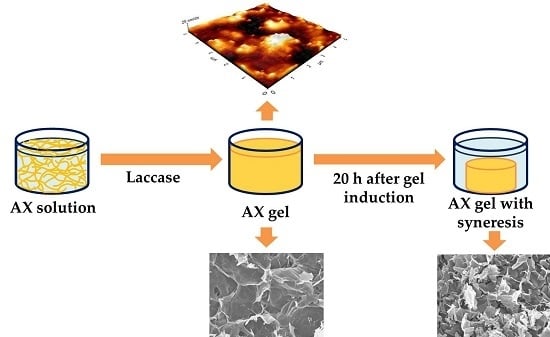
Syneresis in Gels of Highly Ferulated Arabinoxylans: Characterization of Covalent Cross-Linking, Rheology, and Microstructure
Abstract
:1. Introduction
2. Materials and Methods
2.1 Materials
2.2 Methods
2.2.1. Extraction of AXs
2.2.2. Neutral Sugars
2.2.3. Ferulic Acid (FA), Dimers of Ferulic Acid (di-FA), and Trimers of Ferulic Acid (tri-FA) in AXs and AX Gels
2.2.4. Rheological Measurements
2.2.5. Atomic Force Microscopy
2.2.6. Syneresis
2.2.7. Swelling
2.2.8. Scanning Electron Microscopy
2.2.9. Statistical Analysis
3. Results and Discussion
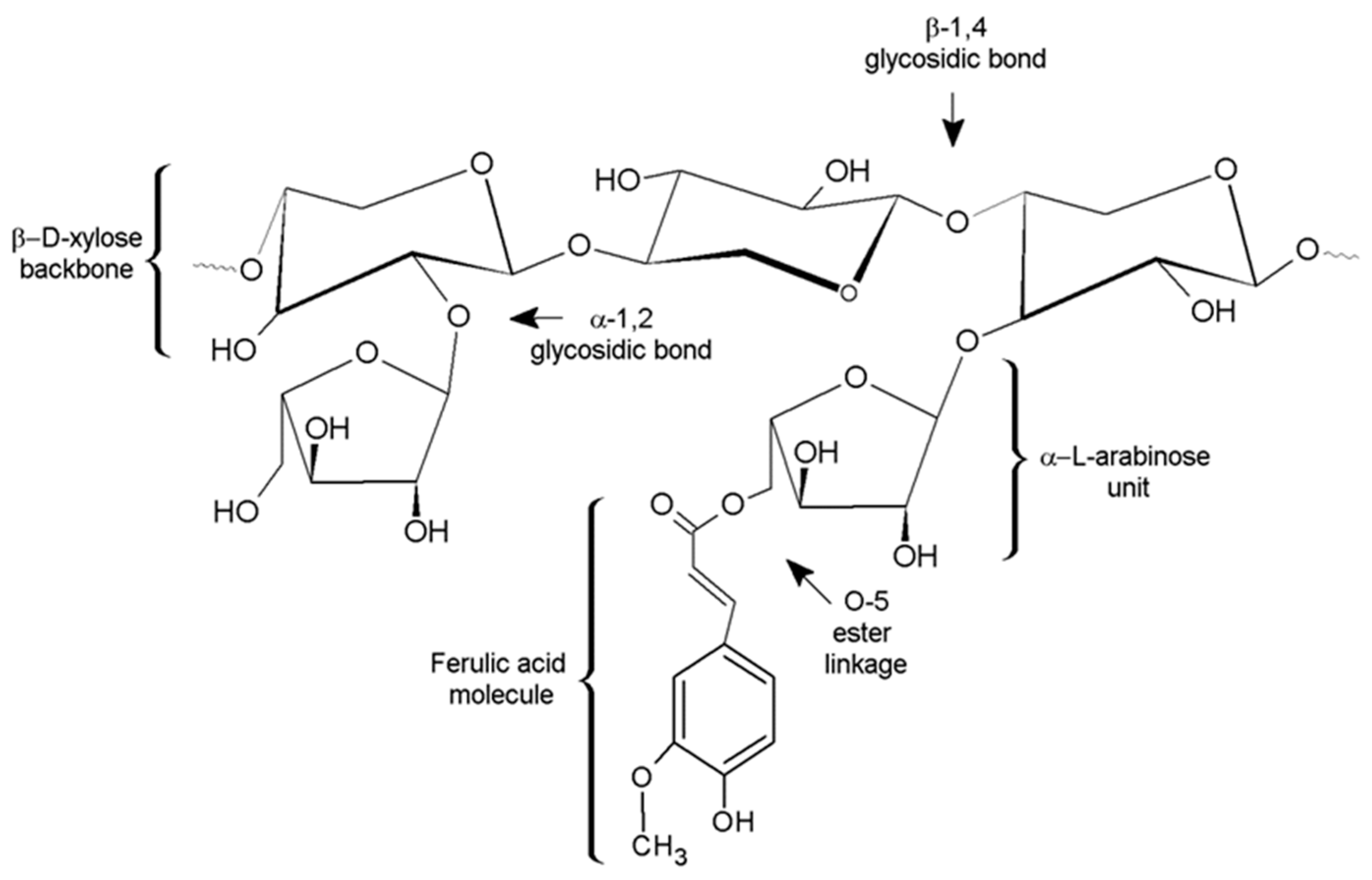
3.1. Extraction and Characterization of AXs
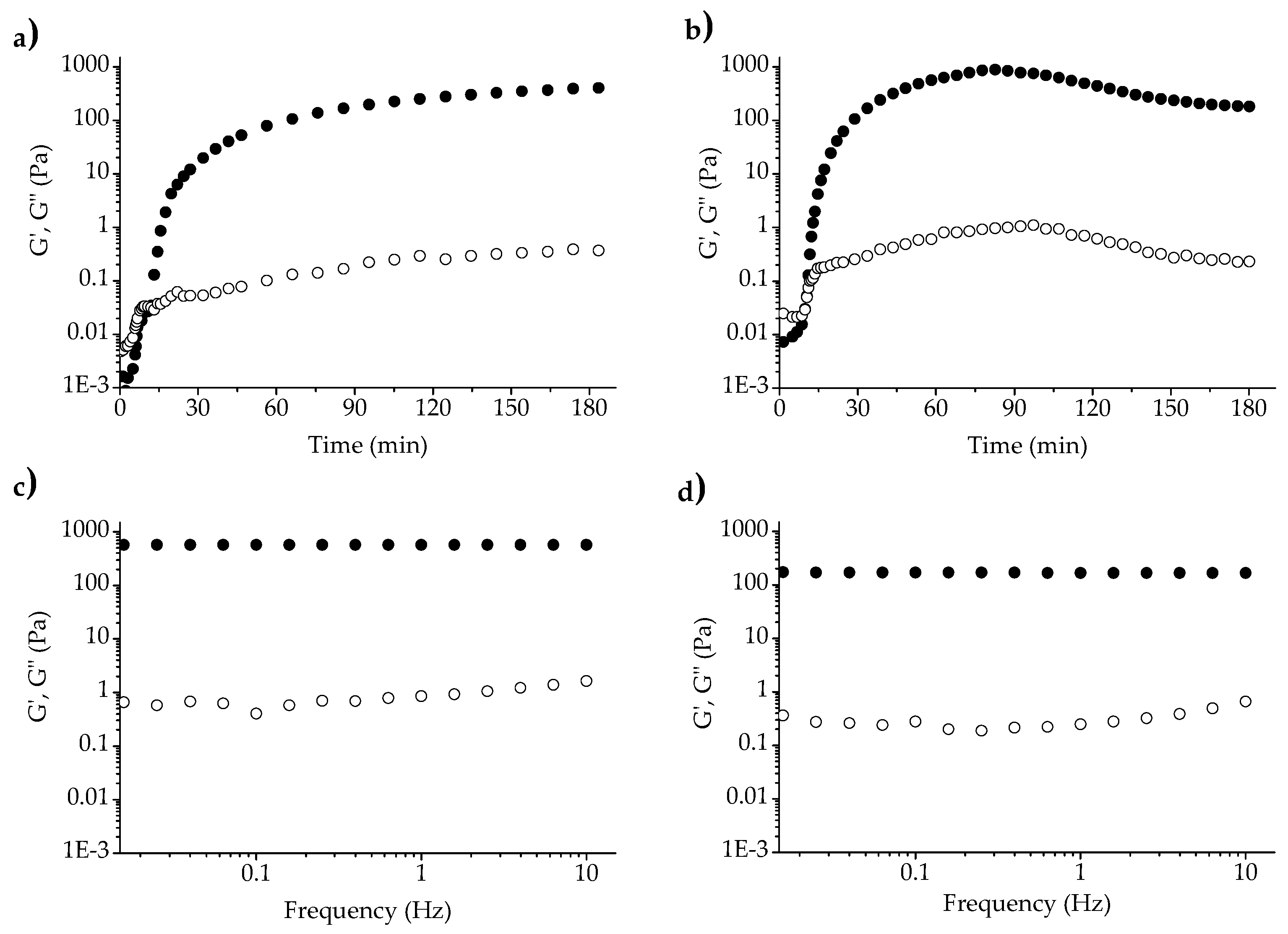
3.2. Rheology
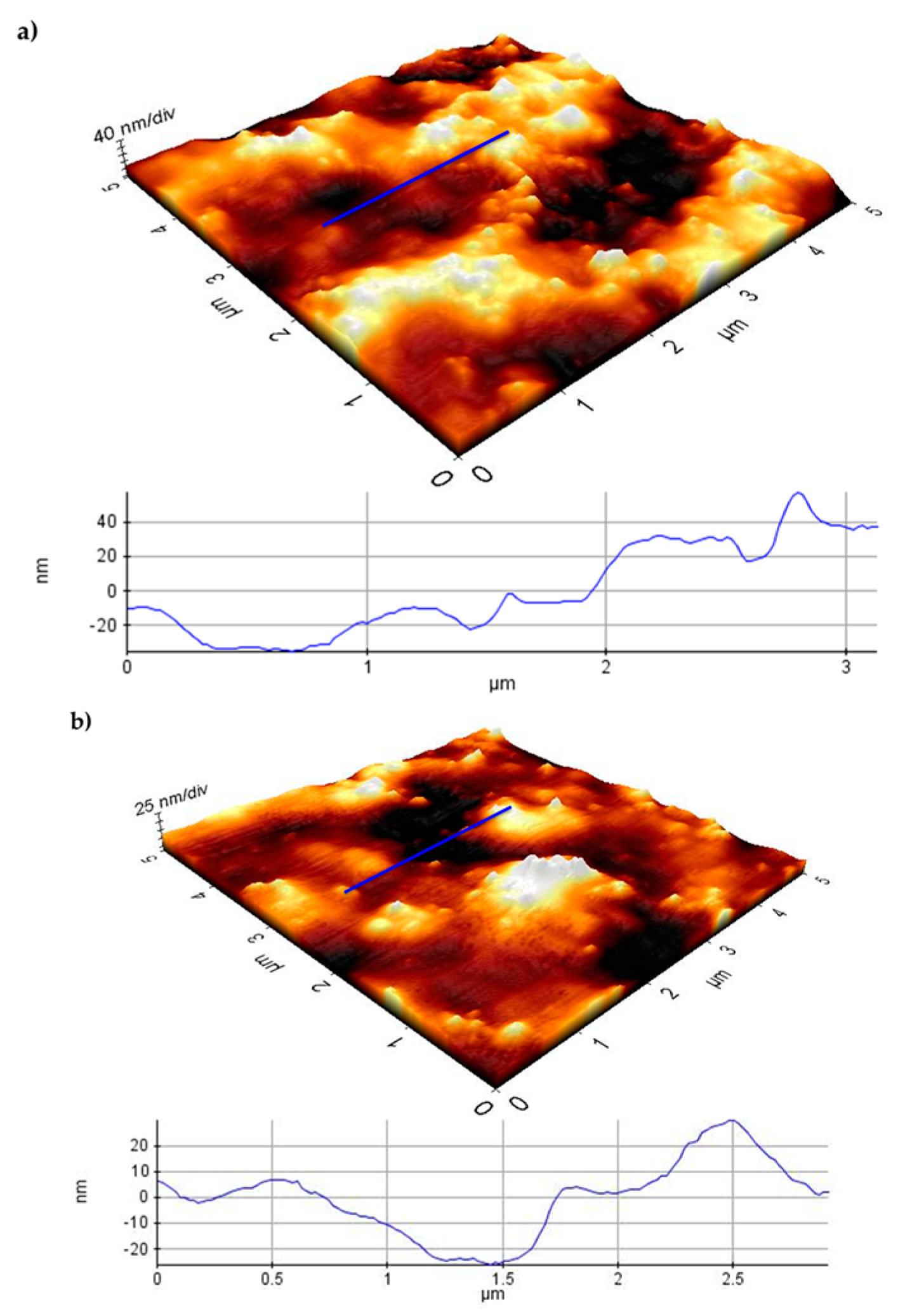
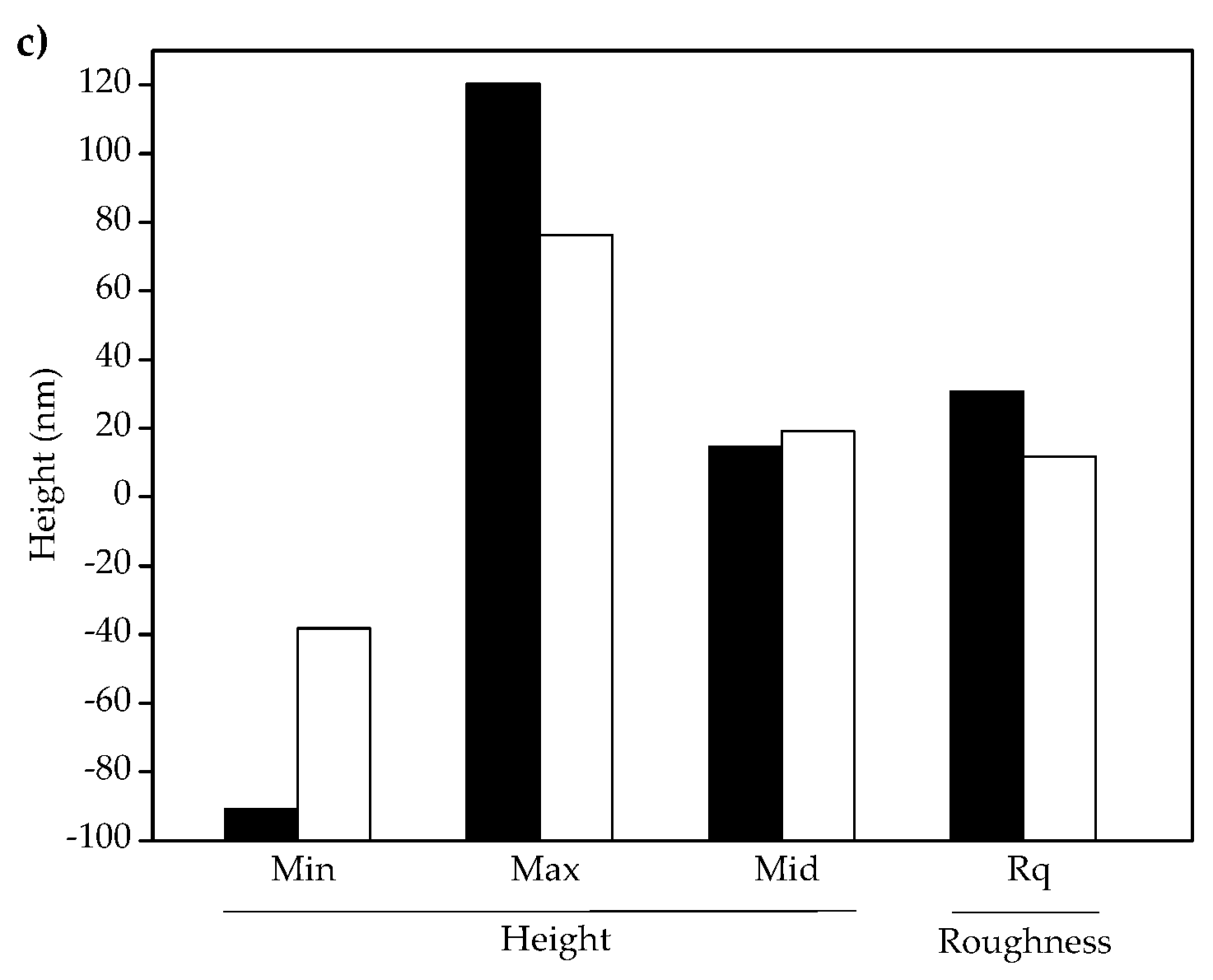
3.3. Atomic Force Microscopy (AFM)
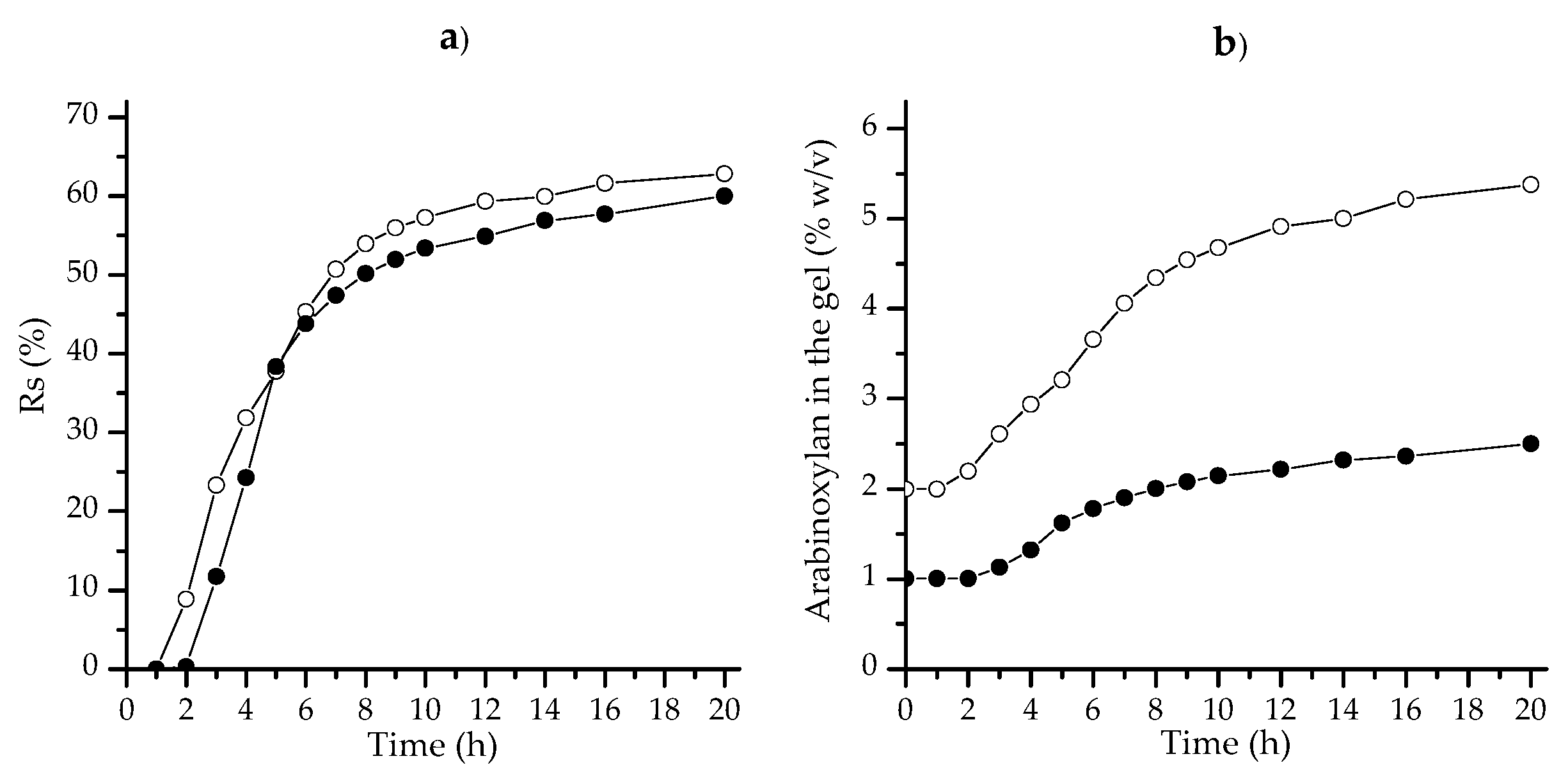
3.4. Syneresis
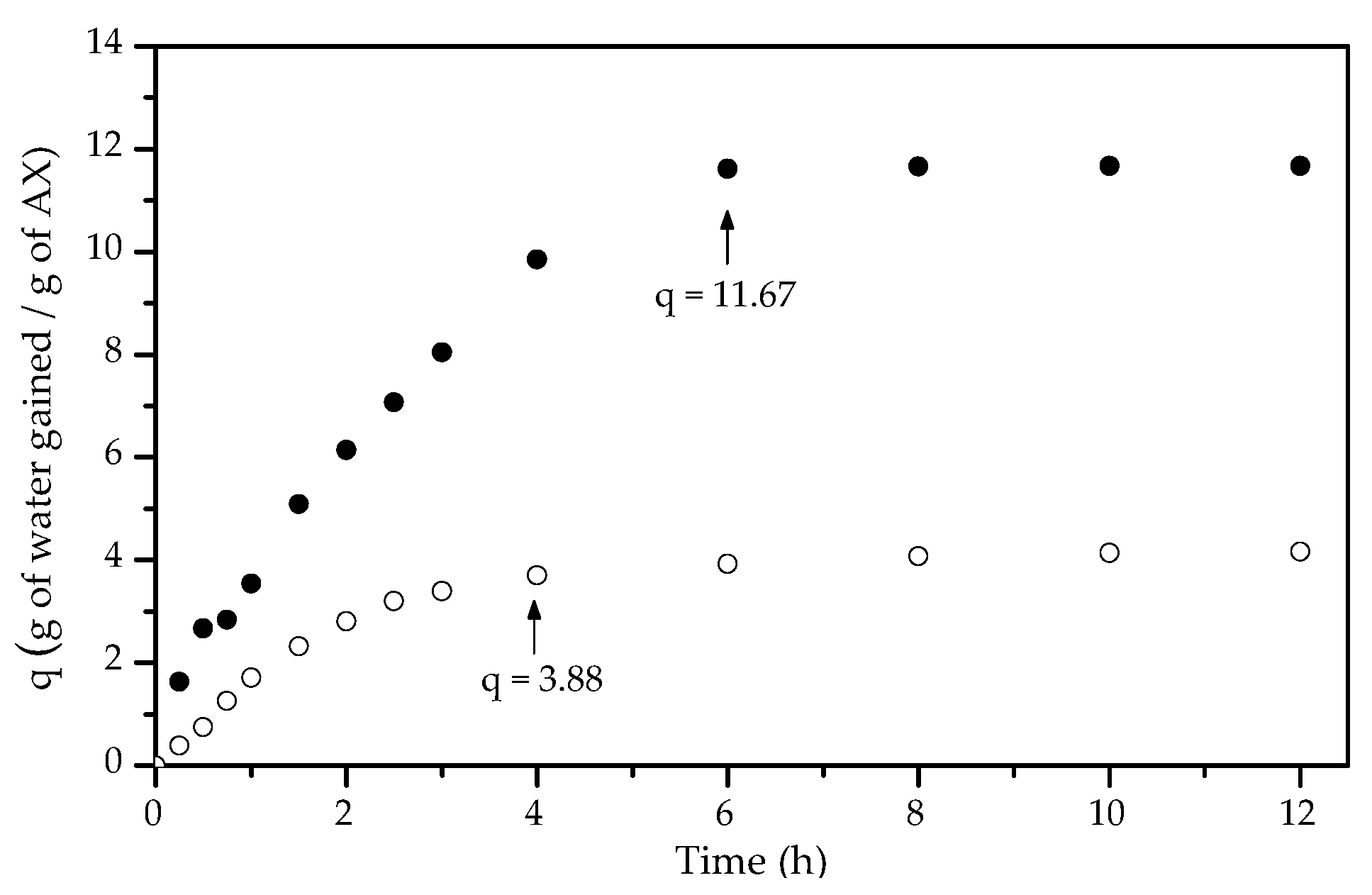
3.5. Swelling
3.6. Covalent Cross-Links (di-FA and tri-FA)
3.7. Scanning Electron Microscopy (SEM)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Izydorczyk, M.; Biliaderis, C. Cereal arabinoxylans: Advances in structure and physicochemical properties. Carbohydr. Polym. 1995, 28, 33–48. [Google Scholar] [CrossRef]
- Niño-Medina, G.; Carvajal-Millán, E.; Lizardi, J.; Rascon-Chu, A.; Marquez-Escalante, J.A.; Gardea, A.; Martinez-Lopez, A.L.; Guerrero, V. Maize processing waste water arabinoxylans: Gelling capability and cross-linking content. Food Chem. 2009, 115, 1286–1290. [Google Scholar] [CrossRef]
- Paz-Samaniego, R.; Carvajal-Millan, E.; Brown-Bojorquez, F.; Rascón-Chu, A.; López-Franco, Y.L.; Sotelo-Cruz, N.; Lizardi-Mendoza, J. Gelation of Arabinoxylans from Maize Wastewater—Effect of Alkaline Hydrolysis Conditions on the Gel Rheology and Microstructure. In Wastewater Treatment Engineering; Samer, M., Ed.; InTech: Rijeka, Croatia, 2015; pp. 101–114. [Google Scholar]
- Ayala-Soto, F.E.; Serna-Saldívar, S.O.; Welti-Chanes, J. Effect of processing time, temperature and alkali concentration on yield extraction, structure and gelling properties of corn fiber arabinoxylans. Food Hydrocoll. 2016, 60, 21–28. [Google Scholar] [CrossRef]
- Carvajal-Millan, E.; Landillon, V.; Morel, M.H.; Rouau, X.; Doublier, J.L.; Micard, V. Arabinoxylan gels: Impact of the feruloylation degree on their structure and properties. Biomacromolecules 2005, 6, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Millan, E.; Guilbert, S.; Morel, M.; Micard, V. Impact of the structure of arabinoxylan gels on their rheological and protein transport properties. Carbohydr. Polym. 2005, 60, 431–438. [Google Scholar] [CrossRef]
- Carvajal-Millan, E.; Guigliarelli, B.; Belle, V.; Rouau, X.; Micard, V. Storage stability of laccase induced arabinoxylan gels. Carbohydr. Polym. 2005, 59, 181–188. [Google Scholar] [CrossRef]
- Martínez-López, A.L.; Carvajal-Millan, E.; Micard, V.; Rascón-Chu, A.; Brown-Bojorquez, F.; Sotelo-Cruz, N.; López-Franco, Y.L.; Lizardi-Mendoza, J. In vitro degradation of covalently cross-linked arabinoxylan hydrogels by bifidobacteria. Carbohydr. Polym. 2016, 144, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Scherer, G.W. Mechanics of syneresis I. Theory. J. Non Cryst. Solids 1989, 108, 18–27. [Google Scholar] [CrossRef]
- Kunitz, M. Syneresis and swelling of gelatin. J. Gen. Physiol. 1928, 12, 289–312. [Google Scholar] [CrossRef] [PubMed]
- Mellema, M.; Walstra, P.; Opheusden, J.; Van-Vliet, T. Effects of structural rearrangements on the rheology of rennet-induced casein particle gels. Adv. Colloid Interface Sci. 2002, 98, 25–50. [Google Scholar] [CrossRef]
- Boral, S.; Saxena, A.; Bohidar, H.B. Syneresis in agar hydrogels. Int. J. Biol. Macromol. 2010, 46, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Divoux, T.; Mao, B.; Snabre, P. Syneresis and delayed detachment in agar plates. Soft Matter 2015, 11, 3677–3685. [Google Scholar] [CrossRef] [PubMed]
- Hermansson, A. Rheological and microstructural evidence for transient states during gelation of kappa-carrageenan in the presence of potassium. Carbohydr. Polym. 1989, 10, 163–181. [Google Scholar] [CrossRef]
- Ako, K. Influence of elasticity on the syneresis properties of κ-carrageenan gels. Carbohydr. Polym. 2015, 115, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Vachoud, L.; Zydowicz, N.; Domard, A. Physicochemical behaviour of chitin gels. Carbohydr. Res. 2000, 326, 295–304. [Google Scholar] [CrossRef]
- Carvajal-Millan, E.; Rascón-Chu, A.; Márquez-Escalante, J.A.; Micard, V.; Ponce de León, N.; Gardea, A. Maize bran gum: Extraction, characterization and functional properties. Carbohydr. Polym. 2007, 69, 280–285. [Google Scholar] [CrossRef]
- Vansteenkiste, E.; Babot, C.; Rouau, X.; Micard, V. Oxidative gelation of feruloylated arabinoxylan as affected by protein. Influence on protein enzymatic hydrolysis. Food Hydrocoll. 2004, 18, 557–564. [Google Scholar] [CrossRef]
- Blakeney, A.B.; Harris, P.J.; Henry, R.J.; Stone, B.A. A simple and rapid preparation of alditol acetates for monosaccharide analysis. Carbohydr. Res. 1983, 113, 291–299. [Google Scholar] [CrossRef]
- Martinez-Lopez, A.L.; Carvajal-Millan, E.; Lizardi-Mendoza, J.; Rascón-Chu, A.; López-Franco, Y.L.; Salas-Muñoz, E. Ferulated arabinoxylans as by-product from maize wet-milling process: Characterization and gelling capability. In Maize: Cultivation, Uses and Health Benefits; Jimenez-Lopez, J.C., Ed.; Nova Science: New York, NY, USA, 2012; pp. 65–74. [Google Scholar]
- Vogel, B.; Gallaher, D.D.; Bunzel, M. Influence of cross-linked arabinoxylans on the postprandial blood glucose response in rats. J. Agric. Food Chem. 2012, 60, 3847–3852. [Google Scholar] [CrossRef] [PubMed]
- Martínez-López, A.L.; Carvajal-Millan, E.; Rascón-Chu, A.; Márquez-Escalante, J.; Martínez-Robinson, K. Gels of ferulated arabinoxylans extracted from nixtamalized and non-nixtamalized maize bran: Rheological and structural characteristics. CyTA J. Food 2013, 11, 22–28. [Google Scholar] [CrossRef]
- Velkova, N.; Doliška, A.; Zemljic, L.; Vesel, A.; Saake, B.; Strnad, S. Influence of carboxymethylation on the surface physical–chemical properties of glucuronoxylan and arabinoxylan films. Polym. Eng. Sci. 2015, 55, 2706–2713. [Google Scholar] [CrossRef]
- Paës, G.; Chabbert, B. Characterization of arabinoxylan/cellulose nanocrystals gels to investigate fluorescent probes mobility in bioinspired models of plant secondary cell wall. Biomacromolecules 2011, 13, 206–214. [Google Scholar] [CrossRef] [PubMed]




| Component | Content |
|---|---|
| Arabinose 1 | 34.50 ± 2.94 |
| Xylose 1 | 47.79 ± 4.50 |
| Galactose 1 | 8.18 ± 0.71 |
| Glucose 1 | 5.26 ± 0.56 |
| Mannose 1 | 0.53 ± 0.03 |
| Ferulic acid 2 | 7.18 ± 0.20 |
| di-FA 2 | 0.44 ± 0.02 |
| tri-FA 2 | 0.01 ± 0.002 |
| Gel | % Rs | FA | di-FA | tri-FA | FA Oxidized (%) | FA Recovered (%) |
|---|---|---|---|---|---|---|
| (µg/mg AX) | ||||||
| AX-1 | 0 | 4.16 ± 0.08 | 2.49 ± 0.48 | 0.16 ± 0.04 | 42 ± 1 | 88 ± 2 |
| 60 * | 2.21 ± 0.20 | 2.71 ± 0.79 | 0.31 ± 0.10 | 69 ± 3 | 61 ± 2 | |
| AX-2 | 0 | 5.00 ± 0.10 | 1.24 ± 0.28 | 0.07 ± 0.03 | 30 ± 1 | 61 ± 3 |
| 62 * | 1.88 ± 0.14 | 2.21 ± 0.65 | 0.28 ± 0.05 | 74 ± 2 | 47 ± 1 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Burgos, A.M.; Carvajal-Millan, E.; López-Franco, Y.L.; Rascón-Chu, A.; Lizardi-Mendoza, J.; Sotelo-Cruz, N.; Brown-Bojórquez, F.; Burgara-Estrella, A.; Pedroza-Montero, M. Syneresis in Gels of Highly Ferulated Arabinoxylans: Characterization of Covalent Cross-Linking, Rheology, and Microstructure. Polymers 2017, 9, 164. https://doi.org/10.3390/polym9050164
Morales-Burgos AM, Carvajal-Millan E, López-Franco YL, Rascón-Chu A, Lizardi-Mendoza J, Sotelo-Cruz N, Brown-Bojórquez F, Burgara-Estrella A, Pedroza-Montero M. Syneresis in Gels of Highly Ferulated Arabinoxylans: Characterization of Covalent Cross-Linking, Rheology, and Microstructure. Polymers. 2017; 9(5):164. https://doi.org/10.3390/polym9050164
Chicago/Turabian StyleMorales-Burgos, Ana M., Elizabeth Carvajal-Millan, Yolanda L. López-Franco, Agustín Rascón-Chu, Jaime Lizardi-Mendoza, Norberto Sotelo-Cruz, Francisco Brown-Bojórquez, Alexel Burgara-Estrella, and Martin Pedroza-Montero. 2017. "Syneresis in Gels of Highly Ferulated Arabinoxylans: Characterization of Covalent Cross-Linking, Rheology, and Microstructure" Polymers 9, no. 5: 164. https://doi.org/10.3390/polym9050164