pH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications
Abstract
:1. Introduction
1.1. Brief History of Hydrogels
1.2. Hydrogels
1.2.1. Unique Properties of Hydrogels
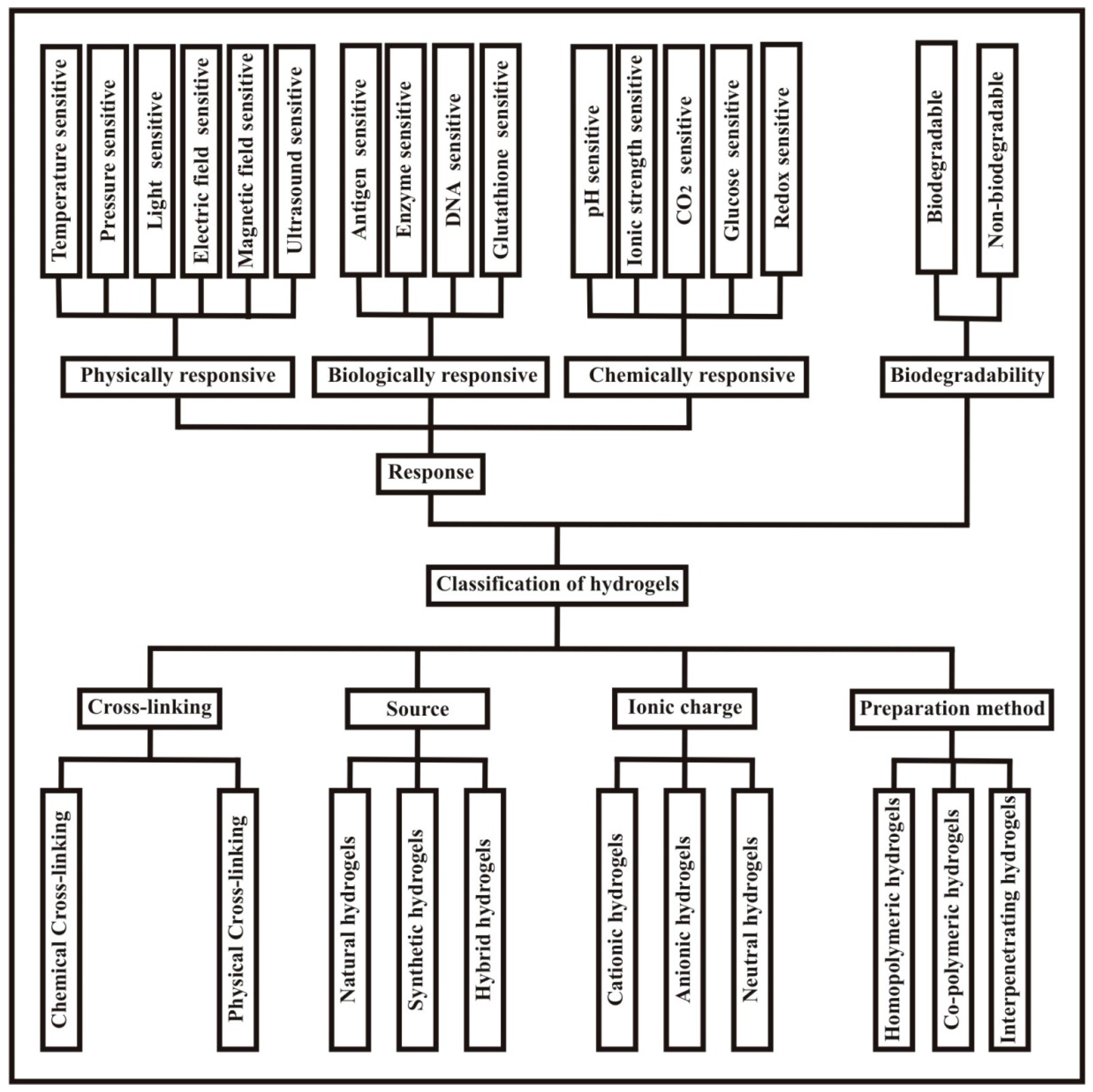
1.2.2. Classification of Hydrogels
1.2.3. Stimuli Sensitive/Responsive Hydrogels
1.2.4. Properties of pH Sensitive Hydrogels
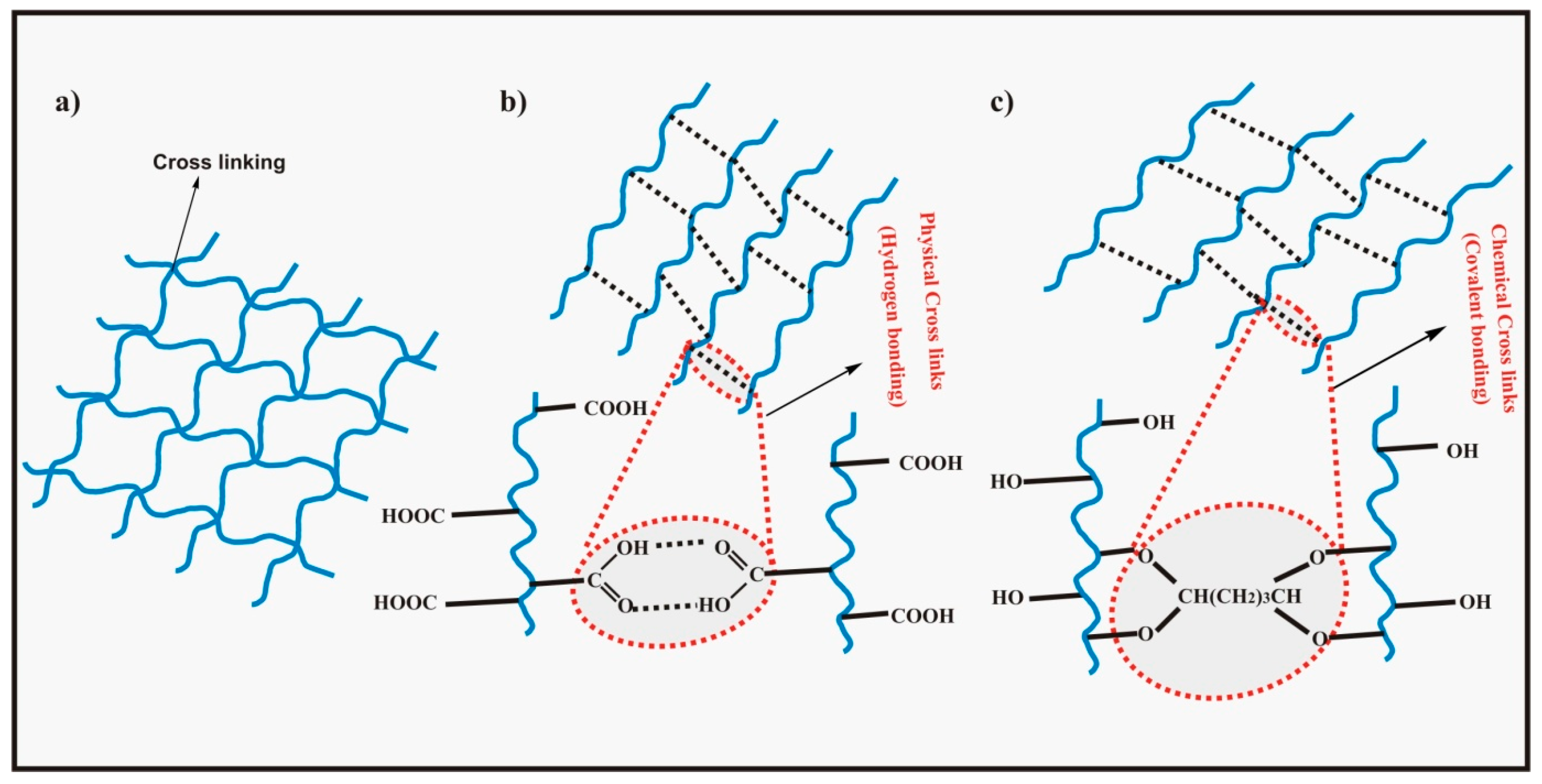
1.2.5. Theory and Swelling Mechanism of pH Sensitive Hydrogels
1.2.6. Drug Release Mechanism of pH Sensitive Hydrogels
2. Base Materials for pH Sensitive Hydrogels
2.1. Natural Hydrogels
2.1.1. Chitosan
2.1.2. Guar Gum
2.1.3. Carrageenan
2.1.4. Dextran
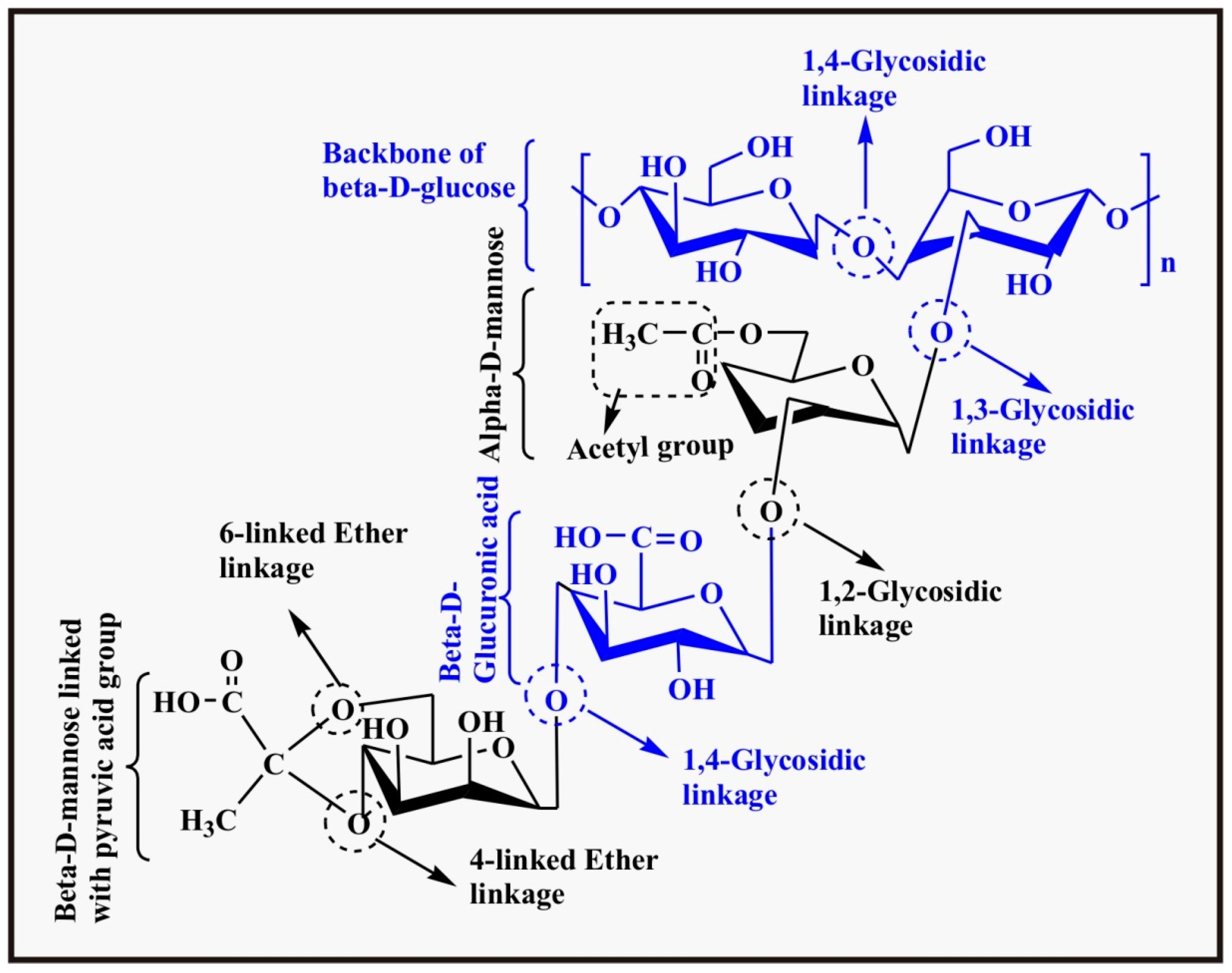
2.1.5. Xanthan
2.1.6. Cellulose
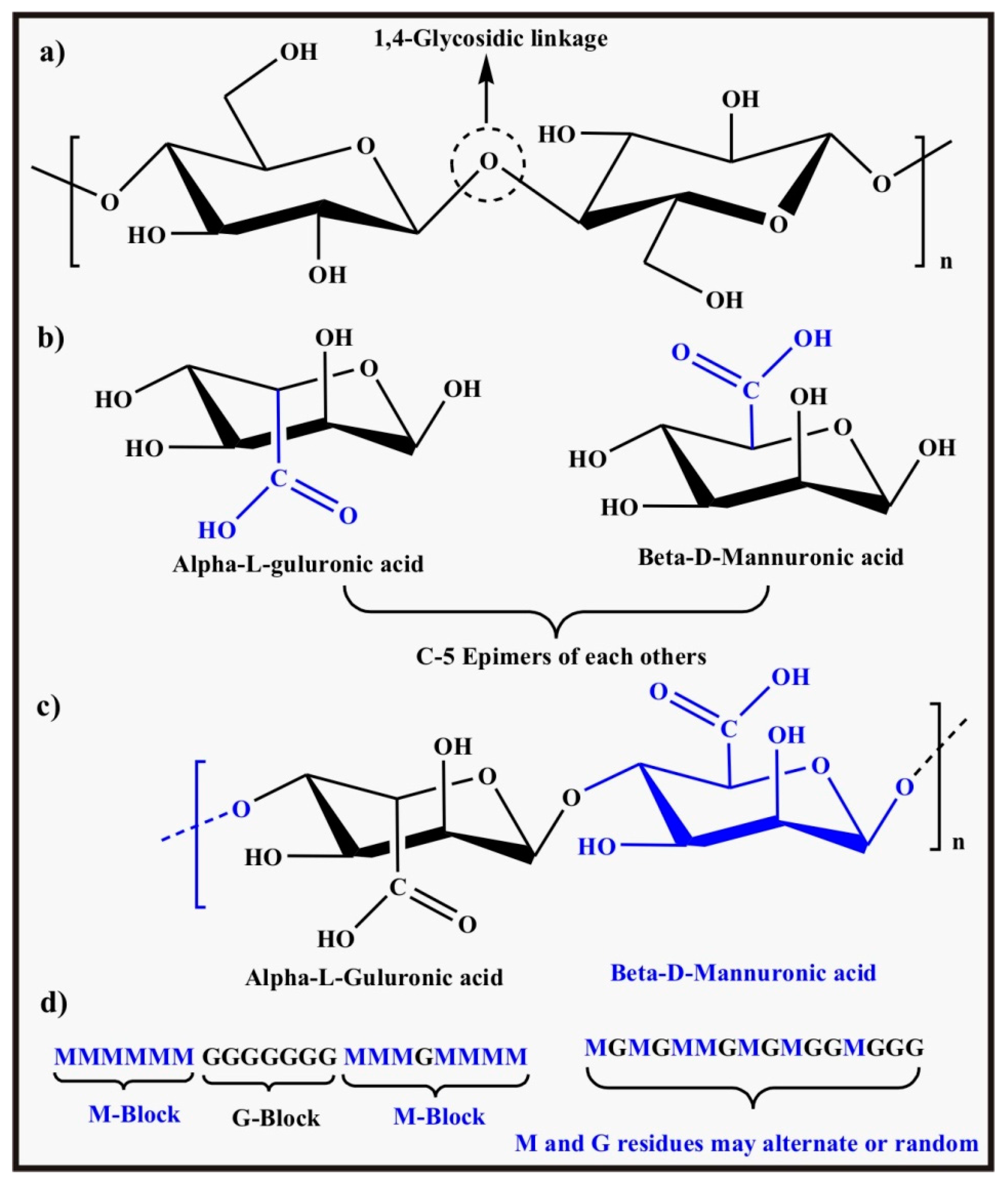
2.1.7. Alginate
2.2. Synthetic Hydrogels
2.2.1. Poly(acrylic acid)
2.2.2. Poly(acrylamide)
2.2.3. Poly(vinyl alcohol)
2.2.4. Poly(ethylene glycol)
2.2.5. Poly(vinyl pyrrolidone)
2.2.6. Poly(lactic acid)
2.3. Hybrid Hydrogels
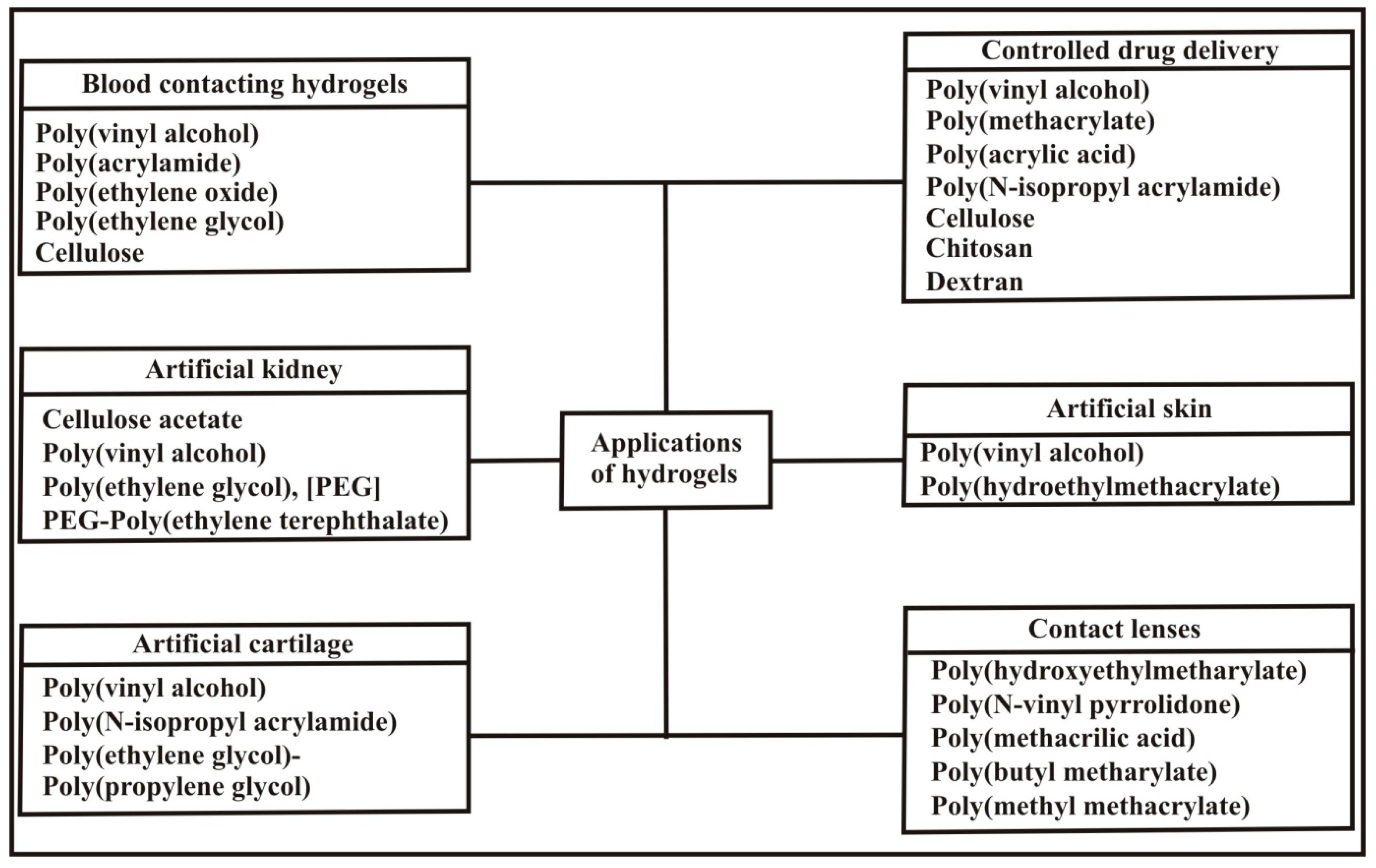
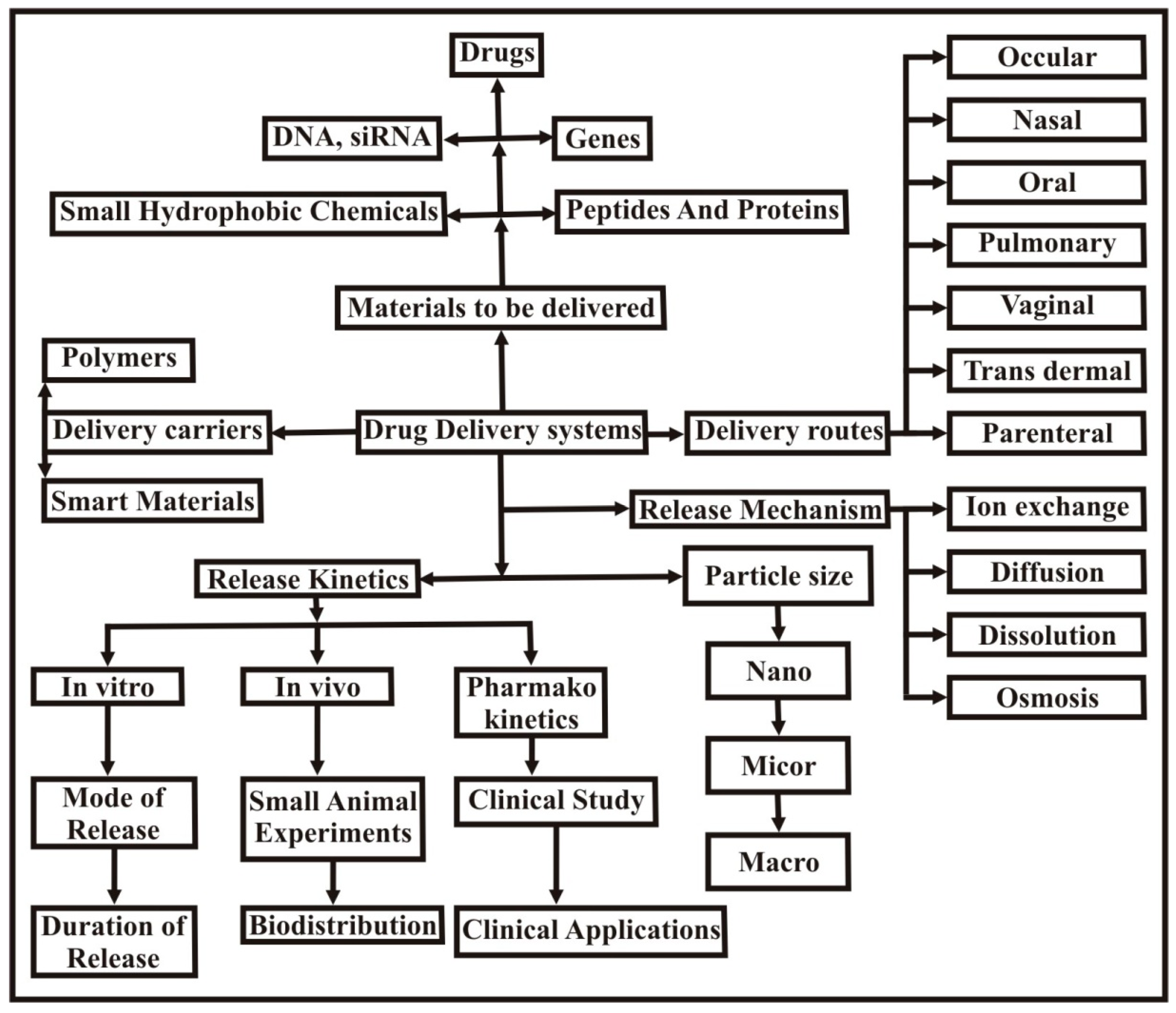
3. Applications of pH Sensitive Hydrogels
3.1. Controlled Drug Delivery
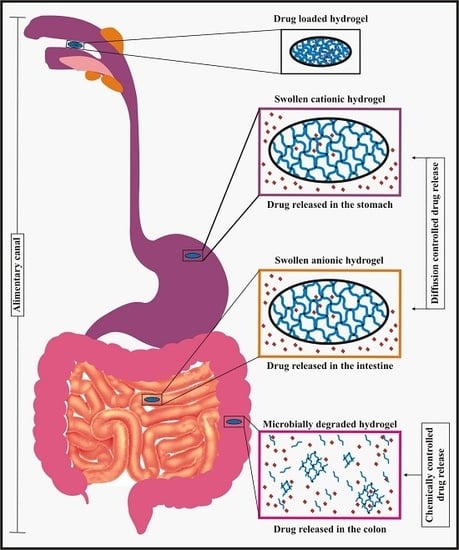
3.2. Drug Delivery in the Stomach
3.3. Drug Delivery in the Intestine
3.4. Drug Delivery in the Colon
3.5. Delivery of Proteins
3.5.1. Delivery of Insulin
3.5.2. Delivery of Bovine Serum Albumin
3.6. Delivery of Genes
3.7. Drug Delivery via Injectable Hydrogels
3.8. Trans Dermal Drug Delivery (pH Sensitive)
4. Challenges and Opportunities
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Yahia, L.; Chirani, N.; Gritsch, L.; Motta, F.L. History and applications of hydrogels. Biomed. Sci. 2015, 4, 1–23. [Google Scholar] [CrossRef]
- Van Bemmelen, J.M. Das hydrogel und das krystallinische hydrat des kupferoxyds. Z. Anorg. Chem. 1894, 5, 466–483. [Google Scholar] [CrossRef]
- Kirschner, C.M.; Anseth, K.S. Hydrogels in healthcare: From static to dynamic material microenvironments. Acta Mater. 2013, 61, 931–944. [Google Scholar] [CrossRef] [PubMed]
- Danno, A. Gel formation of aqueous solution of polyvinyl alcohol irradiated by gamma rays from cobalt-60. J. Phys. Soc. Jpn. 1958, 13, 722–727. [Google Scholar] [CrossRef]
- Nguyen, Q.V.; Huynh, D.P.; Park, J.H.; Lee, D.S. Injectable polymeric hydrogels for the delivery of therapeutic agents: A review. Eur. Polym. J. 2015, 72, 602–619. [Google Scholar] [CrossRef]
- Wichterle, O.; Lim, D. Hydrophilic gels for biological use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine; Elsevier Science: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Kopecek, J. Hydrogels: From soft contact lenses and implants to self-assembled nanomaterials. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 5929–5946. [Google Scholar] [CrossRef] [PubMed]
- Harland, R.S.; Prud’homme, R.K. Polyelectrolyte Gels: Properties, Preparation, and Applications; American Chemical Society: Washington, DC, USA, 1992. [Google Scholar]
- Yannas, I.V.; Lee, E.; Orgill, D.P.; Skrabut, E.M.; Murphy, G.F. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc. Natl. Acad. Sci. USA 1989, 86, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Yar, M.; Shahzad, S.; Siddiqi, S.A.; Mahmood, N.; Rauf, A.; Anwar, M.S.; Chaudhry, A.A.; Rehman, I.U. Triethyl orthoformate mediated a novel crosslinking method for the preparation of hydrogels for tissue engineering applications: Characterization and in vitro cytocompatibility analysis. Mater. Sci. Eng. C 2015, 56, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Samanta, H.S.; Ray, S.K. Controlled release of tinidazole and theophylline from chitosan based composite hydrogels. Carbohydr. Polym. 2014, 106, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Prabaharan, M. Prospective of guar gum and its derivatives as controlled drug delivery systems. Int. J. Biol. Macromol. 2011, 49, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Siegel, R.A.; Rathbone, M.J. Fundamentals and Applications of Controlled Release Drug Delivery; Springer: Heldelberg, Germany, 2011. [Google Scholar]
- Miyata, T.; Uragami, T.; Nakamae, K. Biomolecule-sensitive hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 79–98. [Google Scholar] [CrossRef]
- Ulijn, R.V.; Bibi, N.; Jayawarna, V.; Thornton, P.D.; Todd, S.J.; Mart, R.J.; Smith, A.M.; Gough, J.E. Bioresponsive hydrogels. Mater. Today 2007, 10, 40–48. [Google Scholar] [CrossRef]
- Atta, S.; Khaliq, S.; Islam, A.; Javeria, I.; Jamil, T.; Athar, M.M.; Shafiq, M.I.; Ghaffar, A. Injectable biopolymer based hydrogels for drug delivery applications. Int. J. Biol. Macromol. 2015, 80, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Singh, R.; Sarker, B.; Papageorgiou, D.G.; Juhasz, J.A.; Roether, J.A.; Cicha, I.; Kaschta, J.; Schubert, D.W.; Chrissafis, K. Hybrid hydrogels based on keratin and alginate for tissue engineering. J. Mater. Chem. B 2014, 2, 5441–5451. [Google Scholar] [CrossRef]
- Islam, A.; Yasin, T.; Bano, I.; Riaz, M. Controlled release of aspirin from ph-sensitive chitosan/poly (vinyl alcohol) hydrogel. J. Appl. Polym. Sci. 2012, 124, 4184–4192. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Pillai, O.; Panchagnula, R. Polymers in drug delivery. Curr. Opin. Chem. Biol. 2001, 5, 447–451. [Google Scholar] [CrossRef]
- Karimi, M.; Ghasemi, A.; Sahandi Zangabad, P.; Rahighi, R.; Moosavi Basri, S.M.; Mirshekari, H.; Amiri, M.; Shafaei Pishabad, Z.; Aslani, A.; Bozorgomid, M.; et al. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem. Soc. Rev. 2016, 45, 1457–1501. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Vermani, K.; Garg, S. Hydrogels: From controlled release to ph-responsive drug delivery. Drug Discov. Today 2002, 7, 569–579. [Google Scholar] [CrossRef]
- Khan, A.; Othman, M.B.H.; Razak, K.A.; Akil, H.M. Synthesis and physicochemical investigation of chitosan-pmaa-based dual-responsive hydrogels. J. Polym. Res. 2013, 20, 1–8. [Google Scholar] [CrossRef]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Pan, L.; Chortos, A.; Yu, G.; Wang, Y.; Isaacson, S.; Allen, R.; Shi, Y.; Dauskardt, R.; Bao, Z. An ultra-sensitive resistive pressure sensor based on hollow-sphere microstructure induced elasticity in conducting polymer film. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Bromberg, L.; Concheiro, A. Light-sensitive intelligent drug delivery systems. Photochem. Photobiol. 2009, 85, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.; Gutowska, A. Lessons from nature: Stimuli-responsive polymers and their biomedical applications. Trends Biotechnol. 2002, 20, 305–311. [Google Scholar] [CrossRef]
- Namdeo, M.; Bajpai, S.K.; Kakkar, S. Preparation of a magnetic-field-sensitive hydrogel and preliminary study of its drug release behavior. J. Biomater. Sci. Polym. Ed. 2009, 20, 1747–1761. [Google Scholar] [CrossRef] [PubMed]
- Huebsch, N.; Kearney, C.J.; Zhao, X.; Kim, J.; Cezar, C.A.; Suo, Z.; Mooney, D.J. Ultrasound-triggered disruption and self-healing of reversibly cross-linked hydrogels for drug delivery and enhanced chemotherapy. Proc. Natl. Acad. Sci. USA 2014, 111, 9762–9767. [Google Scholar] [CrossRef] [PubMed]
- Zardad, A.-Z.; Choonara, Y.; du Toit, L.; Kumar, P.; Mabrouk, M.; Kondiah, P.; Pillay, V. A review of thermo- and ultrasound-responsive polymeric systems for delivery of chemotherapeutic agents. Polymers 2016, 8, 359. [Google Scholar] [CrossRef]
- Schmaljohann, D. Thermo- and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- Seeli, D.S.; Prabaharan, M. Guar gum succinate as a carrier for colon-specific drug delivery. Int. J. Biol. Macromol. 2016, 84, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Hezaveh, H.; Muhamad, I.I. Controlled drug release via minimization of burst release in ph-response kappa-carrageenan/polyvinyl alcohol hydrogels. Chem. Eng. Res. Des. 2013, 91, 508–519. [Google Scholar] [CrossRef]
- Rasool, N.; Yasin, T.; Heng, J.Y.Y.; Akhter, Z. Synthesis and characterization of novel ph-, ionic strength and temperature- sensitive hydrogel for insulin delivery. Polymer 2010, 51, 1687–1693. [Google Scholar] [CrossRef]
- Herber, S.; Olthuis, W.; Bergveld, P.; Berg, A. Exploitation of a pH-sensitive hydrogel for CO2 detection. In Proceedings of the Eurosensors XVII, European Conference on Solid-State Transducers, Guimaraes, Portugal, 21–24 September 2003. [Google Scholar]
- Guenther, M.; Wallmersperger, T.; Keller, K.; Gerlach, G. Swelling behaviour of functionalized hydrogels for application in chemical sensors. In Intelligent Hydrogels; Sadowski, G., Richtering, W., Eds.; Springer: Cham, Switzerland, 2013; pp. 265–273. [Google Scholar]
- Yu, J.; Fan, H.; Huang, J.; Chen, J. Fabrication and evaluation of reduction-sensitive supramolecular hydrogel based on cyclodextrin/polymer inclusion for injectable drug-carrier application. Soft Matter 2011, 7, 7386–7394. [Google Scholar] [CrossRef]
- Kim, I.-S.; Oh, I.-J. Drug release from the enzyme-degradable and pH-sensitive hydrogel composed of glycidyl methacrylate dextran and poly(acrylic acid). Arch. Pharm. Res. 2005, 28, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Maeda, M. DNA-responsive hydrogels that can shrink or swell. Biomacromolecules 2005, 6, 2927–2929. [Google Scholar] [CrossRef] [PubMed]
- Buenger, D.; Topuz, F.; Groll, J. Hydrogels in sensing applications. Prog. Polym. Sci. 2012, 37, 1678–1719. [Google Scholar] [CrossRef]
- Guo, W.; Lu, C.H.; Orbach, R.; Wang, F.; Qi, X.J.; Cecconello, A.; Seliktar, D.; Willner, I. pH-stimulated DNA hydrogels exhibiting shape-memory properties. Adv. Mater. 2015, 27, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Hennink, W.E.; Zhong, Z. Reduction-sensitive polymers and bioconjugates for biomedical applications. Biomaterials 2009, 30, 2180–2198. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Liu, M.; Yang, X.; Zhai, G. The design of ph-sensitive chitosan-based formulations for gastrointestinal delivery. Drug Discov. Today 2015, 20, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Zaino, C.; Zambito, Y.; Mollica, G.; Geppi, M.; Serafini, M.; Carelli, V.; Di Colo, G. A novel polyelectrolyte complex (pec) hydrogel for controlled drug delivery to the distal intestine. Open Drug Deliv. J. 2007, 1, 68–75. [Google Scholar]
- Ferreira, L.; Vidal, M.M.; Gil, M.H. Evaluation of poly(2-hydroxyethyl methacrylate) gels as drug delivery systems at different pH values. Int. J. Pharm. 2000, 194, 169–180. [Google Scholar] [CrossRef]
- Gutowska, A.; Bae, Y.H.; Feijen, J.; Kim, S.W. Heparin release from thermosensitive hydrogels. J. Control. Release 1992, 22, 95–104. [Google Scholar] [CrossRef]
- Owens, D.E.; Jian, Y.; Fang, J.E.; Slaughter, B.V.; Chen, Y.-H.; Peppas, N.A. Thermally responsive swelling properties of polyacrylamide/poly(acrylic acid) interpenetrating polymer network nanoparticles. Macromolecules 2007, 40, 7306–7310. [Google Scholar] [CrossRef]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive polymers for biomedical applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef]
- Shibayama, M.; Tanaka, T. Volume phase transition and related phenomena of polymer gels. In Responsive Gels: Volume Transitions I; Dušek, K., Ed.; Springer: Berlin/Heidelberg, Germany, 1993; pp. 1–62. [Google Scholar]
- Lin, C.-C.; Metters, A.T. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef] [PubMed]
- Ranga Rao, K.V.; Padmalatha Devi, K. Swelling controlled-release systems: Recent developments and applications. Int. J. Pharm. 1988, 48, 1–13. [Google Scholar] [CrossRef]
- Bencherif, S.A.; Siegwart, D.J.; Srinivasan, A.; Horkay, F.; Hollinger, J.O.; Washburn, N.R.; Matyjaszewski, K. Nanostructured hybrid hydrogels prepared by a combination of atom transfer radical polymerization and free radical polymerization. Biomaterials 2009, 30, 5270–5278. [Google Scholar] [CrossRef] [PubMed]
- Valderruten, N.E.; Valverde, J.D.; Zuluaga, F.; Ruiz-Durántez, E. Synthesis and characterization of chitosan hydrogels cross-linked with dicarboxylic acids. React. Funct. Polym. 2014, 84, 21–28. [Google Scholar] [CrossRef]
- Cumpstey, I. Chemical modification of polysaccharides. ISRN Org. Chem. 2013, 2013, 417672. [Google Scholar] [CrossRef] [PubMed]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Bostan, M.S.; Senol, M.; Cig, T.; Peker, I.; Goren, A.C.; Ozturk, T.; Eroglu, M.S. Controlled release of 5-aminosalicylicacid from chitosan based ph and temperature sensitive hydrogels. Int. J. Biol. Macromol. 2013, 52, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Elgadir, M.A.; Uddin, M.S.; Ferdosh, S.; Adam, A.; Chowdhury, A.J.K.; Sarker, M.Z.I. Impact of chitosan composites and chitosan nanoparticle composites on various drug delivery systems: A review. J. Food Drug Anal. 2015, 23, 619–629. [Google Scholar] [CrossRef]
- Kammoun, M.; Haddar, M.; Kallel, T.K.; Dammak, M.; Sayari, A. Biological properties and biodegradation studies of chitosan biofilms plasticized with peg and glycerol. Int. J. Biol. Macromol. 2013, 62, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zivanovic, S.; Draughon, F.A.; Conway, W.S.; Sams, C.E. Physicochemical properties and bioactivity of fungal chitin and chitosan. J. Agric. Food. Chem. 2005, 53, 3888–3894. [Google Scholar] [CrossRef] [PubMed]
- Siedenbiedel, F.; Tiller, J.C. Antimicrobial polymers in solution and on surfaces: Overview and functional principles. Polymers 2012, 4, 46. [Google Scholar] [CrossRef]
- Kulbokaite, R.; Ciuta, G.; Netopilik, M.; Makuska, R. N-peg’ylation of chitosan via “click chemistry” reactions. React. Funct. Polym. 2009, 69, 771–778. [Google Scholar] [CrossRef]
- Sashiwa, H.; Aiba, S.-I. Chemically modified chitin and chitosan as biomaterials. Prog. Polym. Sci. 2004, 29, 887–908. [Google Scholar] [CrossRef]
- Chen, S.-H.; Tsao, C.-T.; Chang, C.-H.; Lai, Y.-T.; Wu, M.-F.; Chuang, C.-N.; Chou, H.-C.; Wang, C.-K.; Hsieh, K.-H. Assessment of reinforced poly(ethylene glycol) chitosan hydrogels as dressings in a mouse skin wound defect model. Mater. Sci. Eng. C 2013, 33, 2584–2594. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Larsson, M.; Huang, W.-C.; Hsiao, M.-H.; Wang, Y.-J.; Nydén, M.; Chiou, S.-H.; Liu, D.-M. Biomedical applications and colloidal properties of amphiphilically modified chitosan hybrids. Prog. Polym. Sci. 2013, 38, 1307–1328. [Google Scholar] [CrossRef]
- Hsu, L.-W.; Lee, P.-L.; Chen, C.-T.; Mi, F.-L.; Juang, J.-H.; Hwang, S.-M.; Ho, Y.-C.; Sung, H.-W. Elucidating the signaling mechanism of an epithelial tight-junction opening induced by chitosan. Biomaterials 2012, 33, 6254–6263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, X.H.; Gong, Y.D.; Zhao, N.M.; Zhang, X.F. Properties and biocompatibility of chitosan films modified by blending with peg. Biomaterials 2002, 23, 2641–2648. [Google Scholar] [CrossRef]
- Su, F.-Y.; Lin, K.-J.; Sonaje, K.; Wey, S.-P.; Yen, T.-C.; Ho, Y.-C.; Panda, N.; Chuang, E.-Y.; Maiti, B.; Sung, H.-W. Protease inhibition and absorption enhancement by functional nanoparticles for effective oral insulin delivery. Biomaterials 2012, 33, 2801–2811. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Wang, Z.; Jiang, C.; Kong, M.; Zhou, X.; Li, Y.; Cheng, X.; Chen, X. Chitosan/o-carboxymethyl chitosan nanoparticles for efficient and safe oral anticancer drug delivery: In vitro and in vivo evaluation. Int. J. Pharm. 2013, 457, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Alves, N.M.; Mano, J.F. Chitosan derivatives obtained by chemical modifications for biomedical and environmental applications. Int. J. Biol. Macromol. 2008, 43, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Gujarathi, N.A.; Rane, B.R.; Patel, J.K. pH sensitive polyelectrolyte complex of o-carboxymethyl chitosan and poly(acrylic acid) cross-linked with calcium for sustained delivery of acid susceptible drugs. Int. J. Pharm. 2012, 436, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Bromberg, L.; Alakhov, V. Effects of polyether-modified poly(acrylic acid) microgels on doxorubicin transport in human intestinal epithelial Caco-2 cell layers. J. Control. Release 2003, 88, 11–22. [Google Scholar] [CrossRef]
- Sinha, V.R.; Kumria, R. Polysaccharides in colon-specific drug delivery. Int. J. Pharm. 2001, 224, 19–38. [Google Scholar] [CrossRef]
- Wong, D.; Larrabee, S.; Clifford, K.; Tremblay, J.; Friend, D.R. Usp dissolution apparatus iii (reciprocating cylinder) for screening of guar-based colonic delivery formulations. J. Control. Release 1997, 47, 173–179. [Google Scholar] [CrossRef]
- Campo, V.L.; Kawano, D.F.; Silva, D.B.D., Jr.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Li, L.; Ni, R.; Shao, Y.; Mao, S. Carrageenan and its applications in drug delivery. Carbohydr. Polym. 2014, 103, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Piyakulawat, P.; Praphairaksit, N.; Chantarasiri, N.; Muangsin, N. Preparation and evaluation of chitosan/carrageenan beads for controlled release of sodium diclofenac. AAPS PharmSciTech 2007, 8, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Pacelli, S.; Paolicelli, P.; Casadei, M.A. New biodegradable dextran-based hydrogels for protein delivery: Synthesis and characterization. Carbohydr. Polym. 2015, 126, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, L.; Hovgaard, L.; Mortensen, P.B.; Brøndsted, H. Dextran hydrogels for colon-specific drug delivery. V. Degradation in human intestinal incubation models. Eur. J. Pharm. Sci. 1995, 3, 329–337. [Google Scholar] [CrossRef]
- Hovgaard, L.; Brøndsted, H. Dextran hydrogels for colon-specific drug delivery. J. Control. Release 1995, 36, 159–166. [Google Scholar] [CrossRef]
- Chiu, H.-C.; Hsiue, G.-H.; Lee, Y.-P.; Huang, L.-W. Synthesis and characterization of ph-sensitive dextran hydrogels as a potential colon-specific drug delivery system. J. Biomater. Sci. Polym. Ed. 1999, 10, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Dumitriu, S. Polysaccharides: Structural Diversity and Functional Versatility, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Bueno, V.B.; Bentini, R.; Catalani, L.H.; Petri, D.F.S. Synthesis and swelling behavior of xanthan-based hydrogels. Carbohydr. Polym. 2013, 92, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable cellulose-based hydrogels: Design and applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Lim, L.S.; Ahmad, I.; Lazim, M.A.S.M. pH sensitive hydrogel based on poly (acrylic acid) and cellulose nanocrystals. Sains Malays. 2015, 44, 779–785. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, H.; Yang, X.; Zhang, Y.; Zhang, X.; Cui, K.; Shao, L.; Yao, J. Temperature/pH sensitive cellulose-based hydrogel: Synthesis, characterization, loading, and release of model drugs for potential oral drug delivery. BioResources 2014, 10, 760–771. [Google Scholar] [CrossRef]
- Jabeen, S.; Islam, A.; Ghaffar, A.; Gull, N.; Hameed, A.; Bashir, A.; Jamil, T.; Hussain, T. Development of a novel ph sensitive silane crosslinked injectable hydrogel for controlled release of neomycin sulfate. Int. J. Biol. Macromol. 2017, 97, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Jao, W.-C.; Ho, L.-C.; Chen, Z.-W. Evaluation of the drug release mechanism of ph-sensitive calcium alginate hydrogels in simulated physiological fluids. J. China Univ. Sci. Technol. 2010, 37–61. [Google Scholar]
- Chen, S.-C.; Wu, Y.-C.; Mi, F.-L.; Lin, Y.-H.; Yu, L.-C.; Sung, H.-W. A novel pH-sensitive hydrogel composed of n,o-carboxymethyl chitosan and alginate cross-linked by genipin for protein drug delivery. J. Control. Release 2004, 96, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Sohail, K.; Khan, I.U.; Shahzad, Y.; Hussain, T.; Ranjha, N.M. pH-sensitive polyvinylpyrrolidone-acrylic acid hydrogels: Impact of material parameters on swelling and drug release. Braz. J. Pharm. Sci. 2014, 50, 173–184. [Google Scholar]
- Das, S.; Subuddhi, U. pH-responsive guar gum hydrogels for controlled delivery of dexamethasone to the intestine. Int. J. Biol. Macromol. 2015, 79, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Arunbabu, D.; Shahsavan, H.; Zhang, W.; Zhao, B. Poly(AAC-co-MBA) hydrogel films: Adhesive and mechanical properties in aqueous medium. J. Phys. Chem. B 2013, 117, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Chang, A. pH-sensitive starch-g-poly(acrylic acid)/sodium alginate hydrogels for controlled release of diclofenac sodium. Iran. Polym. J. 2015, 24, 161–169. [Google Scholar] [CrossRef]
- Jabbari, E.; Nozari, S. Swelling behavior of acrylic acid hydrogels prepared by γ-radiation crosslinking of polyacrylic acid in aqueous solution. Eur. Polym. J. 2000, 36, 2685–2692. [Google Scholar] [CrossRef]
- Sheikh, N.; Jalili, L.; Anvari, F. A study on the swelling behavior of poly(acrylic acid) hydrogels obtained by electron beam crosslinking. Radiat. Phys. Chem. 2010, 79, 735–739. [Google Scholar] [CrossRef]
- Yang, T.-H. Recent applications of polyacrylamide as biomaterials. Recent Pat. Mater. Sci. 2008, 1, 29–40. [Google Scholar] [CrossRef]
- Bashir, S.; Teo, Y.Y.; Ramesh, S.; Ramesh, K. Synthesis, characterization, properties of N-succinyl chitosan-g-poly (methacrylic acid) hydrogels and in vitro release of theophylline. Polymer 2016, 92, 36–49. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Sarkar, K.; Bhattacharya, S.; Bhattacharyya, A.; Mishra, R.; Kundu, P.P. pH sensitive n-succinyl chitosan grafted polyacrylamide hydrogel for oral insulin delivery. Carbohydr. Polym. 2014, 112, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.-T.; Gong, J.; Gu, X.-H.; Kim, H.-Y.; Dong, J.; Shen, X.-Y. Fabrication and characterization of poly (vinyl alcohol)/chitosan blend nanofibers produced by electrospinning method. Carbohydr. Polym. 2007, 67, 403–409. [Google Scholar] [CrossRef]
- Rafique, A.; Mahmood Zia, K.; Zuber, M.; Tabasum, S.; Rehman, S. Chitosan functionalized poly(vinyl alcohol) for prospects biomedical and industrial applications: A review. Int. J. Biol. Macromol. 2016, 87, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Bano, I.; Ghauri, M.A.; Yasin, T.; Huang, Q.; Palaparthi, A.D.S. Characterization and potential applications of gamma irradiated chitosan and its blends with poly(vinyl alcohol). Int. J. Biol. Macromol. 2014, 65, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Jun, S.T.; Marsh, K.S. Physical properties of pvoh/chitosan-blended films cast from different solvents. Food Hydrocoll. 2001, 15, 499–502. [Google Scholar] [CrossRef]
- Shahzad, S.; Yar, M.; Siddiqi, S.A.; Mahmood, N.; Rauf, A.; Anwar, M.S.; Afzaal, S. Chitosan-based electrospun nanofibrous mats, hydrogels and cast films: Novel anti-bacterial wound dressing matrices. J. Mater. Sci. Mater. Med. 2015, 26, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Yasin, T. Controlled delivery of drug from pH sensitive chitosan/poly (vinyl alcohol) blend. Carbohydr. Polym. 2012, 88, 1055–1060. [Google Scholar] [CrossRef]
- Kadajji, V.G.; Betageri, G.V. Water soluble polymers for pharmaceutical applications. Polymers 2011, 3, 1972. [Google Scholar] [CrossRef]
- Rajendra, P.P.; Sunil, U.T.; Suresh, U.S.; Jalinder, T.T.; Abraham, J.D. Biomedical applications of poly(lactic acid). Recent Pat. Regen. Med. 2014, 4, 40–51. [Google Scholar]
- Wang, C.; Stewart, R.J.; KopeCek, J. Hybrid hydrogels assembled from synthetic polymers and coiled-coil protein domains. Nature 1999, 397, 417–420. [Google Scholar] [PubMed]
- Kopeček, J. Hydrogel biomaterials: A smart future? Biomaterials 2007, 28, 5185–5192. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ha, W.; Sun, J.-N.; Shi, Y.-P. Supramolecular hybrid hydrogel based on host–guest interaction and its application in drug delivery. ACS Appl. Mater. Interfaces 2014, 6, 19544–19551. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, X.; Tan, G.; Tian, L.; Liu, D.; Liu, Y.; Yang, X.; Pan, W. A novel pH-induced thermosensitive hydrogel composed of carboxymethyl chitosan and poloxamer cross-linked by glutaraldehyde for ophthalmic drug delivery. Carbohydr. Polym. 2017, 155, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Anjum, F.; Lienemann, P.S.; Metzger, S.; Biernaskie, J.; Kallos, M.S.; Ehrbar, M. Enzyme responsive gag-based natural-synthetic hybrid hydrogel for tunable growth factor delivery and stem cell differentiation. Biomaterials 2016, 87, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Zare-Akbari, Z.; Farhadnejad, H.; Furughi-Nia, B.; Abedin, S.; Yadollahi, M.; Khorsand-Ghayeni, M. pH-sensitive bionanocomposite hydrogel beads based on carboxymethyl cellulose/zno nanoparticle as drug carrier. Int. J. Biol. Macromol. 2016, 93(Part A), 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.Z.; Cevenini, A.; Yazdi, I.K.; Parodi, A.; Evangelopoulos, M.; Corbo, C.; Scaria, S.; Hu, Y.; Haddix, S.G.; Corradetti, B.; et al. One-pot synthesis of pH-responsive hybrid nanogel particles for the intracellular delivery of small interfering RNA. Biomaterials 2016, 87, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zou, X.; Ye, L. Controlled pH- and glucose-responsive drug release behavior of cationic chitosan based nano-composite hydrogels by using graphene oxide as drug nanocarrier. J. Ind. Eng. Chem. 2017. [CrossRef]
- Hilt, J.Z.; Gupta, A.K.; Bashir, R.; Peppas, N.A. Ultrasensitive biomems sensors based on microcantilevers patterned with environmentally responsive hydrogels. Biomed. Microdevices 2003, 5, 177–184. [Google Scholar] [CrossRef]
- Nebhani, L.; Choudhary, V.; Adler, H.-J.; Kuckling, D. pH- and metal ion-sensitive hydrogels based on N-[2-(dimethylaminoethyl)acrylamide]. Polymers 2016, 8, 233. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Swaddiwudhipong, S.; Miao, H.; Ding, Z.; Yang, Z. pH-sensitive hydrogel for micro-fluidic valve. J. Funct. Biomater. 2012, 3, 464. [Google Scholar] [CrossRef] [PubMed]
- Kurnia, J.C.; Birgersson, E.; Mujumdar, A.S. Computational study of pH-sensitive hydrogel-based microfluidic flow controllers. J. Funct. Biomater. 2011, 2, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Siddiqui, A.; Datta, M.; Ramchand, D. Interpenetrating polymeric network hydrogel for stomach-specific drug delivery of clarithromycin: Preparation and evaluation. Asian J. Pharm. 2010, 4, 179. [Google Scholar] [CrossRef]
- El-Mahrouk, G.M.; Aboul-Einien, M.H.; Makhlouf, A.I. Design, optimization, and evaluation of a novel metronidazole-loaded gastro-retentive ph-sensitive hydrogel. AAPS PharmSciTech 2015, 17, 1285–1297. [Google Scholar] [CrossRef] [PubMed]
- Risbud, M.V.; Hardikar, A.A.; Bhat, S.V.; Bhonde, R.R. pH-sensitive freeze-dried chitosan–polyvinyl pyrrolidone hydrogels as controlled release system for antibiotic delivery. J. Control. Release 2000, 68, 23–30. [Google Scholar] [CrossRef]
- Sun, X.-F.; Wang, H.-H.; Jing, Z.-X.; Mohanathas, R. Hemicellulose-based pH-sensitive and biodegradable hydrogel for controlled drug delivery. Carbohydr. Polym. 2013, 92, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wan, S.; Chen, B.; Gao, W.; Chen, J.; Liu, M.; He, B.; Wu, H. Dual ph and temperature responsive hydrogels based on β-cyclodextrin derivatives for atorvastatin delivery. Carbohydr. Polym. 2016, 136, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Cevik, O.; Gidon, D.; Kizilel, S. Visible-light-induced synthesis of pH-responsive composite hydrogels for controlled delivery of the anticonvulsant drug pregabalin. Acta Biomater. 2015, 11, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, Z.; Guo, X.; He, Q.; Hao, C.; Ge, C. Ultrasonic-assisted synthesis of sodium lignosulfonate-grafted poly(acrylic acid-co-poly(vinyl pyrrolidone)) hydrogel for drug delivery. RSC Adv. 2016, 6, 35550–35558. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, X.; Zhang, Y.; Shang, Q. Fabrication and evaluation of a novel polymeric hydrogel of carboxymethyl chitosan-g-polyacrylic acid (CMC-g-PAA) for oral insulin delivery. RSC Adv. 2016, 6, 52858–52867. [Google Scholar] [CrossRef]
- Abdel Ghaffar, A.M.; Radwan, R.R.; Ali, H.E. Radiation synthesis of poly(starch/acrylic acid) pH sensitive hydrogel for rutin controlled release. Int. J. Biol. Macromol. 2016, 92, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Seeli, D.S.; Dhivya, S.; Selvamurugan, N.; Prabaharan, M. Guar gum succinate-sodium alginate beads as a ph-sensitive carrier for colon-specific drug delivery. Int. J. Biol. Macromol. 2016, 91, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Dong, K.; Yan, Y.; Xu, W.; Zhang, L.; Zhao, G.; Xing, J. In vitro and in vivo study of a colon-targeting ph-sensitive hydrocortisone sodium succinate hydrogel. RSC Adv. 2015, 5, 80625–80633. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Mohan, A.M. Novel ph switchable gelatin based hydrogel for the controlled delivery of the anti cancer drug 5-fluorouracil. RSC Adv. 2014, 4, 12109–12118. [Google Scholar] [CrossRef]
- Tiwari, G.; Tiwari, R.; Sriwastawa, B.; Bhati, L.; Pandey, S.; Pandey, P.; Bannerjee, S. Drug delivery systems: An updated review. Int. J. Pharm. Investig. 2012, 2, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Park, K. Oral Controlled Release Formulation Design and Drug Delivery: Theory to Practice; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Baliga, S.; Muglikar, S.; Kale, R. Salivary pH: A diagnostic biomarker. J. Indian Soc. Periodontol. 2013, 17, 461. [Google Scholar] [CrossRef] [PubMed]
- Grassi, M.; Grassi, G.; Lapasin, R.; Colombo, I. Understanding Drug Release and Absorption Mechanisms: A Physical and Mathematical Approach; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Rakoff, A.E.; Feo, L.G.; Goldstein, L. The biologic characteristics of the normal vagina. Am. J. Obstet. Gynecol. 1994, 47, 467–494. [Google Scholar] [CrossRef]
- Kwon, S.S.; Kong, B.J.; Park, S.N. Physicochemical properties of pH-sensitive hydrogels based on hydroxyethyl cellulose–hyaluronic acid and for applications as transdermal delivery systems for skin lesions. Eur. J. Pharm. Biopharm. 2015, 92, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Ishak, R.A.; Awad, G.A.; Mortada, N.D.; Nour, S.A. Preparation, in vitro and in vivo evaluation of stomach-specific metronidazole-loaded alginate beads as local anti-helicobacter pylori therapy. J. Control. Release 2007, 119, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Li, J.; Yao, F.; Yin, Y. Chitosan-Based Hydrogels: Functions and Applications; Taylor&Francis: Abingdom, UK, 2011. [Google Scholar]
- George, M.; Abraham, T.E. pH sensitive alginate–guar gum hydrogel for the controlled delivery of protein drugs. Int. J. Pharm. 2007, 335, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Krishnaiah, Y.S.R.; Satyanarayana, V.; Dinesh Kumar, B.; Karthikeyan, R.S. In vitro drug release studies on guar gum-based colon targeted oral drug delivery systems of 5-fluorouracil. Eur. J. Pharm. Sci. 2002, 16, 185–192. [Google Scholar] [CrossRef]
- Fonte, P.; Araújo, F.; Reis, S.; Sarmento, B. Oral insulin delivery: How far are we? J. Diabetes Sci. Technol. 2013, 7, 520–531. [Google Scholar] [CrossRef] [PubMed]
- M Ramesan, R.; P Sharma, C. Recent advances in the oral delivery of insulin. Recent Pat. Drug Deliv. Formul. 2014, 8, 155–159. [Google Scholar] [CrossRef]
- Elsayed, A.M. Oral Delivery of Insulin: Novel Approaches; INTECH Open Access Publisher: Rijeka, Croatia, 2012. [Google Scholar]
- Demirdirek, B.; Uhrich, K.E. Salicylic acid-based pH-sensitive hydrogels as potential oral insulin delivery systems. J. Drug Target. 2015, 23, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Dergunov, S.A.; Mun, G.A. Γ-irradiated chitosan-polyvinyl pyrrolidone hydrogels as ph-sensitive protein delivery system. Radiat. Phys. Chem. 2009, 78, 65–68. [Google Scholar] [CrossRef]
- Gao, X.; He, C.; Xiao, C.; Zhuang, X.; Chen, X. Synthesis and characterization of biodegradable ph-sensitive poly(acrylic acid) hydrogels crosslinked by 2-hydroxyethyl methacrylate modified poly(l-glutamic acid). Mater. Lett. 2012, 77, 74–77. [Google Scholar] [CrossRef]
- Yang, J.; Chen, J.; Pan, D.; Wan, Y.; Wang, Z. pH-sensitive interpenetrating network hydrogels based on chitosan derivatives and alginate for oral drug delivery. Carbohydr. Polym. 2013, 92, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-H.; Zhang, X.-Z.; Zhang, L.; Xu, X.-D.; Zhuo, R.-X. Temperature- and pH-sensitive hydrogels to immobilize heparin-modified pei/DNA complexes for sustained gene delivery. J. Mater. Chem. 2009, 19, 8982–8989. [Google Scholar] [CrossRef]
- Li, P.; Liu, D.; Miao, L.; Liu, C.; Sun, X.; Liu, Y.; Zhang, N. A pH-sensitive multifunctional gene carrier assembled via layer-by-layer technique for efficient gene delivery. Int. J. Nanomed. 2012, 7, 925–939. [Google Scholar]
- Kim, M.S.; Park, K. Injectable hydrogel. In Encyclopedia of Nanotechnology; Bhushan, B., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 1091–1096. [Google Scholar]
- Garbern, J.C.; Hoffman, A.S.; Stayton, P.S. Injectable pH- and temperature-responsive poly(N-isopropylacrylamide-co-propylacrylic acid) copolymers for delivery of angiogenic growth factors. Biomacromolecules 2010, 11, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Huynh, C.T.; Nguyen, M.K.; Lee, D.S. Dually cationic and anionic ph/temperature-sensitive injectable hydrogels and potential application as a protein carrier. Chem. Commun. 2012, 48, 10951–10953. [Google Scholar] [CrossRef] [PubMed]
- Giri, A.; Bhunia, T.; Mishra, S.R.; Goswami, L.; Panda, A.B.; Pal, S.; Bandyopadhyay, A. Acrylic acid grafted guargum–nanosilica membranes for transdermal diclofenac delivery. Carbohydr. Polym. 2013, 91, 492–501. [Google Scholar] [CrossRef] [PubMed]
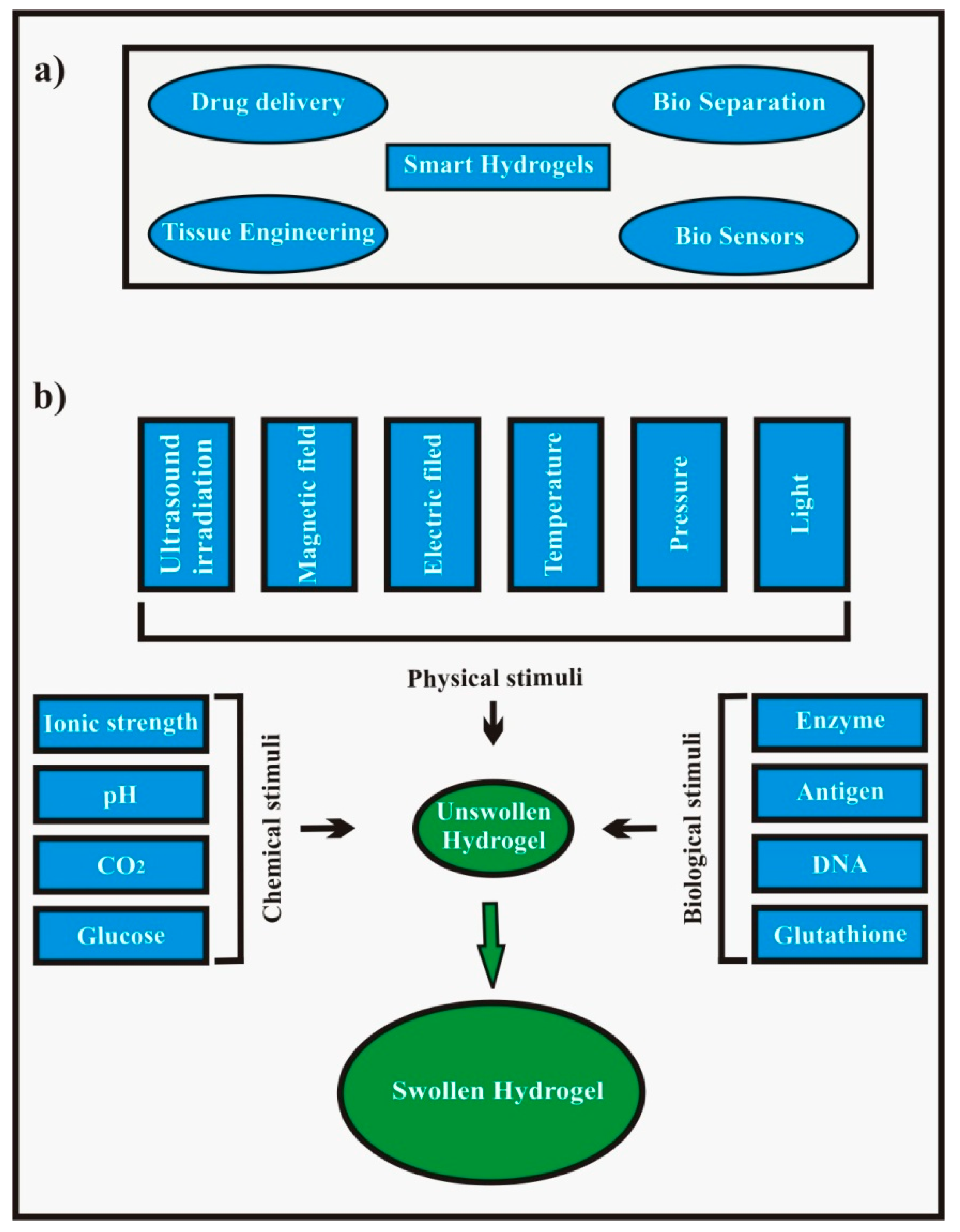





| Nature of Stimulus | Stimulus | Mechanism | Example | Ref. |
|---|---|---|---|---|
| Physical stimuli | Temperature | Shift in temperature changes polymer-polymer and polymer-water interaction responsible for swelling and drug release. | Chitosan-Poly(acrylamide) | [27] |
| Pressure | Swelling under increased pressure and vice versa. This fact is due to an increase in lower critical solution temperature (LCST) value of hydrogels with pressure. LCST is the temperature below which negative thermoresponsive hydrogels swell. | Poly(N-isopropylacrylamide), poly(N,N-diethylacrylamide) | [28,29] | |
| Light | Exposure to light (UV and visible light) reversibly changes the hydrogel from its flowable form to non-flowable form and vice versa. | Poly(trimethylenium iminium trifluorosulfonimide) and 2,6-bis(benzoxal-2-yl)pyridine blend | [3,30] | |
| Electric field | Changes in electrical charge distribution within the hydrogels matrix on the application of electric field cause swelling–deswelling and is consequently responsible for the on demand drug release. | Polythiophene and polypyrrole | [28,31] | |
| Magnetic Field | When a magnetic field is applied, it causes pores in the gel to swell leading to drug release. | Magnetite nanoparticles and poly(acrylamide) composite hydrogels | [32] | |
| Ultrasound irradiation | Exposure to ultrasound temporarily breaks the ionic cross-links in the hydrogels and the drug is released but cross-links are reformed on discontinuation of the ultrasound waves. This facilitates on-demand drug release. | Calcium alginate Poly(lactic acid) | [33,34] | |
| Chemical stimuli | pH | Shift in pH causes change in the charge on the polymer chains leading to swelling and drug release. | Poly(acrylic acid), Guar gum succinate, Kappa-carrageenan/poly(vinyl alcohol) | [35,36,37] |
| Ionic strength | Change in ion concentration also causes swelling and drug release. | Kappa carrageenan-g-poly(acrylic acid) hydrogels | [38] | |
| CO2 | In CO2 sensors, a pH sensitive hydrogel disc comes in contact with bicarbonate solution. On exposure to CO2, the pH of solution changes resulting in swelling or deswelling of the hydrogel which causes a change in pressure which is a measure of the partial pressure of CO2. | Poly(2-hydroxyethylmethacrylate)-co-(2-dimethylaminoethylmethacrylate) | [39] | |
| Glucose | Hydrogels show swelling in response to increased glucose concentration. The complex formed between glucose and phenylboronic acid drives the swelling of the hydrogels and consequently insulin release. | Poly(acrylamide)-co-(3-acrylamidophenylboronic acid) | [40] | |
| Redox | Disulfide linkages in reduction sensitive hydrogels cleave in the reductive environment (high level of glutathione concentration = 0.5–10 mM) in intracellular matrix and release bioactive molecules/drugs. | [poly(ethylene glycol) monomethyl ether]-graft-[disulfide linked poly(amido-amine)] and α-cyclodextrin | [41] | |
| Biological stimuli | Enzyme | Enzymes cause hydrogel degradation and consequently the drug release. This is called a chemically controlled drug release mechanism. | Glycidylmethacrylate dextran-g-poly(acrylic acid) | [42] |
| Antigen | Hydrogels sense the free antigen and undergo swelling followed by drug release. | N-succinimidylacrylate based antigen-antibody entrapment hydrogel | [13,18] | |
| DNA | Single stranded (ss) DNA grafted hydrogel probes show swelling in the presence of ssDNA. | Single stranded DNA probe-g-poly(acrylamide) hydrogels | [43] |
| Target | Compositions/Carrier | Drugs | Disease | Ref. | |
|---|---|---|---|---|---|
| Stomach | Chitosan | Acrylic acid grafted chitosan/poly(vinyl pyrrolidone) cross-linked with glutaraldehyde and N,N-Methylene (bisacrylamide) | Clarithromycine | Peptic ulcer | [125] |
| Chitosan cross-linked with citrate or tripolyphosphate | Metronidazole | [126] | |||
| Chitosan/poly(vinyl pyrrolidone) blend cross-linked with glutaraldehyde | Amoxicilin | [127] | |||
| Intestine | Hemicellulose | Hemicellulose-co-acrylic acid | Theophylline | Respiratory tract diseases | [128] |
| Guar gum | Acrylic acid grafted Guar gum blended with β-cyclodextrin and cross-linked with tetraethyl orthosilicate | Dexamethasone | Ulcerative colitis, arthritis. | [97] | |
| Cyclodextrin | β-cyclodextrin-co-methacrylic acid | Atorvastatin | Various hyperlipidemias | [129] | |
| Poly(ethylene glycol) | Styrene-butadiene-styrene incorporated into methacrylic acid-co-poly(ethylene glycol) | Pregabalin | Epilepsy, neuropathic pain, etc. | [130] | |
| Poly(vinyl pyrrolidone) | Lignosulfonate grafted poly(acrylic acid)-co-poly(vinyl pyrrolidone) | Amoxicilin | Bacterial infections | [131] | |
| Chitosan | Acrylic acid grafted chitosan | Insulin | Diabetes | [132] | |
| Colon | Starch | Acrylic acid grafted starch | Rutin | Inflammatory bowel disease, allergy, etc. | [133] |
| Guar gum | Guar gum succinate blended sodium alginate cross-linked with barium ions | Ibuprofen | Anti-inflammatory/anti-analgesic drug | [134] | |
| Alginate | Sodium alginate cross-linked with calcium chloride | Hydrocortisone | Allergy, arthritis, asthma. | [135] | |
| Gelatin | β-cyclodextrin grafted gelatin cross-linked with oxidized dextrin | 5-Fluorouracil | Cancer | [136] | |
| Dextran | Glycidyl methacrylate dextran and poly(acrylic acid) | 5-Aminosalicylic acid | Ulcerative colitis and Crohn’s disease | [42] | |
| Chitosan | Chitosan blended with poly(vinyl alcohol) cross-linked with tetraethyl orthosilicate | Dexamethasone | Ulcerative colitis and arthritis | [110] | |
| Fluids Tissue/Cellular Compartment | pH Ranges | References |
|---|---|---|
| Saliva in baccul cavity | 6.7–7.3 | [139] |
| Stomach | 2.0 | [35] |
| Duodenum | 5.0–8.0 | |
| Jejunum | 6.0–7.0 | [140] |
| Ileum | 7.0 | |
| Cecum | 6.4 | [123,138] |
| Colon | 7.0–7.5 | [35] |
| Rectum | 7.0 | [140] |
| Vagina | 4.0–5.0 | [141] |
| Chronic wounds | 5.4–7.4 | [35] |
| Extracellular matrix in cancerous tissue | 6.5–7.2 | |
| Lysosomes | 4.5–5.0 | |
| Golgi bodies | 6.4 | |
| Early endosome | 6.0–6.5 | |
| Late endosome | 5.0–6.0 | |
| Blood | 7.35–7.45 | |
| Stratum corneum | 5.0–6.0 | [142] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N. pH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications. Polymers 2017, 9, 137. https://doi.org/10.3390/polym9040137
Rizwan M, Yahya R, Hassan A, Yar M, Azzahari AD, Selvanathan V, Sonsudin F, Abouloula CN. pH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications. Polymers. 2017; 9(4):137. https://doi.org/10.3390/polym9040137
Chicago/Turabian StyleRizwan, Muhammad, Rosiyah Yahya, Aziz Hassan, Muhammad Yar, Ahmad Danial Azzahari, Vidhya Selvanathan, Faridah Sonsudin, and Cheyma Naceur Abouloula. 2017. "pH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications" Polymers 9, no. 4: 137. https://doi.org/10.3390/polym9040137