Stimuli-Regulated Smart Polymeric Systems for Gene Therapy
Abstract
:1. Introduction
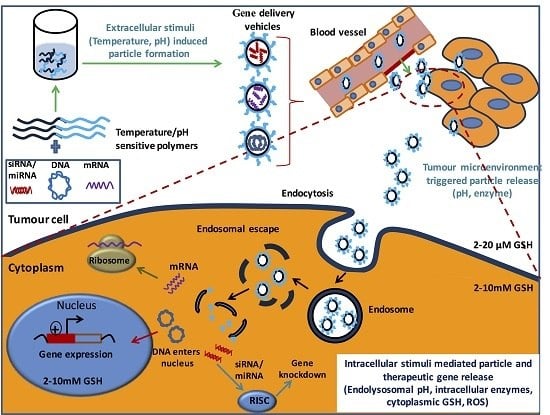
2. Intelligent/Smart Polymers for Stimuli-Responsive Polymeric Gene Carriers
3. Internal Stimuli-Responsive Polymeric Systems for Gene Therapy
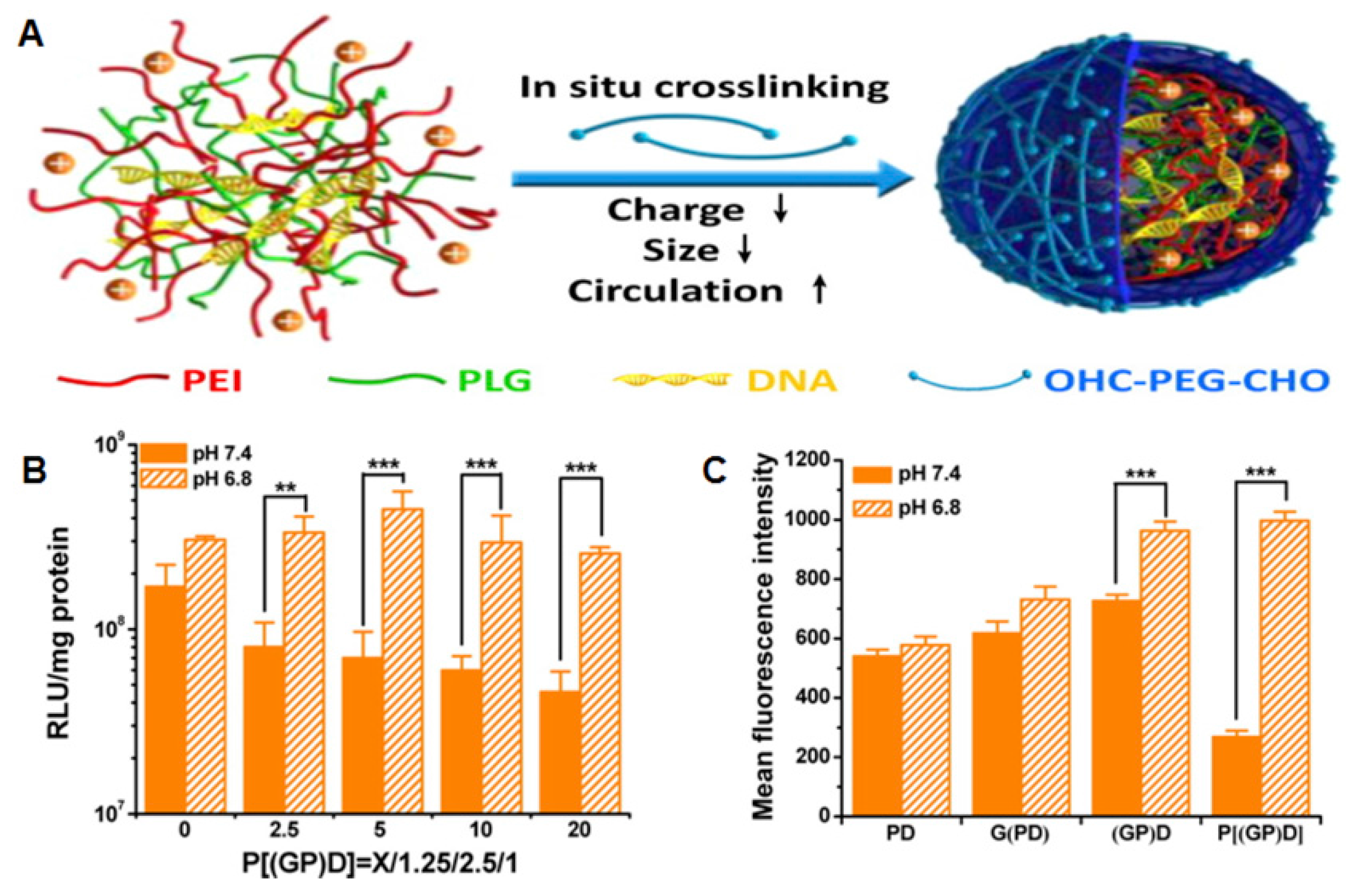
3.1. pH-Responsive Systems
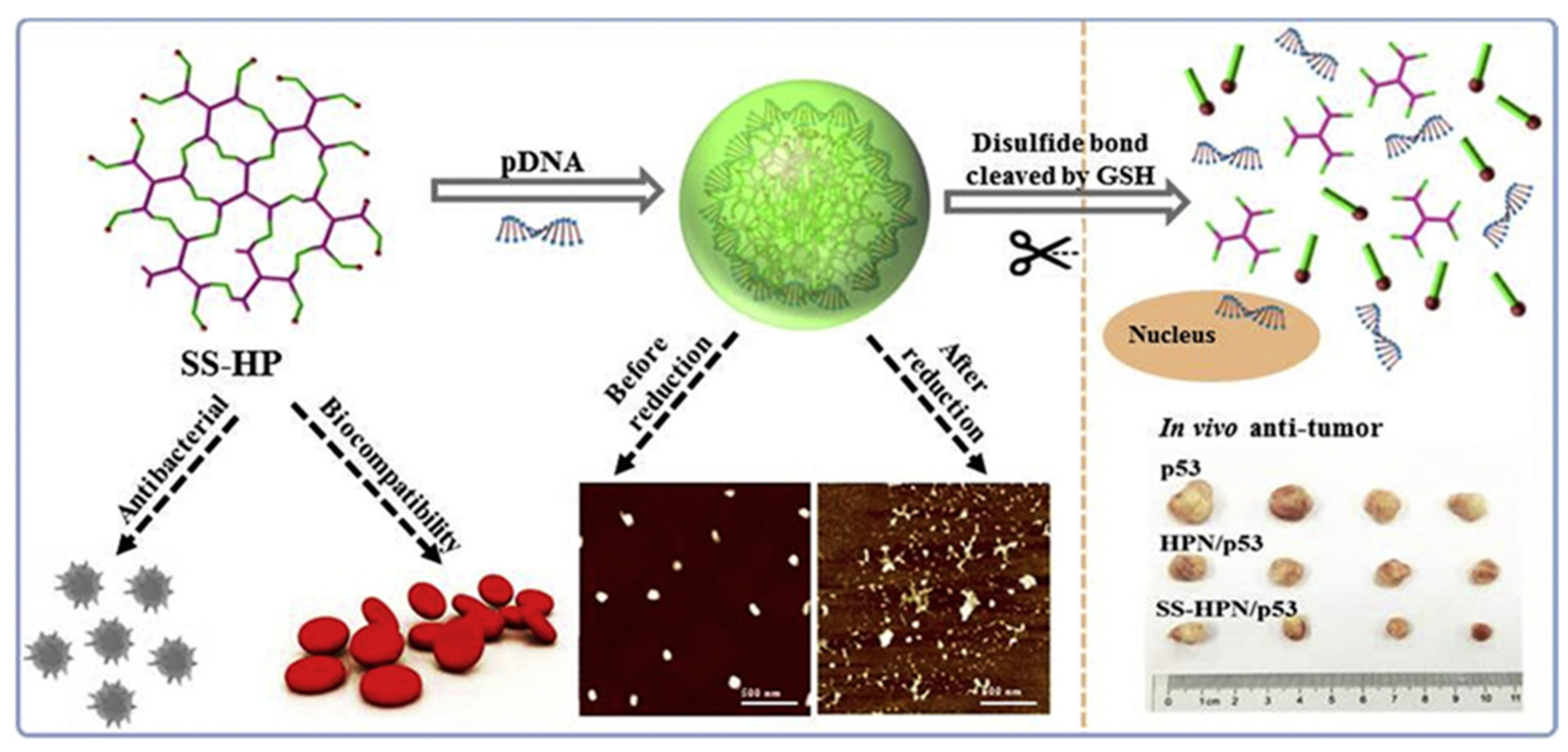
3.2. Redox-Responsive System
3.3. ROS-Responsive Systems
3.4. Enzyme-Responsive Systems
3.5. Osmotic-Responsive System
3.6. Other Strategies Involved in Internal Stimuli-Responsive Systems
3.6.1. Thermo-Responsive Systems
3.6.2. Hypoxia- and Inflammation-Induced Gene Delivery Systems
3.6.3. Multi-Stimuli-Responsive Gene Carriers
4. Conclusions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Giacca, M. Introduction to gene therapy. In Gene Therapy; Springer Milan: Milano, Italy, 2010; pp. 1–7. [Google Scholar]
- Massadeh, S.; Alaamery, M. Polymer nanoparticles for targeted gene delivery. Nanotechnol. Drug Deliv. 2013, 4, 1–20. [Google Scholar]
- Nayerossadat, N.; Maedeh, T.; Ali, P.A. Viral and nonviral delivery systems for gene delivery. Adv. Biomed. Res. 2012, 1, 27. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-D.; Pofali, P.; Park, T.-E.; Singh, B.; Cho, K.; Maharjan, S.; Dandekar, P.; Jain, R.; Choi, Y.-J.; Arote, R. Gene therapy for bone tissue engineering. Tissue Eng. Regener. Med. 2016, 13, 111–125. [Google Scholar] [CrossRef]
- Gardlík, R.; Pálffy, R.; Hodosy, J.; Lukács, J.; Turna, J.; Celec, P. Vectors and delivery systems in gene therapy. Med. Sci. Mon. 2005, 11, 110–121. [Google Scholar]
- Wang, Y.; Shim, M.S.; Levinson, N.S.; Sung, H.W.; Xia, Y. Stimuli-responsive materials for controlled release of theranostic agents. Adv. Funct. Mater. 2014, 24, 4206–4220. [Google Scholar] [CrossRef] [PubMed]
- Du, F.-S.; Wang, Y.; Zhang, R.; Li, Z.-C. Intelligent nucleic acid delivery systems based on stimuli-responsive polymers. Soft Matter 2010, 6, 835–848. [Google Scholar] [CrossRef]
- Schaffert, D.; Wagner, E. Gene therapy progress and prospects: Synthetic polymer-based systems. Gene Ther. 2008, 15, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Luten, J.; van Nostrum, C.F.; De Smedt, S.C.; Hennink, W.E. Biodegradable polymers as non-viral carriers for plasmid DNA delivery. J. Control. Release 2008, 126, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Park, T.G.; Jeong, J.H.; Kim, S.W. Current status of polymeric gene delivery systems. Adv. Drug Deliv. Rev. 2006, 58, 467–486. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, M.; Elvira, C.; Gallardo, A.; Vázquez, B.; Román, J. Smart polymers and their applications as biomaterials. Topic. Tissue Eng. 2007, 3, 256–270. [Google Scholar]
- Stuart, M.A.C.; Huck, W.T.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Aggarwal, G. Smart polymers: Innovations in novel drug delivery. Int. J. Drug Dev. Res. 2011, 3, 16–30. [Google Scholar]
- Cabane, E.; Zhang, X.; Langowska, K.; Palivan, C.G.; Meier, W. Stimuli-responsive polymers and their applications in nanomedicine. Biointerphases 2012, 7, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.; Gutowska, A. Lessons from nature: Stimuli-responsive polymers and their biomedical applications. Trend Biotechnol. 2002, 20, 305–311. [Google Scholar] [CrossRef]
- Roy, D.; Cambre, J.N.; Sumerlin, B.S. Future perspectives and recent advances in stimuli-responsive materials. Prog. Polym. Sci. 2010, 35, 278–301. [Google Scholar] [CrossRef]
- Yao, J.; Feng, J.; Chen, J. External-stimuli responsive systems for cancer theranostic. Asian J. Pharm. Sci. 2016, 11, 585–595. [Google Scholar] [CrossRef]
- Meng, F.; Cheng, R.; Deng, C.; Zhong, Z. Intracellular drug release nanosystems. Mater. Today 2012, 15, 436–442. [Google Scholar] [CrossRef]
- Zhu, Y.; Meng, W.; Gao, H.; Hanagata, N. Hollow mesoporous silica/poly(l-lysine) particles for codelivery of drug and gene with enzyme-triggered release property. J. Phys. Chem. C 2011, 115, 13630–13636. [Google Scholar] [CrossRef]
- Son, S.; Namgung, R.; Kim, J.; Singha, K.; Kim, W.J. Bioreducible polymers for gene silencing and delivery. Acc. Chem. Res. 2011, 45, 1100–1112. [Google Scholar] [CrossRef] [PubMed]
- Shim, M.S.; Xia, Y. A reactive oxygen species (ros)-responsive polymer for safe, efficient, and targeted gene delivery in cancer cells. Angew. Chem. Int. Ed. 2013, 52, 6926–6929. [Google Scholar] [CrossRef] [PubMed]
- Glunde, K.; Guggino, S.E.; Solaiyappan, M.; Pathak, A.P.; Ichikawa, Y.; Bhujwalla, Z.M. Extracellular acidification alters lysosomal trafficking in human breast cancer cells. Neoplasia 2003, 5, 533–545. [Google Scholar] [CrossRef]
- Höckel, M.; Vaupel, P. Tumor hypoxia: Definitions and current clinical, biologic, and molecular aspects. J. Natl. Cancer Inst. 2001, 93, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Pouyssegur, J.; Dayan, F.; Mazure, N.M. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature 2006, 441, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Concheiro, A. Smart drug delivery systems: From fundamentals to the clinic. Chem. Commun. 2014, 50, 7743–7765. [Google Scholar] [CrossRef] [PubMed]
- Dissemond, J.; Witthoff, M.; Brauns, T.; Haberer, D.; Goos, M. pH values in chronic wounds. Evaluation during modern wound therapy. Hautarzt 2003, 54, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Concheiro, A. From drug dosage forms to intelligent drug-delivery systems: A change of paradigm. In Smart Materials for Drug Delivery; Alvarez-Lorenzo, C., Concheiro, A., Eds.; Royal Society of Chemistry: Philadelphia, PA, USA, 2013; pp. 1–32. [Google Scholar]
- Rofstad, E.K.; Mathiesen, B.; Kindem, K.; Galappathi, K. Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer Res. 2006, 66, 6699–6707. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Kallinowski, F.; Okunieff, P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: A review. Cancer Res. 1989, 49, 6449–6465. [Google Scholar] [PubMed]
- Wike-Hooley, J.; Haveman, J.; Reinhold, H. The relevance of tumour pH to the treatment of malignant disease. Radiother. Oncol. 1984, 2, 343–366. [Google Scholar] [CrossRef]
- Volk, T.; Jähde, E.; Fortmeyer, H.; Glüsenkamp, K.; Rajewsky, M. pH in human tumour xenografts: Effect of intravenous administration of glucose. Br. J. Cancer 1993, 68, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Gillies, E.R.; Fréchet, J.M. pH-responsive copolymer assemblies for controlled release of doxorubicin. Bioconjug. Chem. 2005, 16, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.H.; Min, K.H.; Lee, H.J.; Nam, H.Y.; Choi, G.H.; Kim, B.J.; Jeong, S.Y.; Lee, S.C. pH-responsive robust polymer micelles with metal–ligand coordinated core cross-links. Chem. Commun. 2014, 50, 4351–4353. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Jang, W.-D.; Nishiyama, N.; Fukushima, S.; Kataoka, K. Multifunctional polymeric micelles with folate-mediated cancer cell targeting and pH-triggered drug releasing properties for active intracellular drug delivery. Mol. BioSyst. 2005, 1, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Gu, W.; Chen, L.; Gao, Y.; Zhang, Z.; Li, Y. A smart nanoassembly consisting of acid-labile vinyl ether PEG-dope and protamine for gene delivery: Preparation and in vitro transfection. Biomacromolecules 2008, 9, 3119–3126. [Google Scholar] [CrossRef] [PubMed]
- Du, F.-S.; Huang, X.-N.; Chen, G.-T.; Lin, S.-S.; Liang, D.; Li, Z.-C. Aqueous solution properties of the acid-labile thermoresponsive poly(meth) acrylamides with pendent cyclic orthoester groups. Macromolecules 2010, 43, 2474–2483. [Google Scholar] [CrossRef]
- Liu, F.; Eisenberg, A. Preparation and pH triggered inversion of vesicles from poly(acrylic acid)-block-polystyrene-block-poly(4-vinyl pyridine). J. Am. Chem. Soc. 2003, 125, 15059–15064. [Google Scholar] [CrossRef] [PubMed]
- Soppimath, K.S.; Kulkarni, A.R.; Aminabhavi, T.M. Chemically modified polyacrylamide-g-guar gum-based crosslinked anionic microgels as pH-sensitive drug delivery systems: Preparation and characterization. J. Control. Release 2001, 75, 331–345. [Google Scholar] [CrossRef]
- Park, S.Y.; Bae, Y.H. Novel pH-sensitive polymers containing sulfonamide groups. Macromol. Rapid Commun. 1999, 20, 269–273. [Google Scholar] [CrossRef]
- You, J.-O.; Almeda, D.; George, J.; Auguste, D.T. Bioresponsive matrices in drug delivery. J. Biol. Eng. 2010, 4, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Shim, J.K. Preparation of pH/temperature responsive polymer membrane by plasma polymerization and its riboflavin permeation. Polymer 1997, 38, 1227–1232. [Google Scholar] [CrossRef]
- Gil, E.S.; Hudson, S.M. Stimuli-reponsive polymers and their bioconjugates. Prog. Polym. Sci. 2004, 29, 1173–1222. [Google Scholar] [CrossRef]
- Kang, S.I.; Bae, Y.H. pH-induced solubility transition of sulfonamide-based polymers. J. Control. Release 2002, 80, 145–155. [Google Scholar] [CrossRef]
- Park, H.-J.; Yang, F.; Cho, S.-W. Nonviral delivery of genetic medicine for therapeutic angiogenesis. Adv. Drug Deliv. Rev. 2012, 64, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Green, J.J.; Langer, R.; Anderson, D.G. A combinatorial polymer library approach yields insight into nonviral gene delivery. Acc. Chem. Res. 2008, 41, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Guo, Z.; Lin, L.; Chen, J.; Tian, H.; Chen, X. Ultrasensitive pH triggered charge/size dual-rebound gene delivery system. Nano Lett. 2016, 16, 6823–6831. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K. Smart polymeric micelles as nanocarriers for gene and drug delivery. In Proceedings of the MEMS, NANO and Smart Systems (ICMENS) Conference, Banff, AB, Canada, 25–27 August 2004. [Google Scholar]
- Lee, Y.; Miyata, K.; Oba, M.; Ishii, T.; Fukushima, S.; Han, M.; Koyama, H.; Nishiyama, N.; Kataoka, K. Charge-conversion ternary polyplex with endosome disruption moiety: A technique for efficient and safe gene delivery. Angew. Chem. 2008, 120, 5241–5244. [Google Scholar] [CrossRef]
- Gupta, B.; Levchenko, T.S.; Torchilin, V.P. Intracellular delivery of large molecules and small particles by cell-penetrating proteins and peptides. Adv. Drug Deliv. Rev. 2005, 57, 637–651. [Google Scholar] [CrossRef] [PubMed]
- Jeon, O.; Yang, H.S.; Lee, T.-J.; Kim, B.-S. Heparin-conjugated polyethylenimine for gene delivery. J. Control. Release 2008, 132, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Coelho, J.F.; Ferreira, P.C.; Alves, P.; Cordeiro, R.; Fonseca, A.C.; Góis, J.R.; Gil, M.H. Drug delivery systems: Advanced technologies potentially applicable in personalized treatments. EPMA J. 2010, 1, 164–209. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, H.; Tanaka, E.; Fukuyama, N.; Sato, E.; Sakamoto, H.; Tabata, Y.; Ando, K.; Iseki, H.; Shinozaki, Y.; Kimura, K. Biodegradable gelatin hydrogel potentiates the angiogenic effect of fibroblast growth factor 4 plasmid in rabbit hindlimb ischemia. J. Am. Coll. Cardiol. 2003, 41, 1056–1062. [Google Scholar] [CrossRef]
- Kang, S.-W.; Lim, H.-W.; Seo, S.-W.; Jeon, O.; Lee, M.; Kim, B.-S. Nanosphere-mediated delivery of vascular endothelial growth factor gene for therapeutic angiogenesis in mouse ischemic limbs. Biomaterials 2008, 29, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-K.; Thompson, D.H.; Jang, H.S.; Chung, Y.J.; Van den Bossche, J. pH-responsive biodegradable assemblies containing tunable phenyl-substituted vinyl ethers for use as efficient gene delivery vehicles. ACS Appl. Mater. Interfaces 2013, 5, 5648–5658. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Fricke, J.; Kavanagh, D.G.; Irvine, D.J. In vitro and in vivo mrna delivery using lipid-enveloped pH-responsive polymer nanoparticles. Mol. Pharm. 2011, 8, 774–787. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, M.; Neuberg, P.; Kichler, A.; Tounsi, N.; Wagner, A.; Remy, J.-S. p-responsive nanometric polydiacetylenic micelles allow for efficient intracellular siRNA delivery. ACS Appl. Mater. Interfaces 2016, 8, 30665–30670. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zou, Y.; Wang, Y.; Huang, X.; Huang, G.; Sumer, B.D.; Boothman, D.A.; Gao, J. Overcoming endosomal barrier by amphotericin B-loaded dual pH-responsive PDMA-b-PDPA micelleplexes for siRNA delivery. ACS Nano 2011, 5, 9246–9255. [Google Scholar] [CrossRef] [PubMed]
- Gallon, E.; Matini, T.; Sasso, L.; Mantovani, G.; Armiñan de Benito, A.; Sanchis, J.; Caliceti, P.; Alexander, C.; Vicent, M.J.; Salmaso, S. Triblock copolymer nanovesicles for pH-responsive targeted delivery and controlled release of siRNA to cancer cells. Biomacromolecules 2015, 16, 1924–1937. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Acharya, R.; Chatterji, U.; De, P. Side-chain amino-acid-based pH-responsive self-assembled block copolymers for drug delivery and gene transfer. Langmuir 2013, 29, 15375–15385. [Google Scholar] [CrossRef] [PubMed]
- Benoit, D.S.; Henry, S.M.; Shubin, A.D.; Hoffman, A.S.; Stayton, P.S. pH-responsive polymeric siRNA carriers sensitize multidrug resistant ovarian cancer cells to doxorubicin via knockdown of polo-like kinase 1. Mol. Pharm. 2010, 7, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Parhiz, H.; Hashemi, M.; Hatefi, A.; Shier, W.T.; Amel Farzad, S.; Ramezani, M. Arginine-rich hydrophobic polyethylenimine: Potent agent with simple components for nucleic acid delivery. Int. J. Biol. Macromol. 2013, 60, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Lai, J.; Tang, R.; Ji, W.; Wang, R.; Wang, J.; Wang, C. Synthesis and characterization of homopolymers bearing acid-cleavable cationic side-chains for pH-modulated release of DNA. Macromol. Biosci. 2014, 14, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Saito, G.; Swanson, J.A.; Lee, K.-D. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: Role and site of cellular reducing activities. Adv. Drug Deliv. Rev. 2003, 55, 199–215. [Google Scholar] [CrossRef]
- Quinn, J.F.; Whittaker, M.R.; Davis, T.P. Glutathione responsive polymers and their application in drug delivery systems. Polym. Chem. 2017, 8, 97–126. [Google Scholar] [CrossRef]
- Koo, H.; Jin, G.-W.; Kang, H.; Lee, Y.; Nam, K.; Bai, C.Z.; Park, J.-S. Biodegradable branched poly(ethylenimine sulfide) for gene delivery. Biomaterials 2010, 31, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Kwoh, D.Y.; Coffin, C.C.; Lollo, C.P.; Jovenal, J.; Banaszczyk, M.G.; Mullen, P.; Philips, A.; Amini, A.; Fabrycki, J.; Bartholomew, R.M. Stabilization of poly-l-lysine/DNA polyplexes for in vivo gene delivery to the liver. Biochim. Biophys. Acta 1999, 1444, 171–190. [Google Scholar] [CrossRef]
- Cavallaro, G.; Campisi, M.; Licciardi, M.; Ogris, M.; Giammona, G. Reversibly stable thiopolyplexes for intracellular delivery of genes. J. Control. Release 2006, 115, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Kommareddy, S.; Amiji, M. Preparation and evaluation of thiol-modified gelatin nanoparticles for intracellular DNA delivery in response to glutathione. Bioconjug. Chem. 2005, 16, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Kommareddy, S.; Amiji, M. Poly(ethylene glycol)–modified thiolated gelatin nanoparticles for glutathione-responsive intracellular DNA delivery. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, P.; Shen, J. The development and characterization of a glutathione-sensitive cross-linked polyethylenimine gene vector. Biomaterials 2006, 27, 5292–5298. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Lee, D.; Gujrati, V.; Rejinold, N.S.; Lekshmi, K.M.; Uthaman, S.; Jeong, C.; Park, I.-K.; Jon, S.; Kim, Y.-C. Bioreducible branched poly(modified nona-arginine) cell-penetrating peptide as a novel gene delivery platform. J. Control. Release 2017, 246, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, T.; Bravo-Osuna, I.; Vauthier, C.; Ponchel, G.; Loretz, B.; Bernkop-Schnürch, A. Development and in vitro evaluation of a thiomer-based nanoparticulate gene delivery system. Biomaterials 2007, 28, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Che, H.-L.; Bae, I.-H.; Lim, K.S.; Uthaman, S.; Song, I.T.; Lee, H.; Lee, D.; Kim, W.J.; Ahn, Y.; Park, I.-K. Novel fabrication of microRNA nanoparticle-coated coronary stent for prevention of post-angioplasty restenosis. J. Korean Circ. 2016, 46, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Uthaman, S.; Moon, M.J.; Lee, D.; Kim, W.J.; Park, I.-K. Di-sulfide linked polyethylenimine coated gold nanoparticles as a non-viral gene delivery agent in nih-3T3 mouse embryonic fibroblast. J. Nanosci. Nanotechnol. 2015, 15, 7895–7899. [Google Scholar] [CrossRef] [PubMed]
- Muthiah, M.; Che, H.-L.; Kalash, S.; Jo, J.; Choi, S.-Y.; Kim, W.J.; Cho, C.S.; Lee, J.Y.; Park, I.-K. Formulation of glutathione responsive anti-proliferative nanoparticles from thiolated AKT1 siRNA and disulfide-crosslinked pei for efficient anti-cancer gene therapy. Colloids Surf. B 2015, 126, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Li, W.; MacKay, J.A.; Szoka, F.C. Thiocholesterol-based lipids for ordered assembly of bioresponsive gene carriers. Mol. Ther. 2005, 11, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Kirpotin, D.; Hong, K.; Mullah, N.; Papahadjopoulos, D.; Zalipsky, S. Liposomes with detachable polymer coating: Destabilization and fusion of dioleoylphosphatidylethanolamine vesicles triggered by cleavage of surface-grafted poly(ethylene glycol). FEBS Lett. 1996, 388, 115–118. [Google Scholar] [CrossRef]
- Ma, D.; Tian, S.; Baryza, J.; Luft, J.C.; DeSimone, J.M. Reductively responsive hydrogel nanoparticles with uniform size, shape, and tunable composition for systemic siRNA delivery in vivo. Mol. Pharm. 2015, 12, 3518–3526. [Google Scholar] [CrossRef] [PubMed]
- Dunn, S.S.; Tian, S.; Blake, S.; Wang, J.; Galloway, A.L.; Murphy, A.; Pohlhaus, P.D.; Rolland, J.P.; Napier, M.E.; DeSimone, J.M. Reductively responsive siRNA-conjugated hydrogel nanoparticles for gene silencing. J. Am. Chem. Soc. 2012, 134, 7423–7430. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ding, X.; Qi, Y.; Yu, B.; Xu, F.-J. Reduction-responsive multifunctional hyperbranched polyaminoglycosides with excellent antibacterial activity, biocompatibility and gene transfection capability. Biomaterials 2016, 106, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z. Supramolecular biomaterials based on cyclodextrin-oligoethylenimine star polymers for drug and gene delivery. Ph.D. Thesis, National University of Singapore, Singapore, 2013. [Google Scholar]
- Liu, J.; Hennink, W.E.; van Steenbergen, M.J.; Zhuo, R.; Jiang, X. Versatile supramolecular gene vector based on host–guest interaction. Bioconjug. Chem. 2016, 27, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, X.; Hennink, W.E.; Zhuo, R. A modular approach toward multifunctional supramolecular nanopolyplexes for targeting gene delivery. J. Control. Release 2015, 213, 123–124. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, X.; Xu, L.; Wang, X.; Hennink, W.E.; Zhuo, R. Novel reduction-responsive cross-linked polyethylenimine derivatives by click chemistry for nonviral gene delivery. Bioconjug. Chem. 2010, 21, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Chen, X.; Ren, X.; Zhang, X.; Fang, X.; Sha, X. Cd44-targeted hyaluronic acid-coated redox-responsive hyperbranched poly(amido amine)/plasmid DNA ternary nanoassemblies for efficient gene delivery. Bioconjug. Chem. 2016, 27, 1723–1736. [Google Scholar] [CrossRef] [PubMed]
- Ping, Y.; Wu, D.; Kumar, J.N.; Cheng, W.; Lay, C.L.; Liu, Y. Redox-responsive hyperbranched poly(amido amine)s with tertiary amino cores for gene delivery. Biomacromolecules 2013, 14, 2083–2094. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cheng, D.; Yin, T.; Chen, W.; Lin, Y.; Chen, J.; Li, R.; Shuai, X. Copolymer of poly(ethylene glycol) and poly(l-lysine) grafting polyethylenimine through a reducible disulfide linkage for siRNA delivery. Nanoscale 2014, 6, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.O.; Magnusson, J.P.; Moradi, E.; Soliman, M.; Wang, W.; Stolnik, S.; Thurecht, K.J.; Howdle, S.M.; Alexander, C. Modular construction of multifunctional bioresponsive cell-targeted nanoparticles for gene delivery. Bioconjug. Chem. 2011, 22, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, J.; Yang, Q.; Zhuo, R.; Jiang, X. Disulfide-containing brushed polyethylenimine derivative synthesized by click chemistry for nonviral gene delivery. Bioconjug. Chem. 2012, 23, 1290–1299. [Google Scholar] [CrossRef] [PubMed]
- Manickam, D.S.; Oupický, D. Multiblock reducible copolypeptides containing histidine-rich and nuclear localization sequences for gene delivery. Bioconjug. Chem. 2006, 17, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Tang, X.; Pulli, B.; Lin, C.; Zhao, P.; Cheng, J.; Lv, Z.; Yuan, X.; Luo, Q.; Cai, H. Theranostic nanoparticles based on bioreducible polyethylenimine-coated iron oxide for reduction-responsive gene delivery and magnetic resonance imaging. Int. J. Nanomed. 2014, 9, 3347–3356. [Google Scholar]
- Kim, S.H.; Jeong, J.H.; Kim, T.-I.; Kim, S.W.; Bull, D.A. Vegf siRNA delivery system using arginine-grafted bioreducible poly (disulfide amine). Mol. Pharm. 2008, 6, 718–726. [Google Scholar] [CrossRef] [PubMed]
- D’Autréaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Gupta, M.K.; Bang, J.B.; Bae, H.; Sung, H.J. Current progress in reactive oxygen species (ROS)-responsive materials for biomedical applications. Adv. Healthc. Mater. 2013, 2, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xiang, J.; Zhu, D.; Jiang, L.; Zhou, Z.; Tang, J.; Liu, X.; Huang, Y.; Shen, Y. Fusogenic reactive oxygen species triggered charge-reversal vector for effective gene delivery. Adv. Mater. 2016, 28, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.S.; Dalmasso, G.; Wang, L.; Sitaraman, S.V.; Merlin, D.; Murthy, N. 193 orally delivered thioketal-nanoparticles loaded with tnfα-siRNA target inflammation and inhibit gene expression in the intestines. Gastroenterology 2010, 138, 35–36. [Google Scholar] [CrossRef]
- Yu, L.-Y.; Su, G.-M.; Chen, C.-K.; Chiang, Y.-T.; Lo, C.-L. Specific cancer cytosolic drug delivery triggered by reactive oxygen species-responsive micelles. Biomacromolecules 2016, 17, 3040–3047. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, G.; Liu, S. Enzyme-responsive polymeric assemblies, nanoparticles and hydrogels. Chem. Soc. Rev. 2012, 41, 5933–5949. [Google Scholar] [CrossRef] [PubMed]
- Hovgaard, L.; Brøndsted, H. Dextran hydrogels for colon-specific drug delivery. J. Control. Release 1995, 36, 159–166. [Google Scholar] [CrossRef]
- Jain, A.; Gupta, Y.; Jain, S.K. Perspectives of biodegradable natural polysaccharides for site-specific drug delivery to the colon. J. Pharm. Pharm. Sci. 2007, 10, 86–128. [Google Scholar] [PubMed]
- Thornton, P.D.; Mart, R.J.; Webb, S.J.; Ulijn, R.V. Enzyme-responsive hydrogel particles for the controlled release of proteins: Designing peptide actuators to match payload. Soft Matter 2008, 4, 821–827. [Google Scholar] [CrossRef]
- McDonald, T.O.; Qu, H.; Saunders, B.R.; Ulijn, R.V. Branched peptide actuators for enzyme responsive hydrogel particles. Soft Matter 2009, 5, 1728–1734. [Google Scholar] [CrossRef]
- Wang, Z.; Qian, L.; Wang, X.; Zhu, H.; Yang, F.; Yang, X. Hollow DNA/PLL microcapsules with tunable degradation property as efficient dual drug delivery vehicles by α-chymotrypsin degradation. Colloids Surf. A 2009, 332, 164–171. [Google Scholar] [CrossRef]
- Chien, M.-P.; Thompson, M.P.; Lin, E.C.; Gianneschi, N.C. Fluorogenic enzyme-responsive micellar nanoparticles. Chem. Sci. 2012, 3, 2690–2694. [Google Scholar] [CrossRef] [PubMed]
- Ku, T.-H.; Chien, M.-P.; Thompson, M.P.; Sinkovits, R.S.; Olson, N.H.; Baker, T.S.; Gianneschi, N.C. Controlling and switching the morphology of micellar nanoparticles with enzymes. J. Am. Chem. Soc. 2011, 133, 8392–8395. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, A.J.; Mastrobattista, E.; Vermonden, T.; van Nostrum, C.F.; Rijkers, D.T.; Liskamp, R.M.; Hennink, W.E. Thermosensitive peptide-hybrid abc block copolymers obtained by atrp: Synthesis, self-assembly, and enzymatic degradation. Macromolecules 2012, 45, 842–851. [Google Scholar] [CrossRef]
- Li, J.; Ge, Z.; Liu, S. Peg-sheddable polyplex micelles as smart gene carriers based on mmp-cleavable peptide-linked block copolymers. Chem. Commun. 2013, 49, 6974–6976. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, J.; Palmer, L.; Ossanlou, M.; MacLachlan, I.; Graham, R.; Zhang, Y.; Hope, M.; Scherrer, P.; Cullis, P. Stabilized plasmid-lipid particles: Construction and characterization. Gene Ther. 1999, 6, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Song, S.J.; Lee, S.; Lee, Y.; Choi, J.S. Enzyme-responsive destabilization of stabilized plasmid-lipid nanoparticles as an efficient gene delivery. Eur. J. Pharm. Sci. 2016, 91, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Katayama, Y.; Fujii, K.; Ito, E.; Sakakihara, S.; Sonoda, T.; Murata, M.; Maeda, M. Intracellular signal-responsive artificial gene regulation for novel gene delivery. Biomacromolecules 2002, 3, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Arote, R.B.; Lee, E.-S.; Jiang, H.-L.; Kim, Y.-K.; Choi, Y.-J.; Cho, M.-H.; Cho, C.-S. Efficient gene delivery with osmotically active and hyperbranched poly(ester amine)s. Bioconjug. Chem. 2009, 20, 2231–2241. [Google Scholar] [CrossRef] [PubMed]
- Maiti, K.K.; Lee, W.S.; Takeuchi, T.; Watkins, C.; Fretz, M.; Kim, D.C.; Futaki, S.; Jones, A.; Kim, K.T.; Chung, S.K. Guanidine-containing molecular transporters: Sorbitol-based transporters show high intracellular selectivity toward mitochondria. Angew. Chem. Int. Ed. 2007, 46, 5880–5884. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Khalil, I.A.; Maiti, K.K.; Lee, W.S.; Akita, H.; Harashima, H.; Chung, S.-K. Novel lipidated sorbitol-based molecular transporters for non-viral gene delivery. J. Control. Release 2009, 136, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Kiss, A.L.; Botos, E. Endocytosis via caveolae: Alternative pathway with distinct cellular compartments to avoid lysosomal degradation? J. Cell. Mol. Med. 2009, 13, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Yun, C.-H.; Choi, Y.-J.; Shin, J.-Y.; Arote, R.; Jiang, H.-L.; Kang, S.-K.; Nah, J.-W.; Park, I.-K.; Cho, M.-H. Accelerated gene transfer through a polysorbitol-based transporter mechanism. Biomaterials 2011, 32, 9908–9924. [Google Scholar] [CrossRef] [PubMed]
- Luu, Q.-P.; Shin, J.-Y.; Kim, Y.-K.; Islam, M.A.; Kang, S.-K.; Cho, M.-H.; Choi, Y.-J.; Cho, C.-S. High gene transfer by the osmotic polysorbitol-mediated transporter through the selective caveolae endocytic pathway. Mol. Pharm. 2012, 9, 2206–2218. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.C.T.; Muthiah, M.; Islam, M.A.; Kalash, R.S.; Cho, C.-S.; Park, H.; Lee, I.-K.; Kim, H.-J.; Park, I.-K.; Cho, K.A. Selective transfection with osmotically active sorbitol modified pei nanoparticles for enhanced anti-cancer gene therapy. Colloid Surf. B 2014, 119, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Shin, J.-Y.; Firdous, J.; Park, T.-E.; Choi, Y.-J.; Cho, M.-H.; Yun, C.-H.; Cho, C.-S. The role of osmotic polysorbitol-based transporter in rnai silencing via caveolae-mediated endocytosis and cox-2 expression. Biomaterials 2012, 33, 8868–8880. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Shin, J.-Y.; Yun, C.-H.; Cho, C.-S.; Seo, H.W.; Chae, C.; Cho, M.-H. The effect of rnai silencing of p62 using an osmotic polysorbitol transporter on autophagy and tumorigenesis in lungs of k-ras la1 mice. Biomaterials 2014, 35, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Kouri, F.M.; Ritner, C.; Stegh, A.H. Mirna-182 and the regulation of the glioblastoma phenotype-toward mirna-based precision therapeutics. Cell Cycle 2015, 14, 3794–3800. [Google Scholar] [CrossRef] [PubMed]
- Muthiah, M.; Islam, M.A.; Cho, C.S.; Hwang, J.E.; Chung, I.-J.; Park, I.K. Substrate-mediated delivery of microrna-145 through a polysorbitol-based osmotically active transporter suppresses smooth muscle cell proliferation: Implications for restenosis treatment. J. Biomed. Nanotechnol. 2014, 10, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Park, T.-E.; Kang, B.; Kim, Y.-K.; Zhang, Q.; Lee, W.-S.; Islam, M.A.; Kang, S.-K.; Cho, M.-H.; Choi, Y.-J.; Cho, C.-S. Selective stimulation of caveolae-mediated endocytosis by an osmotic polymannitol-based gene transporter. Biomaterials 2012, 33, 7272–7281. [Google Scholar] [CrossRef] [PubMed]
- Park, T.-E.; Singh, B.; Li, H.; Lee, J.-Y.; Kang, S.-K.; Choi, Y.-J.; Cho, C.-S. Enhanced bbb permeability of osmotically active poly (mannitol-co-pei) modified with rabies virus glycoprotein via selective stimulation of caveolar endocytosis for rnai therapeutics in alzheimer's disease. Biomaterials 2015, 38, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, D.; Jain, R.K. Tumor microenvironment abnormalities: Causes, consequences, and strategies to normalize. J. Cell. Biochem. 2007, 101, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, T.; Lin, Z. Controlled release of ionic drug through the positively charged temperature-responsive membranes. J. Membr. Sci. 2006, 281, 491–499. [Google Scholar] [CrossRef]
- Gandhi, A.; Paul, A.; Sen, S.O.; Sen, K.K. Studies on thermoresponsive polymers: Phase behaviour, drug delivery and biomedical applications. Asian J. Pharm. Sci. 2015, 10, 99–107. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, L.; Lu, X.; Zhang, L.; He, Y. Thermo and pH sensitive fluorescent polymer sensor for metal cations in aqueous solution. Polym. Adv. Technol. 2008, 19, 137–143. [Google Scholar] [CrossRef]
- Dincer, S.; Türk, M.; Pişkin, E. Intelligent polymers as nonviral vectors. Gene Ther. 2005, 12, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Ohya, S.; Sonoda, H.; Nakayama, Y.; Matsuda, T. The potential of poly(n-isopropylacrylamide)(PNIPAM)-grafted hyaluronan and pnipam-grafted gelatin in the control of post-surgical tissue adhesions. Biomaterials 2005, 26, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Suwa, K.; Morishita, K.; Kishida, A.; Akashi, M. Synthesis and functionalities of poly(n-vinylalkylamide). J. Polym. Sci. 1997, 35, 3087–3094. [Google Scholar] [CrossRef]
- Na, K.; Lee, K.H.; Lee, D.H.; Bae, Y.H. Biodegradable thermo-sensitive nanoparticles from poly(l-lactic acid)/poly(ethylene glycol) alternating multi-block copolymer for potential anti-cancer drug carrier. Eur. J. Pharm. Sci. 2006, 27, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Ruel-Gariepy, E.; Leroux, J.-C. In situ-forming hydrogels—Review of temperature-sensitive systems. Eur. J. Pharm. Biopharm. 2004, 58, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, W.; Schuurmans-Nieuwenbroek, N.; Van De Wetering, P.; Hennink, W. Thermosensitive polymers as carriers for DNA delivery. J. Control. Release 1999, 60, 249–259. [Google Scholar] [CrossRef]
- Kurisawa, M.; Yokoyama, M.; Okano, T. Gene expression control by temperature with thermo-responsive polymeric gene carriers. J. Control. Release 2000, 69, 127–137. [Google Scholar] [CrossRef]
- Özdemir, N.; Tuncel, A.; Kang, M.; Denkbş, E.B. Preparation and characterization of thermosensitive submicron particles for gene delivery. J. Nanosci. Nanotechnol. 2006, 6, 2804–2810. [Google Scholar] [CrossRef] [PubMed]
- Bouchemal, K.; Aka-Any-Grah, A.; Dereuddre-Bosquet, N.; Martin, L.; Lievin-Le-Moal, V.; Le Grand, R.; Nicolas, V.; Gibellini, D.; Lembo, D.; Poüs, C. Thermosensitive and mucoadhesive pluronic-hydroxypropylmethylcellulose hydrogel containing the mini-cd4 m48u1 is a promising efficient barrier against hiv diffusion through macaque cervicovaginal mucus. Antimicrob. Agents Chemother. 2015, 59, 2215–2222. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Lee, S.H.; Park, T.G. Temperature-sensitive pluronic/poly (ethylenimine) nanocapsules for thermally triggered disruption of intracellular endosomal compartment. Biomacromolecules 2006, 7, 1864–1870. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhao, W.; Liu, K.; Yu, Q.; Mao, Y.; Lu, Z.; Zhang, Y.; Zhu, M. Low-molecular weight polyethylenimine modified with pluronic 123 and rgd-or chimeric rgd-nls peptide: Characteristics and transfection efficacy of their complexes with plasmid DNA. Molecules 2016, 21, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Perche, F.; Biswas, S.; Wang, T.; Zhu, L.; Torchilin, V.P. Hypoxia-targeted siRNA delivery. Angew. Chem. Int. Ed. 2014, 53, 3362–3366. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.A. Role of oxygen free radicals in retinal damage associated with experimental uveitis. Trans. Am. Ophthalmol. Soc. 1990, 88, 797–803. [Google Scholar] [PubMed]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Cho, H.; Cho, Y.; Kang, S.; Kwak, M.; Huh, K.; Bae, Y.; Kang, H. Tempo-spatial activation of sequential quadruple stimuli for high gene expression of polymeric gene nanocomplexes. Mol. Pharm. 2017, 14, 842–855. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-Y.; Wan, S.; Zheng, D.-W.; Lei, Q.; Zhuo, R.-X.; Feng, J.; Zhang, X.-Z. Propelled transnuclear gene transport achieved through intracellularly redox-responsive and acidity-accelerative decomposition of supramolecular florescence-quenchable vectors. ACS Appl. Mater. Interfaces 2016, 9, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Shao, K.; Liu, Y.; Kuang, Y.; Li, J.; An, S.; Guo, Y.; Ma, H.; Jiang, C. Tumor-targeting and microenvironment-responsive smart nanoparticles for combination therapy of antiangiogenesis and apoptosis. ACS Nano 2013, 7, 2860–2871. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhang, Y.; Zhuo, R.; Jiang, X. A light and reduction dual sensitive supramolecular self-assembly gene delivery system based on poly(cyclodextrin) and disulfide-containing azobenzene-terminated branched polycations. J. Mater. Chem. B 2016, 4, 7731–7740. [Google Scholar] [CrossRef]
- Wilson, D.S.; Dalmasso, G.; Wang, L.; Sitaraman, S.V.; Merlin, D.; Murthy, N. Orally delivered thioketal nanoparticles loaded with TNF-α-siRNA target inflammation and inhibit gene expression in the intestines. Nat. Mater. 2010, 9, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.K.; Lee, S.H.; Crowder, S.W.; Wang, X.; Hofmeister, L.H.; Nelson, C.E.; Bellan, L.M.; Duvall, C.L.; Sung, H.-J. Oligoproline-derived nanocarrier for dual stimuli-responsive gene delivery. J. Mater. Chem. B 2015, 3, 7271–7280. [Google Scholar] [CrossRef]
- Yingyuad, P.; Mével, M.; Prata, C.; Furegati, S.; Kontogiorgis, C.; Thanou, M.; Miller, A.D. Enzyme-triggered pegylated pdna-nanoparticles for controlled release of pdna in tumors. Bioconjug. Chem. 2013, 24, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Perche, F.; Wang, T.; Torchilin, V.P. Matrix metalloproteinase 2-sensitive multifunctional polymeric micelles for tumor-specific co-delivery of siRNA and hydrophobic drugs. Biomaterials 2014, 35, 4213–4222. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yuan, Y.; Cheng, D.; Chen, J.; Wang, L.; Shuai, X. Co-delivery of doxorubicin and siRNA with reduction and pH dually sensitive nanocarrier for synergistic cancer therapy. Small 2014, 10, 2678–2687. [Google Scholar] [CrossRef] [PubMed]


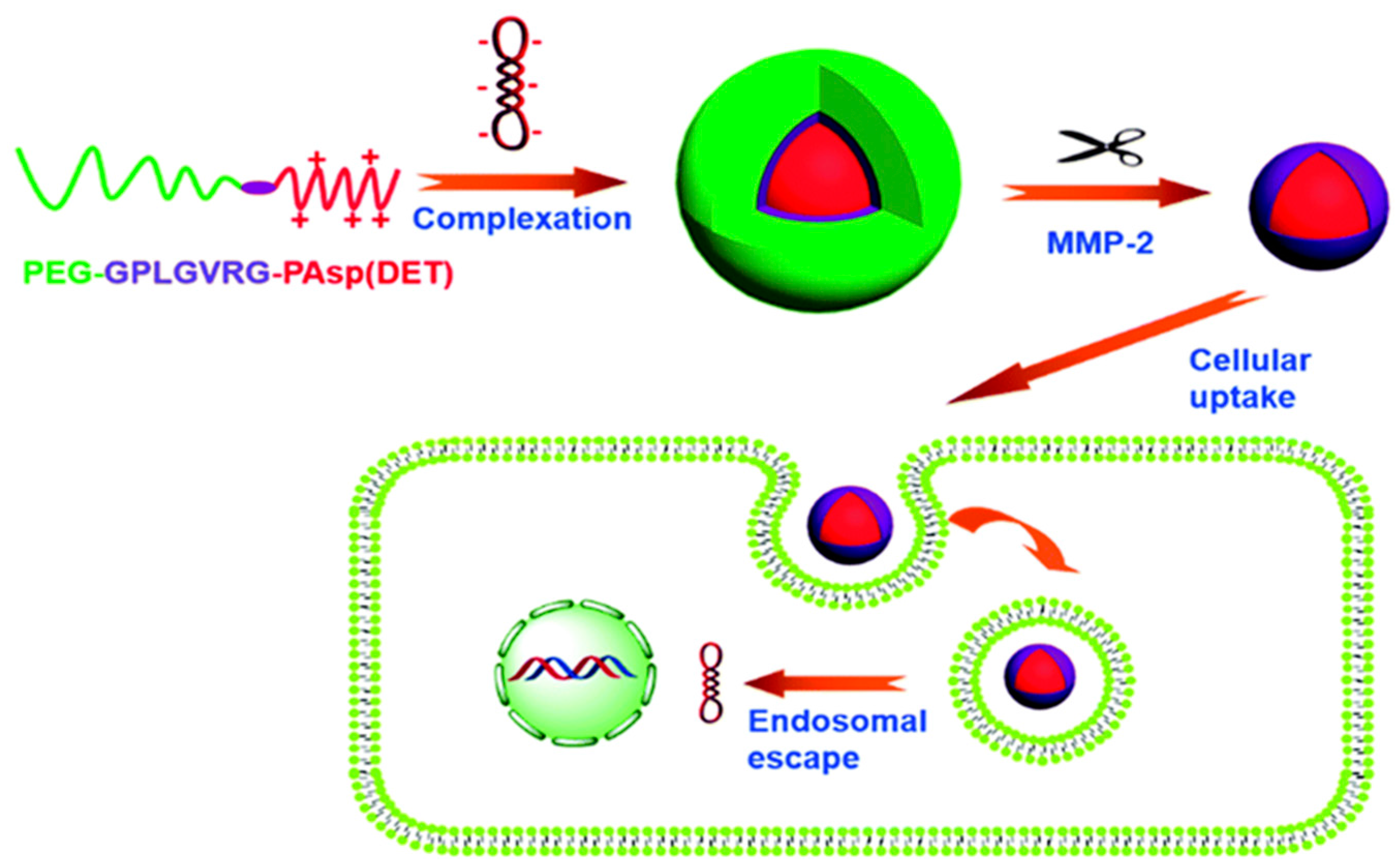

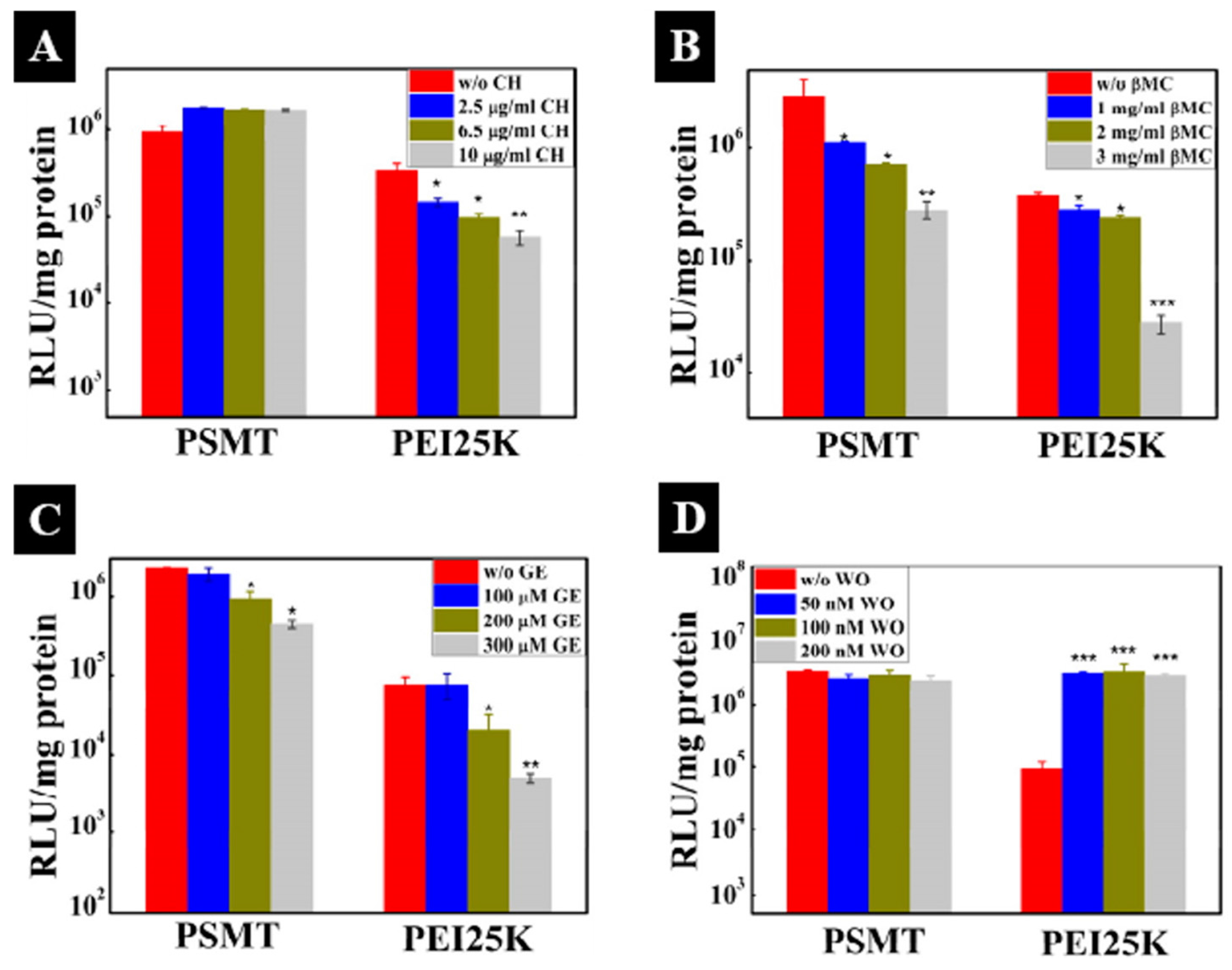
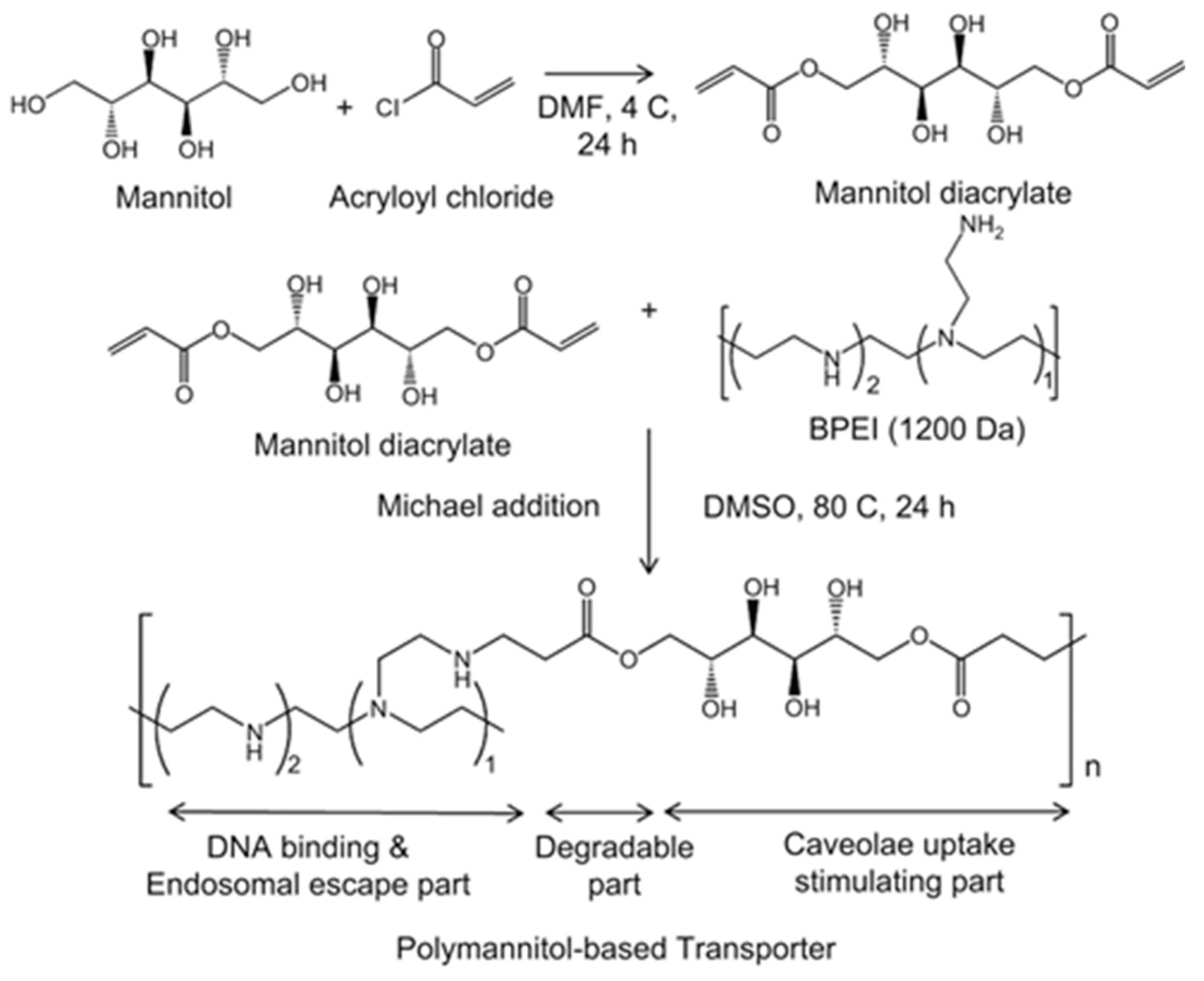
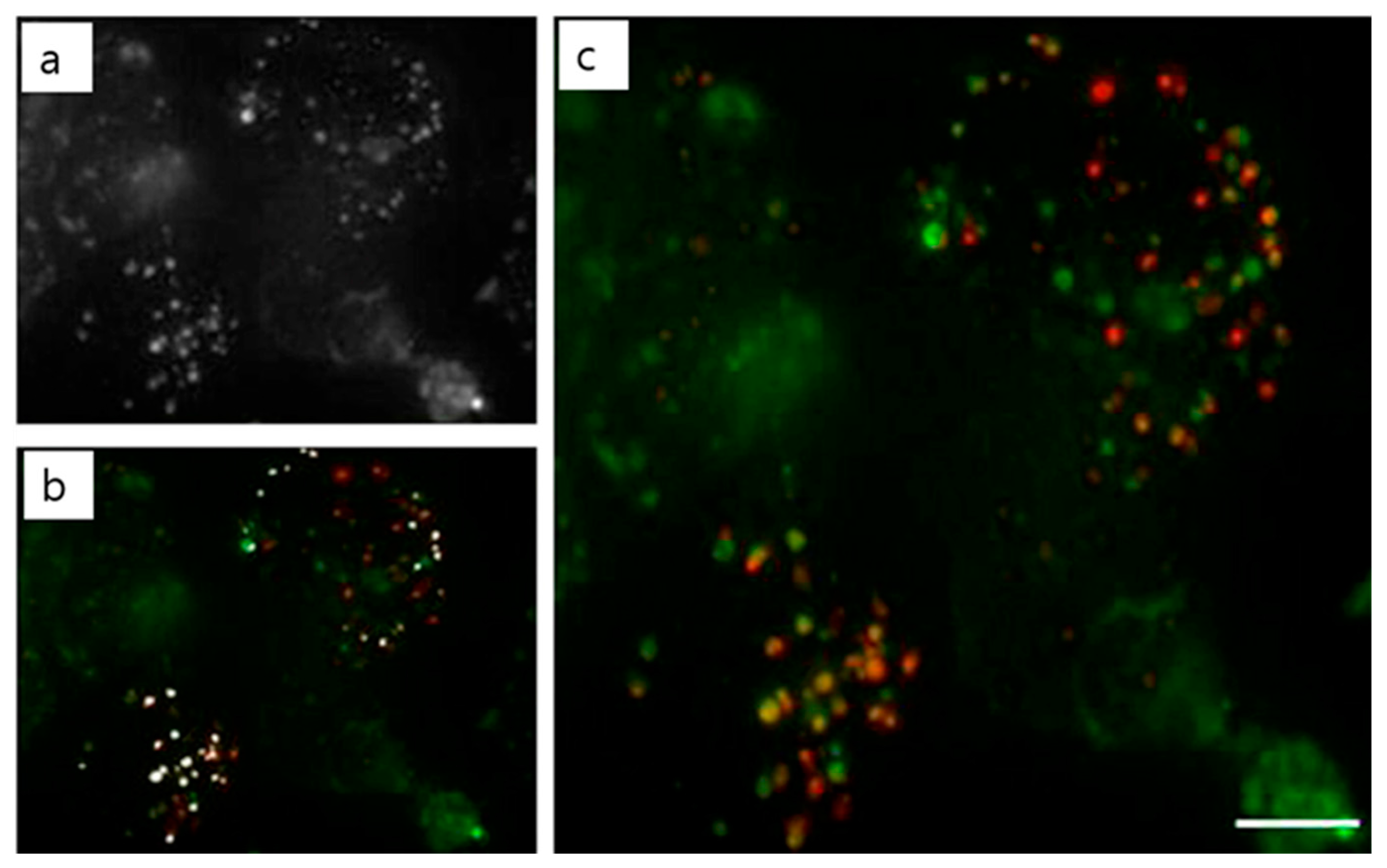
| Polymer used | pH-responsive moiety | Drug used | Ref. |
|---|---|---|---|
| PEG-lipid:DOPE liposomes | Phenyl-substituted vinyl ether (PIVE) | Plasmid DNA | [54] |
| Lipid-coated PBAE nanoparticles | Poly(β-amino ester) (PBAE) | mRNA | [55] |
| Polymerized diacetylenic amphiphile (PDA) micelles | Imidazole group of histidine | siRNA | [56] |
| Poly(2(dimethylamino) ethyl methacrylate)-block poly(2(diisopropylamino)ethyl methacrylate) (PDMA-b-PDPA) micelles | PDPA protonation | siRNA and Amphotericin B | [57] |
| PEG-pImHeMA-pGMA copolymer (polymersome) | Polyimidazole-hexyl methacrylate (poly-ImHeMA)) | siRNA | [58] |
| Monomethoxy poly(ethylene glycol)-b-poly(amino acid methacryloyloxyethyl ester) (mPEGn-b-P(H2N-AA-EMA)) | Poly((meth)acrylamide) | Plasmid DNA | [59] |
| Poly(dimethylaminoethyl methacrylate-block-butyl methacrylate) copolymers (pDbB) (micelle) | Poly(styrene-alt-maleic anhydride) (pSMA) | siRNA | [60] |
| PEI and hexanoate-PEI | Arginine rich peptides | pDNA, siRNA | [61] |
| Poly(N-((2-(2-(dimethylamino)ethoxy)-1,3-dioxolan-4-yl)methyl)methacrylamide (PMAOE) | Acid-cleavable side chain | pDNA | [62] |
| Polymer used | Type of system | Gene used | Ref. |
|---|---|---|---|
| Disulfide-containing cross-linked polyethylenimines (PEI-SS-CLs) | Polyplexes | pDNA | [84] |
| Hyaluronic acid coated reducible hyperbranched poly-(amidoamine) (RHB) (HA/RHB/pDNA) | Nanoassembly | pDNA | [85] |
| Hyperbranched PAAs with tertiary amino cores and amine, poly(ethylene glycol) (PEG) and hydroxyl terminal groups | Polyplexes | pDNA | [86] |
| mPEG-b-PLL-g-(ss-lPEI) (PLI) | Polyplexes | XIAP siRNA | [87] |
| Bock co-polymer from PLGA-s-s-PEGMA | Core-shell nanoparticle | pDNA | [88] |
| Polyaspartamide-based disulfide-containing brushed polyethylenimine (P(Asp-Az)X-SS-PEIs) | Polyplexes | pDNA | [89] |
| Reducible copolypeptides (rCPP) with HRP(histidine-rich peptide (HRP)) and NLS sequences | Polyplexes | p DNA | [90] |
| SSPEI | Polyplexes | siRNA | [91] |
| Arginine-conjugated poly(cystamine-bis-acrylamide-diaminohexane) (poly(CBA-DAH-R)) | Polyplexes | siRNA | [92] |
| RPMI + 10% FBS | 276 | |
| Mannitol | 1% | 348 |
| 3% | 441 | |
| 5% | 531 | |
| PMT/DNA (N/P 20) | 1% mannitol (wt %) | 297 |
| 3% mannitol (wt %) | 395 | |
| 5% mannitol (wt %) | 421 |
| Polymer used | Type of trigger | Type of linkage/sensitive moieties | Gene used | Ref. |
|---|---|---|---|---|
| Poly-(1,4-phenyleneacetone dimethylene thioketal) | ROS | Thioketal | siRNA | [146] |
| mPEG113-b-CP5K-b-PDMAEMA42-b-P(DMAEMA22-co-BMA40-co-PAA24) (PPDDBP) | ROS and pH | CP5K peptide linker, PAA | pDNA | [147] |
| PEG2000-peptidyl lipids | Enzyme | Elastase or MMP-2-mediated digestion | pDNA | [148] |
| PEG-pp-PEI-PE | Enzyme | MMP-2-mediated digestion | siRNA | [149] |
| PEG-PAsp(AED)-PDPA | pH and redox potential | Di-sulfide linkage | siRNA | [150] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathew, A.P.; Cho, K.-H.; Uthaman, S.; Cho, C.-S.; Park, I.-K. Stimuli-Regulated Smart Polymeric Systems for Gene Therapy. Polymers 2017, 9, 152. https://doi.org/10.3390/polym9040152
Mathew AP, Cho K-H, Uthaman S, Cho C-S, Park I-K. Stimuli-Regulated Smart Polymeric Systems for Gene Therapy. Polymers. 2017; 9(4):152. https://doi.org/10.3390/polym9040152
Chicago/Turabian StyleMathew, Ansuja Pulickal, Ki-Hyun Cho, Saji Uthaman, Chong-Su Cho, and In-Kyu Park. 2017. "Stimuli-Regulated Smart Polymeric Systems for Gene Therapy" Polymers 9, no. 4: 152. https://doi.org/10.3390/polym9040152