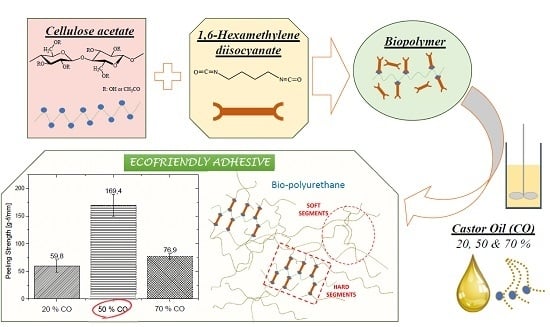
Preparation, Characterization and Mechanical Properties of Bio-Based Polyurethane Adhesives from Isocyanate-Functionalized Cellulose Acetate and Castor Oil for Bonding Wood
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
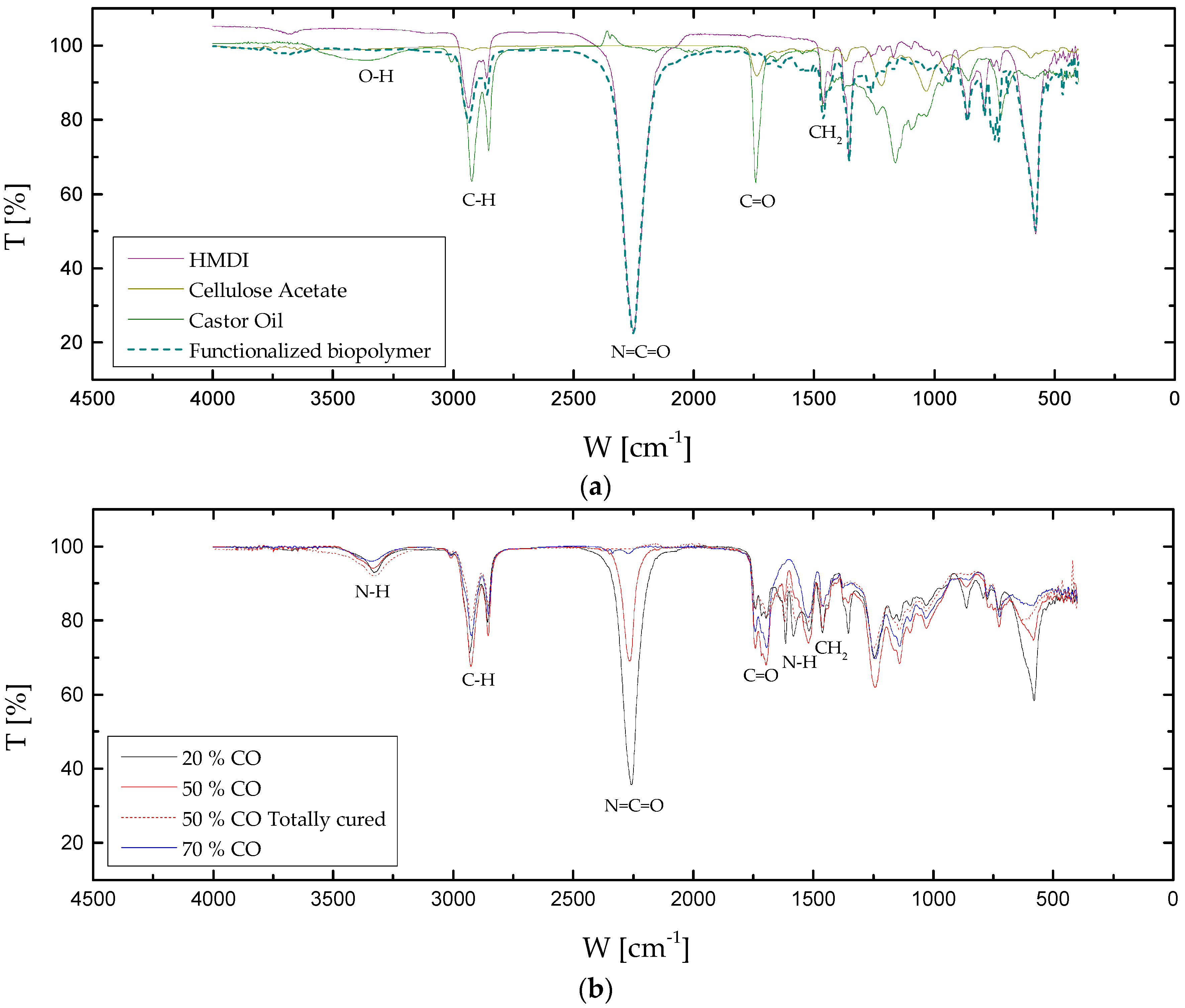
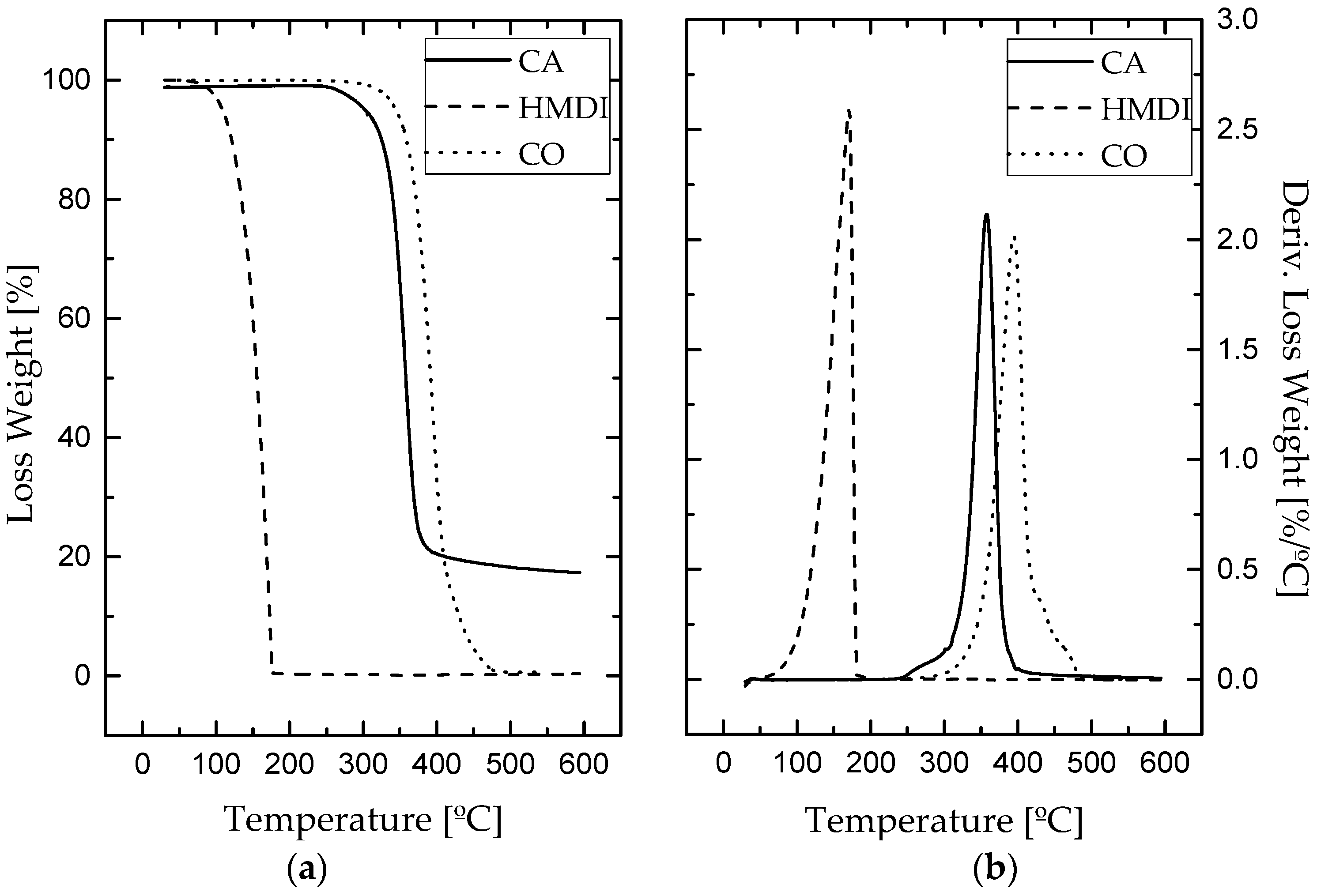
3.1. Chemical and Thermal Characterization
3.2. Rheological Properties
3.3. Adhesion Performance on Wood Substrates
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moubarik, A.; Allal, A.; Pizzi, A.; Charrier, F.; Charrier, B. Characterization of a formaldehyde-free cornstarch-tannin wood adhesive for interior plywood. Eur. J. Wood Wood Prod. 2010, 68, 427–433. [Google Scholar] [CrossRef]
- Desroches, M.; Escouvois, M.; Auvergne, R.; Caillol, S.; Boutevin, B. From vegetable oils to polyurethanes: Synthetic routes to polyols and main industrial products. Polym. Rev. 2012, 52, 38–79. [Google Scholar] [CrossRef]
- Kong, X.; Liu, G.; Curtis, J.M. Characterization of canola oil based polyurethane wood adhesives. Int. J. Adhes. Adhes. 2011, 31, 559–564. [Google Scholar] [CrossRef]
- Liu, Y.; Li, K. Development and characterization of adhesives from soy protein for bonding wood. Int. J. Adhes. Adhes. 2007, 27, 59–67. [Google Scholar] [CrossRef]
- Gogoi, S.; Karak, N. Biobased biodegradable waterborne hyperbranched polyurethane as an ecofriendly sustainable material. ACS Sustain. Chem. Eng. 2014, 2, 2730–2738. [Google Scholar] [CrossRef]
- Pizzi, A. Advanced Wood Adhesives Technology; CRC Press: New York, NY, USA, 1994. [Google Scholar]
- Ferdosian, F.; Pan, Z.; Gao, G.; Zhao, B. Bio-based adhesives and evaluation for wood composites application. Polymers 2017, 9, 70. [Google Scholar] [CrossRef]
- Pizzi, A. Recent developments in eco-efficient bio-based adhesives for wood bonding: Opportunities and issues. J. Adhes. Sci. Technol. 2006, 20, 829–846. [Google Scholar] [CrossRef]
- Santiago-Medina, F.; Foyer, G.; Pizzi, A.; Caillol, S.; Delmotte, L. Lignin-derived non-toxic aldehydes for ecofriendly tannin adhesives for wood panels. Int. J. Adhes. Adhes. 2016, 70, 239–248. [Google Scholar] [CrossRef]
- Muttil, N.; Ravichandra, G.; Bigger, S.W.; Thorpe, G.R.; Shailaja, D.; Singh, S.K. Comparative study of bond strength of formaldehyde and soya based adhesive in wood fibre plywood. Procedia Mater. Sci. 2014, 6, 2–9. [Google Scholar] [CrossRef]
- Bayer, O. Das Di-Isocyanat-Polyadditionsverfahren (Polyurethane). Angew. Chem. 1947, 59, 257–272. [Google Scholar] [CrossRef]
- Ji, D.; Fang, Z.; He, W.; Zhang, K.; Luo, Z.; Wang, T.; Guo, K. Synthesis of soy-polyols using a continuous microflow system and preparation of soy-based polyurethane rigid foams. ACS Sustain. Chem. Eng. 2015, 3, 1197–1204. [Google Scholar] [CrossRef]
- Pan, Y.; Zhan, J.; Pan, H.; Wang, W.; Tang, G.; Song, L.; Hu, Y. Effect of fully biobased coatings constructed via layer-by-layer assembly of chitosan and lignosulfonate on the thermal, flame retardant, and mechanical properties of flexible polyurethane foam. ACS Sustain. Chem. Eng. 2016, 4, 1431–1438. [Google Scholar] [CrossRef]
- Bakhshi, H.; Yeganeh, H.; Yari, A.; Nezhad, S.K. Castor oil-based polyurethane coatings containing benzyl triethanol ammonium chloride: Synthesis, characterization, and biological properties. J. Mater. Sci. 2014, 49, 5365–5377. [Google Scholar] [CrossRef]
- Ali, A.; Yusoh, K.; Hasany, S. Synthesis and physicochemical behaviour of polyurethane-multiwalled carbon nanotubes nanocomposites based on renewable castor oil polyols. J. Nanomater. 2014, 2014, 564384. [Google Scholar] [CrossRef]
- Abdalla, S.; Al-Aama, N.; Al-Ghamdi, M.A. A bio polymeric adhesive produced by photo cross-linkable technique. Polymers 2016, 8, 292. [Google Scholar] [CrossRef]
- Daniel da Silva, A.L.; Martín-Martínez, J.M.; Bordado, J.C.M. Influence of the free isocyanate content in the adhesive properties of reactive trifunctional polyether urethane quasi-prepolymers. Int. J. Adhes. Adhes. 2006, 26, 355–362. [Google Scholar] [CrossRef]
- Manjula, K.; Kumar, S.; Soare, B.G.; Picciani, P. Biobased chain extended polyurethane and its composites with silk fiber. Polym. Eng. Sci. 2010, 50, 851–856. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Raju, K. Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci. 2007, 32, 352–418. [Google Scholar] [CrossRef]
- Silva, B.B.; Santana, R.M.; Forte, M.M. A solventless castor oil-based PU adhesive for wood and foam substrates. Int. J. Adhes. Adhes. 2010, 30, 559–565. [Google Scholar] [CrossRef]
- Sharma, V.; Kundu, P. Condensation polymers from natural oils. Prog. Polym. Sci. 2008, 33, 1199–1215. [Google Scholar] [CrossRef]
- Xu, Y.; Petrovic, Z.; Das, S.; Wilkes, G.L. Morphology and properties of thermoplastic polyurethanes with dangling chains in ricinoleate-based soft segments. Polymer 2008, 49, 4248–4258. [Google Scholar] [CrossRef]
- Calvo-Correas, T.; Martin, M.D.; Retegi, A.; Gabilondo, N.; Corcuera, M.A.; Eceiza, A. Synthesis and characterization of polyurethanes with high renewable carbon content and tailored properties. ACS Sustain. Chem. Eng. 2016, 4, 5684–5692. [Google Scholar] [CrossRef]
- Ronda, J.C.; Lligadas, G.; Galià, M.; Cádiz, V. Vegetable oils as platform chemicals for polymer synthesis. Eur. J. Lipid Sci. Technol. 2011, 113, 46–58. [Google Scholar] [CrossRef]
- Gallego, R.; González, M.; Arteaga, J.F.; Valencia, C.; Franco, J.M. Influence of functionalization degree on the rheological properties of isocyanate-functionalized chitin-and chitosan-based chemical oleogels for lubricant applications. Polymers 2014, 6, 1929–1947. [Google Scholar] [CrossRef]
- Gallego, R.; Arteaga, J.; Valencia, C.; Díaz, M.; Franco, J. Gel-like dispersions of HMDI-cross-linked lignocellulosic materials in castor oil: Toward completely renewable lubricating grease formulations. ACS Sustain. Chem. Eng. 2015, 3, 2130–2141. [Google Scholar] [CrossRef]
- Allauddin, S.; Narayan, R.; Raju, K. Synthesis and properties of alkoxysilane castor oil and their polyurethane/urea–silica hybrid coating films. ACS Sustain. Chem. Eng. 2013, 1, 910–918. [Google Scholar] [CrossRef]
- Gallego, R.; Arteaga, J.; Valencia, C.; Franco, J. Thickening properties of several NCO-functionalized cellulose derivatives in castor oil. Chem. Eng. Sci. 2015, 134, 260–268. [Google Scholar] [CrossRef]
- Głowińska, E.; Datta, J. Bio polyetherurethane composites with high content of natural ingredients: Hydroxylated soybean oil based polyol, bio glycol and microcrystalline cellulose. Cellulose 2016, 23, 581–592. [Google Scholar] [CrossRef]
- Deka, H.; Karak, N. Vegetable oil-based hyperbranched thermosetting polyurethane/clay nanocomposites. Nanoscale Res. Lett. 2009, 4, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, T.; Chung, J.S. Physicochemical oroperties of amino–silane-terminated vegetable oil-based waterborne polyurethane nanocomposites. ACS Sustain. Chem. Eng. 2016, 4, 4645–4653. [Google Scholar] [CrossRef]
- Campanella, A.; Bonnaillie, L.; Wool, R. Polyurethane foams from soyoil-based polyols. J. Appl. Polym. Sci. 2009, 112, 2567–2578. [Google Scholar] [CrossRef]
- Ferrer, M.C.C.; Babb, D.; Ryan, A.J. Characterisation of polyurethane networks based on vegetable derived polyol. Polymer 2008, 49, 3279–3287. [Google Scholar] [CrossRef]
- Rojek, P.; Prociak, A. Effect of different rapeseed-oil-based polyols on mechanical properties of flexible polyurethane foams. J. Appl. Polym. Sci. 2012, 125, 2936–2945. [Google Scholar] [CrossRef]
- Das, B.; Konwar, U.; Mandal, M.; Karak, N. Sunflower oil based biodegradable hyperbranched polyurethane as a thin film material. Ind. Crops Prod. 2013, 44, 396–404. [Google Scholar] [CrossRef]
- Kong, X.; Liu, G.; Curtis, J.M. Novel polyurethane produced from canola oil based poly (ether ester) polyols: Synthesis, characterization and properties. Eur. Polym. J. 2012, 48, 2097–2106. [Google Scholar] [CrossRef]
- Ogunniyi, D. Castor oil: A vital industrial raw material. Bioresour. Technol. 2006, 97, 1086–1091. [Google Scholar] [CrossRef] [PubMed]
- Somani, K.P.; Kansara, S.S.; Patel, N.K.; Rakshit, A.K. Castor oil based polyurethane adhesives for wood-to-wood bonding. Int. J. Adhes. Adhes. 2003, 23, 269–275. [Google Scholar] [CrossRef]
- Gallego, R.; Arteaga, J.; Valencia, C.; Franco, J. Chemical modification of methyl cellulose with HMDI to modulate the thickening properties in castor oil. Cellulose 2013, 20, 495–507. [Google Scholar] [CrossRef]
- Stuart, B.H. Organic molecules. In Infrared Spectroscopy: Fundamentals and Applications; Ando, D.J., Ed.; University of Technology: Sidney, Australia, 2004; pp. 71–93. [Google Scholar]
- Corcuera, M.; Rueda, L.; d’Arlas, B.F.; Arbelaiz, A.; Marieta, C.; Mondragon, I.; Eceiza, A. Microstructure and properties of polyurethanes derived from castor oil. Polym. Degrad. Stab. 2010, 95, 2175–2184. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Zhang, L.; Tan, T.; Fong, H. Nonisocyanate biobased poly (ester urethanes) with tunable properties synthesized via an environment-friendly route. ACS Sustain. Chem. Eng. 2016, 4, 2762–2770. [Google Scholar] [CrossRef]
- Ugarte, L.; Gómez-Fernández, S.; Peña-Rodrı́uez, C.; Prociak, A.; Corcuera, M.A.; Eceiza, A. Tailoring mechanical properties of rigid polyurethane foams by sorbitol and corn derived biopolyol mixtures. ACS Sustain. Chem. Eng. 2015, 3, 3382–3387. [Google Scholar] [CrossRef]
- Gallego, R.; Arteaga, J.; Valencia, C.; Franco, J. Rheology and thermal degradation of isocyanate-functionalized methyl cellulose-based oleogels. Carbohydr. Polym. 2013, 98, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. Isocyanate terminated castor oil-based polyurethane prepolymer: Synthesis and characterization. Prog. Org. Coat. 2015, 80, 39–48. [Google Scholar] [CrossRef]
- Bagdi, K.; Molnár, K.; Sajó, I.; Pukánszky, B. Specific interactions, structure and properties in segmented polyurethane elastomers. Express Polym. Lett. 2011, 5, 417–427. [Google Scholar] [CrossRef]
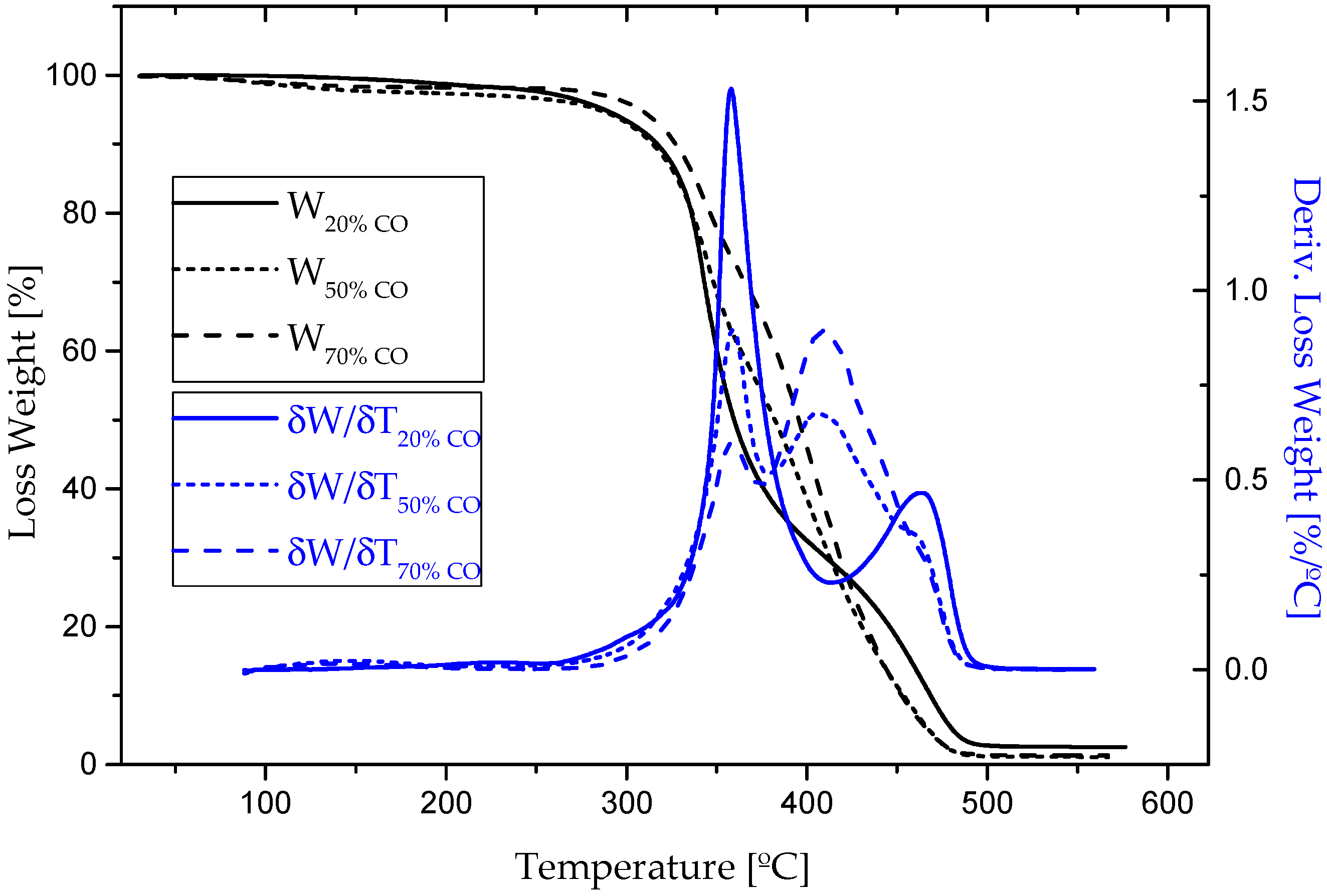
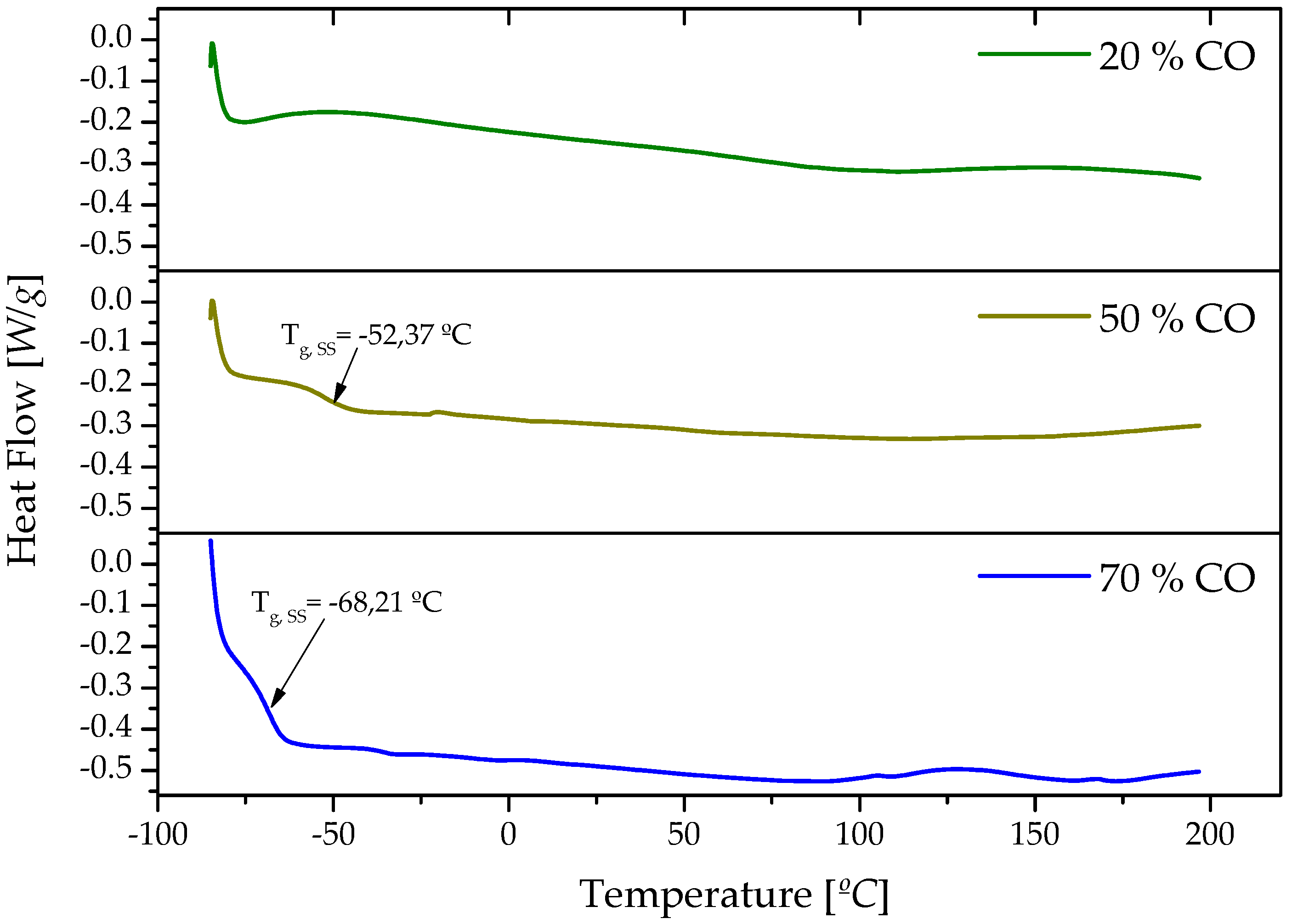
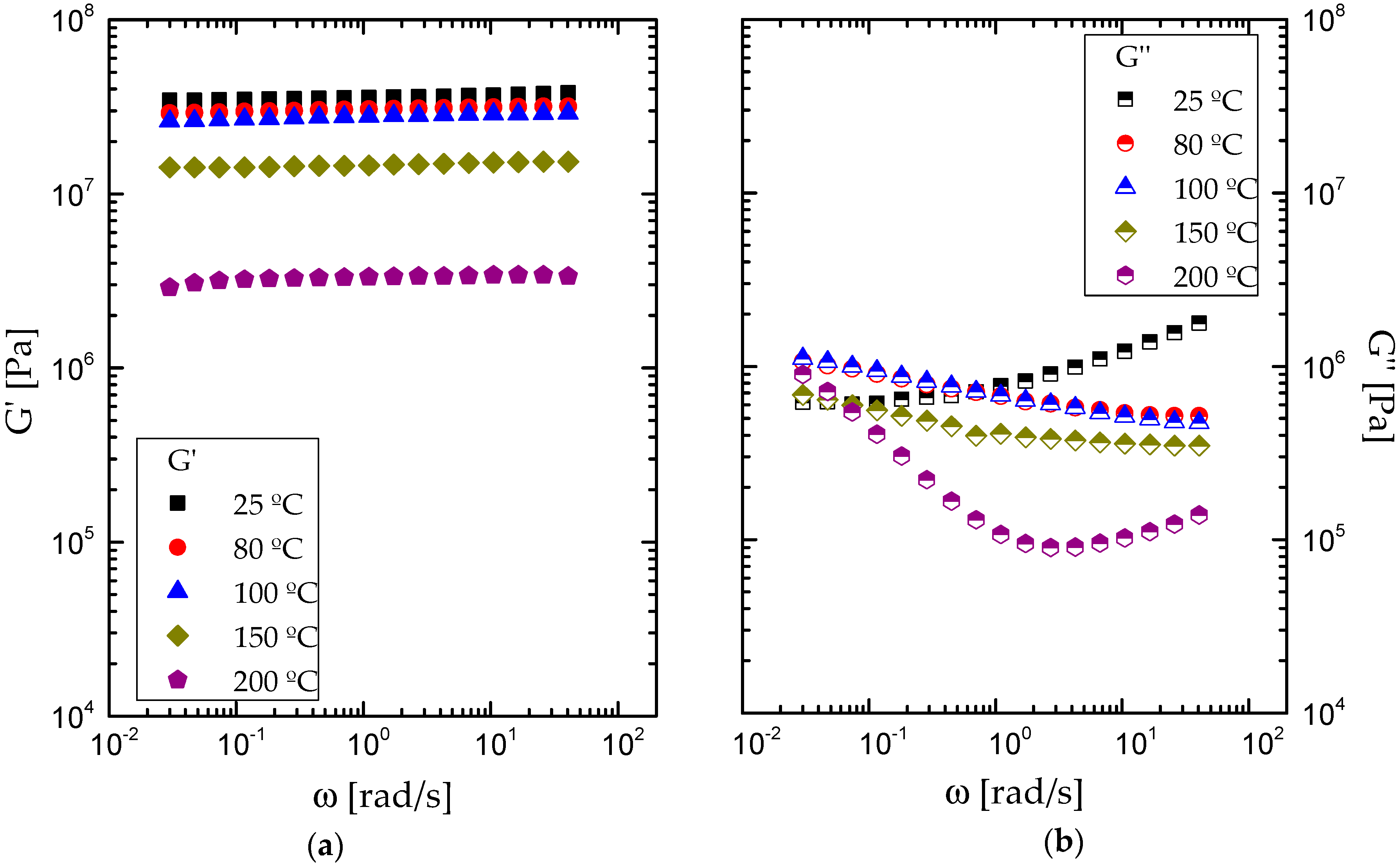

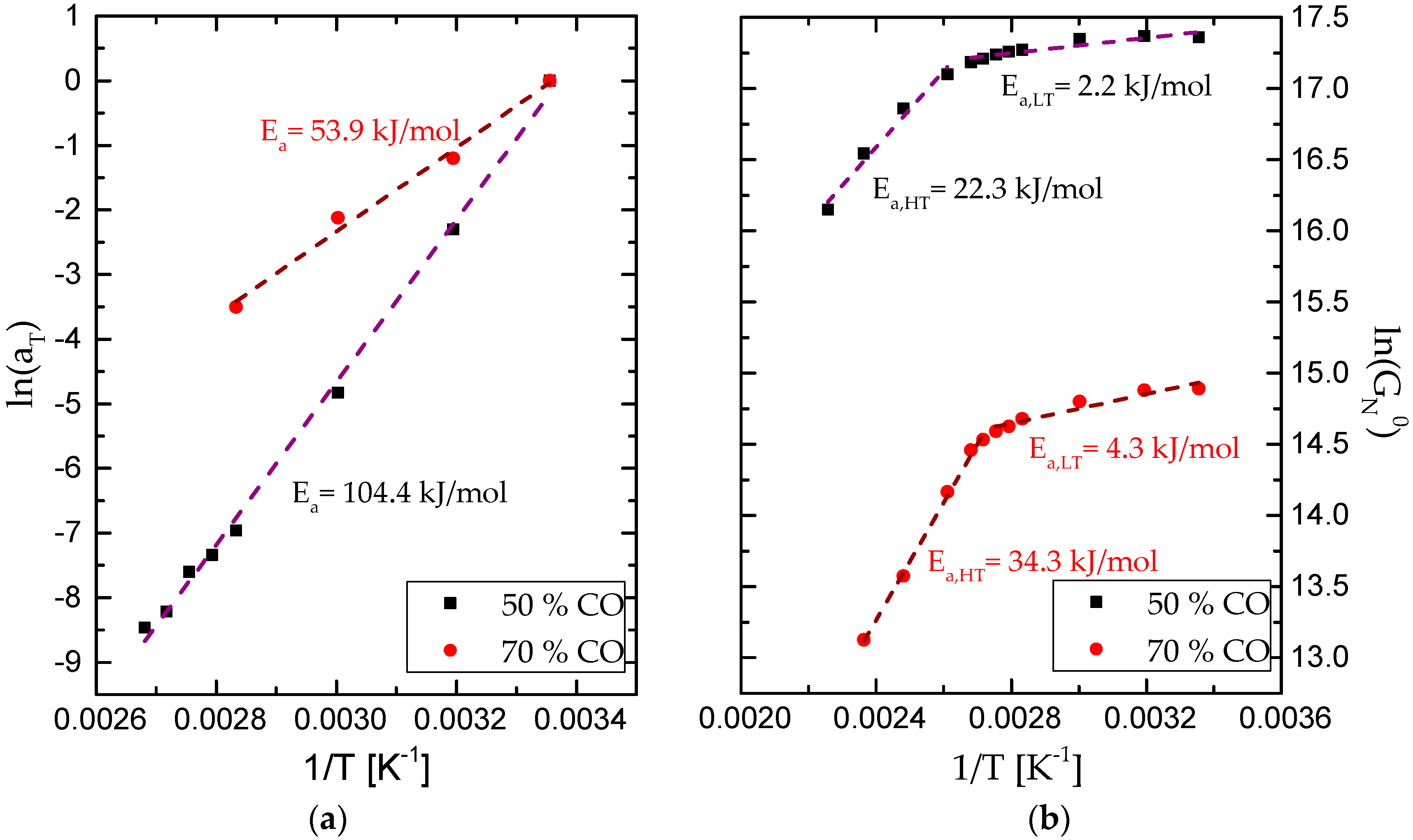
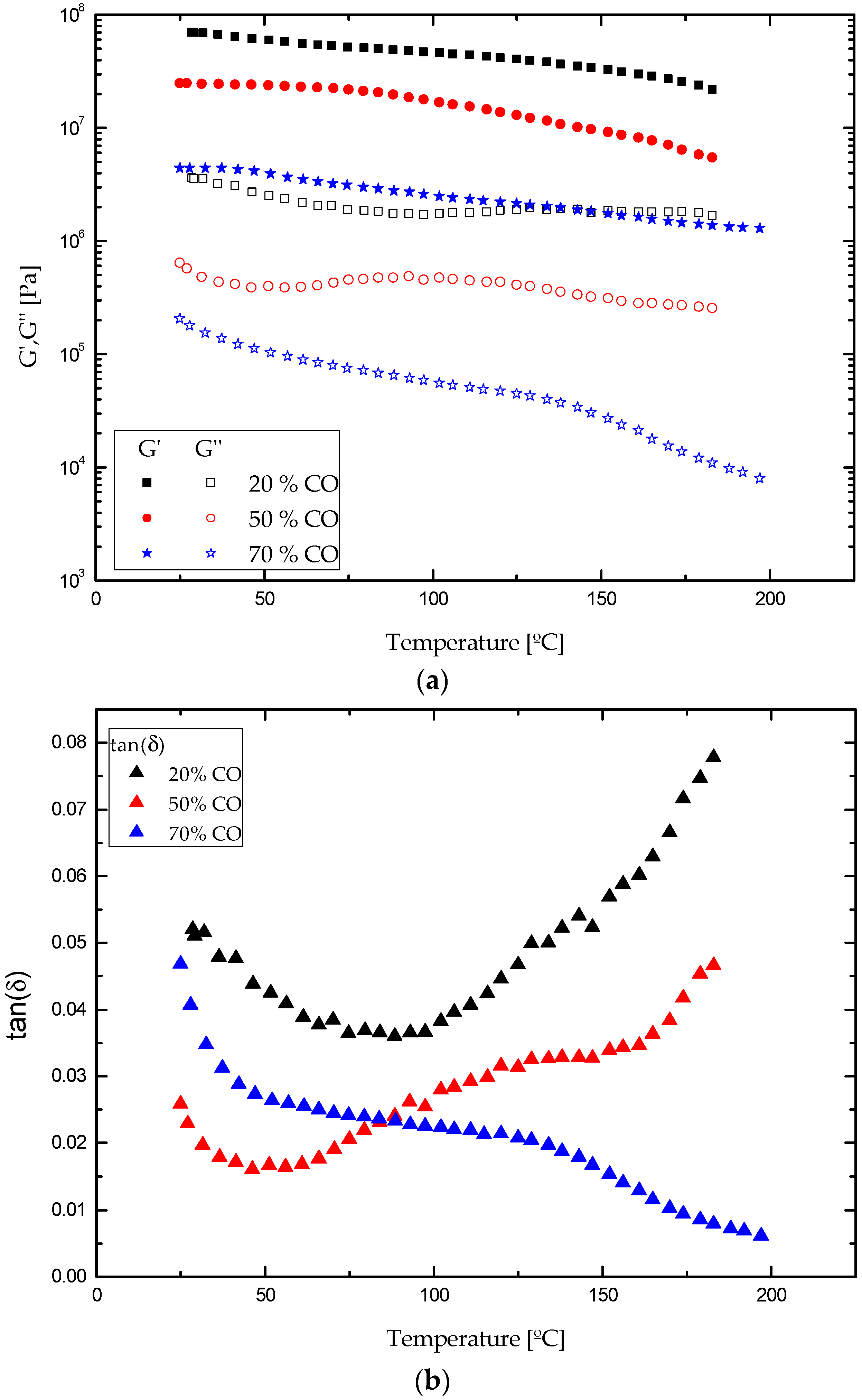
| Sample | Tonset [°C] | Tmax [°C] | Tfinal [°C] | Weight loss [%] | Residue [%] |
|---|---|---|---|---|---|
| 20% CO | 130/326/426 | 193/343/465 | 204/372/493 | 1.6/67.5/28.5 | 2.6 |
| 50% CO | 63/314/374 | 98/344/397 | 158/358/442 | 2.3/39.2/47.2 | 1.2 |
| 70% CO | 60/307/370 | 93/343/402 | 155/357/453 | 1.6/26.9/63.1 | 1.4 |
| HMDI | 138 | 171 | 176 | 99.7 | 0.3 |
| Cellulose Acetate | 335 | 358 | 373 | 82 | 18 |
| Castor Oil | 367 | 395 | 416 | 99.4 | 0.6 |
| Sample | Peeling strength [g–f/mm] | Shear strength [MPa] | Flexural strength [MPa] |
|---|---|---|---|
| 20% CO | 59.8 ± 10.9 a | 2.37 ± 0.03 b,c | 21.9 ± 6.0 d,e |
| 50% CO | 169.4 ± 19.6 b | 2.84 ± 0.36 b,c | 21.6 ± 4.9 d,e |
| 70% CO | 76.9 ± 4.9 b | 0.94 ± 0.24 b | 14.5 ± 0.9 d |
| Commercial polyurethane adhesive | 228.6 ± 28.3 b | 2.50 ± 0.40 b | 11.0 ± 3.0 d,e |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tenorio-Alfonso, A.; Sánchez, M.C.; Franco, J.M. Preparation, Characterization and Mechanical Properties of Bio-Based Polyurethane Adhesives from Isocyanate-Functionalized Cellulose Acetate and Castor Oil for Bonding Wood. Polymers 2017, 9, 132. https://doi.org/10.3390/polym9040132
Tenorio-Alfonso A, Sánchez MC, Franco JM. Preparation, Characterization and Mechanical Properties of Bio-Based Polyurethane Adhesives from Isocyanate-Functionalized Cellulose Acetate and Castor Oil for Bonding Wood. Polymers. 2017; 9(4):132. https://doi.org/10.3390/polym9040132
Chicago/Turabian StyleTenorio-Alfonso, Adrián, María Carmen Sánchez, and José M. Franco. 2017. "Preparation, Characterization and Mechanical Properties of Bio-Based Polyurethane Adhesives from Isocyanate-Functionalized Cellulose Acetate and Castor Oil for Bonding Wood" Polymers 9, no. 4: 132. https://doi.org/10.3390/polym9040132