Novel Magnet and Thermoresponsive Chemosensory Electrospinning Fluorescent Nanofibers and Their Sensing Capability for Metal Ions
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
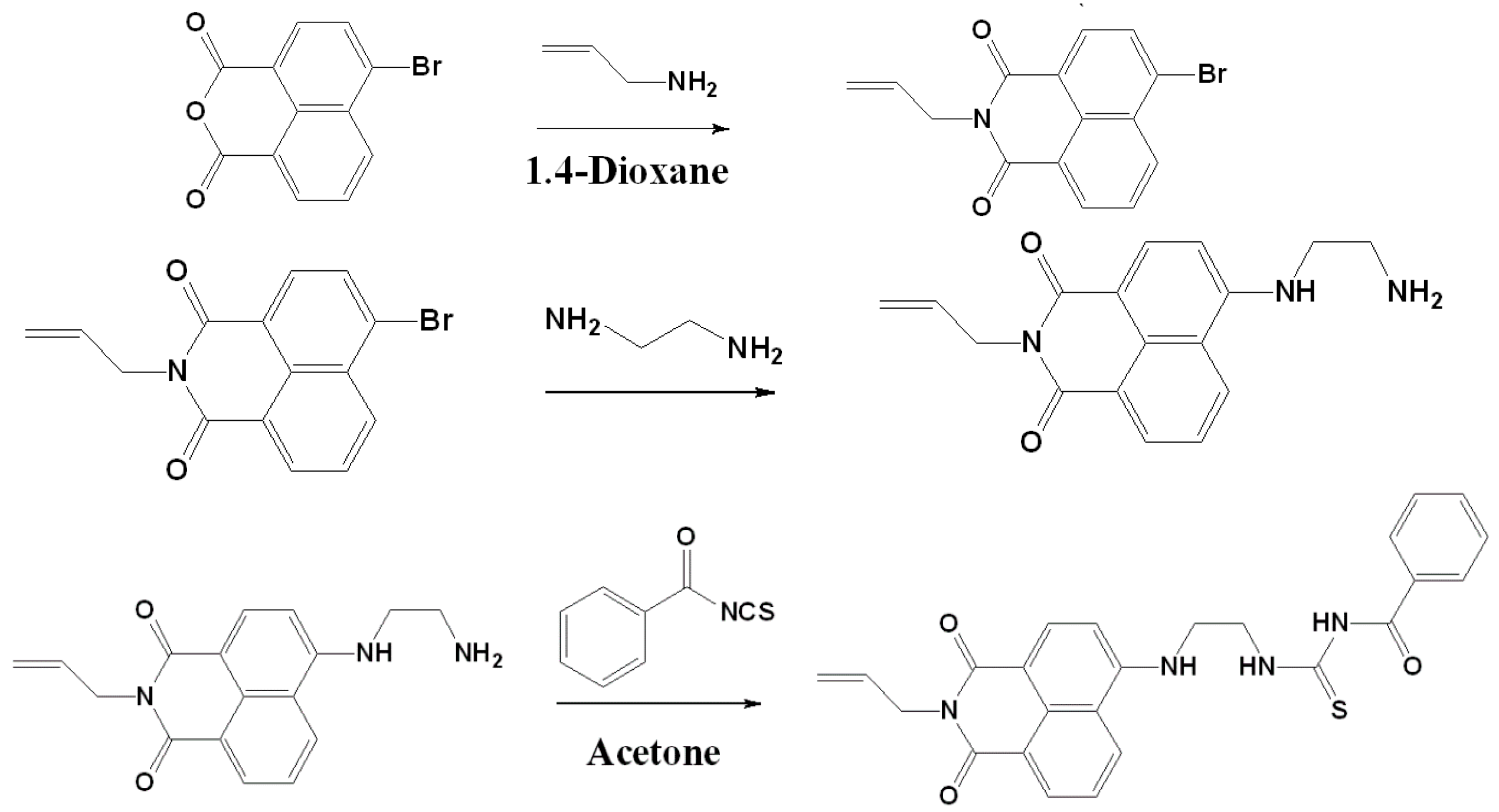
2.2. Synthesis of 4-Bromo-N-allyl-1,8-naphthalimide
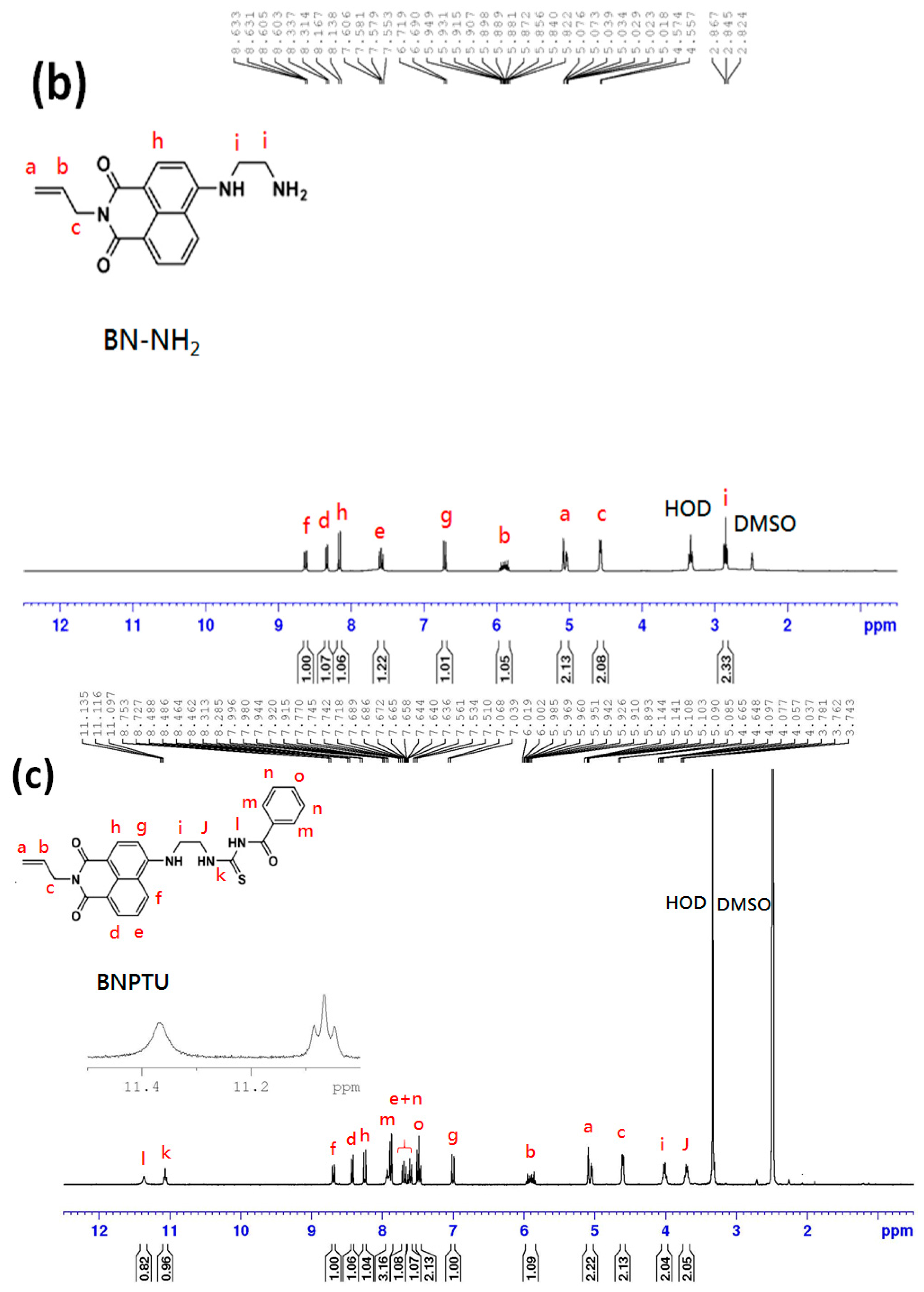
2.3. Synthesis of 4-(Aminoethylene)amino-N-allyl-1,8-naphthalimide
2.4. Synthesis of 1-Benzoyl-3-[2-(2-allyl-1,3-dioxo-2,3-dihydro-1Hbenzo[de]isoquinolin-6-ylamino)-ethyl]-thiourea (BNPTU)
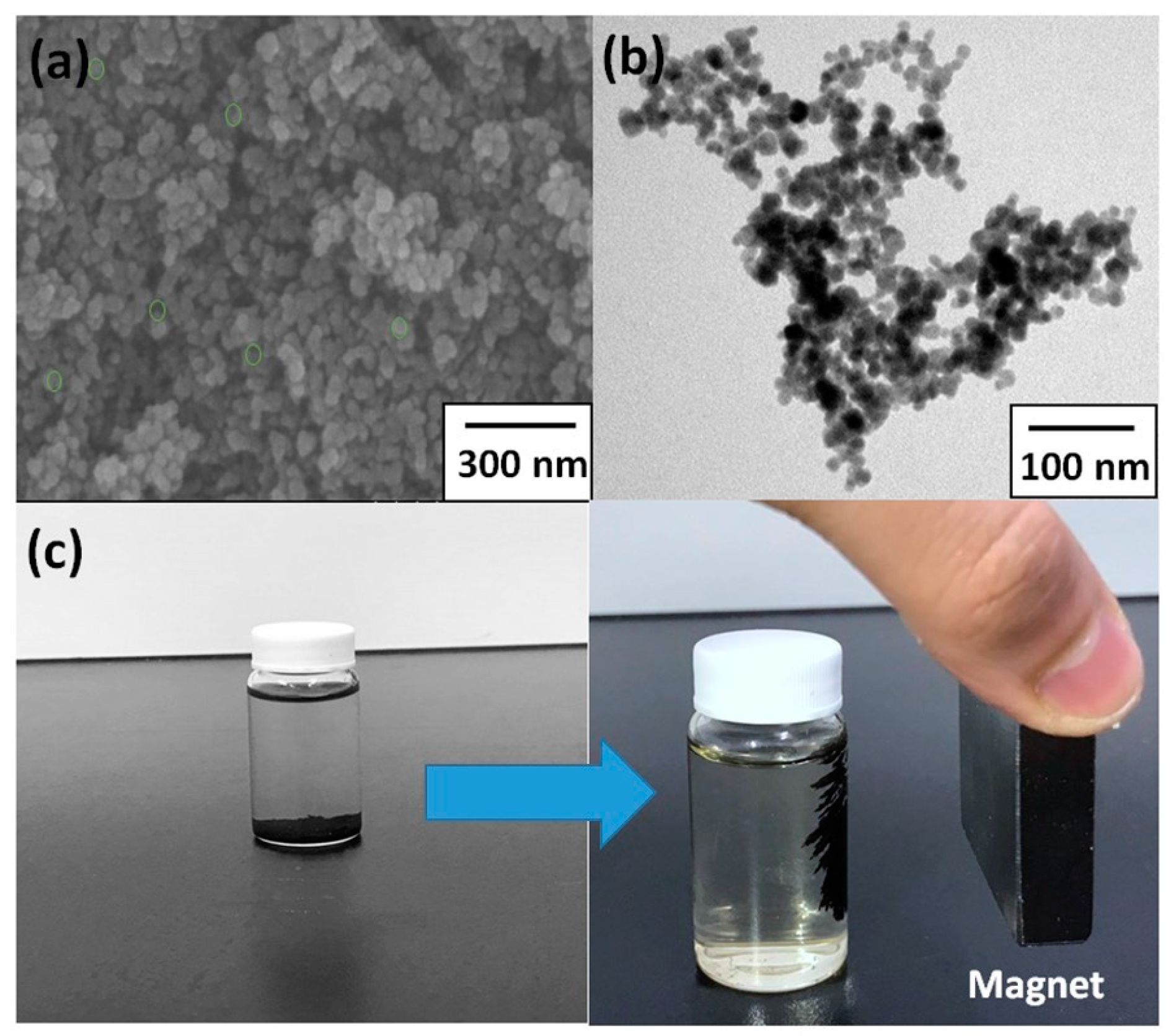
2.5. Synthesis of Magnetic Iron Oxide (Fe3O4) Nanoparticles (NPs)
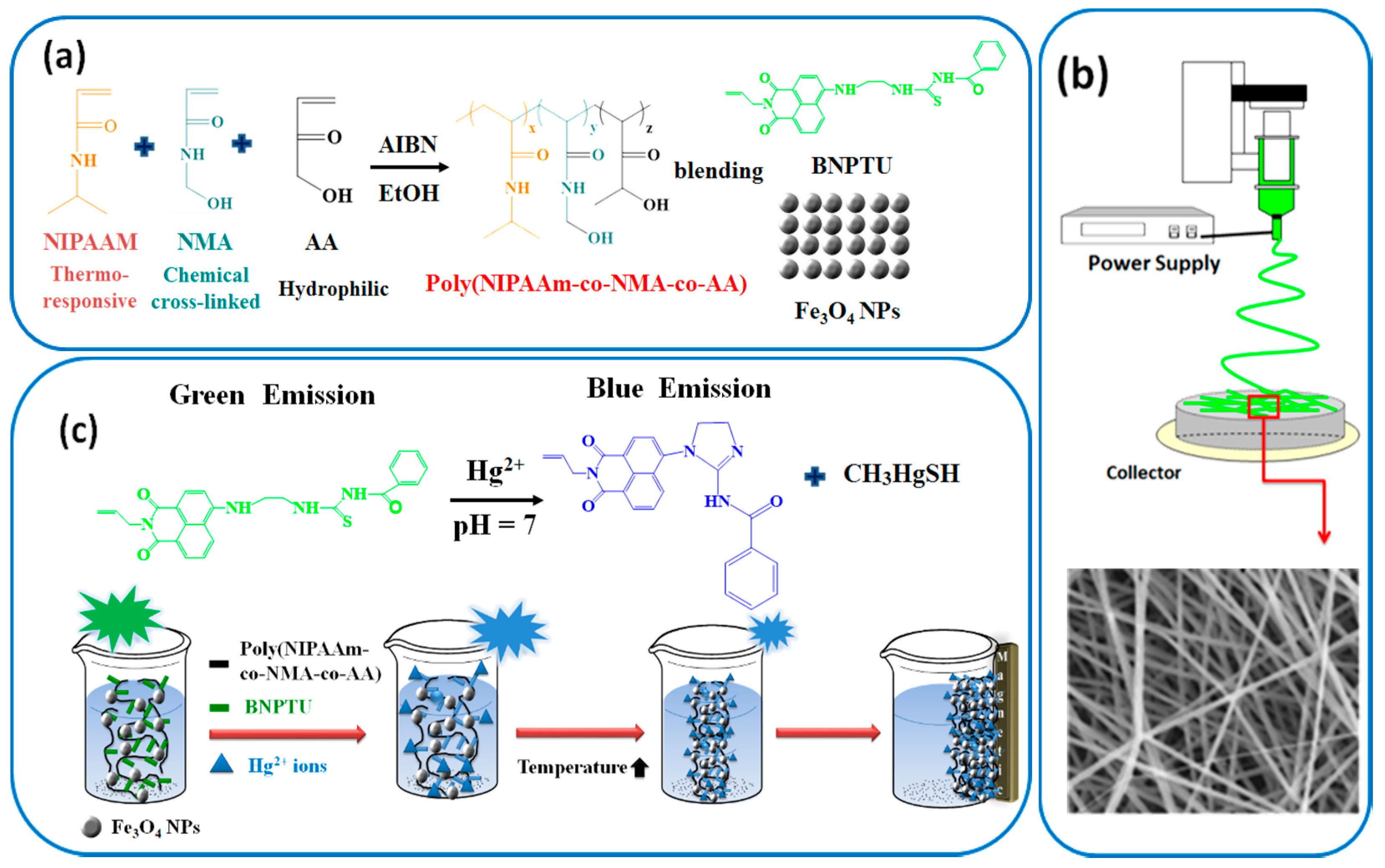
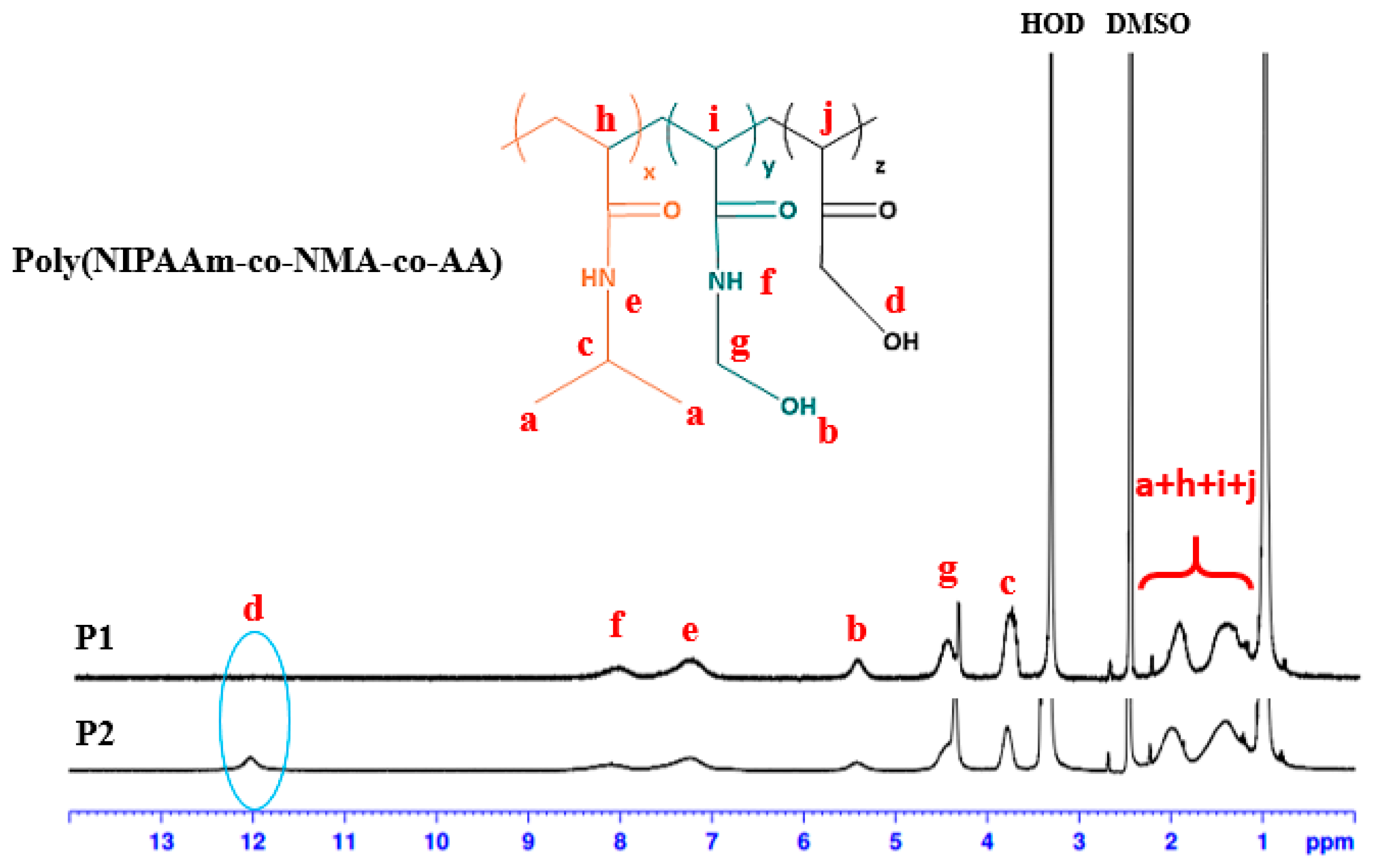
2.6. Synthesis of Poly(NIPAAm-co-NMA-co-AA)
2.7. Synthesis of Poly(NIPAAm-co-NMA) Random Copolymers (P1)
2.8. Synthesis of Poly(NIPAAm-co-NMA-co-AA) Random Copolymers (P2)
2.9. Preparation of Electrospun (ES) Nanofibers
2.10. Characterization
3. Results and Discussion
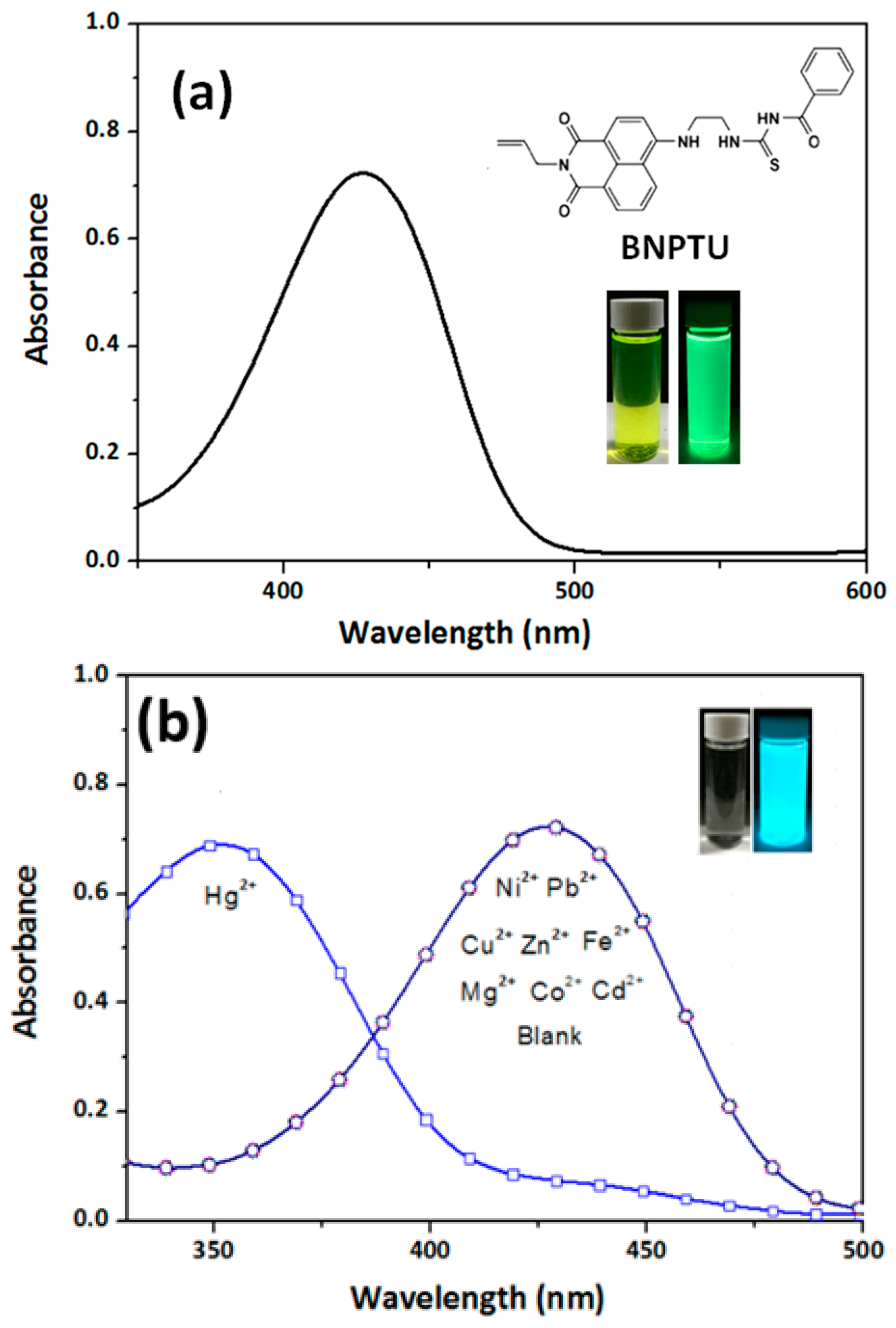
3.1. Characterization of BNPTU and Poly(NIPAAm-co-NMA-co-AA)
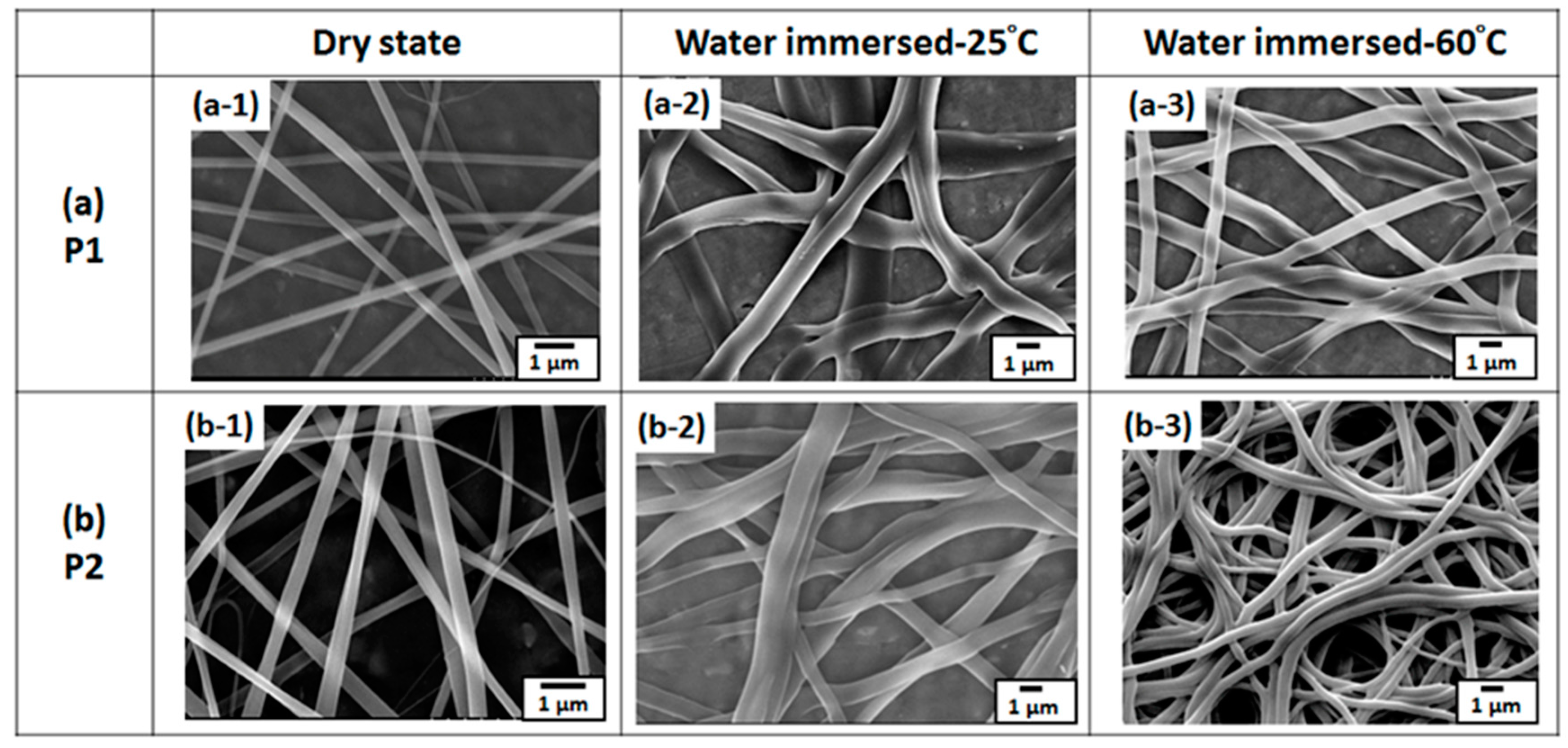
3.2. Morphology and Characterization of ES Nanofibers
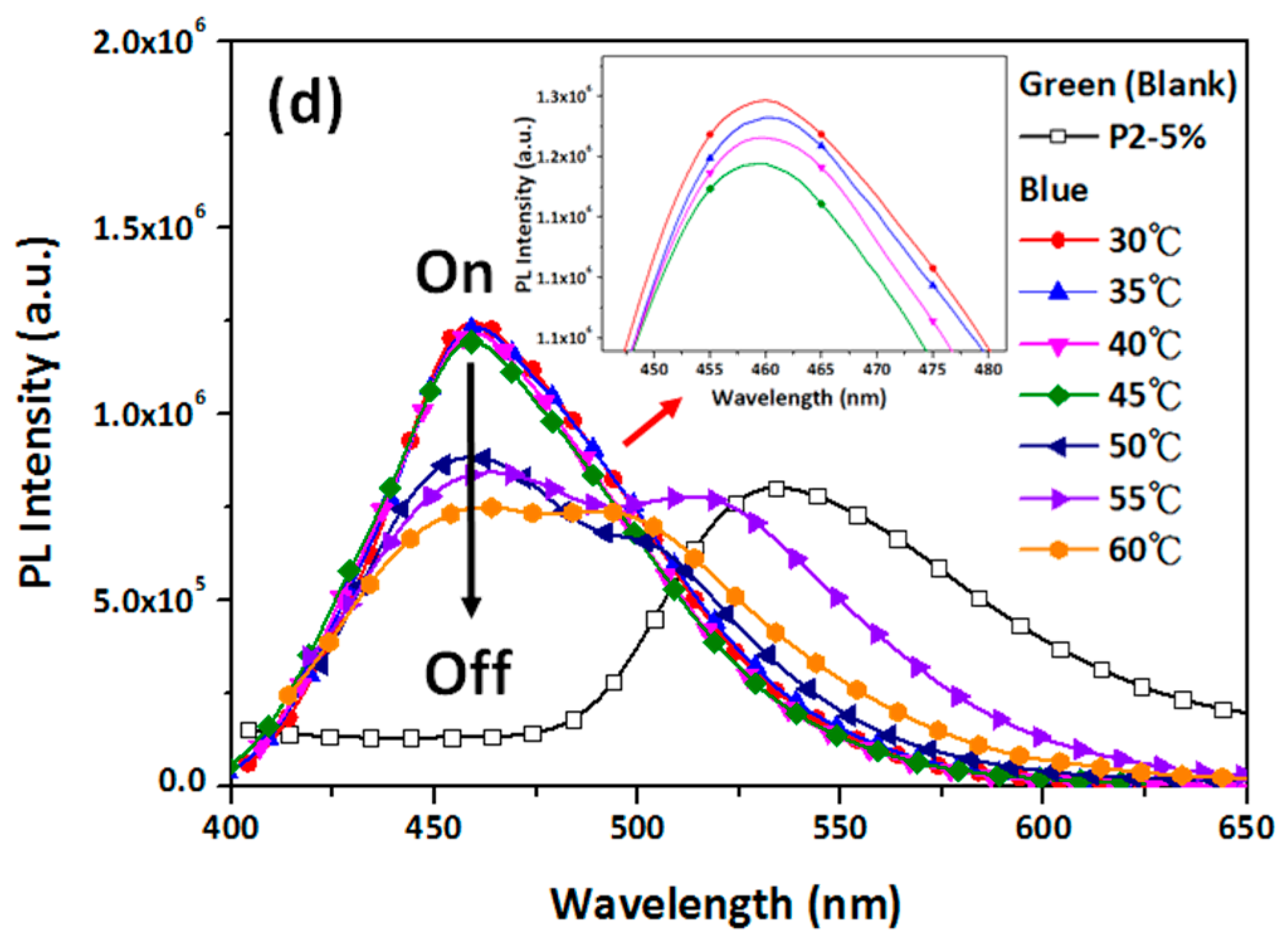
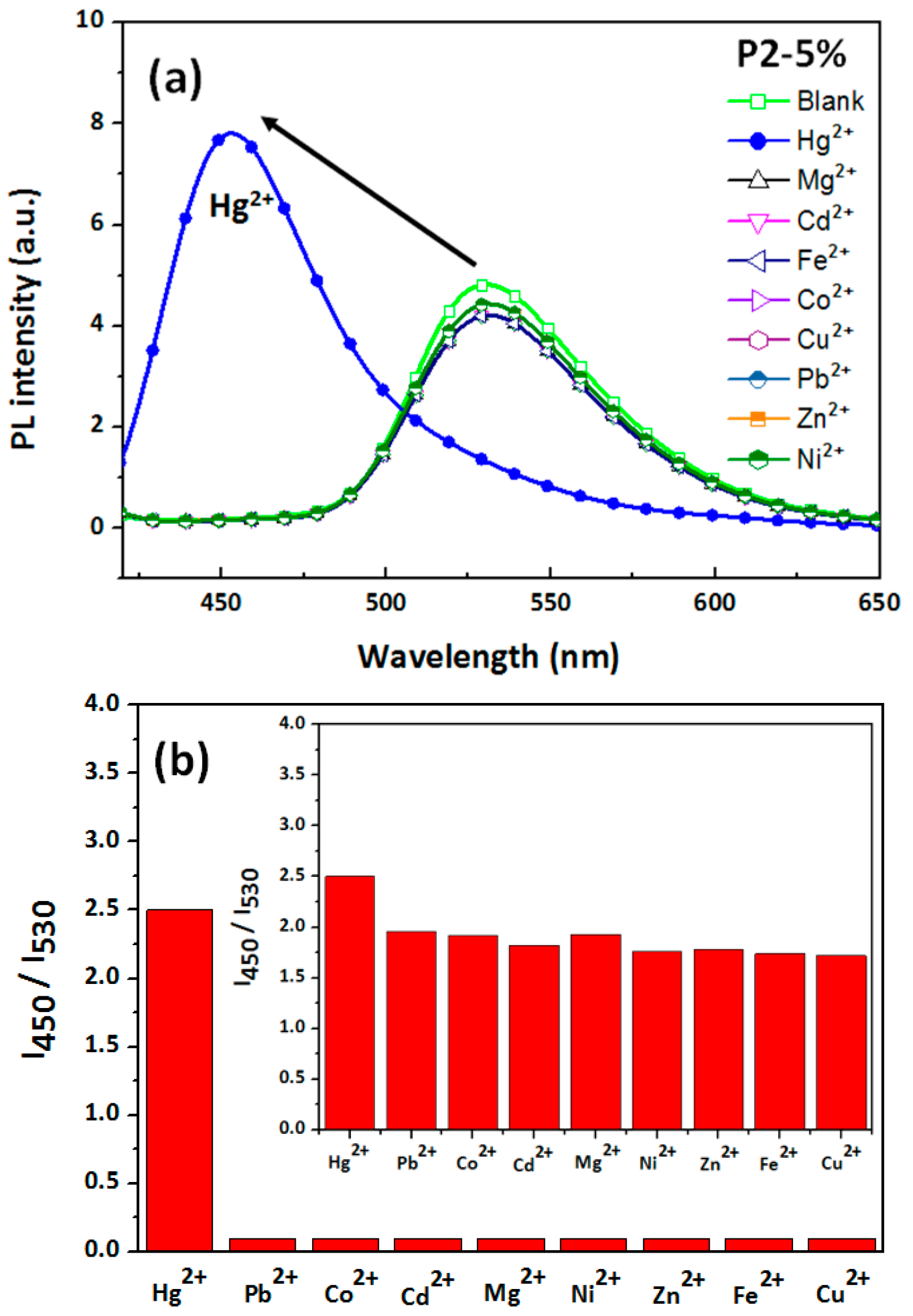
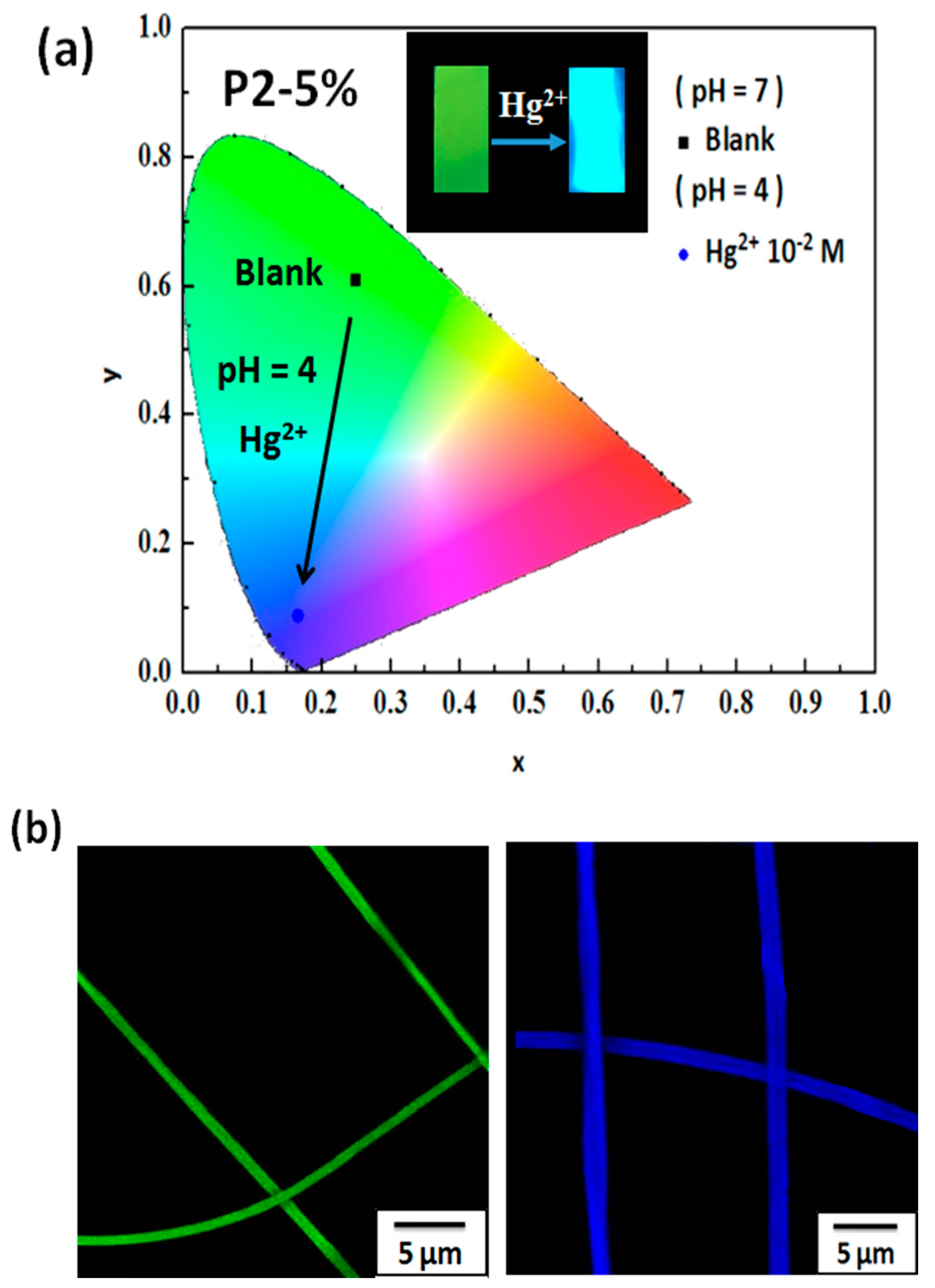
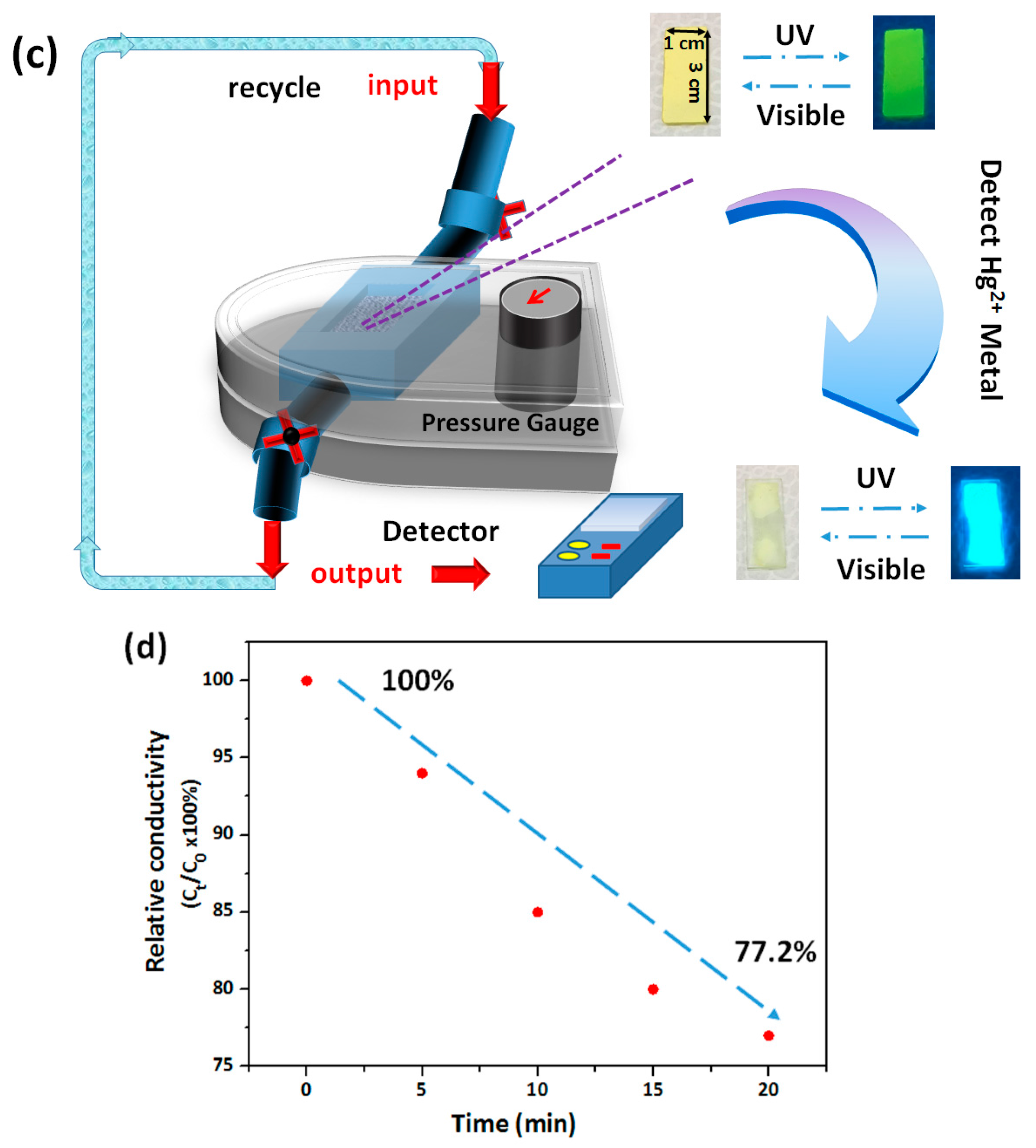
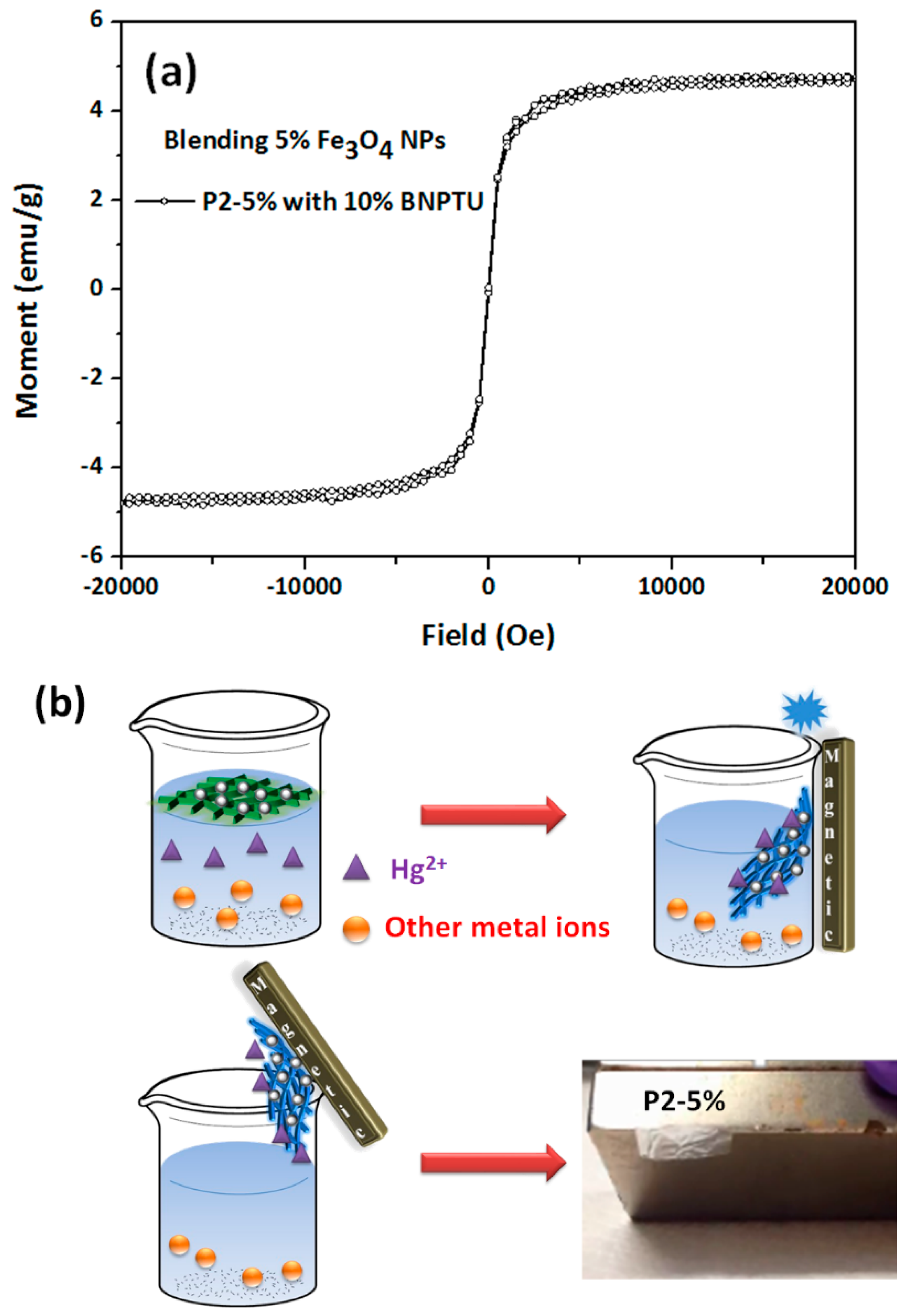
3.3. Hg2+ Sensing, Thermoresponsiveness, and Magnetic Properties of ES Nanofibers
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rurack, K.; Resch-Genger, U. Rigidization, Preorientation and Electronic Decoupling—The ‘Magic Triangle’ for the Design of Highly Efficient Fluorescent Sensors and Switches. Chem. Soc. Rev. 2002, 31, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Fabbrizzi, L.; Foti, F.; Licchelli, M.; Mangano, C.; Pallavicini, P.; Poggi, A.; Sacchi, D.; Taglietti, A. Light-emitting molecular devices based on transition metals. Coord. Chem. Rev. 2006, 250, 273–299. [Google Scholar] [CrossRef]
- Boening, D.W. Ecological Effects, Transport, and Fate of Mercury: A General Review. Chemosphere 2000, 40, 1335–1351. [Google Scholar] [CrossRef]
- Milaeva, E.R. The Role of Radical Reactions in Organomercurials Impact on Lipid Peroxidation. J. Inorg. Biochem. 2006, 100, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Tian, H. A Selective Fluorescent Ratiometric Chemodosimeter for Mercury Ion. Chem. Commun. 2005, 3156–3158. [Google Scholar] [CrossRef] [PubMed]
- Leng, B.; Zou, L.; Jiang, J.; Tian, H. Colorimetric Detection of Mercuric Ion (Hg2+) in Aqueous Media Using Chemodosimeter-Functionalized Gold Nanoparticles. Sens. Actuators B 2009, 140, 162–169. [Google Scholar] [CrossRef]
- Zou, Q.; Tian, H. Chemodosimeters for Mercury(II) and Methylmercury(I) Based on 2, 1, 3-Benzothiadiazole. Sens. Actuators B 2010, 149, 20–27. [Google Scholar] [CrossRef]
- Lua, Z.J.; Wang, P.N.; Zhang, Y.; Chen, J.Y.; Zhen, S.; Leng, B.; Tian, H. Tracking of Mercury Ions in Living Cells with a Fluorescent Chemodosimeter under Single- or Two-Photon Excitation. Anal. Chim. Acta 2007, 597, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wen, Y.; Zhang, Y.; Fei, X.; Li, Y.; Yang, Q.; Xu, X. Simple Naphthalimide-Based Fluorescent Sensor for Highly Sensitive and Selective Detection of Cd2+ and Cu2+ in Aqueous Solution and Living Cells. Dalton Trans. 2013, 42, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xu, J.; Yang, F.; Zhou, W.; Li, Z.; Wei, M.; Yu, M. Nanomolar Cu2+ and F− Naked-Eye Detection with a 1,8-Naphthalimide-Based Colorimetric Probe. Sens. Actuators B 2015, 212, 364–370. [Google Scholar] [CrossRef]
- Patrick, L.G.F.; Whiting, A. Synthesis of Some Polymerisable Fluorescent Dyes. Dyes Pigment. 2002, 55, 123–132. [Google Scholar] [CrossRef]
- Gil, E.S.; Hudson, S.M. Stimuli-Reponsive Polymers and Their Bioconjugates. Prog. Polym. Sci. 2004, 29, 1173–1222. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, L.; Dan, Y. Preparation and Thermal Response Behavior of Poly(N-isopropylacrylamide-co-acrylic acid) Microgels via Soap-Free Emulsion Polymerization Based on AIBN Initiator. J. Appl. Polym. Sci. 2011, 121, 3322–3331. [Google Scholar] [CrossRef]
- Babel, A.; Li, D.; Xia, Y.; Jenekhe, S.A. Electrospun Nanofibers of Blends of Conjugated Polymers: Morphology, Optical Properties, and Field-Effect Transistors. Macromolecules 2005, 38, 4705–4711. [Google Scholar] [CrossRef]
- Kuo, C.C.; Lin, C.H.; Chen, W.C. Morphology and Photophysical Properties of Light-Emitting Electrospun Nanofibers Prepared from Poly(fluorene) Derivative/PMMA Blends. Macromolecules 2007, 40, 6959–6966. [Google Scholar] [CrossRef]
- Kuo, C.C.; Tung, Y.C.; Lin, C.H.; Chen, W.C. Novel Luminescent Electrospun Fibers Prepared from Conjugated Rod–Coil Block Copolymer of Poly[2,7-(9,9-dihexylfluorene)]-block-Poly(methyl methacrylate). Macromol. Rapid Commun. 2008, 29, 1711–1715. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Kuo, C.C.; Chen, B.U.; Chiu, P.C.; Tsai, P.C. Multifunctional Polyacrylonitrile-ZnO/Ag Electrospun Nanofiber Membranes with Various ZnO Morphologies for Photocatalytic, UV-shielding, and Antibacterial Applications. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 262–269. [Google Scholar] [CrossRef]
- Kuo, C.C.; Tung, Y.C.; Chen, W.C. Morphology and pH Sensing Characteristics of New Luminescent Electrospun Fibers Prepared from Poly(phenylquinoline)-block-Polystyrene/Polystyrene Blends. Macromol. Rapid Commun. 2010, 31, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.C.; Kuo, C.C.; Hsu, J.C.; Chen, W.C. Thermoresponsive Luminescent Electrospun Fibers Prepared From Poly(DMAEMA-co-SA-co-StFl) Multifunctional Random Copolymers. ACS Appl. Mater. Interfaces 2010, 11, 3340–3347. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.C.; Chen, Y.; Kuo, C.C.; Tung, S.H.; Kakuchi, T.; Chen, W.C. Synthesis, Morphology, and Sensory Applications of Multifunctional Rod–Coil–Coil Triblock Copolymers and Their Electrospun Nanofibers. ACS Appl. Mater. Interfaces 2012, 4, 3387–3395. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.N.; Chiu, Y.C.; Hung, J.J.; Kuo, C.C.; Chen, W.C. Multifunctional Electrospun Nanofibers Prepared from Poly((N-isopropylacrylamide)-co-(N-hydroxymethylacrylamide)) and Their Blends with 1,2-Diaminoanthraquinone for NO Gas Detection. Macromol. Chem. Phys. 2014, 215, 286–294. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Q.; Sun, L.; Wang, H.; Zhang, C.; Fei, X.; Sun, M.; Li, Y. Preparation of Fluorescent Nanofibrous Film as a Sensing Material and Adsorbent for Cu2+ in Aqueous Solution via Copolymerization and Electrospinning. J. Hazard. Mater. 2011, 194, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.N.; Kuo, C.C.; Chiu, Y.C.; Chen, W.C. Ultra Metal Ions and pH Sensing Characteristics of Thermoresponsive Luminescent Electrospun Nanofibers Prepared from Poly(HPBO-co-NIPAAm-co-SA). RSC Adv. 2014, 4, 45345–45353. [Google Scholar] [CrossRef]
- Chen, B.Y.; Kuo, C.C.; Huang, Y.S.; Lu, S.T.; Liang, F.C.; Jiang, D.H. Novel Highly Selective and Reversible Chemosensors Based on Dual-Ratiometric Fluorescent Electrospun Nanofibers with pH- and Fe3+-Modulated Multicolor Fluorescence Emission. ACS Appl. Mater. Interfaces 2015, 7, 2797–2808. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.J.; Lu, S.T.; Kuo, C.C.; Liang, F.C.; Chen, B.Y.; Chu, C.C. Pyrene or Rhodamine Derivative–Modified Surfaces of Electrospun Nanofibrous Chemosensors for Colorimetric and Fluorescent Determination of Cu2+, Hg2+, and pH. React. Funct. Polym. 2016, 137–147. [Google Scholar] [CrossRef]
- Hung, C.C.; Kuo, C.C.; Weng, N.K.; Wu, W.C.; Chen, B.Y.; Cho, C.J.; Hsu, I.J.; Chiu, Y.C.; Chen, C.C. Novel Highly Sensitive and Reversible Electrospun Nanofibrous Chemosensor-Filters Composed of poly(HEMA-co-MNA) and Bpy-F-Bpy with Metal-Ionmodulated Multicolor Fluorescence Emission. Polym. J. 2016, 48, 439–449. [Google Scholar]
- Kuo, C.Y.; Liu, T.Y.; Hardiansyah, A.; Lee, C.F.; Wang, M.S.; Chiu, W.Y. Self-Assembly Behaviors of Thermal- and pH Sensitive Magnetic Nanocarriers for Stimulitriggered Release. Nanoscale Res. Lett. 2014, 9, 520. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jiang, W.; Liu, L.; Chen, B.; Wu, S.; Sun, D.; Li, F. One-Step Hydrothermal Synthesis of Highly Water-Soluble Secondary Structural Fe3O4 Nanoparticles. J. Magn. Magn. Mater. 2012, 324, 2249–2257. [Google Scholar] [CrossRef]
- Si, S.; Kotal, A.; Mandal, T.K.; Giri, S.; Nakamura, H.; Kohara, T. Size-Controlled Synthesis of Magnetite Nanoparticles in the Presence of Polyelectrolytes. Chem. Mater. 2004, 16, 3489–3496. [Google Scholar] [CrossRef]
- Zhao, C.; Jiang, Z.; Zhao, J.; Cao, K.; Zhang, Q.; Pan, F. High Pervaporation Dehydration Performance of the Composite Membrane with an Ultrathin Alginate/Poly(acrylic acid)–Fe3O4 Active Layer. Ind. Eng. Chem. Res. 2014, 53, 1606–1616. [Google Scholar] [CrossRef]
- Jiang, X.; Zhai, S.; Jiang, X.; Lu, G.; Huang, X. Synthesis of PAA-g-PNIPAM Well-Defined Graft Oolymer by Sequential RAFT and SET-LRP and its Application in Preparing Size-Controlled Super-Paramagnetic Fe3O4 Nanoparticles as a Stabilizer. Polymer 2014, 55, 3703–3712. [Google Scholar] [CrossRef]
- Liu, F.; Ni, Q.Q.; Murakami, Y. Preparation of Magnetic Polyvinyl Alcohol Composite Nanofibers with Homogenously Dispersed Nanoparticles and High Water Resistance. Text. Res. J. 2013, 83, 510–518. [Google Scholar] [CrossRef]
- Xu, L.; Liu, N.; Cao, Y.; Lu, F.; Chen, Y.; Zhang, X.; Feng, L.; Wei, L. Mercury Ion Responsive Wettability and Oil/Water Separation. ACS Appl. Mater. Interfaces 2014, 6, 13324–13329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, W.; Zhou, N.; Cheng, Z.; Zhu, J.; Zhu, X. Synthesis and Self-Assembly Behaviors of Three-Armed Amphiphilic Block Copolymers via RAFT Polymerization. Polymer 2008, 49, 4569–4575. [Google Scholar] [CrossRef]
- Manimala, J.C.; Anslyn, E.V. Solid-Phase Synthesis of Guanidinium Derivatives from Thiourea and Isothiourea Functionalities. Eur. J. Org. Chem. 2002, 3909–3922. [Google Scholar] [CrossRef]
- Kim, K.S.; Qian, L. Improved Method for the Preparation of Guanidines. Tetrahedron Lett. 1993, 34, 7677–7680. [Google Scholar] [CrossRef]
- Batey, R.A.; Powell, D.A. A General Synthetic Method for the Formation of Substituted 5-Aminotetrazoles from Thioureas: A Strategy for Diversity Amplification. Org. Lett. 2000, 2, 3237–3240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Niu, Y.; Meng, X.; Li, Y.; Zhao, J. Structural Elution and characteristics of the phase transformations between a-Fe2O3, Fe3O4 and c-Fe2O3nanoparticles under reducing and oxidizing atmospheres. CrystEngComm 2013, 15, 8166–8172. [Google Scholar] [CrossRef]



| Polymer No. | Composition a NIPAAm:NMA:AA | Mn b | PDI | Td (°C) | LCST (°C) (in pH = 7) |
|---|---|---|---|---|---|
| P1 | 10:5:0 | 26,856 | 2.01 | 375 | 54.5 |
| P2 | 10:5:3 | 18,041 | 1.76 | 374 | 58.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, F.-C.; Luo, Y.-L.; Kuo, C.-C.; Chen, B.-Y.; Cho, C.-J.; Lin, F.-J.; Yu, Y.-Y.; Borsali, R. Novel Magnet and Thermoresponsive Chemosensory Electrospinning Fluorescent Nanofibers and Their Sensing Capability for Metal Ions. Polymers 2017, 9, 136. https://doi.org/10.3390/polym9040136
Liang F-C, Luo Y-L, Kuo C-C, Chen B-Y, Cho C-J, Lin F-J, Yu Y-Y, Borsali R. Novel Magnet and Thermoresponsive Chemosensory Electrospinning Fluorescent Nanofibers and Their Sensing Capability for Metal Ions. Polymers. 2017; 9(4):136. https://doi.org/10.3390/polym9040136
Chicago/Turabian StyleLiang, Fang-Cheng, Yi-Ling Luo, Chi-Ching Kuo, Bo-Yu Chen, Chia-Jung Cho, Fan-Jie Lin, Yang-Yen Yu, and Redouane Borsali. 2017. "Novel Magnet and Thermoresponsive Chemosensory Electrospinning Fluorescent Nanofibers and Their Sensing Capability for Metal Ions" Polymers 9, no. 4: 136. https://doi.org/10.3390/polym9040136






