A Strategy to Enhance the Electrode Performance of Novel Three-Dimensional PEDOT/RVC Composites by Electrochemical Deposition Method
Abstract
:1. Introduction
2. Materials, Methods, and Experimental
2.1. Chemicals and Materials
2.2. Pre-Treatment of the RVC Electrode
2.3. Electrochemical Polymerization of PEDOT on RVC Electrode
2.4. Physical Characterization
2.5. Electrochemical Characterization
3. Results and Discussion
3.1. PEDOT Deposited on RVC Electrode
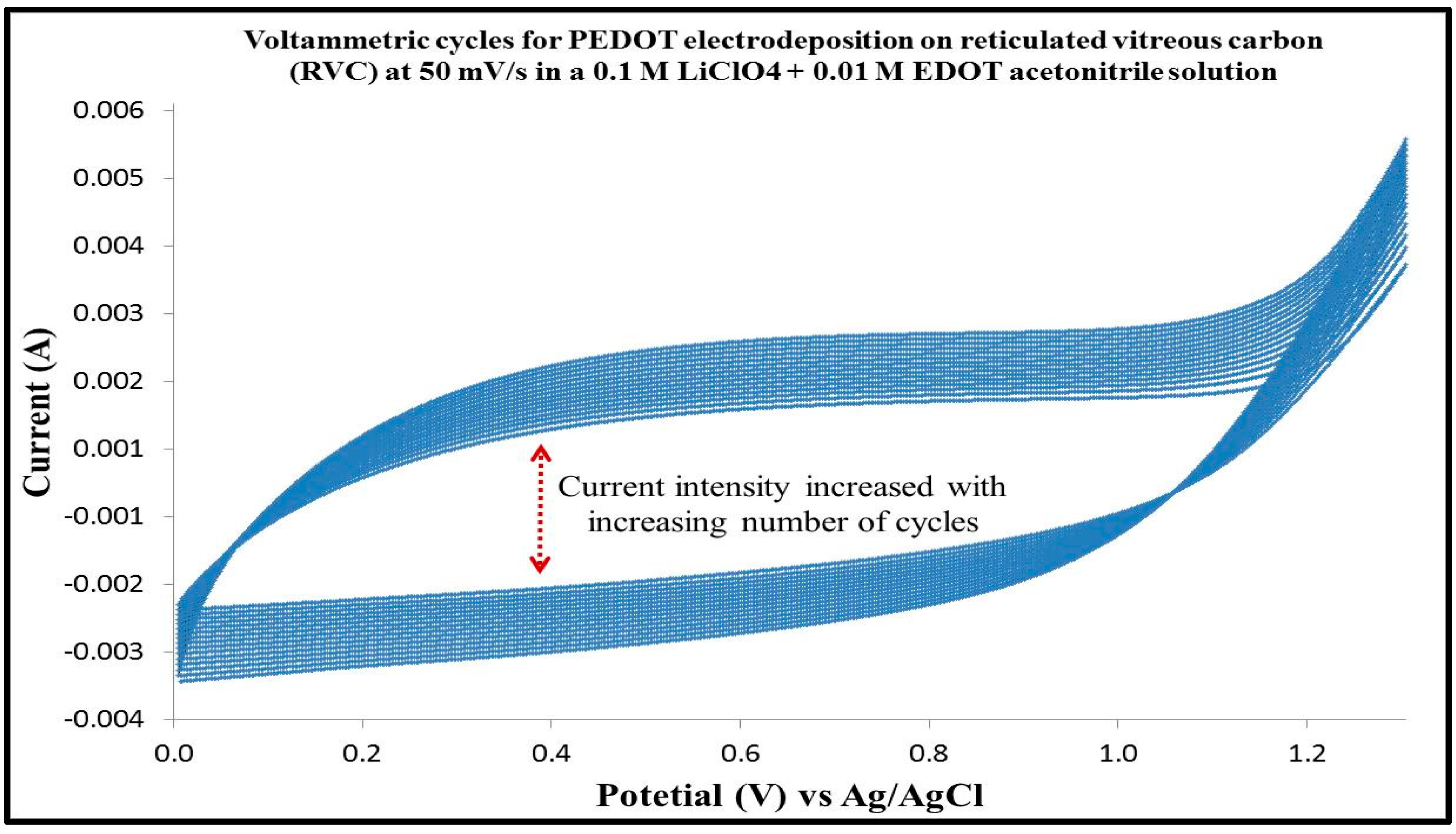
3.2. Cyclic Voltammetry
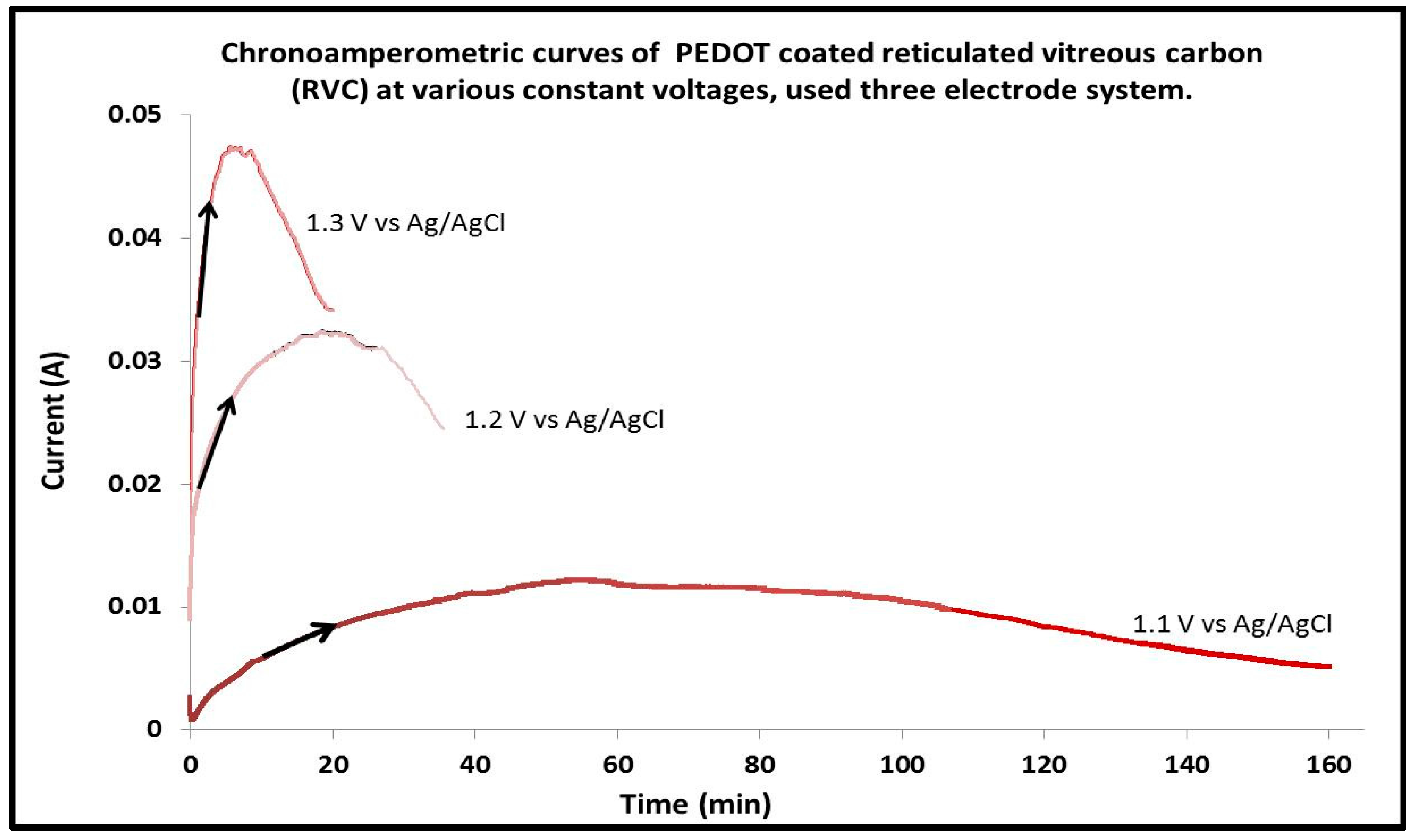
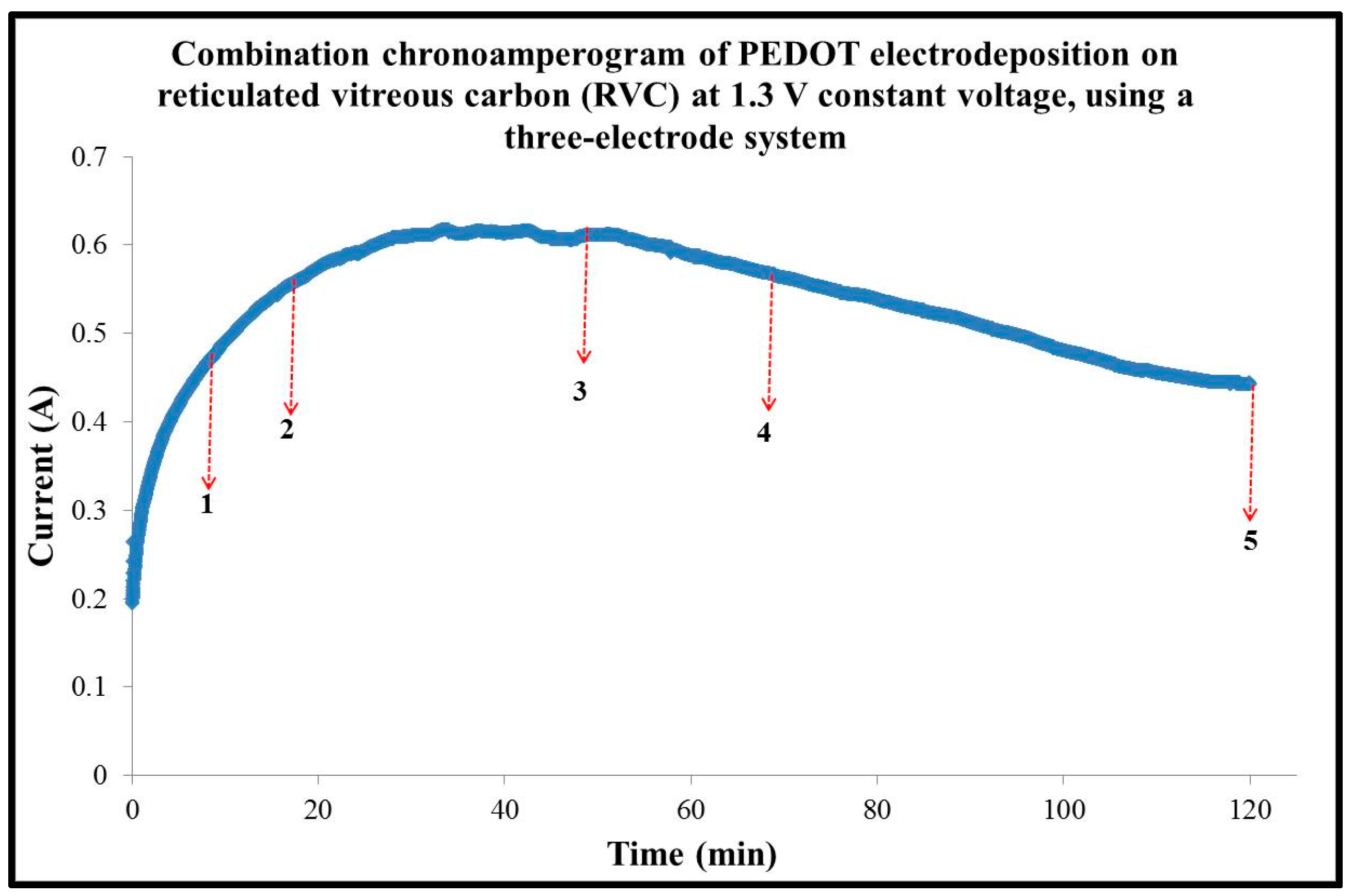
3.3. Effect of Applied Constant Potential on PEDOT Electrosynthesis
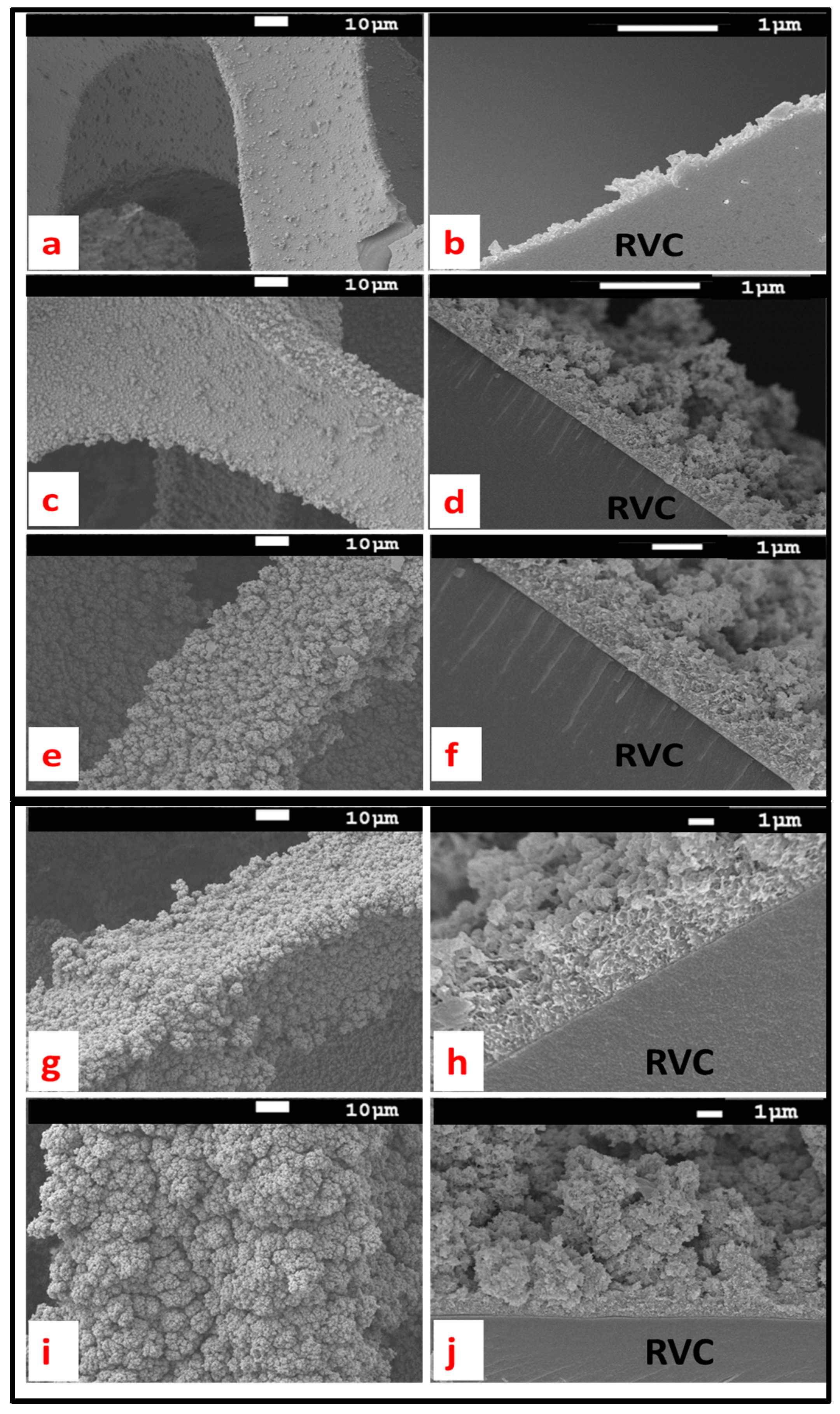
3.4. Electrodeposition of Different Amounts of PEDOT on RVC Electrodes
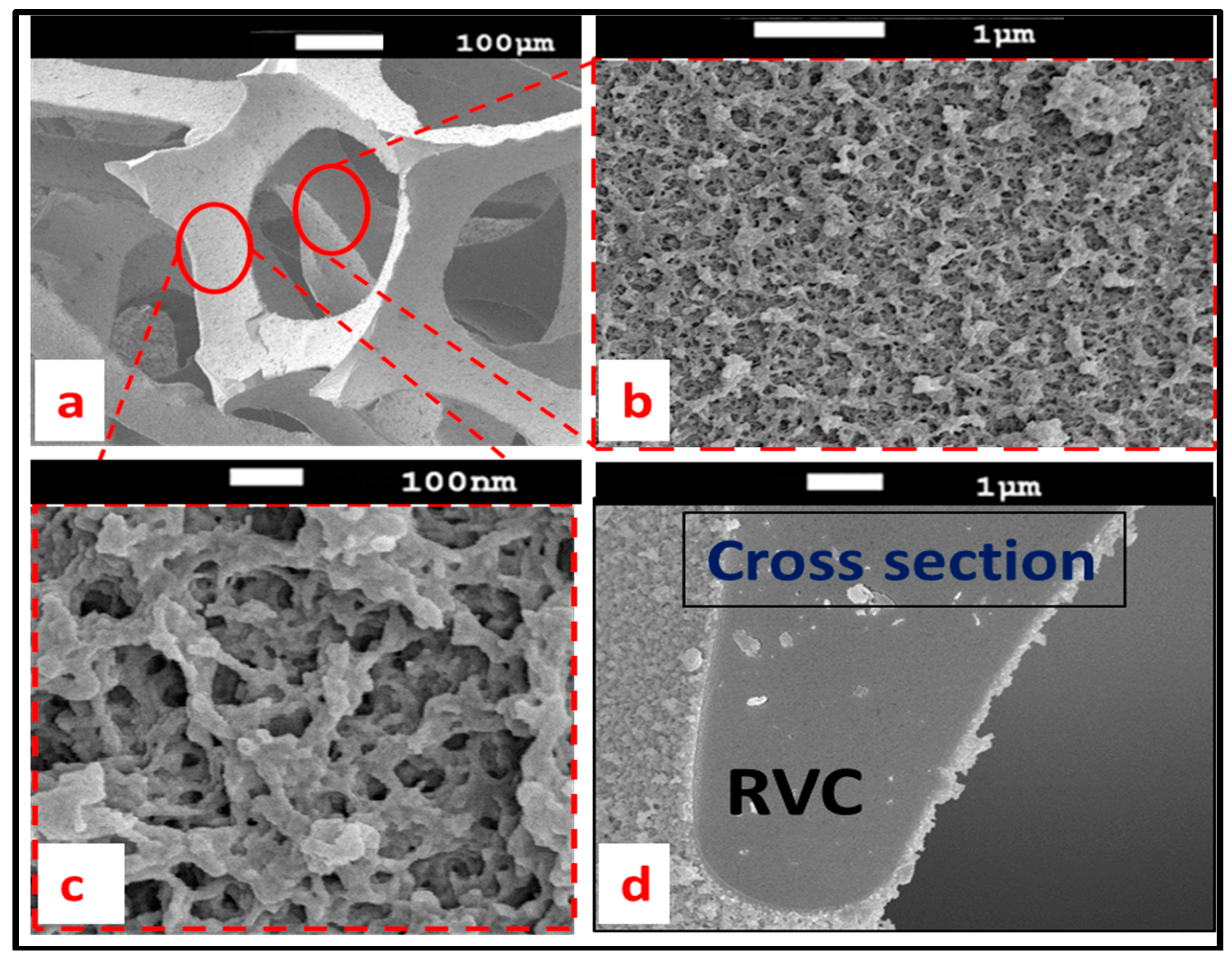
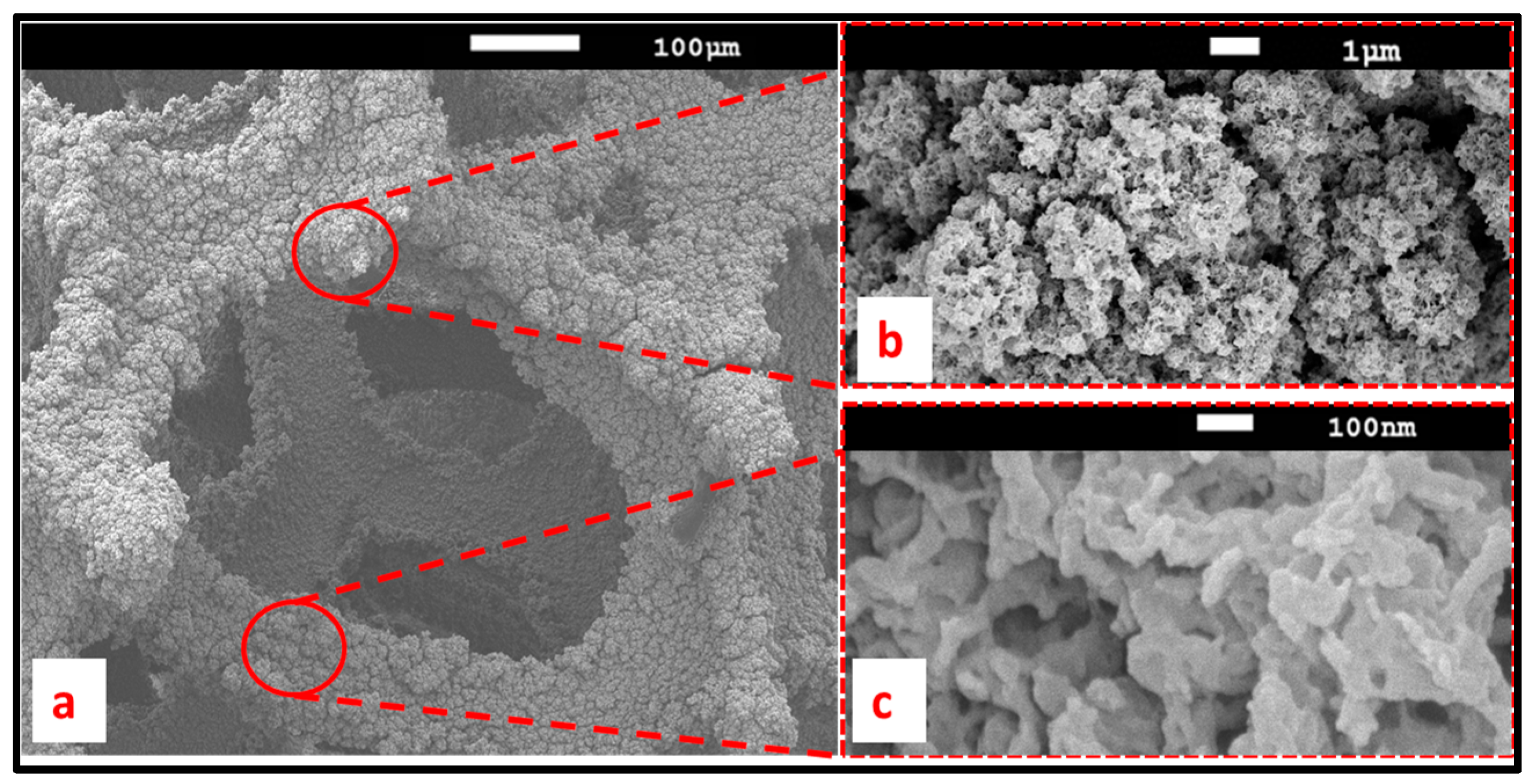
3.5. PEDOT Surface Properties
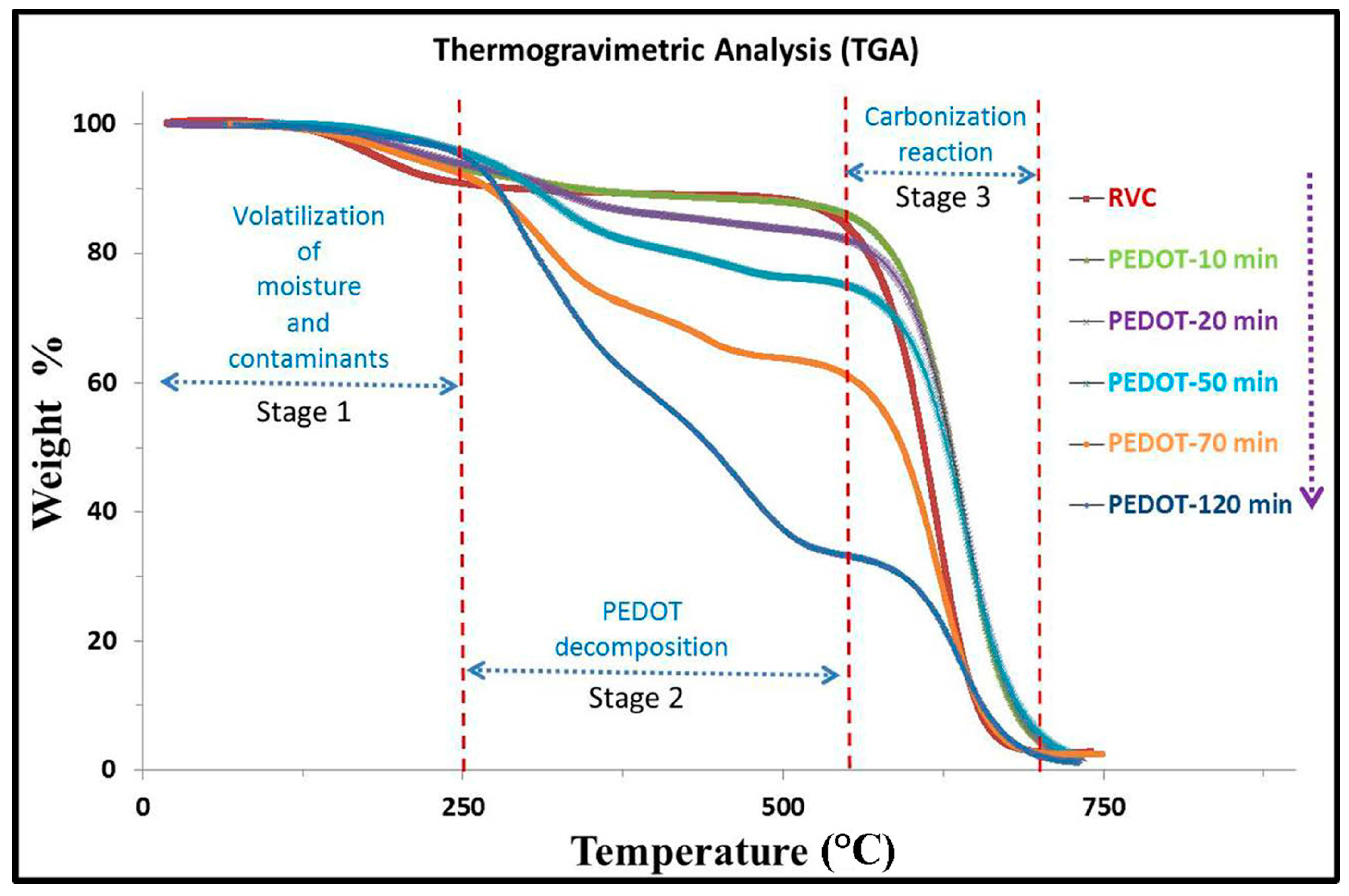
3.6. Thermogravimetric Analysis
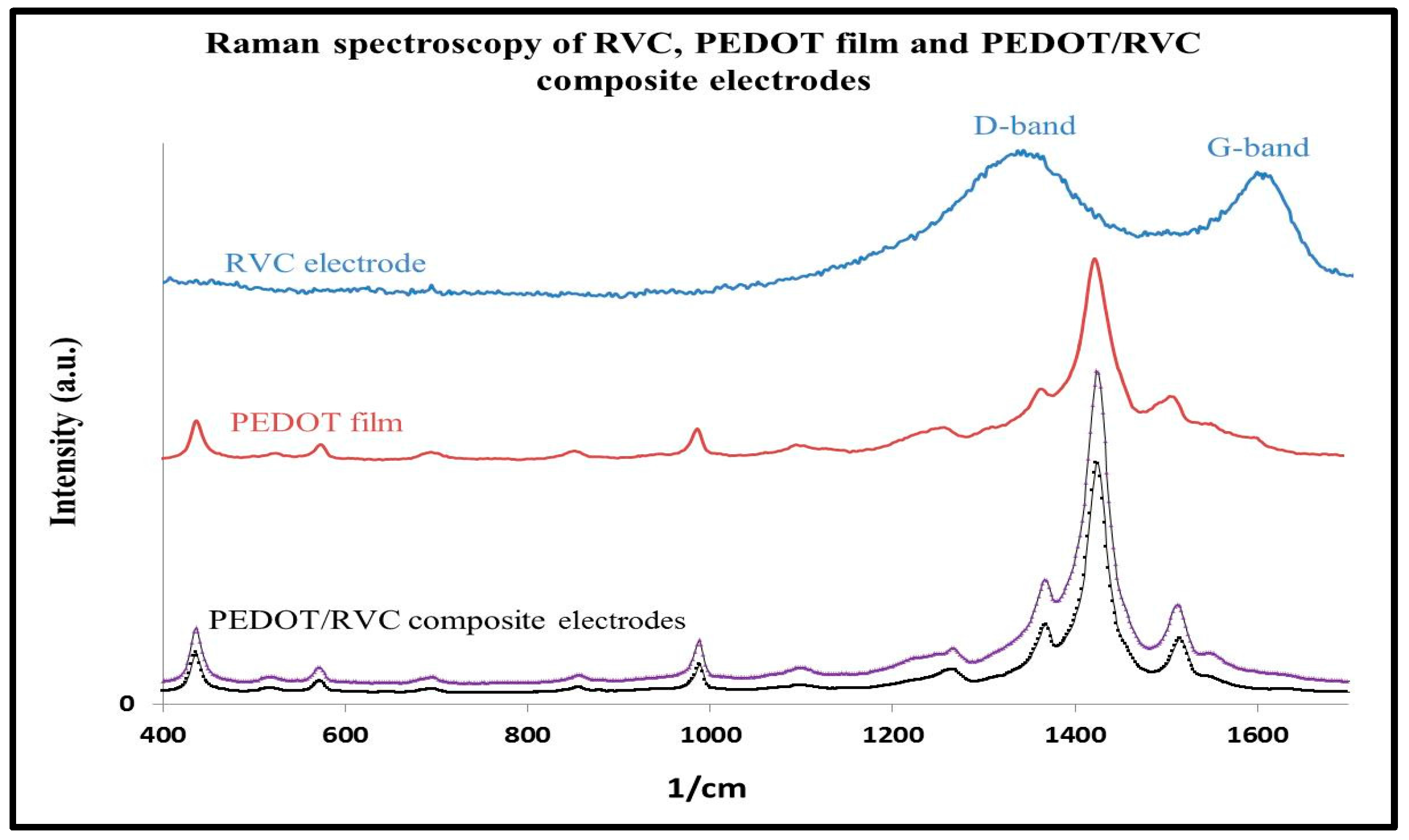
3.7. Raman Spectroscopy
3.8. Electrochemical Characterizations
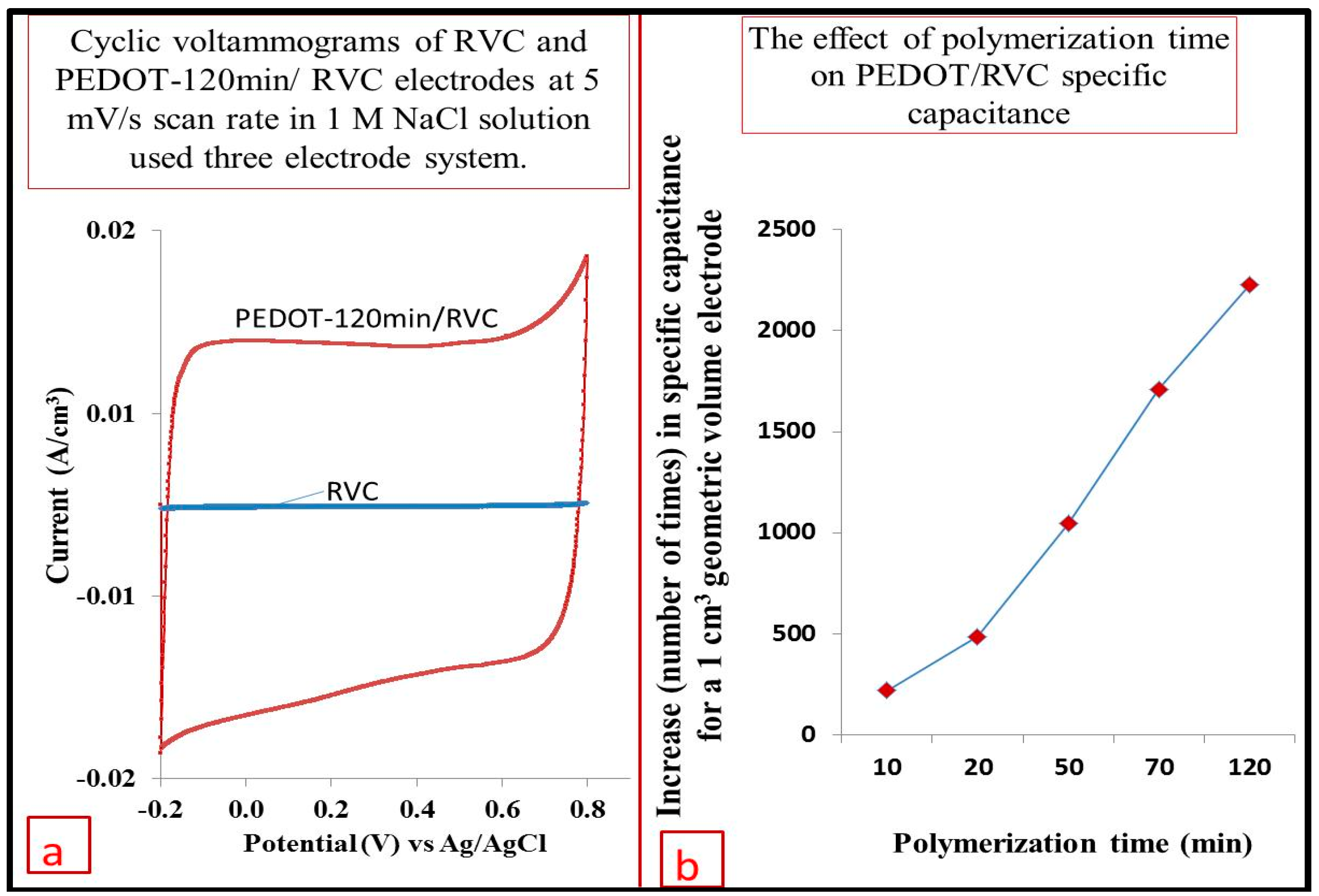
3.9. Comparison between RVC before and after PEDOT Coating
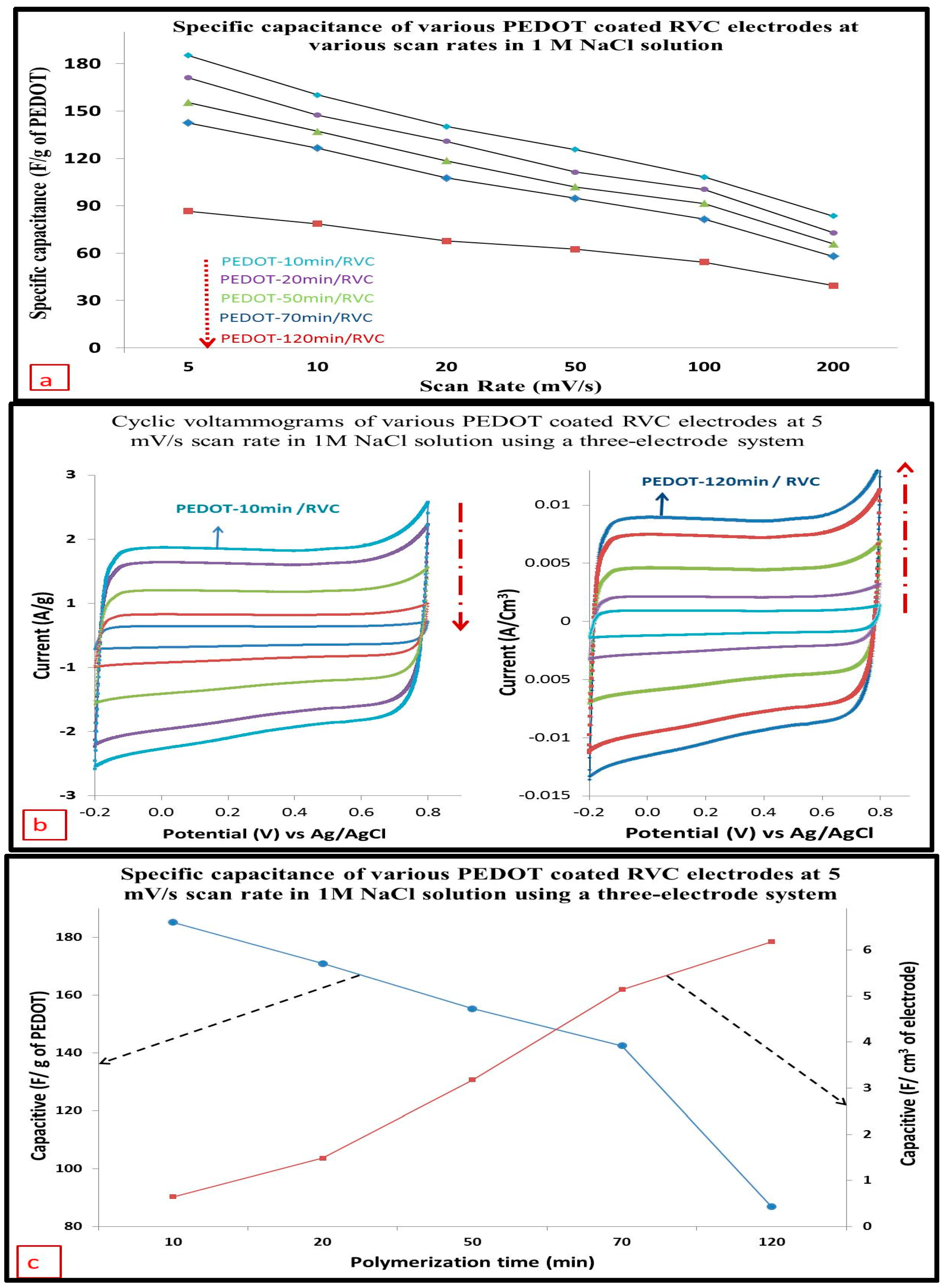
3.10. Effect of Increasing Scan Rate on the Electrode Capacitance
3.11. Capacitance
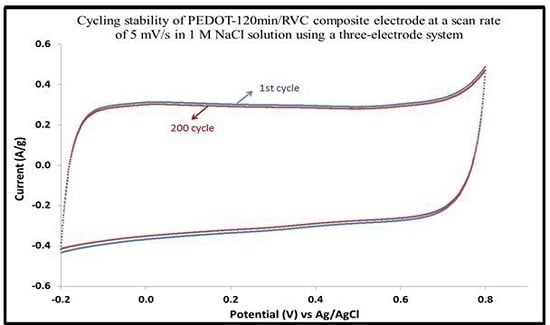
3.12. Cycling Stability of PEDOT/RVC Electrodes
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Oh, H.-J.; Lee, J.-H.; Ahn, H.-J.; Jeong, Y.; Kim, Y.-J.; Chi, C.-S. Nanoporous activated carbon cloth for capacitive deionization of aqueous solution. Thin Solid Films 2006, 515, 220–225. [Google Scholar] [CrossRef]
- Dai, K.; Shi, L.; Fang, J.; Zhang, D.; Yu, B. NaCl adsorption in multi-walled carbon nanotubes. Mater. Lett. 2005, 59, 1989–1992. [Google Scholar] [CrossRef]
- Jung, H.; Hwang, S.; Hyun, S.; Lee, K.; Kim, G. Capacitive deionization characteristics of nanostructured carbon aerogel electrodes synthesized via ambient drying. Desalination 2007, 216, 377–385. [Google Scholar] [CrossRef]
- Pekala, R.W.; Farmer, J.C.; Alviso, C.T.; Tran, T.D.; Mayer, S.T.; Miller, J.M.; Dunn, B. Carbon aerogels for electrochemical applications. J. Non Cryst. Solids 1998, 225, 74–80. [Google Scholar] [CrossRef]
- Li, H.; Lu, T.; Pan, L.; Zhang, Y.; Sun, Z. Electrosorption behavior of graphene in NaCl solutions. J. Mater. Chem. 2009, 19, 6773–6779. [Google Scholar] [CrossRef]
- Wang, G.; Pan, C.; Wang, L.; Dong, Q.; Yu, C.; Zhao, Z.; Qiu, J. Activated carbon nanofiber webs made by electrospinning for capacitive deionization. Electrochim. Acta 2012, 69, 65–70. [Google Scholar] [CrossRef]
- Peng, Z.; Zhang, D.; Shi, L.; Yan, T. High performance ordered mesoporous carbon/carbon nanotube composite electrodes for capacitive deionization. J. Mater. Chem. 2012, 22, 6603–6612. [Google Scholar] [CrossRef]
- Villar, I.; Suarez-De La Calle, D.J.; González, Z.; Granda, M.; Blanco, C.; Menéndez, R.; Santamaría, R. Carbon materials as electrodes for electrosorption of NaCl in aqueous solutions. Adsorption 2011, 17, 467–471. [Google Scholar] [CrossRef]
- Li, H.; Zou, L.; Pan, L.; Sun, Z. Using graphene nano-flakes as electrodes to remove ferric ions by capacitive deionization. Sep. Purif. Technol. 2010, 75, 8–14. [Google Scholar] [CrossRef]
- Yan, C.; Zou, L.; Short, R. Single-walled carbon nanotubes and polyaniline composites for capacitive deionization. Desalination 2012, 290, 125–129. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Xu, S.; Wang, J.; Wang, Z.; Wang, S. Polypyrrole nanowire modified graphite (PPy/G) electrode used in capacitive deionization. Synth. Met. 2010, 160, 1392–1396. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Zhang, Y.; Xu, S.; Wang, J. Effect of dopants on the adsorbing performance of polypyrrole/graphite electrodes for capacitive deionization process. Synth. Met. 2012, 162, 655–661. [Google Scholar] [CrossRef]
- Wallace, G.G. Conductive Electroactive Polymers: Intelligent Polymer Systems; Taylor & Francis: Hoboken, NJ, USA, 2008. [Google Scholar]
- Frydrychewicz, A.; Czerwiński, A.; Jackowska, K. Electrochemistry of multilayer electrodes RVCPaniPdPani. Synth. Met. 2001, 121, 1401–1402. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, D.; Shi, L.; Yan, T.; Wang, H.; Zhang, J. Three-dimensional hierarchical porous carbon with a bimodal pore arrangement for capacitive deionization. J. Mater. Chem. 2012, 22, 23835–23844. [Google Scholar] [CrossRef]
- Groenendaal, L.; Jonas, F.; Freitag, D.; Pielartzik, H.; Reynolds, J.R. Poly(3,4-ethylenedioxythiophene) and Its Derivatives: Past, Present, and Future. Adv. Mater. 2000, 12, 481–494. [Google Scholar] [CrossRef]
- Wei, B.; Liu, J.; Ouyang, L.; Kuo, C.C.; Martin, D.C. Significant Enhancement of PEDOT Thin Film Adhesion to Inorganic Solid Substrates with EDOT-Acid. ACS Appl. Mater. Interfaces 2015, 7, 15388–15394. [Google Scholar] [CrossRef] [PubMed]
- Rivnay, J.; Leleux, P.; Ferro, M.; Sessolo, M.; Williamson, A.; Koutsouras, D.A.; Khodagholy, D.; Ramuz, M.; Strakosas, X.; Owens, R.M. High-performance transistors for bioelectronics through tuning of channel thickness. Sci. Adv. 2015, 1, 1400251. [Google Scholar] [CrossRef] [PubMed]
- Rolison, D.R. Catalytic nanoarchitectures—The importance of nothing and the unimportance of periodicity. Science 2003, 299, 1698–1701. [Google Scholar] [CrossRef] [PubMed]
- Carriazo, D.; Pico, F.; Gutierrez, M.C.; Rubio, F.; Rojo, J.M.; del Monte, F. Block-copolymer assisted synthesis of hierarchical carbon monoliths suitable as supercapacitor electrodes. J. Mater. Chem. 2010, 20, 773–780. [Google Scholar] [CrossRef]
- Yuan, Y.; Kim, S. Polypyrrole-coated reticulated vitreous carbon as anode in microbial fuel cell for higher energy output. Bull. Korean Chem. Soc. 2008, 29, 168–172. [Google Scholar]
- Dalmolin, C.; Biaggio, S.R.; Rocha, R.C.; Bocchi, N. Reticulated vitreous carbon/polypyrrole composites as electrodes for lithium batteries: Preparation, electrochemical characterization and charge-discharge performance. Synth. Met. 2010, 160, 173–179. [Google Scholar] [CrossRef]
- Frydrychewicz, A.; Vassiliev, S.Y.; Tsirlina, G.A.; Jackowska, K. Reticulated vitreous carbon-polyaniline-palladium composite electrodes. Electrochim. Acta 2005, 50, 1885–1893. [Google Scholar] [CrossRef]
- Wang, D.W.; Li, F.; Liu, M.; Lu, G.Q.; Cheng, H.-M. 3D aperiodic hierarchical porous graphitic carbon material for high-rate electrochemical capacitive energy storage. Angew. Chem. 2008, 120, 379–382. [Google Scholar] [CrossRef]
- Xu, F.; Cai, R.; Zeng, Q.; Zou, C.; Wu, D.; Li, F.; Lu, X.; Liang, Y.; Fu, R. Fast ion transport and high capacitance of polystyrene-based hierarchical porous carbon electrode material for supercapacitors. J. Mater. Chem. 2011, 21, 1970–1976. [Google Scholar] [CrossRef]
- Friedrich, J.M. Reticulated vitreous carbon as an electrode material. J. Electroanal. Chem. Interfacial Electrochem. 2004, 561, 203–217. [Google Scholar] [CrossRef]
- Czerwiski, A.; Rogulski, Z.; Obrecbowski, S.; Siwek, H.; Paleska, I.; Chotkowski, M.; Aukaszewski, M. RVC as new carbon material for batteries. J. Appl. Electrochem. 2009, 39, 559–567. [Google Scholar] [CrossRef]
- Frysz, C.A.; Shui, X.; Chung, D.D.L. Electrochemical behavior of porous carbons. Carbon 1997, 35, 893–916. [Google Scholar] [CrossRef]
- Wang, J. Reticulated vitreous carbon-a new versatile electrode material. Electrochim. Acta 1981, 26, 1721–1726. [Google Scholar] [CrossRef]
- Shedge, H.Y. Specific and Non-Specific Binding of Proteins and Nucleic Acids on Chemically Modified Reticulated Vitreous Carbon Electrodes. Ph.D. Thesis, Clemson University, Clemson, SC, USA, 2009. [Google Scholar]
- Otero, T.F.; Romero, M.C. Conformational energy from the oxidation kinetics of poly(3,4-ethylenedioxythiophene) films. Polym. Int. 2010, 59, 329–336. [Google Scholar] [CrossRef]
- Seshadri, V.; Wu, L.; Sotzing, G.A. Conjugated polymers via electrochemical polymerization of thieno[3,4-b]thiophene (T34bT) and 3,4-ethylenedioxythiophene (EDOT). Langmuir 2003, 19, 9479–9485. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Hwang, B.-J. Identification of oxidized polypyrrole on Raman spectrum. Synth. Met. 2000, 113, 203–207. [Google Scholar] [CrossRef]
- Tamburri, E.; Orlanducci, S.; Toschi, F.; Terranova, M.L.; Passeri, D. Growth mechanisms, morphology, and electroactivity of PEDOT layers produced by electrochemical routes in aqueous medium. Synth. Met. 2009, 159, 406–414. [Google Scholar] [CrossRef]
- Downard, A.J.; Pletcher, D. The influence of water on the electrodeposition of polypyrrole in acetonitrile. J. Electroanal. Chem. 1986, 206, 139–145. [Google Scholar] [CrossRef]
- Heinze, J.; Rasche, A.; Pagels, M.; Geschke, B. On the origin of the so-called nucleation loop during electropolymerization of conducting polymers. J. Phys. Chem. B 2007, 111, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Sakmeche, N.; Aeiyach, S.; Aaron, J.J.; Jouini, M.; Lacroix, J.C.; Lacaze, P.C. Improvement of the electrosynthesis and physicochemical properties of poly(3,4-ethylenedioxythiophene) using a sodium dodecyl sulfate micellar aqueous medium. Langmuir 1999, 15, 2566–2574. [Google Scholar] [CrossRef]
- Wang, L.; Wang, M.; Huang, Z.-H.; Cui, T.; Gui, X.; Kang, F.; Wang, K.; Wu, D. Capacitive deionization of NaCl solutions using carbon nanotube sponge electrodes. J. Mater. Chem. 2011, 21, 18295–18299. [Google Scholar] [CrossRef]
- Zainal, M.F.; Mohd, Y. Characterization of PEDOT films for electrochromic applications. Polym. Plast. Technol. Eng. 2015, 54, 276–281. [Google Scholar] [CrossRef]
- Chao, F.; Costa, M.; Tian, C. Different steps in electrodeposition of poly(3-methylthiophene) films on platinum electrodes studied by ellipsometry, SEM and AFM techniques. Synth. Met. 1993, 53, 127–147. [Google Scholar] [CrossRef]
- Schrebler, R.; Grez, P.; Cury, P.; Veas, C.; Merino, M.; Gomez, H.; Cordova, R.; Del Valle, M.A. Nucleation and growth mechanisms of poly(thiophene) part 1. Effect of electrolyte and monomer concentration in dichloromethane. J. Electroanal. Chem. 1997, 430, 77–90. [Google Scholar] [CrossRef]
- Liu, J.; Wei, B.; Sloppy, J.D.; Ouyang, L.; Ni, C.; Martin, D.C. Direct imaging of the electrochemical deposition of poly(3,4-ethylenedioxythiophene) by transmission electron microscopy. ACS Macro Lett. 2015, 4, 897–900. [Google Scholar] [CrossRef]
- Patra, S.; Barai, K.; Munichandraiah, N. Scanning electron microscopy studies of PEDOT prepared by various electrochemical routes. Synth. Met. 2008, 158, 430–435. [Google Scholar] [CrossRef]
- Snook, G.A.; Peng, C.; Fray, D.J.; Chen, G.Z. Achieving high electrode specific capacitance with materials of low mass specific capacitance: Potentiostatically grown thick micro-nanoporous PEDOT films. Electrochem. Commun. 2007, 9, 83–88. [Google Scholar] [CrossRef]
- Randriamahazaka, H.; Noël, V.; Chevrot, C. Nucleation and growth of poly(3,4-ethylenedioxythiophene) in acetonitrile on platinum under potentiostatic conditions. J. Electroanal. Chem. 1999, 472, 103–111. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, H.; Huang, Y.; Wang, Y. Porous carbon/silica composite monoliths derived from resorcinol-formaldehyde/TEOS. J. Cryst. Solids 2010, 356, 971–976. [Google Scholar] [CrossRef]
- Shin, H.-J.; Jeon, S.S.; Im, S.S. CNT/PEDOT core/shell nanostructures as a counter electrode for dye-sensitized solar cells. Synth. Met. 2011, 161, 1284–1288. [Google Scholar] [CrossRef]
- Yuvaraj, H.; Jeong, Y.T.; Lee, W.K.; Lim, K.T. Synthesis of MWNT/PEDOT Composites for the Application of Organic Light Emitting Diodes. Mol. Cryst. Liq. Cryst. 2009, 514, 366–374. [Google Scholar] [CrossRef]
- Goncalves, E.S.; Rezende, M.C.; Ferreira, N.G. Dynamics of defects and surface structure formation in reticulated vitreous carbon. Braz. J. Phys. 2006, 36, 264–266. [Google Scholar] [CrossRef]
- Pesin, L.A. Review Structure and properties of glass-like carbon. J. Mater. Sci. 2002, 37, 1–28. [Google Scholar] [CrossRef]
- Garreau, S.; Louarn, G.; Buisson, J.P.; Froyer, G.; Lefrant, S. In situ spectroelectrochemical raman studies of poly(3,4-ethylenedioxythiophene) (PEDT). Macromolecules 1999, 32, 6807–6812. [Google Scholar] [CrossRef]
- Tamburri, E.; Sarti, S.; Orlanducci, S.; Terranova, M.L.; Rossi, M. Study of PEDOT conductive polymer films by admittance measurements. Mater. Chem. Phys. 2011, 125, 397–404. [Google Scholar] [CrossRef]
- Selvaganesh, S.V.; Mathiyarasu, J.; Phani, K.L.N.; Yegnaraman, V. Chemical Synthesis of PEDOT–Au Nanocomposite. Nanoscale Res. Lett. 2007, 2, 546–549. [Google Scholar] [CrossRef]
- Endut, Z.; Hamdi, M.; Basirun, W.J. Pseudocapacitive performance of vertical copper oxide nanoflakes. Thin Solid Films 2013, 528, 213–216. [Google Scholar] [CrossRef]
- Chen, J.H.; Li, W.Z.; Wang, D.Z.; Yang, S.X.; Wen, J.G.; Ren, Z.F. Electrochemical characterization of carbon nanotubes as electrode in electrochemical double-layer capacitors. Carbon 2002, 40, 1193–1197. [Google Scholar] [CrossRef]
- Bard, A. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Allen, J., Bard, R., Faulkner, A., Eds.; John Wiley: New York, NY, USA, 2001. [Google Scholar]
- Wang, J.; Xu, Y.; Sun, X.; Li, X.; Du, X. Electrochemical capacitance of the composite of poly(3,4-ethylenedioxythiophene) and functionalized single-walled carbon nanotubes. J. Solid State Electrochem. 2008, 12, 947–952. [Google Scholar] [CrossRef]
- Ran, L.; Seung Il, C.; Sang Bok, L. Poly(3,4-ethylenedioxythiophene) nanotubes as electrode materials for a high-powered supercapacitor. Nanotechnology 2008, 19, 215710. [Google Scholar]
- Sen, P.; De, A. Electrochemical performances of poly(3,4-ethylenedioxythiophene)–NiFe2O4 nanocomposite as electrode for supercapacitor. Electrochim. Acta 2010, 55, 4677–4684. [Google Scholar] [CrossRef]
- Si, W.; Lei, W.; Han, Z.; Zhang, Y.; Hao, Q.; Xia, M. Electrochemical sensing of acetaminophen based on poly(3,4- ethylenedioxythiophene)/graphene oxide composites. Sens. Actuators B 2014, 193, 823–829. [Google Scholar] [CrossRef]
- Lota, K.; Khomenko, V.; Frackowiak, E. Capacitance properties of poly(3,4-ethylenedioxythiophene)/carbon nanotubes composites. J. Phys. Chem. Solids 2004, 65, 295–301. [Google Scholar] [CrossRef]
- Czardybon, A.; Lapkowski, M. Synthesis and electropolymerisation of 3,4-ethylenedioxythiophene functionalised with alkoxy groups. Synth. Met. 2001, 119, 161–162. [Google Scholar] [CrossRef]
- Anothumakkool, B.; Bhange, S.N.; Unni, S.M.; Kurungot, S. 1-Dimensional confinement of porous polyethylenedioxythiophene using carbon nanofibers as a solid template: an efficient charge storage material with improved capacitance retention and cycle stability. RSC Adv. 2013, 3, 11877–11887. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Chen, X.; Du, X. Electrochemical supercapacitor electrode material based on poly(3,4-ethylenedioxythiophene)/polypyrrole composite. J. Power Sources 2007, 163, 1120–1125. [Google Scholar] [CrossRef]
- Pandey, G.P.; Rastogi, A.C. Synthesis and characterization of pulsed polymerized poly(3,4-ethylenedioxythiophene) electrodes for high-performance electrochemical capacitors. Electrochim. Acta 2013, 87, 158–168. [Google Scholar] [CrossRef]
- Li, L.; Zou, L.; Song, H.; Morris, G. Ordered mesoporous carbons synthesized by a modified sol–gel process for electrosorptive removal of sodium chloride. Carbon 2009, 47, 775–781. [Google Scholar] [CrossRef]
- Lu, Y.; Li, T.; Zhao, X.; Li, M.; Cao, Y.; Yang, H.; Duan, Y.Y. Electrodeposited polypyrrole/carbon nanotubes composite films electrodes for neural interfaces. Biomaterials 2010, 31, 5169–5181. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yuan, C.; Dou, H.; Gao, B.; Chen, S.; Zhang, X. Synthesis and electrochemical capacitance of core–shell poly(3,4-ethylenedioxythiophene)/poly(sodium 4-styrenesulfonate)-modified multiwalled carbon nanotube nanocomposites. Electrochim. Acta 2009, 54, 2335–2341. [Google Scholar] [CrossRef]


| Sample | Polymerization time (min) | Charge consumed (C) | Mass of PEDOT-ClO4 (mg) |
|---|---|---|---|
| PEDOT-10 min/RVC | 10 | 20.64 | 13 |
| PEDOT-20 min/RVC | 20 | 46.04 | 29 |
| PEDOT-50 min/RVC | 50 | 112.71 | 71 |
| PEDOT-70 min/RVC | 70 | 185.73 | 117 |
| PEDOT-120 min/RVC | 120 | 380.98 | 240 |
| Description of the vibration | cm−1 | Description of the vibration | cm−1 |
|---|---|---|---|
| asym C= Cstr | 1509 | C–O–C def | 1152, 1120 and 1085 |
| CH2 Scissoring | 1477 | Oxyethylene ring def | 988 |
| Sym Cα=Cβ(–O) str | 1426 | ClO4– | 933 |
| Cβ=Cβ str | 1365 | C–H bending | 806 |
| Cα=Cα str | 1252 | Sym C–S–C def | 690 |
| Cα=Cα str | 1236 | Oxyethylene ring def | 572 |
| Composite electrode | PEDOT-10 min/RVC | PEDOT-20 min/RVC | PEDOT-50 min/RVC | PEDOT-70 min/RVC | PEDOT-120 min/RVC |
| Scan rate (mV/s) | Capacitance F/g | ||||
| 5 | 185.29 | 171.04 | 155.36 | 142.53 | 86.56 |
| 10 | 160.12 | 147.41 | 137.16 | 126.51 | 78.62 |
| 20 | 140.15 | 130.90 | 118.63 | 107.59 | 67.77 |
| 50 | 125.66 | 111.32 | 102.04 | 94.78 | 62.41 |
| 100 | 108.23 | 100.36 | 91.52 | 81.47 | 54.34 |
| 200 | 83.57 | 72.95 | 65.81 | 57.96 | 39.45 |
| Composite electrode | PEDOT-10 min/RVC | PEDOT-20 min/RVC | PEDOT-50 min/RVC | PEDOT-70 min/RVC | PEDOT-120 min/RVC |
| Scan rate (mV/s) | Capacitance F/cm2 | ||||
| 5 | 0.08 | 0.19 | 0.41 | 0.66 | 0.80 |
| 10 | 0.07 | 0.17 | 0.36 | 0.59 | 0.73 |
| 20 | 0.06 | 0.15 | 0.31 | 0.50 | 0.63 |
| 50 | 0.06 | 0.12 | 0.27 | 0.44 | 0.58 |
| 100 | 0.05 | 0.11 | 0.24 | 0.38 | 0.50 |
| 200 | 0.04 | 0.08 | 0.17 | 0.27 | 0.36 |
| Composite electrode | PEDOT-10 min/RVC | PEDOT-20 min/RVC | PEDOT-50 min/RVC | PEDOT-70 min/RVC | PEDOT-120 min/RVC |
| Scan rate (mV/s) | Capacitance F/cm3 | ||||
| 5 | 0.65 | 1.48 | 3.18 | 5.14 | 6.18 |
| 10 | 0.56 | 1.28 | 2.81 | 4.57 | 5.62 |
| 20 | 0.49 | 1.14 | 2.43 | 3.89 | 4.84 |
| 50 | 0.44 | 0.97 | 2.09 | 3.42 | 4.46 |
| 100 | 0.38 | 0.87 | 1.87 | 2.94 | 3.88 |
| 200 | 0.29 | 0.63 | 1.35 | 2.09 | 2.82 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldalbahi, A.; Rahaman, M.; Almoiqli, M. A Strategy to Enhance the Electrode Performance of Novel Three-Dimensional PEDOT/RVC Composites by Electrochemical Deposition Method. Polymers 2017, 9, 157. https://doi.org/10.3390/polym9050157
Aldalbahi A, Rahaman M, Almoiqli M. A Strategy to Enhance the Electrode Performance of Novel Three-Dimensional PEDOT/RVC Composites by Electrochemical Deposition Method. Polymers. 2017; 9(5):157. https://doi.org/10.3390/polym9050157
Chicago/Turabian StyleAldalbahi, Ali, Mostafizur Rahaman, and Mohammed Almoiqli. 2017. "A Strategy to Enhance the Electrode Performance of Novel Three-Dimensional PEDOT/RVC Composites by Electrochemical Deposition Method" Polymers 9, no. 5: 157. https://doi.org/10.3390/polym9050157