G. Copolymerization of ethylene with styrene catalyzed by the [η1:η5-tert-butyl(dimethylfluorenylsilyl)amido] metthyltitanium “Cation”. Macromolecules 1998, 31, 2395–2402. [Google Scholar] [CrossRef]
- Kirillov, E.; Razavi, A.; Carpentier, J. Syndiotactic-enriched propylene-styrene copolymer using fluorenyl-based half-titanocene catalysts. J. Mol. Catal. Part A 2006, 249, 230–235. [Google Scholar] [CrossRef]
- Shiono, T.; Sugimoto, M.; Hasan, T. Random copolymerization of norbornene with higher 1-alkene with ansa-fluorenylamidodimethyltitanium catalyst. Macromolecules 2008, 41, 8292–8294. [Google Scholar] [CrossRef]
- Shiono, T.; Suginoto, M.; Hasan, T.; Cai, Z. Facile synthesis of hydroxy-functionalized cycloolefin copolymer using ω-alkenylaluminium as a comonomer. Macromol. Chem. Phys. 2013, 214, 2239–2244. [Google Scholar] [CrossRef]
- Tanaka, R.; Ikeda, T.; Nakayama, Y.; Shiono, T. Pseudo-living copolymerization of norbornene and ω-alkynelborane—Synthesis of monodisperse functionalized cycloolefin copolymer. Polymer 2015, 56, 218–222. [Google Scholar] [CrossRef]
- Razavi, A.; Thewalt, U. Preparation and crystal structures of the complexes (η5-C5H3TMS-CMe2-η5-C13H8)MCl2(M = Hf, Zr or Ti): Mechanistic aspects of the catalytic formation of a isotactic-syndiotactic stereoblock-type polypropylene. J. Organomet. Chem. 2001, 621, 267–276. [Google Scholar] [CrossRef]
- Busico, V.; Cipullo, R.; Cutillo, F.; Talarico, G.; Razavi, A. Syndiotactic poly(propylene) from [Me2Si(3,6-di-tert-butyl-9-fluorenyl) (N-tert-butyl)]TiCl2-based catalysts: Chain-end or enantiotopic-sites stereocontrol? Macromol. Chem. Phys. 2003, 204, 1269–1274. [Google Scholar] [CrossRef]
- Irwin, L.J.; Miller, S.A. Unprecedented syndioselectivity and syndiotactic polyolefin melting temperature: Polypropylene and poly(4-methyl-1-pentene) from a highly active, sterically expanded η1-fluorenyl-η1-amido zirconium complex. J. Am. Chem. Soc. 2005, 127, 9972–9973. [Google Scholar] [CrossRef] [PubMed]
- Nishii, K.; Hagihara, H.; Ikeda, T.; Akita, M.; Shiono, T. Stereospecific polymerization of propylene with group 4 ansa-fluorenylamidodimethyl complexes. J. Organomet. Chem. 2006, 691, 193–201. [Google Scholar] [CrossRef]
- Miller, S.A.; Bercaw, J.E. Highly Stereoregular syndiotactic polypropylen formation with metallocene catalysts via influence of distal ligand substituents. Organometallics 2004, 23, 1777–1789. [Google Scholar] [CrossRef]
- Bader, M.; Marquet, N.; Kirillov, E.; Roisnel, T.; Razavi, A.; Lhost, O.; Carpentier, J.F. Old and new C1-symmetric group 4 metallocenes {(R1R2C)-(R2’R3’R6’R7-Flu)(3-R3-5-R4-C5H2)}ZrCl2: From highly isotactic polypropylenes to vinyl end capped isotactic-enriched oligomers. Organometallics 2012, 31, 8375–8387. [Google Scholar] [CrossRef]
- Esteb, J.J.; Chien, J.C.C.W.; Rausch, M.D. Novel C1 symmetric zirconocenes containing substituted indenyl moieties for the stereoregular polymerization of propylene. J. Organomet. Chem. 2003, 688, 153–160. [Google Scholar] [CrossRef]
- Kleinschmidt, R.; Griebenow, Y.; Fink, G. Stereospecific propylene polymerization using half-sandwich metallocene/MAO systems: A mechanistic insight. J. Mol. Catal. A Chem. 2000, 157, 83–90. [Google Scholar] [CrossRef]
- Cai, Z.; Nakayama, Y.; Shiono, T. Living random copolymerization of propylene and norbornene with ansa-fluorenylamidodimethyltitanium complex: Synthesis of novel syndiotactic polypropylene-b-poly(propylene-ran-norbornene). Macromolecules 2006, 39, 2031–2033. [Google Scholar] [CrossRef]
- Cai, Z.; Harada, R.; Nakayama, Y.; Shiono, T. Highly active living random copolymerization of norbornene and 1-alkene with ansa-fluorenylamidodimethyltitanium derivative: Substituent effects on fluorenyl ligand. Macromolecules 2010, 43, 4527–4531. [Google Scholar] [CrossRef]
- Cai, Z.; Ikeda, T.; Akita, M.; Shiono, T. Substituent effects of tert-butyl groups on flurenyl ligand in syndiospecific living polymerization of propylene with ansa-fluorenylamidodimethyltitanium complex. Macromolecules 2005, 38, 8135–8139. [Google Scholar] [CrossRef]
- Cai, Z.; Ohmagari, M.; Nakayama, Y.; Shiono, T. Highly active syndiospecific living polymerization of higher 1-alkene with ansa-fluorenylamidodimethyltitanium complex. Macromol. Rapid Commun. 2009, 30, 1812–1816. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Su, H.; Nakayama, Y.; Shiono, T.; Akita, M. Synthesis of C1 symmetrical ansa-cyclopentadienylamidotitanium complexes and their application for living polymerization of propylene. J. Organomet. Chem. 2014, 770, 136–141. [Google Scholar] [CrossRef]
- Tanaka, R.; Yanase, C.; Cai, Z. Structure-stereospecificity relationships of propylene polymerization using substituted ansa-silylene(fluorenyl)(amido) titanium complexes. J. Organomet. Chem. 2016, 804, 95–100. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS: An Empirical Absorption Correction Program for Area Detector Data; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- SAINT+, Vdrsion 6.22a, Bruker AXS Inc.: Madison, WI, USA, 2002.
- SAINT+, Vdrsion v7.68A, Bruker AXS Inc.: Madison, WI, USA, 2009.
- SHELXTL NT/2000, Version 6.1, Bruker AXS Inc.: Madison, WI, USA, 2002.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Tolman, C.A.; Seidel, W.C.; Gosser, L.W. Formation of three-coordinate Nike(0) complexes by phosphorus ligand dissociation from NiL4. J. Am. Chem. Soc. 1974, 96, 53–60. [Google Scholar] [CrossRef]
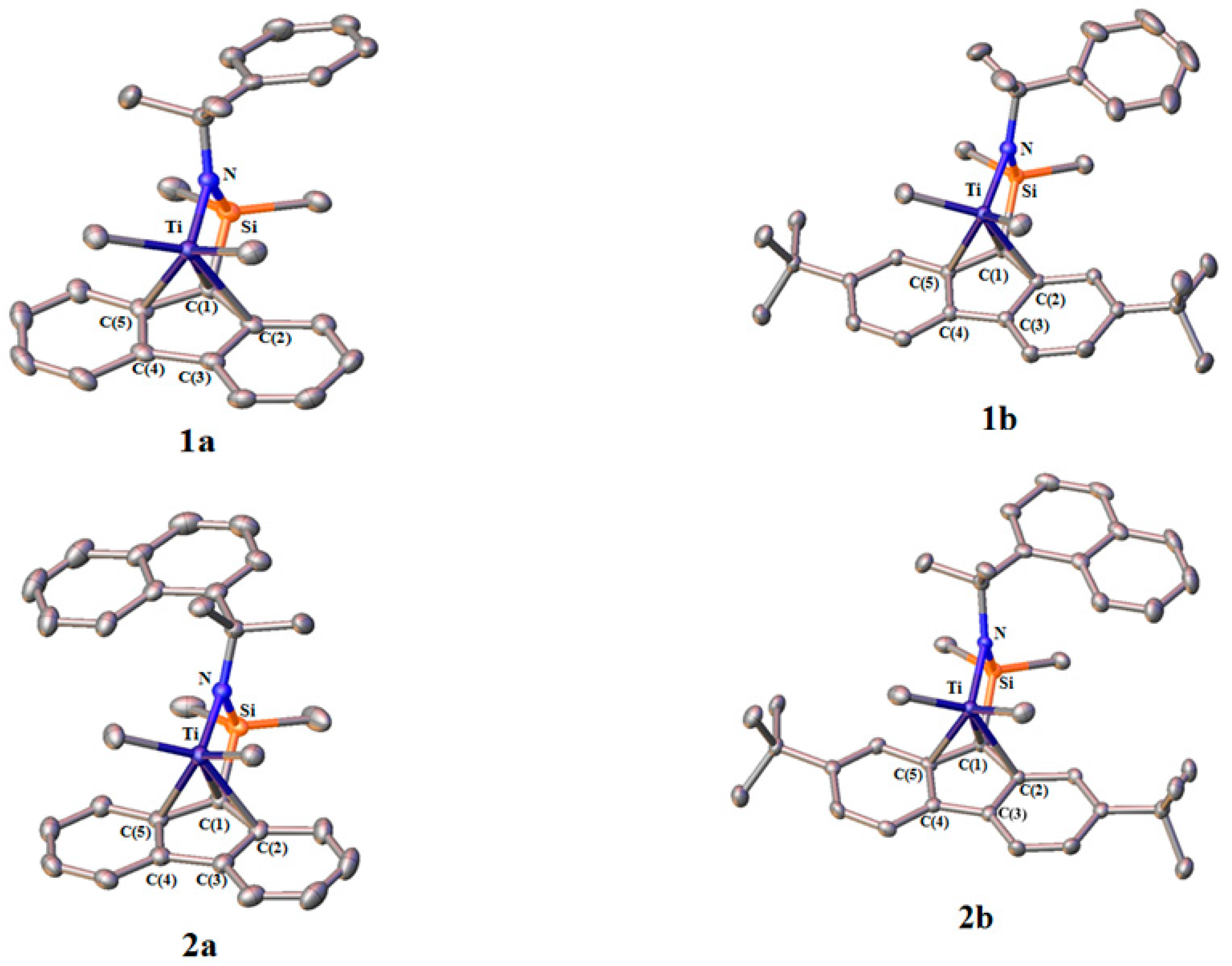
| Complex | 1a | 1b | 2a | 2b |
|---|---|---|---|---|
| deposition numbers | CCDC 1539490 | CCDC 1539499 | CCDC 1540563 | CCDC 1539502 |
| formula | C26H31NSiTi | C34H47NSiTi | C30H33NSiTi | C38H49NSiTi |
| formula weight | 433.51 | 545.71 | 483.56 | 595.77 |
| crystal system | Monoclinic | Triclinic | Monoclinic | Triclinic |
| space group | P1 21/c1 | P | P1 21/c1 | P |
| a (Å) | 8.8160(15) | 10.0609(13) | 12.742(3) | 10.3445(13) |
| b (Å) | 27.430(5) | 11.5953(15) | 17.562(4) | 11.8424(16) |
| c (Å) | 9.9361(18) | 15.4910(3) | 11.404(2) | 15.4920(3) |
| β(deg) | 108.372(4) | 100.124(3) | 95.269(4) | 99.822(4) |
| V(Å3) | 2280.3(7) | 1561.1(4) | 2541.1(9) | 1636.8(4) |
| Z | 4 | 2 | 4 | 2 |
| F(000) | 920 | 588 | 1024 | 640 |
| Dcalcd. (g·cm−3) | 1263 | 1161 | 1264 | 1209 |
| μ (mm−1) | 0.440 | 0.334 | 0.402 | 0.325 |
| theta range for data collection | 2.284° to 30.611° | 2.002° to 30.542° | 1.980° to 30.772° | 1.965° to 25.499° |
| reflections collected | 23,087 | 15,856 | 25,451 | 11,599 |
| independent reflections | 7002 | 9413 | 7920 | 6105 |
| final R indices [I > 2δ(I)] | [R(int) = 0.1147] | [R(int) = 0.0235] | [R(int) = 0.0610] | [R(int) = 0.0569] |
| R1 = 0.0512, wR2 = 0.0963 | R1 = 0.0398, wR2 = 0.0940 | R1 = 0.0455, wR2 = 0.1040 | R1 = 0.0508, wR2 = 0.1051 |
| Parameter | 1a | 1b | 2a | 2b |
|---|---|---|---|---|
| Ti(1)–C(1) | 2.241(2) | 2.250(14) | 2.260(19) | 2.267(3) |
| Ti(1)–C(2) | 2.409(3) | 2.384(14) | 2.414(19) | 2.413(3) |
| Ti(1)–C(3) | 2.586(3) | 2.599(14) | 2.556 | 2.576(3) |
| Ti(1)–C(4) | 2.594(3) | 2.615(15) | 2.538 | 2.569(3) |
| Ti(1)–C(5) | 2.398(2) | 2.430(15) | 2.396(19) | 2.394(3) |
| Ti(1)–N(1) | 1.919(2) | 1.922(12) | 1.922(17) | 1.920(2) |
| Ti(1)–Si(1) | 2.836(9) | 2.828(6) | 2.850 | 2.848(11) |
| N(1)–Ti(1)–C(1) | 78.35(9) | 78.56(5) | 78.01(7) | 77.74(11) |
| Ti(1)–N(1)–Si(1) | 100.88(10) | 100.40(6) | 101.30(8) | 101.76(12) |
| N(1)–Si(1)–C(1) | 93.66(10) | 94.20(6) | 93.75(8) | 93.88(13) |
| Entry | Cat. | Temp. (°C) | Time (min) | Yield (g) | Activity b | Mn c (×104) | Mw/Mn c | N d (μmol) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1a | 0 | 5 | 3.21 | 1930 | 25.2 | 1.49 | 13 |
| 2 | 1b | 0 | 5 | 3.46 | 2080 | 23.9 | 1.37 | 14 |
| 3 | 1c | 0 | 5 | 3.59 | 2150 | 26.1 | 1.58 | 14 |
| 4 | 1a | 25 | 5 | 4.05 | 2430 | 16.8 | 2.05 | 24 |
| 5 | 1b | 25 | 5 | 4.06 | 2430 | 19.4 | 1.70 | 21 |
| 6 | 1c | 25 | 5 | 4.21 | 2530 | 16.6 | 1.93 | 25 |
| 7 | 2a | 0 | 12 | 0.271 | 69 | 5.18 | 1.18 | 5.2 |
| 8 | 2b | 0 | 12 | 0.084 | 21 | 1.73 | 1.16 | 4.9 |
| 9 | 2a | 25 | 12 | 0.474 | 119 | 4.81 | 1.43 | 9.9 |
| 10 | 2b | 25 | 12 | 0.362 | 91 | 4.38 | 1.27 | 8.3 |
| Catalyst | Fluorenyl | N-alkyl | θ (°) |
|---|---|---|---|
| 1a | Flu | Ph | 86 |
| 1b | 2,7-di-tBu-Flu | Ph | 90 |
| 2a | Flu | Naphthyl | 103 |
| 2b | 2,7-di-tBu-Flu | Naphthyl | 102 |
| Entry | Stereosequence distribution a | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| mmmm | mmmr | rmmr | mmrr | mmrm + rmrr | rmrm | rrrr | mrrr | mrrm | |
| 4 | 0.04 | 0.05 | 0.06 | 0.22 | 0.22 | 0.13 | 0.09 | 0.14 | 0.05 |
| 5 | 0.02 | 0.03 | 0.04 | 0.18 | 0.22 | 0.11 | 0.15 | 0.19 | 0.06 |
| 6 | 0.03 | 0.05 | 0.03 | 0.13 | 0.20 | 0.14 | 0.16 | 0.21 | 0.05 |
| 10 | 0.12 | 0.10 | 0.08 | 0.29 | 0.16 | 0.05 | 0.03 | 0.08 | 0.09 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wang, X.; Sun, Y.; Cheng, H.; Shiono, T.; Cai, Z. Living Polymerization of Propylene with ansa-Dimethylsilylene(fluorenyl)(cumylamido) Titanium Complexes. Polymers 2017, 9, 131. https://doi.org/10.3390/polym9040131
Wang H, Wang X, Sun Y, Cheng H, Shiono T, Cai Z. Living Polymerization of Propylene with ansa-Dimethylsilylene(fluorenyl)(cumylamido) Titanium Complexes. Polymers. 2017; 9(4):131. https://doi.org/10.3390/polym9040131
Chicago/Turabian StyleWang, Huajin, Xinwei Wang, Yanjie Sun, Hailong Cheng, Takeshi Shiono, and Zhengguo Cai. 2017. "Living Polymerization of Propylene with ansa-Dimethylsilylene(fluorenyl)(cumylamido) Titanium Complexes" Polymers 9, no. 4: 131. https://doi.org/10.3390/polym9040131