Synthesis and Functionalization of Periodic Copolymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Methods
2.2.1. NMR Spectroscopy
2.2.2. Size Exclusion Chromatography (SEC)
2.2.3. Microwave-Assisted Post-Polymerization Functionalization
2.2.4. MALDI-TOF-MS
2.2.5. ESI-MS
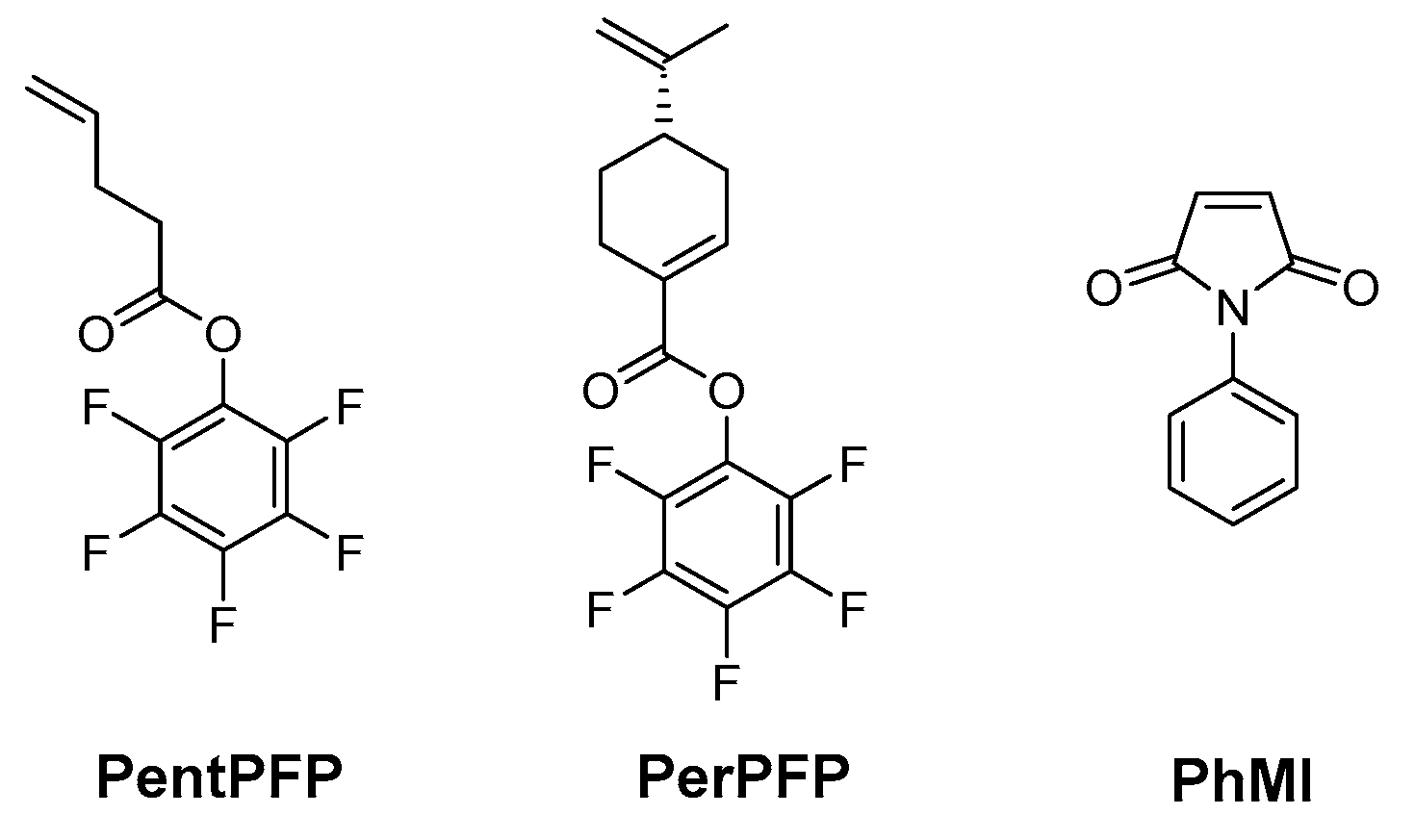
2.3. Monomer Synthesis
2.3.1. 4-Pentenoic Acid Pentafluorophenyl Ester (PentPFP)
2.3.2. (S)-Perillic Acid Pentafluorophenyl Ester (PerPFP)
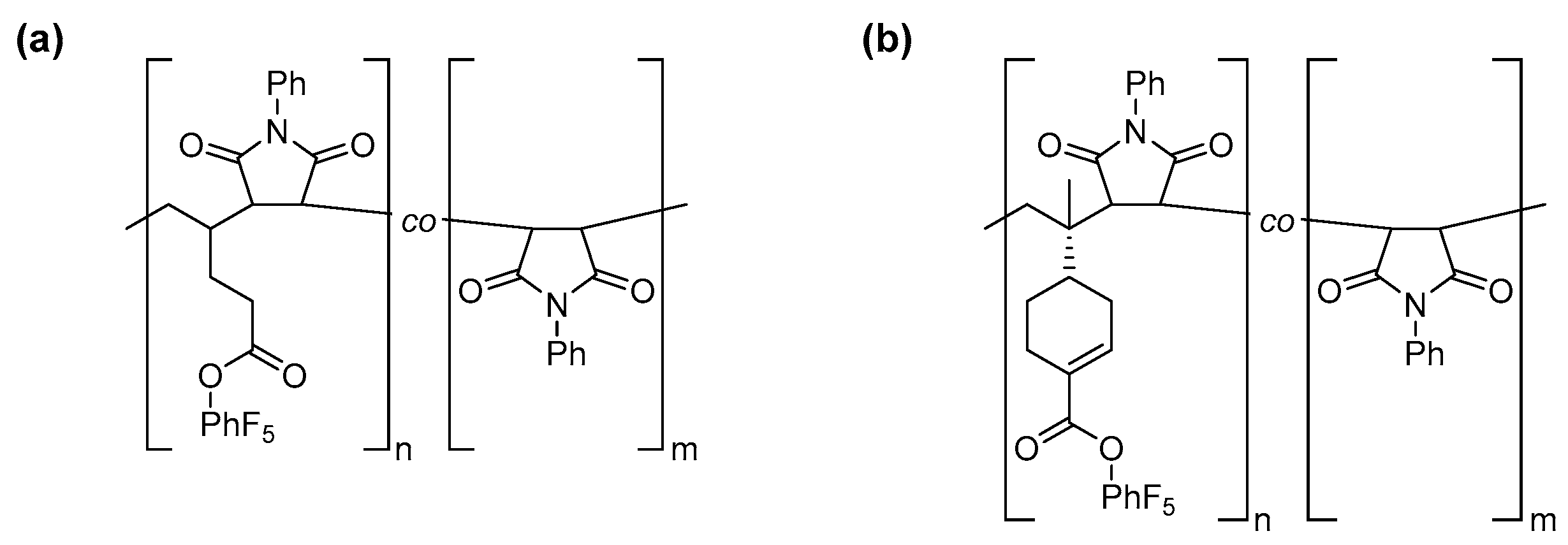
2.4. Polymer Synthesis
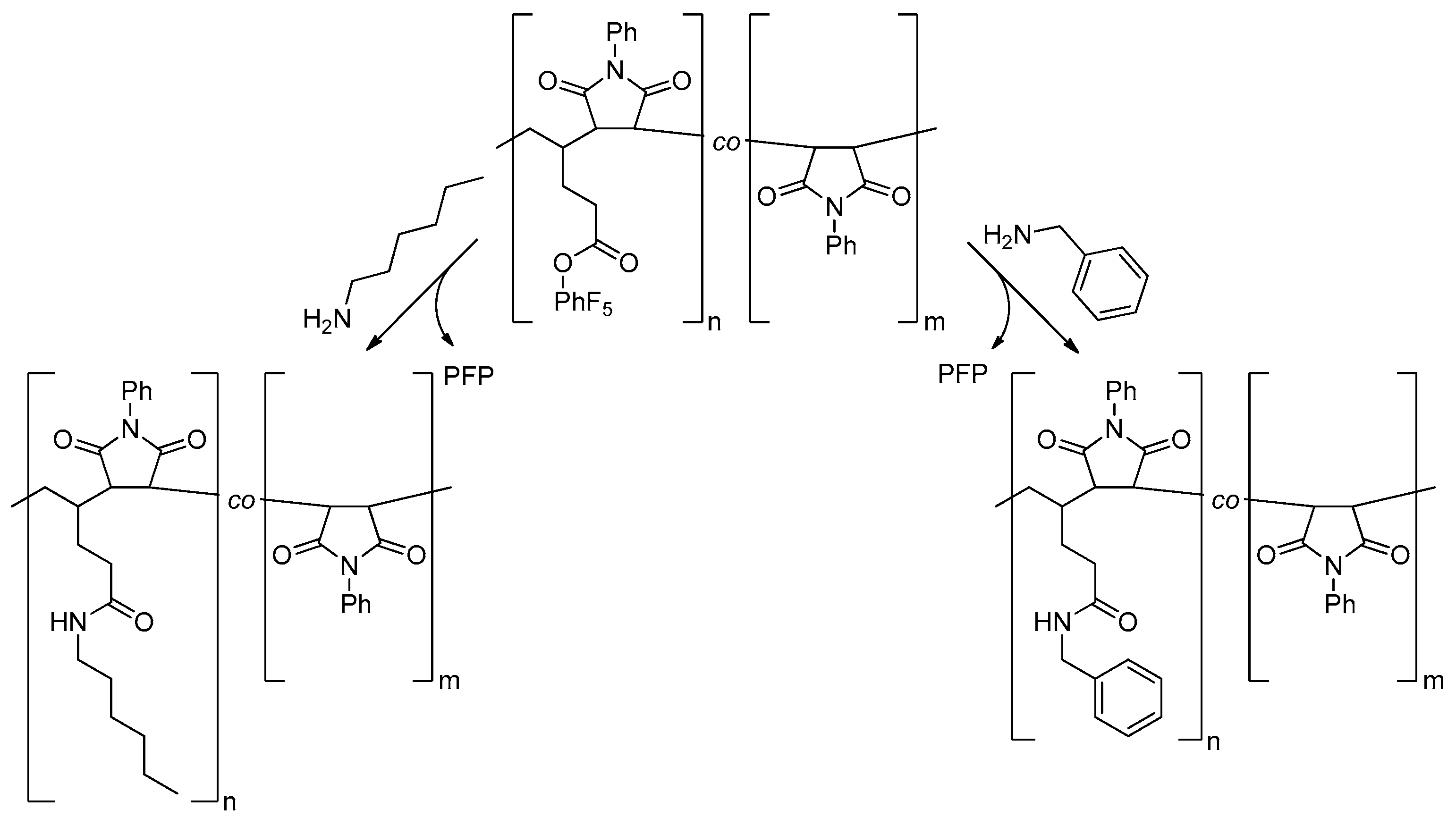
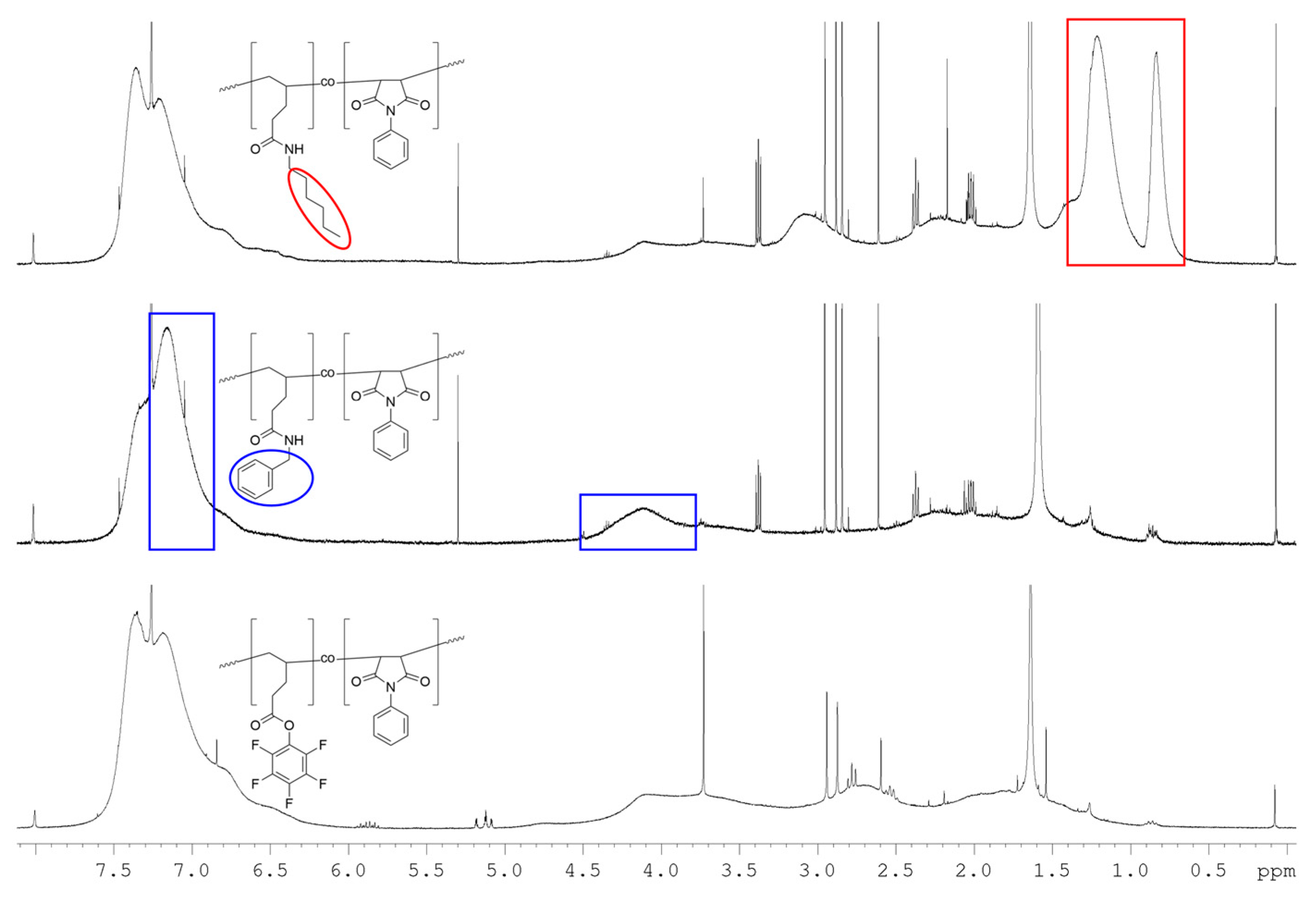
2.5. Post-Polymerization Functionalization
3. Results and Discussion
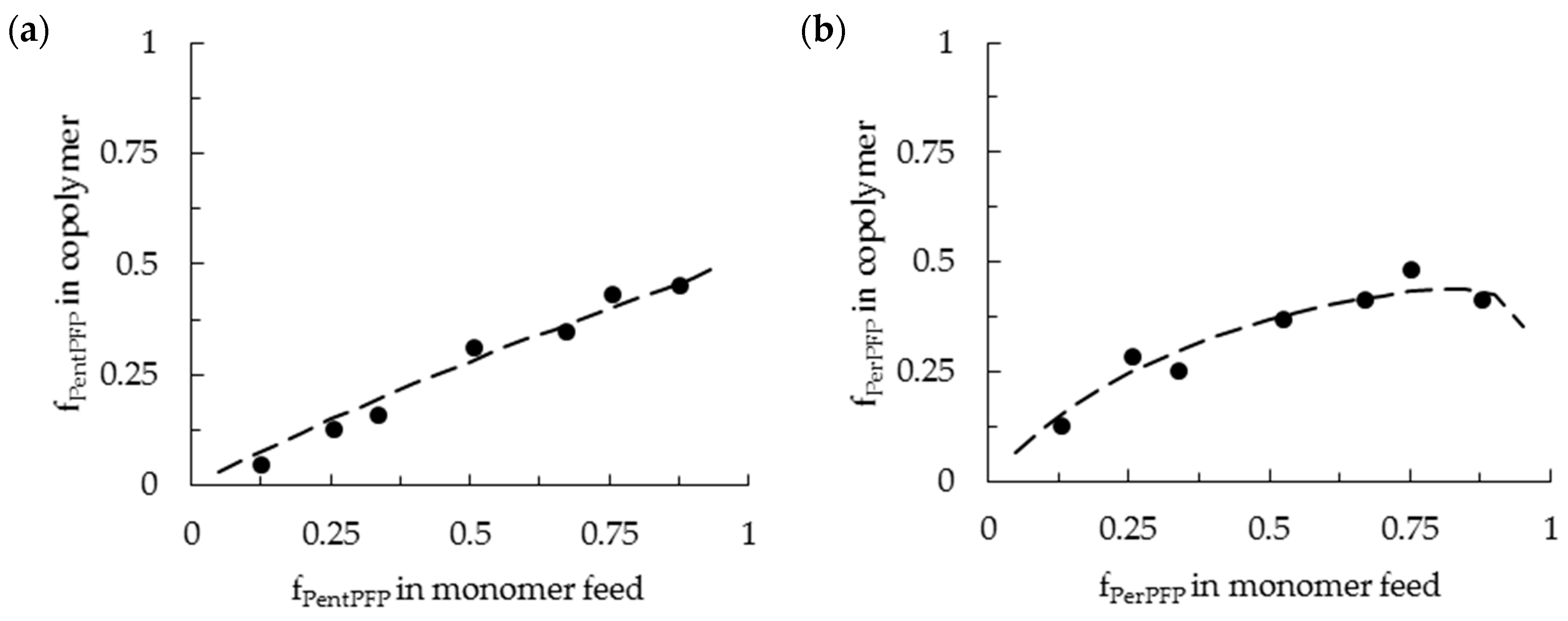
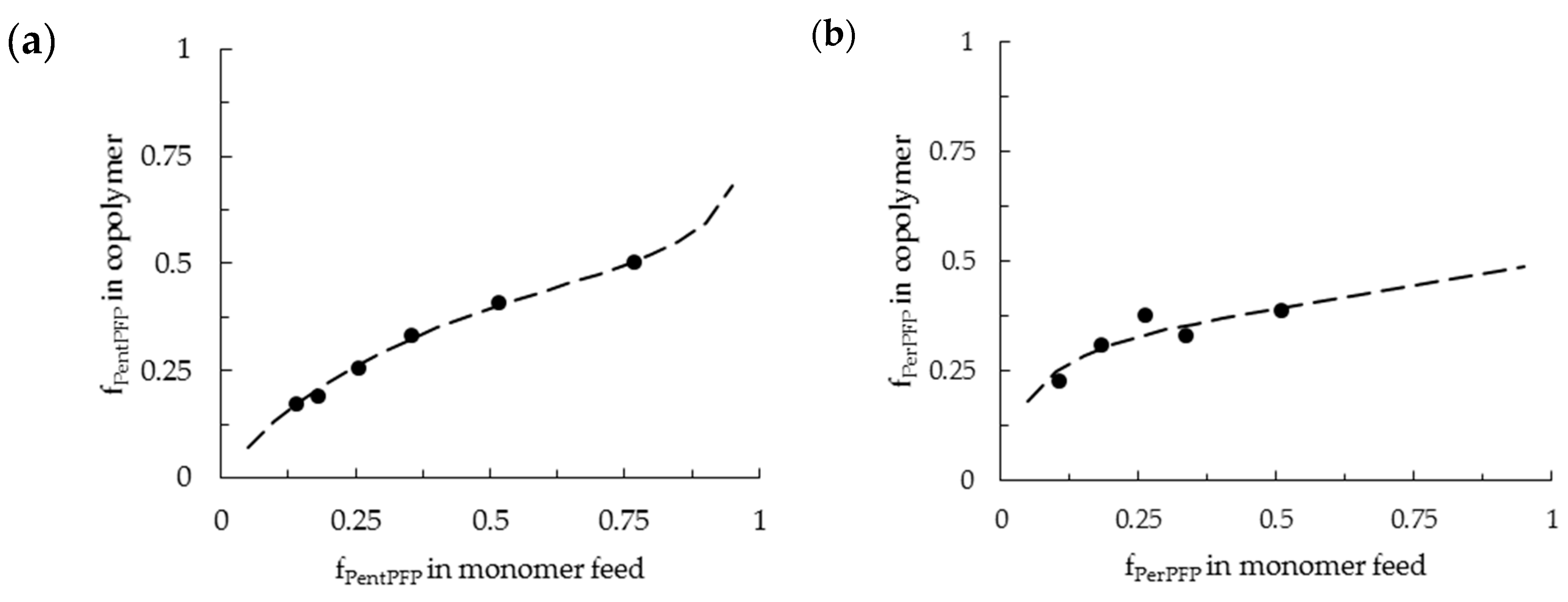
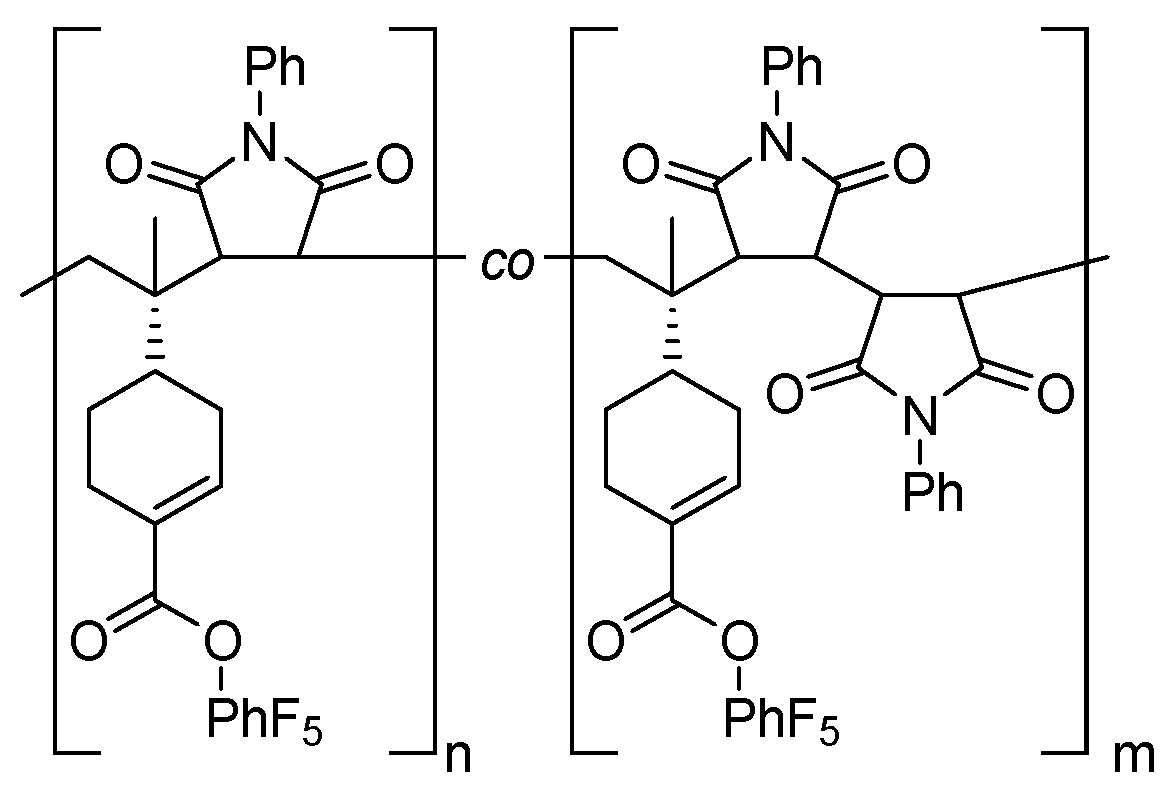
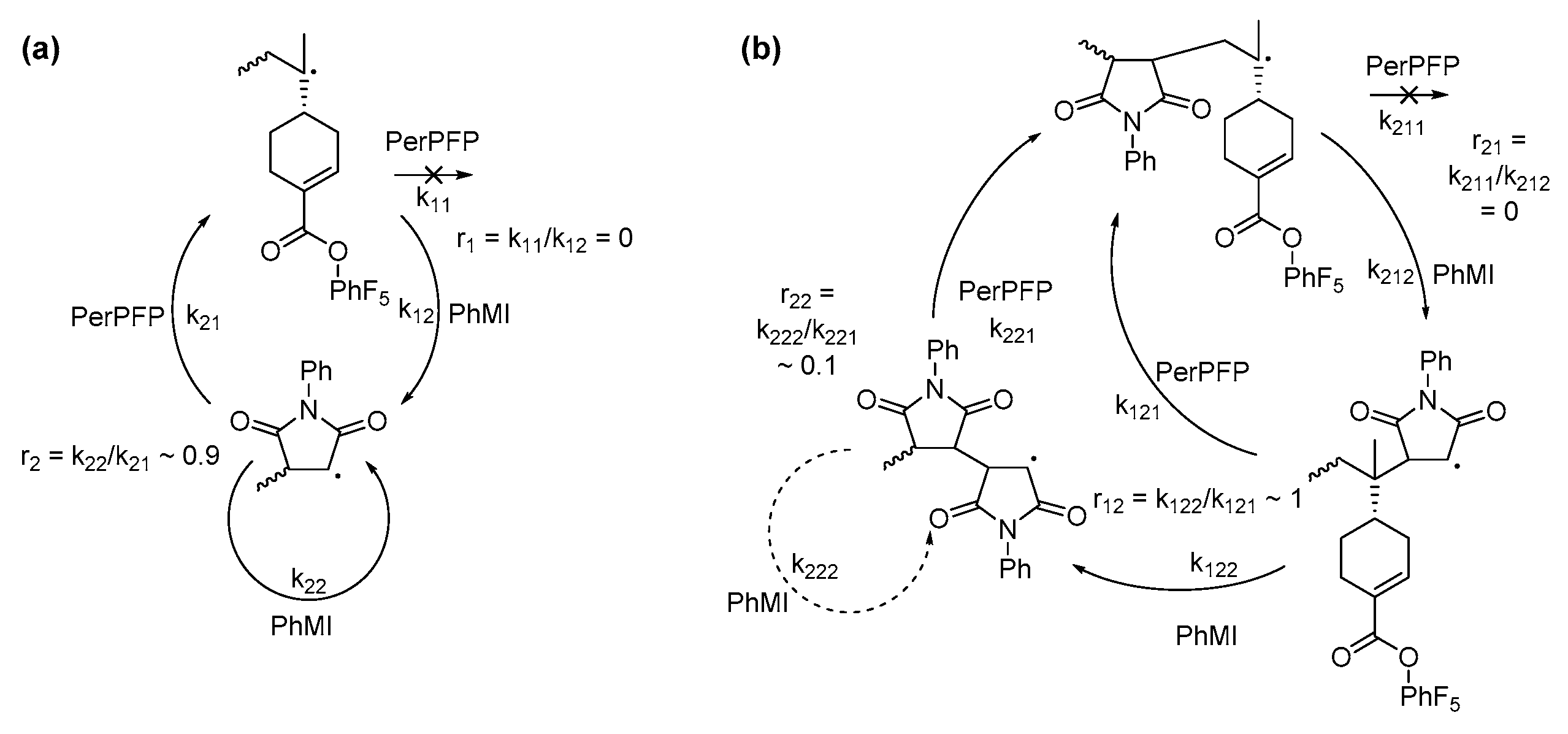
3.1. Copolymerization in DCE
3.2. Copolymerization in HFPP
3.3. Post-Polymerization Functionalization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lutz, J.-F.; Ouchi, M.; Liu, D.R.; Sawamoto, M. Sequence-controlled polymers. Science 2013, 341, 1238149. [Google Scholar] [CrossRef] [PubMed]
- Ten Brummelhuis, N. Controlling monomer-sequence using supramolecular templates. Polym. Chem. 2015, 6, 654–667. [Google Scholar] [CrossRef]
- Schattling, P.; Jochum, F.D.; Theato, P. Multi-responsive copolymers: Using thermo-, light- and redox stimuli as three independent inputs towards polymeric information processing. Chem. Commun. 2011, 47, 8859–8861. [Google Scholar] [CrossRef] [PubMed]
- Van Krevelen, D.W.; te Nijenhuis, K. Properties of Polymers: Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions; Elsevier Science: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Schacher, F.H.; Rupar, P.A.; Manners, I. Functional block copolymers: Nanostructured materials with emerging applications. Angew. Chem. Int. Ed. 2012, 51, 7898–7921. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.; Guillaneuf, Y.; Lefay, C.; Bertin, D.; Gigmes, D.; Charleux, B. Nitroxide-mediated polymerization. Prog. Polym. Sci. 2013, 38, 63–235. [Google Scholar] [CrossRef]
- Gregory, A.; Stenzel, M.H. Complex polymer architectures via raft polymerization: From fundamental process to extending the scope using click chemistry and nature's building blocks. Prog. Polym. Sci. 2012, 37, 38–105. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Living radical polymerization by the raft process—A second update. Aust. J. Chem. 2009, 62, 1402–1472. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Atom transfer radical polymerization (ATRP): Current status and future perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- Coates, G.W. Precise control of polyolefin stereochemistry using single-site metal catalysts. Chem. Rev. 2000, 100, 1223–1252. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Kamigaito, M. Stereospecific living radical polymerization: Dual control of chain length and tacticity for precision polymer synthesis. Chem. Rev. 2009, 109, 5120–5156. [Google Scholar] [CrossRef] [PubMed]
- Ten Brummelhuis, N.; Weck, M. Orthogonal multifunctionalization of random and alternating copolymers. ACS Macro Lett. 2012, 1, 1216–1218. [Google Scholar] [CrossRef]
- Ten Brummelhuis, N.; Weck, M. Raft polymerization of alternating styrene-pentafluorostyrene copolymers. J. Polym. Sci. Part A 2014, 52, 1555–1559. [Google Scholar] [CrossRef]
- Hirai, H. Mechanism of alternating copolymerization of acrylic monomers with donor monomers in the presence of lewis acid. J. Polym. Sci. Macromol. Rev. 1976, 11, 47–91. [Google Scholar] [CrossRef]
- Rzaev, Z.M.O. Complex-radical alternating copolymerization. Prog. Polym. Sci. 2000, 25, 163–217. [Google Scholar] [CrossRef]
- Hibi, Y.; Ouchi, M.; Sawamoto, M. Sequence-regulated radical polymerization with a metal-templated monomer: Repetitive ABA sequence by double cyclopolymerization. Angew. Chem. Int. Ed. 2011, 50, 7434–7437. [Google Scholar] [CrossRef] [PubMed]
- Ten Brummelhuis, N.; Heilmann, M.T. Polymerization of ternary inclusion complexes of interacting monomer pairs with γ-cyclodextrin. Macromolecules 2016, 49, 6879–6887. [Google Scholar] [CrossRef]
- Hibi, Y.; Tokuoka, S.; Terashima, T.; Ouchi, M.; Sawamoto, M. Design of AB divinyl “template monomers” toward alternating sequence control in metal-catalyzed living radical polymerization. Polym. Chem. 2011, 2, 341–347. [Google Scholar] [CrossRef]
- Matsuda, M.; Satoh, K.; Kamigaito, M. 1:2-sequence-regulated radical copolymerization of naturally occurring terpenes with maleimide derivatives in fluorinated alcohol. J. Polym. Sci. Part A 2013, 51, 1774–1785. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Q.; Liang, H.; Lu, J. Conventional and raft radical copolymerizations of β-pinene with N-substituted maleimides. Polym. Int. 2007, 56, 1514–1520. [Google Scholar] [CrossRef]
- Yamamoto, D.; Matsumoto, A. Penultimate unit and solvent effects on 2:1 sequence control during radical copolymerization of N-phenylmaleimide with β-pinene. Macromol. Chem. Phys. 2012, 213, 2479–2485. [Google Scholar] [CrossRef]
- Satoh, K.; Matsuda, M.; Nagai, K.; Kamigaito, M. AAB-sequence living radical chain copolymerization of naturally occurring limonene with maleimide: An end-to-end sequence-regulated copolymer. J. Am. Chem. Soc. 2010, 132, 10003–10005. [Google Scholar] [CrossRef] [PubMed]
- Ten Brummelhuis, N.; Stutz, M.B. Penultimate effects in the copolymerization of maleic anhydride with electron-rich styrene derivatives. J. Polym. Sci. Part A 2016, 54, 2932–2939. [Google Scholar] [CrossRef]
- Gauthier, M.A.; Gibson, M.I.; Klok, H.-A. Synthesis of functional polymers by post-polymerization modification. Angew. Chem. Int. Ed. 2009, 48, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, A.S.; Glassner, M.; Inglis, A.J.; Barner-Kowollik, C. Post-functionalization of polymers via orthogonal ligation chemistry. Macromol. Rapid Commun. 2013, 34, 810–849. [Google Scholar] [CrossRef] [PubMed]
- Gentsch, R.; Pippig, F.; Nilles, K.; Theato, P.; Kikkeri, R.; Maglinao, M.; Lepenies, B.; Seeberger, P.H.; Börner, H.G. Modular approach toward bioactive fiber meshes carrying oligosaccharides. Macromolecules 2010, 43, 9239–9247. [Google Scholar] [CrossRef]
- Tesch, M.; Hepperle, J.A.M.; Klaasen, H.; Letzel, M.; Studer, A. Alternating copolymerization by nitroxide-mediated polymerization and subsequent orthogonal functionalization. Angew. Chem. Int. Ed. 2015, 54, 5054–5059. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.; Fang, F.; Zhou, G.Q.; Coltart, D.M. Direct carbon-carbon bond formation via soft enolization: A facile and efficient synthesis of 1,3-diketones. Org. Lett. 2007, 9, 4139–4142. [Google Scholar] [CrossRef] [PubMed]
- Nilles, K.; Theato, P. Sequential conversion of orthogonally functionalized diblock copolymers based on pentafluorophenyl esters. J. Polym. Sci. Part A. 2010, 48, 3683–3692. [Google Scholar] [CrossRef]
- Joshi, R.M.; Joshi, S.G. New analytical solution of binary copolymer composition equation and suggested procedure for deriving monomer reactivity ratios. J. Macromol. Sci. Chem. 1971, 5, 1329–1338. [Google Scholar] [CrossRef]
- Fineman, M.; Ross, S.D. Linear method for determining monomer reactivity ratios in copolymerization. J. Polym. Sci. 1950, 5, 259–262. [Google Scholar] [CrossRef]
- Kelen, T.; Tudos, F. Analysis of linear methods for determining copolymerization reactivity ratios. 1. New improved linear graphic method. J. Macromol. Sci. Chem. 1975, 9, 1–27. [Google Scholar] [CrossRef]
- Tudos, F.; Kelen, T.; Foldesberezsnich, T.; Turcsanyi, B. Analysis of linear methods for determining copolymerization reactivity ratios. 3. Linear graphic method for evaluating data obtained at high conversion levels. J. Macromol. Sci. Chem. 1976, 10, 1513–1540. [Google Scholar] [CrossRef]
- Tidwell, P.W.; Mortimer, G.A. An improved method of calculating copolymerization reactivity ratios. J. Polym. Sci. Part A 1965, 3, 369–387. [Google Scholar] [CrossRef]
- Mooibroek, T.J.; Gamez, P. Directional character of solvent- and anion-pentafluorophenyl supramolecular interactions. Crystengcomm 2012, 14, 3902–3906. [Google Scholar] [CrossRef]
- Zhao, E.; Lam, J.W.Y.; Meng, L.M.; Hong, Y.; Deng, H.Q.; Bai, G.X.; Huang, X.H.; Hao, J.H.; Tang, B.Z. Poly[(maleic anhydride)-alt-(vinyl acetate)]: A pure oxygenic nonconjugated macromolecule with strong light emission and solvatochromic effect. Macromolecules 2015, 48, 64–71. [Google Scholar] [CrossRef]
| PentPFP/PhMI | LLS | J–J | F–R | Inv. F–R | K–T | Ext. K–T | T–M |
|---|---|---|---|---|---|---|---|
| r1 | 0.00 | 0.19 | 0.01 | 0.32 | 0.10 | −0.02 | −0.01 |
| r2 | 1.57 | 2.52 | 1.92 | 2.79 | 2.32 | 2.40 | 3.48 |
| r1·r2 | 0.01 | 0.49 | 0.02 | 0.89 | 0.24 | −0.04 | −0.021 |
| PerPFP/PhMI | LLS | J–J | F–R | Inv. F–R | K–T | Ext. K–T | T–M |
| r1 | 0.00 | −0.07 | −0.03 | 0.03 | −0.06 | 0.00 a | −0.01 |
| r2 | 0.85 | 0.75 | 1.04 | 0.83 | 0.75 | 0.75 a | 0.87 |
| r1·r2 | 0.00 | −0.05 | −0.03 | 0.2 | −0.05 | 0.00 a | −0.01 |
| Terminal model | LLS | J–J | F–R | Inv. F–R | K–T | Ext. K–T | T–M | |
|---|---|---|---|---|---|---|---|---|
| PentPFP/PhMI | r1 | 0.06 | 0.09 | 0.06 | 0.03 | 0.12 | 0.00 | 0.01 |
| r2 | 0.63 | 0.66 | 0.64 | 0.64 | 0.64 | 0.55 | 0.62 | |
| r1∙r2 | 0.04 | 0.06 | 0.04 | 0.02 | 0.05 | 0.08 | 0.01 | |
| Penultimate model | LLS | Ext. K–T | ||||||
| PerPFP/PhMI | r11 | 0 a | 0 a | |||||
| r21 | 0 a | 0 a | ||||||
| r12 | 0.92 | 1.16 | ||||||
| r22 | 0.14 | 0.11 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubatzki, F.; Al-Shok, L.; Ten Brummelhuis, N. Synthesis and Functionalization of Periodic Copolymers. Polymers 2017, 9, 166. https://doi.org/10.3390/polym9050166
Kubatzki F, Al-Shok L, Ten Brummelhuis N. Synthesis and Functionalization of Periodic Copolymers. Polymers. 2017; 9(5):166. https://doi.org/10.3390/polym9050166
Chicago/Turabian StyleKubatzki, Falk, Lucas Al-Shok, and Niels Ten Brummelhuis. 2017. "Synthesis and Functionalization of Periodic Copolymers" Polymers 9, no. 5: 166. https://doi.org/10.3390/polym9050166







