Study on the Mechanism of a Side Coupling Reaction during the Living Anionic Copolymerization of Styrene and 1-(Ethoxydimethylsilyphenyl)-1-phenylethylene (DPE-SiOEt)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Polymerization Procedures
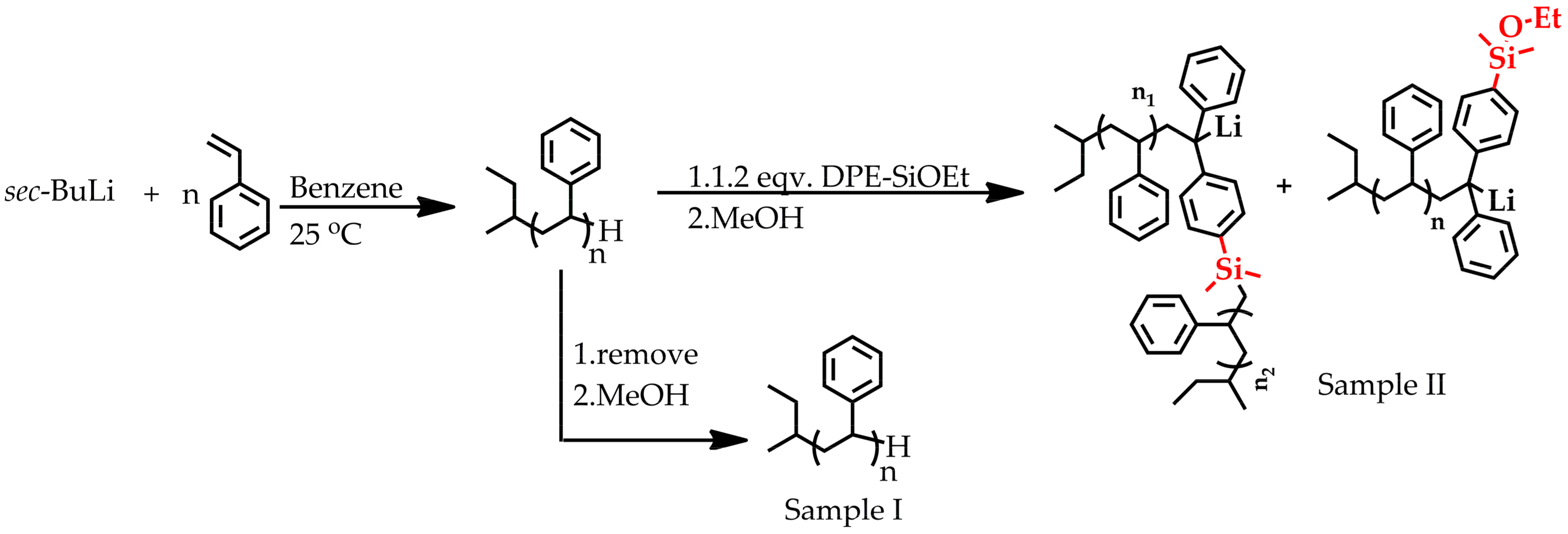
2.3.1. End-Capping PSLi with DPE-SiOEt
2.3.2. Copolymerization of Styrene and DPE-SiOEt
3. Results and Discussion
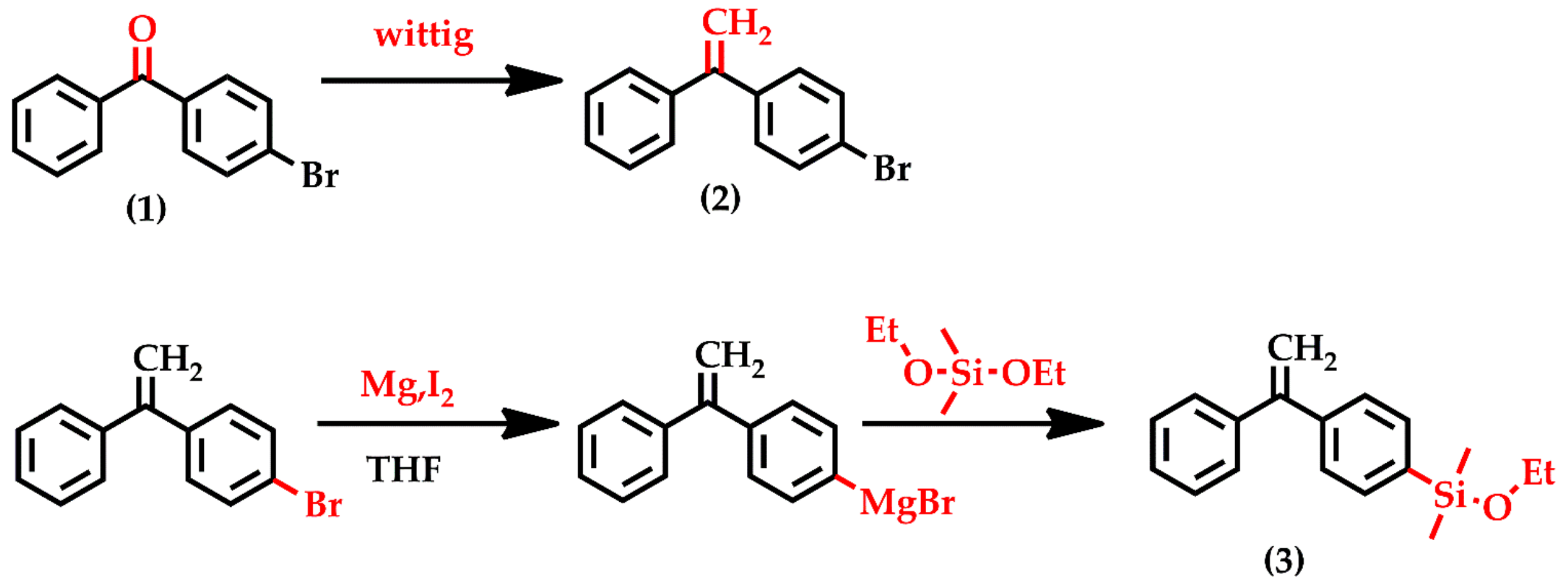
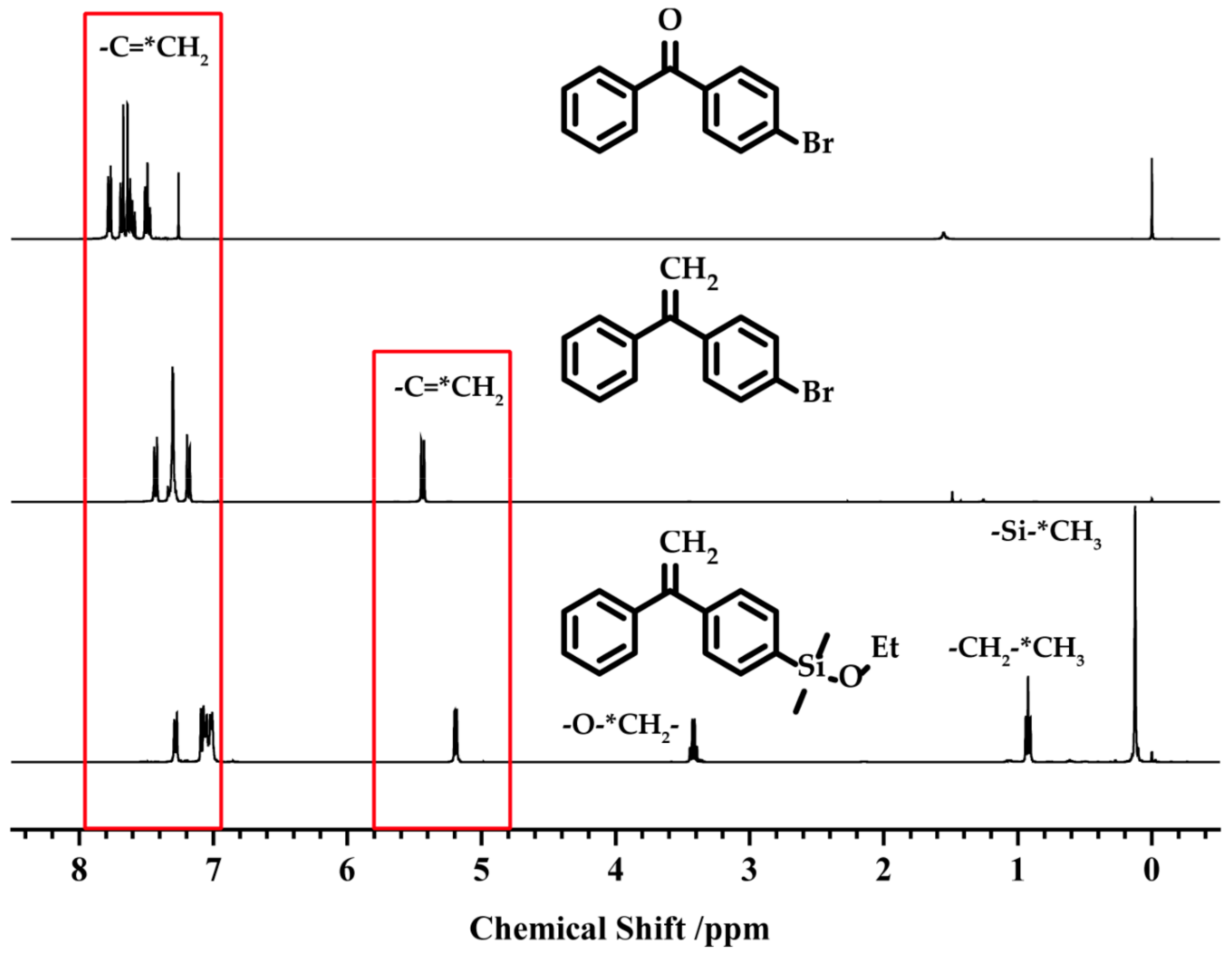
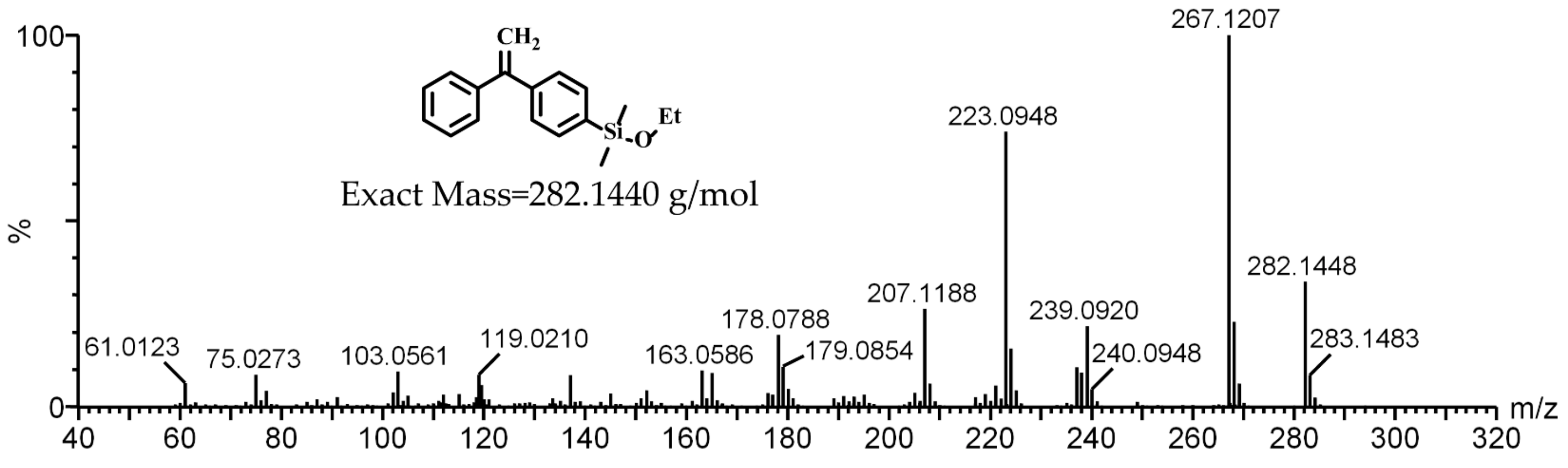
3.1. Synthesis of DPE-SiOEt
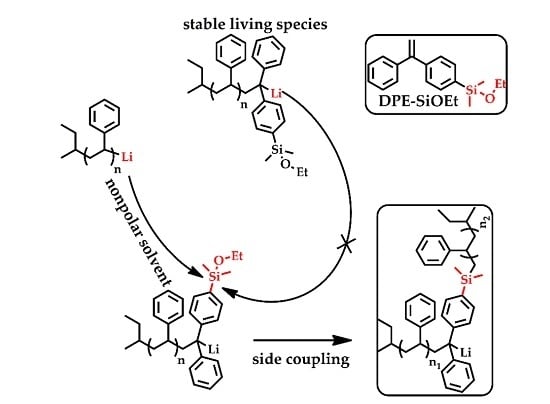
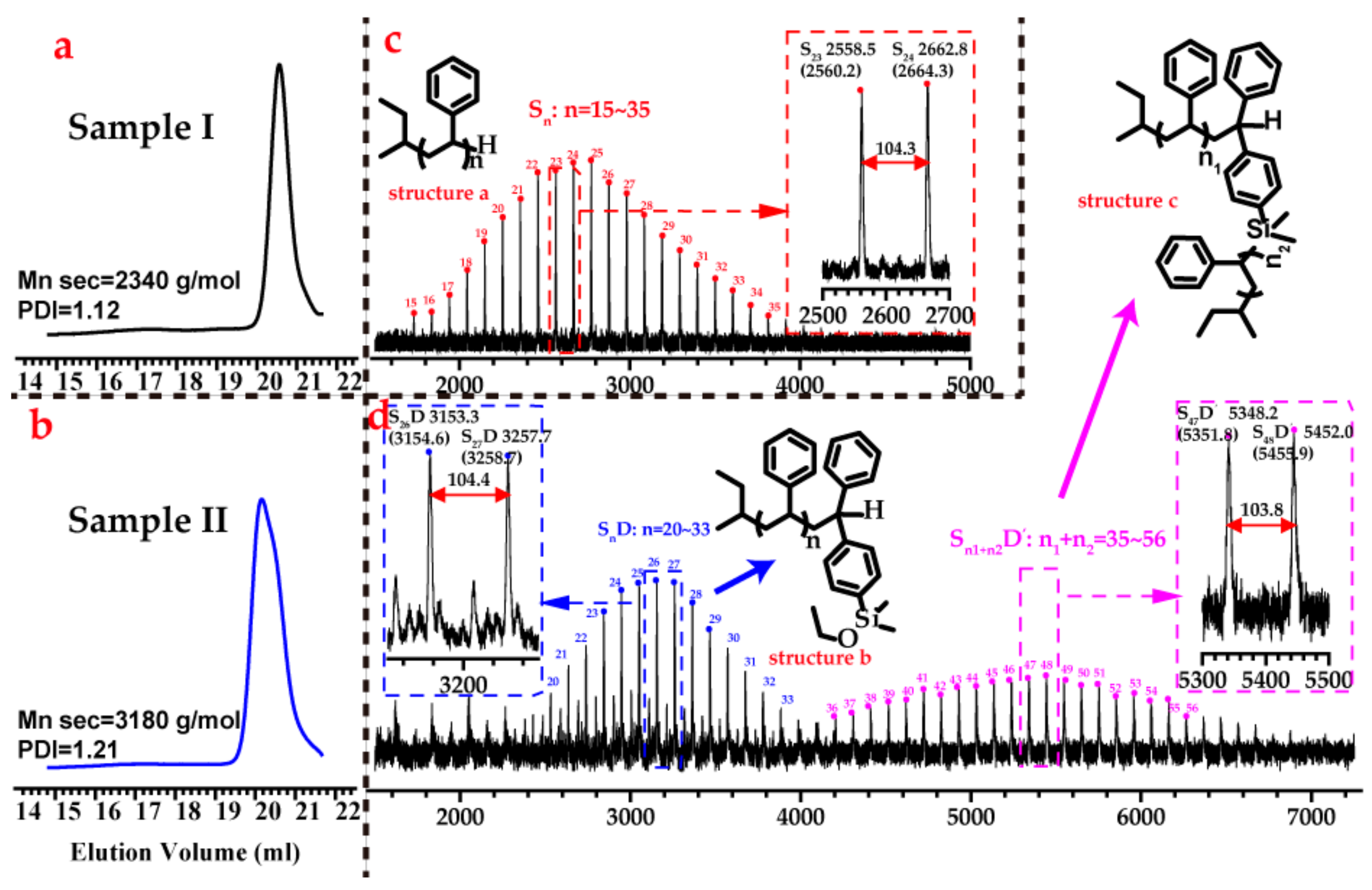
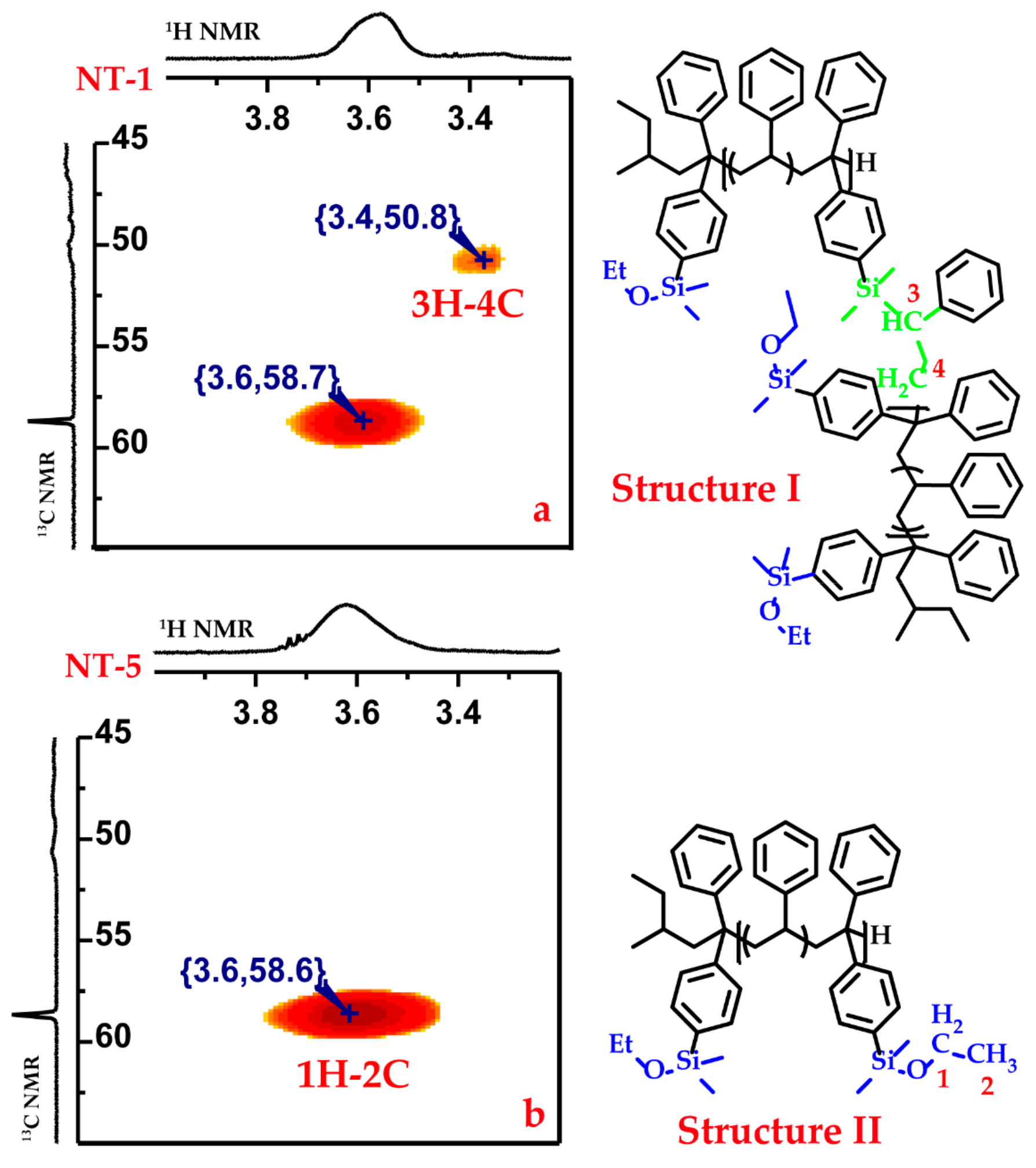
3.2. Side Reaction between Alkoxysilyl Groups and Active Center during LAP
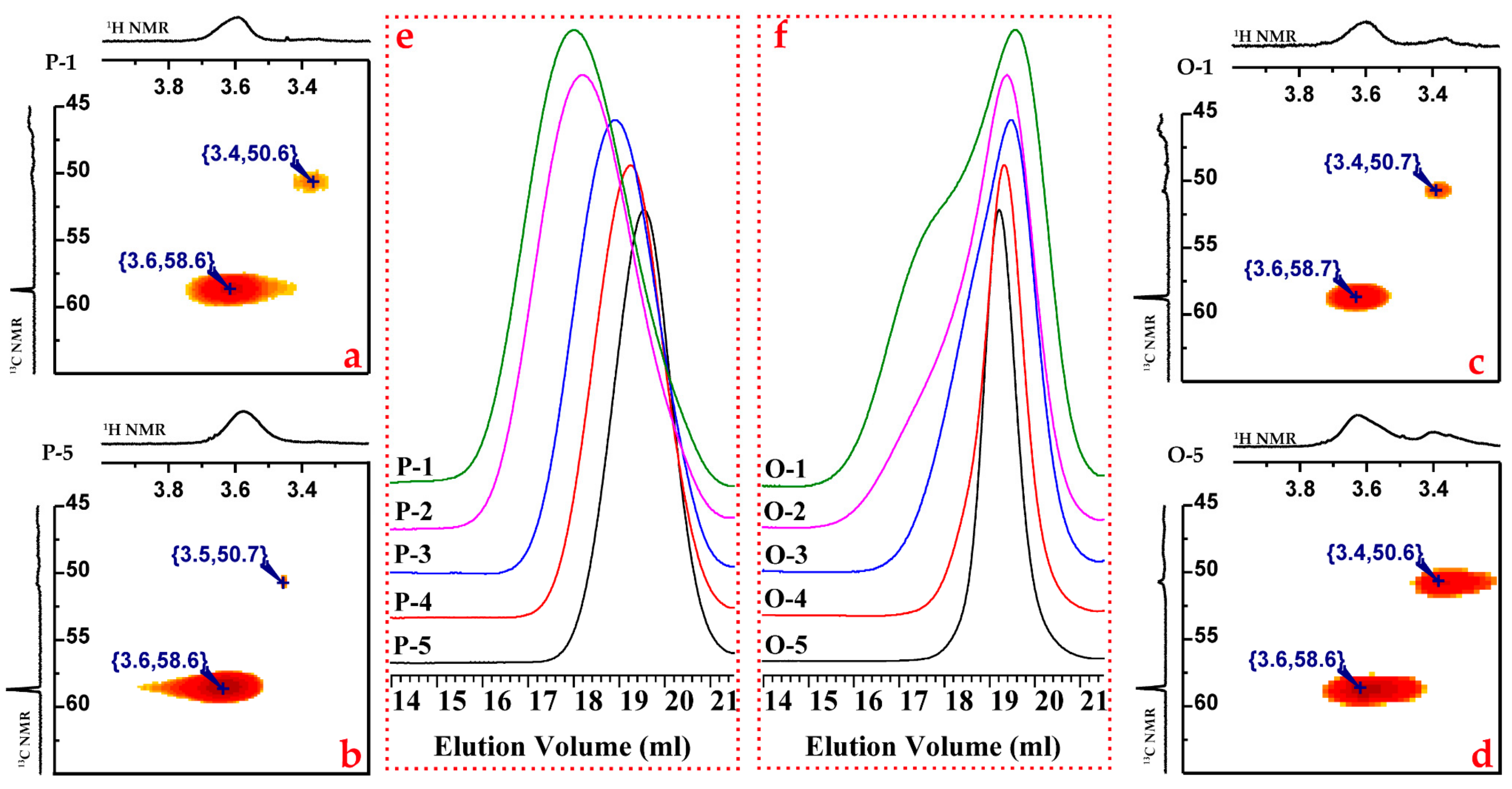
3.3. Kinetic Control of the Side Coupling Reaction
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wu, L.L.; Ma, H.W.; Lu, X.Y.; Wang, Y.S.; Wang, Y.R.; Li, Y. Synthesis and thermal analysis of in-chain multi-functionalized polybutadiene using 1,1-bis(4-dimethylaminophenyl)ethylene. J. Macromol. Sci. 2014, 51, 27–32. [Google Scholar] [CrossRef]
- Wu, L.; Ma, H.; Wang, Q.; Li, L.; Wang, Y.; Li, Y. In-chain multi-functionalized random butadiene-styrene copolymer via anionic copolymerization with 1,1-bis(4-dimethylaminophenyl)ethylene: Synthesis and its application as a rubber matrix of carbon black-based composite. J. Mater. Sci. 2014, 49, 5171–5181. [Google Scholar] [CrossRef]
- Wu, L.; Wang, Y.; Wang, Y.; Shen, K.; Li, Y. In-chain multi-functionalized polystyrene by living anionic copolymerization with 1,1-bis(4-dimethylaminophenyl)ethylene: Synthesis and effect on the dispersity of carbon black in polymer-based composites. Polymer 2013, 54, 2958–2965. [Google Scholar] [CrossRef]
- He, J.; Yue, K.; Liu, Y.; Yu, X.; Ni, P.; Cavicchi, K.A.; Quirk, R.P.; Chen, E.-Q.; Cheng, S.Z.D.; Zhang, W.-B. Fluorinated polyhedral oligomeric silsesquioxane-based shape amphiphiles: Molecular design, topological variation, and facile synthesis. Polym. Chem. 2012, 3, 2112–2120. [Google Scholar] [CrossRef]
- Ito, S.; Senda, S.; Ishizone, T.; Hirao, A. Syntheses of exactly-defined multi-graft polymers with two or more graft chains per branch point by a new iterative methodology. Polym. Chem. 2016, 7, 2078–2086. [Google Scholar] [CrossRef]
- Ito, S.; Goseki, R.; Manners, I.; Ishizone, T.; Hirao, A. Successive synthesis of multiarmed and multicomponent star-branched polymers by new iterative methodology based on linking reaction between block copolymer in-chain anion and α-phenylacrylate-functionalized polymer. Macromol. Chem. Phys. 2015, 216, 1523–1533. [Google Scholar] [CrossRef]
- Hirao, A.; Haraguchi, N.; Sugiyama, K. Synthesis of functionalized polymers by means of anionic living polymerization. 1. Synthesis of functionalized polymers with α-methylstyryl groups by anionic reactions with use of 1-{4-[3-(4-isopropenylphenyl)propyl]phenyl}-1-phenylethylene. Macromolecules 1999, 32, 48–54. [Google Scholar] [CrossRef]
- Hirao, A.; Hatayama, T.; Nakahama, S. Polymerization of monomers containing functional silyl groups. 3. Anionic living polymerization of (4-vinylphenyl)dimethylsilane. Macromolecules 1987, 20, 1505–1509. [Google Scholar] [CrossRef]
- Hirao, A.; Nakahama, S. Anionic living polymerization of functionalized monomers. Acta Polym. 1998, 49, 133–144. [Google Scholar] [CrossRef]
- Ding, J.; Li, Y.; Shen, K.H.; Wang, B.; Wang, Y.R. Anionic synthesis of binary random in-chain multi-functionalized poly(styrene/butadiene/isoprene and dimethyl [4-phenylvinyl)-phenyl]silane) (ps-dpesih, pb-dpesih, pi-dpesih) copolymers. Chin. Chem. Lett. 2012, 23, 749–752. [Google Scholar] [CrossRef]
- Kobayashi, M.; Hirata, M.; Takeishi, M. Synthesis of well-defined poly(n,n-diethylacrylamide)s end-functionalized with methacrylamide structure by means of anionic polymerization. Polym. J. 2004, 36, 238–247. [Google Scholar] [CrossRef]
- Freyss, D.; Rempp, P.; Benoît, H. Polydispersity of anionically prepared block copolymers. J. Polym. Sci. Part B 1964, 2, 217–222. [Google Scholar] [CrossRef]
- Se, K. Anionic living polymerization of useful monomers that can provide intermolecular chemical links. Prog. Polym. Sci. 2003, 28, 583–618. [Google Scholar] [CrossRef]
- Liu, P.; Ma, H.W.; Huang, W.; Han, L.; Hao, X.Y.; Shen, H.Y.; Bai, Y.; Li, Y. Sequence regulation in the living anionic copolymerization of styrene and 1-(4-dimethylaminophenyl)-1-phenylethylene by modification with different additives. Polym. Chem. 2017, 8, 1778–1789. [Google Scholar] [CrossRef]
- Liu, P.; Ma, H.; Huang, W.; Shen, H.; Wu, L.; Li, Y.; Wang, Y. The determination of sequence distribution in the living anionic copolymerization of styrene and strong electron-donating dpe derivative-1,1-bis(4-N,N-dimethylanimophenyl)ethylene. Polymer 2016, 97, 167–173. [Google Scholar] [CrossRef]
- Agostini, S.; Hutchings, L.R. Synthesis and temperature gradient interaction chromatography of model asymmetric star polymers by the “macromonomer” approach. Eur. Polym. J. 2013, 49, 2769–2784. [Google Scholar] [CrossRef]
- Zhang, W.B.; Sun, B.; Li, H.; Ren, X.; Janoski, J.; Sahoo, S.; Dabney, D.E.; Wesdemiotis, C.; Quirk, R.P.; Cheng, S.Z.D. Synthesis of in-chain-functionalized polystyrene-block-poly(dimethylsiloxane) diblock copolymers by anionic polymerization and hydrosilylation using dimethyl-4-(1-phenylvinyl)phenyl silane. Macromolecules 2009, 42, 7258–7262. [Google Scholar] [CrossRef]
- Hirao, A.; Hayashi, M.; Negishi, Y.; Haraguchi, N.; Loykulnant, S. Synthesis of well-defined chain-end- and in-chain-functionalized polystyrenes with a definite number of benzyl chloride moieties and d-glucose residues. Macromol. Symp. 2002, 181, 73–94. [Google Scholar] [CrossRef]
- Quirk, R.P.; Yoo, T.; Lee, Y.; Kim, J.; Lee, B. Applications of 1,1-diphenylethylene chemistry in anionic synthesis of polymers with controlled structures. Biopolym. PVA Hydrog. Anionic Polym. Nanocompos. 2000, 153, 67–162. [Google Scholar]
- Vorobii, M.; de los Santos Pereira, A.; Pop-Georgievski, O.; Kostina, N.Y.; Rodriguez-Emmenegger, C.; Percec, V. Synthesis of non-fouling poly N-(2-hydroxypropyl)-methacrylamide brushes by photoinduced SET-LRP. Polym. Chem. 2015, 6, 4210–4220. [Google Scholar] [CrossRef]
- Zengin, A.; Tamer, U.; Caykara, T. A new plasmonic device made of gold nanoparticles and temperature responsive polymer brush on a silicon substrate. J. Colloid Interface Sci. 2015, 448, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Lin, Y.; Zheng, J.; Tang, T. A novel strategy to synthesize well-defined ps brushes on silica particles by combination of lithium-iodine exchange (lie) and surface-initiated living anionic polymerization (si-lap). Chem. Commun. 2015, 51, 5921–5924. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, K.; Oie, T.; Elmagd, A.A.; Hirao, A. Synthesis of well-defined (ab)n multiblock copolymers composed of polystyrene and poly(methyl methacrylate) segments using specially designed living ab diblock copolymer anion. Macromolecules 2010, 43, 1403–1410. [Google Scholar] [CrossRef]
- Wiles, D.M.; Bywater, S. The electronic spectra of poly(methyl methacrylate) anions †. J. Polym. Sci. Part B 1964, 2, 1175–1179. [Google Scholar] [CrossRef]
- Quirk, R.P.; Garcés, C.; Collins, S.; Dabney, D.; Wesdemiotis, C.; Dudipala, V. Quantitative analysis and characterization of the products of the model reaction of n-butyllithium with excess 1,1-diphenylethylene: 1,1-diphenylhexane and 1,1,3,3-tetraphenyloctane. Polymer 2012, 53, 2162–2167. [Google Scholar] [CrossRef]
- Hutchings, L.R.; Brooks, P.P.; Parker, D.; Mosely, J.A.; Sevinc, S. Monomer sequence control via living anionic copolymerization: Synthesis of alternating, statistical, and telechelic copolymers and sequence analysis by maldi tof mass spectrometry. Macromolecules 2015, 48, 610–628. [Google Scholar] [CrossRef]
- Brooks, P.P.; Natalello, A.; Hall, J.N.; Eccles, E.A.L.; Kimani, S.M.; Bley, K.; Hutchings, L.R. Monomer sequencing in living anionic polymerization using kinetic control. Macromol. Symp. 2013, 323, 42–50. [Google Scholar] [CrossRef]
- Ma, H.; Han, L.; Li, Y. Sequence determination and regulation in the living anionic copolymerization of styrene and 1,1-diphenylethylene (dpe) derivatives. Macromol. Chem. Phys. 2016. [Google Scholar] [CrossRef]
- Gilman, H.; Smart, G.N.R. Steric hindrance in highly-substituted organosilicon compounds. I. The reaction of aryllithium compounds with some chlorosilanes, ethoxysilanes, and related compounds. J. Org. Chem. 1950, 15, 720–740. [Google Scholar] [CrossRef]
- Hirao, A.; Nagawa, T.; Hatayama, T.; Yamaguchi, K.; Nakahama, S. Polymerization of monomers containing functional silyl groups. 1. Anionic living polymerization of (4-vinylphenyl)dimethyl-2-propoxysilane. Macromolecules 1985, 18, 2101–2105. [Google Scholar] [CrossRef]
- Hirao, A.; Hatayama, T.; Nagawa, T.; Yamaguchi, M.; Yamaguchi, K.; Nakahama, S. Polymerization of monomers containing functional silyl groups. 2. Anionic living polymerization of (4-alkoxysilyl)styrenes. Macromolecules 1987, 20, 242–247. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, L.; Gu, X.; Zhang, W.; Zhou, N.; Zhang, Z.; Zhu, X. Sequence-controlled polymers with furan-protected maleimide as a latent monomer. Angew. Chem. Int. Ed. 2017, 56, 2328–2333. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ma, H.; Sang, W.; Han, L.; Liu, P.; Shen, H.; Huang, W.; Gong, X.; Yang, L.; Wang, Y.; et al. Synthesis of sequence-determined bottlebrush polymers based on sequence determination in living anionic copolymerization of styrene and dimethyl(4-(1-phenylvinyl)phenyl)silane. Polym. Chem. 2016, 7, 3090–3099. [Google Scholar] [CrossRef]
- Quirk, R.P.; Lee, B. Experimental criteria for living polymerization. Polym. Int. 1992, 27, 359–367. [Google Scholar] [CrossRef]
- Gausepohl, H.; Oepen, S.; Knoll, K.; Schneider, M.; McKee, G.; Loth, W. Super-polystyrene: A new class of engineering plastics with versatile properties. Des. Monomers Polym. 2000, 3, 299–315. [Google Scholar] [CrossRef]
- Quirk, R.P.; Lee, B. Adducts of polymeric organolithiums with 1,1-diphenylethylene. Rates of formation and crossover to styrene and diene monomers. Macromol. Chem. Phys. 2003, 204, 1719–1737. [Google Scholar] [CrossRef]
- Sang, W.; Ma, H.; Wang, Q.; Hao, X.; Zheng, Y.; Wang, Y.; Li, Y. Monomer sequence determination in the living anionic copolymerization of styrene and asymmetric bi-functionalized 1,1-diphenylethylene derivatives. Polym. Chem. 2016, 7, 219–234. [Google Scholar] [CrossRef]
- Hofmans, J.; Maeseele, L.; Wang, G.; Janssens, K.; Beylen, M.V. The influence of π-complexing additives on the dissociation of organolithium compounds in non-polar medium: A long time unexplored area in anionic polymerization. Polymer 2003, 44, 4109–4115. [Google Scholar] [CrossRef]
- Hsieh, H.L.; Quirk, R.P. Anionic Polymerization: Principles and Practical Applications; Marcel Dekker: New York, NY, USA, 1996; pp. 591–621. [Google Scholar]
- Wofford, C.F.; Hsieh, H.L. Copolymerization of butadiene and styrene by initiation with alkyllithium and alkali metal tert-butoxides. J. Polym. Sci. Part A 1969, 7, 461–469. [Google Scholar] [CrossRef]


| No. a | [MS]0/[MD]0 b | [MS]/[MD] c | Mn d | PDI d | ND e | rSt f |
|---|---|---|---|---|---|---|
| (mol/mol) | (mol/mol) | (kg/mol) | ||||
| NT-1 | 8:1.0 | 6.03 | 11.6 | 1.40 | 12.8 | N/A |
| NT-2 | 6:1.0 | 4.68 | 9.6 | 1.39 | 12.5 | N/A |
| NT-3 | 4:1.0 | 3.85 | 10.3 | 1.38 | 15.0 | N/A |
| NT-4 | 2:1.0 | 2.31 | 12.0 | 1.32 | 23.0 | N/A |
| NT-5 | 1:1.0 | 1.72 | 12.8 | 1.30 | 25.5 | 1.3 |
| NT-6 | 1:1.2 | 1.64 | 11.4 | 1.33 | 25.2 | 1.5 |
| NT-7 | 1:1.4 | 1.56 | 10.6 | 1.33 | 23.9 | 1.5 |
| NT-8 | 1:1.6 | 1.59 | 10.2 | 1.33 | 22.8 | 1.9 |
| NT-9 | 1:1.8 | 1.43 | 11.2 | 1.32 | 26.0 | 1.5 |
| No. a | Additive | [MS]0/[MD]0 b | [MS]/[MD] c | Mn d | PDI d | ND e |
|---|---|---|---|---|---|---|
| (mol/mol) | (mol/mol) | (kg/mol) | ||||
| P-1 | THF/Li = 10 | 8.0 | 9.32 | 22.0 | 1.49 | 17.7 |
| P-2 | 6.0 | 6.41 | 20.7 | 1.42 | 21.9 | |
| P-3 | 4.0 | 4.87 | 13.9 | 1.39 | 17.6 | |
| P-4 | 2.0 | 2.63 | 11.7 | 1.36 | 21.1 | |
| P-5 | 1.0 | 2.14 | 9.6 | 1.31 | 19.2 | |
| O-1 | NaODP/Li = 2 | 8.0 | 5.62 | 13.7 | 1.83 | 15.8 |
| O-2 | 6.0 | 4.73 | 14.0 | 1.81 | 18.3 | |
| O-3 | 4.0 | 3.41 | 12.5 | 1.44 | 19.7 | |
| O-4 | 2.0 | 1.66 | 12.6 | 1.25 | 28.2 | |
| O-5 | 1.0 | 1.08 | 13.2 | 1.20 | 32.9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Ma, H.; Shen, H.; Han, L.; Chang, S.; Zang, L.; Bian, Y.; Bai, Y.; Li, Y. Study on the Mechanism of a Side Coupling Reaction during the Living Anionic Copolymerization of Styrene and 1-(Ethoxydimethylsilyphenyl)-1-phenylethylene (DPE-SiOEt). Polymers 2017, 9, 171. https://doi.org/10.3390/polym9050171
Liu P, Ma H, Shen H, Han L, Chang S, Zang L, Bian Y, Bai Y, Li Y. Study on the Mechanism of a Side Coupling Reaction during the Living Anionic Copolymerization of Styrene and 1-(Ethoxydimethylsilyphenyl)-1-phenylethylene (DPE-SiOEt). Polymers. 2017; 9(5):171. https://doi.org/10.3390/polym9050171
Chicago/Turabian StyleLiu, Pibo, Hongwei Ma, Heyu Shen, Li Han, Shuang Chang, Long Zang, Yiyu Bian, Yu Bai, and Yang Li. 2017. "Study on the Mechanism of a Side Coupling Reaction during the Living Anionic Copolymerization of Styrene and 1-(Ethoxydimethylsilyphenyl)-1-phenylethylene (DPE-SiOEt)" Polymers 9, no. 5: 171. https://doi.org/10.3390/polym9050171