Synthesis, Characterization and Application of Four Novel Electrochromic Materials Employing Nitrotriphenylamine Unit as the Acceptor and Different Thiophene Derivatives as the Donor
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Equipments
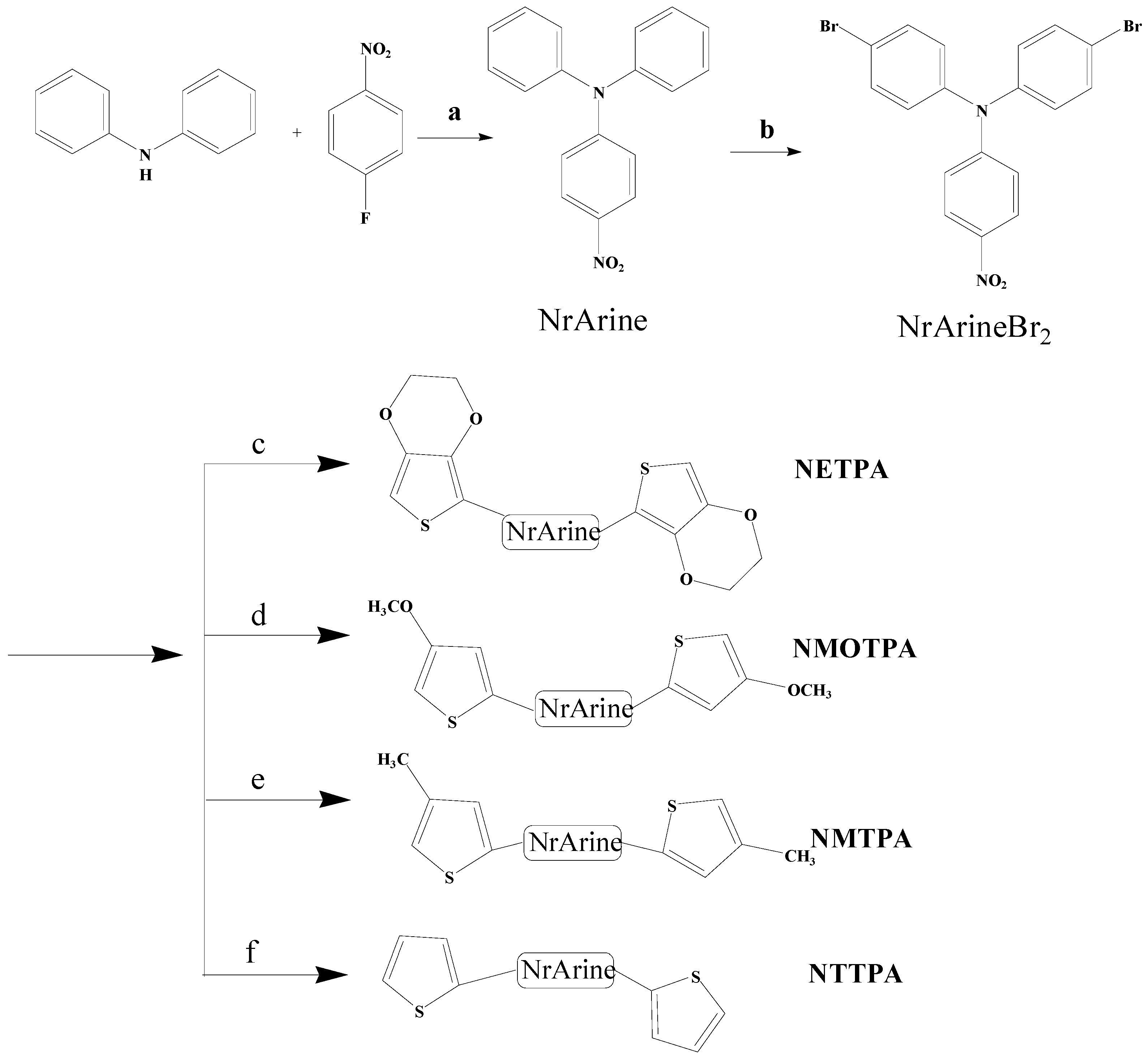
2.3. Synthesis
2.3.1. 4-bromo-N-(4-bromophenyl)-N-(4-nitrophenyl)aniline (NrArineBr2)
2.3.2. Synthesis of Monomers by Stille Coupling Reaction
2.4. Electrochemistry
2.5. Spectroelectrochemistry and Dynamic Switching Tests
3. Result and Discussion
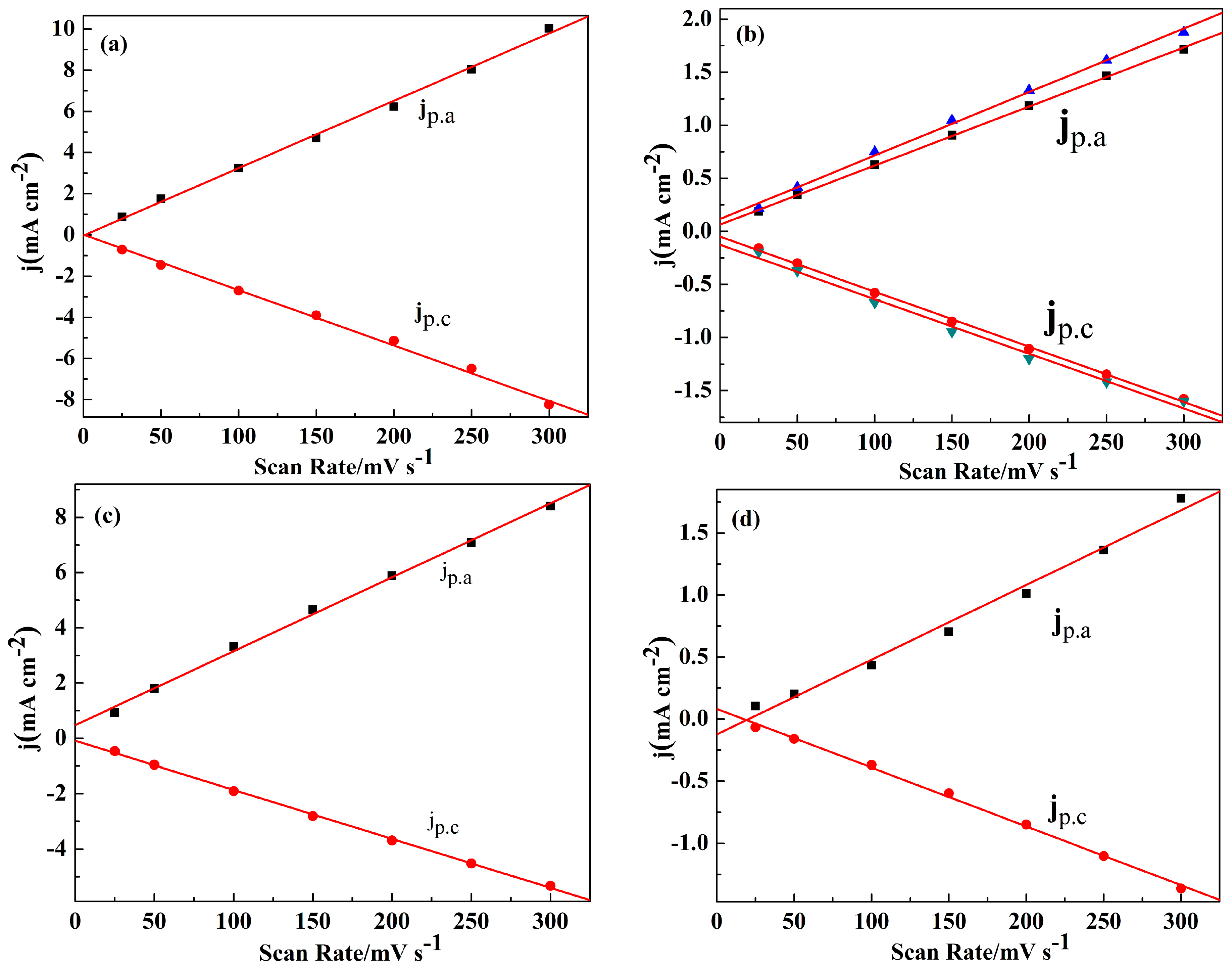
3.1. Electrochemical Polymerization
3.2. Electrochemistry Behavior of the Polymer Films
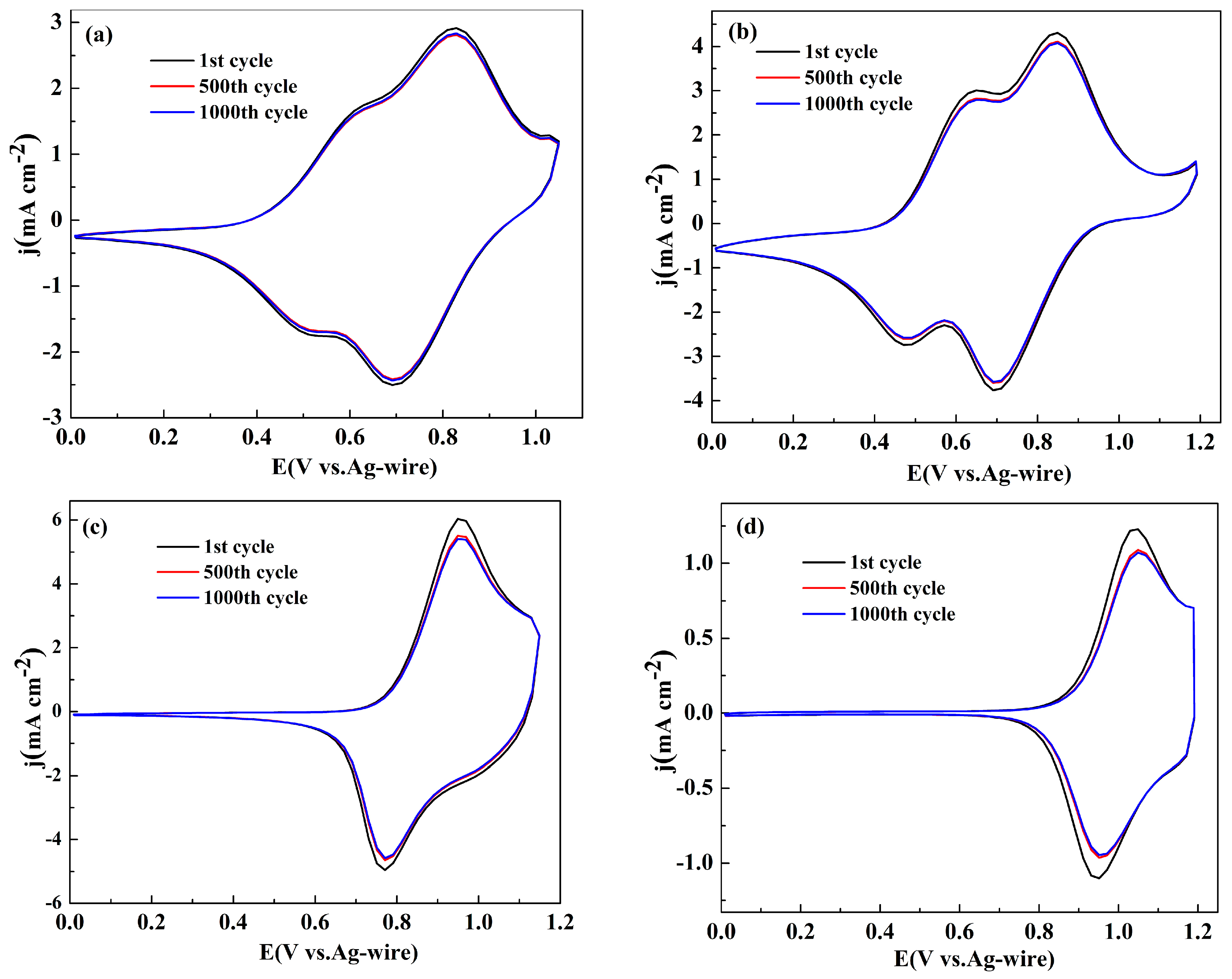
3.3. Electrochemical Stability of the Polymer Films
3.4. Optical Properties of the Monomers and Films
3.5. Electrochromic Properties of the Polymer Films
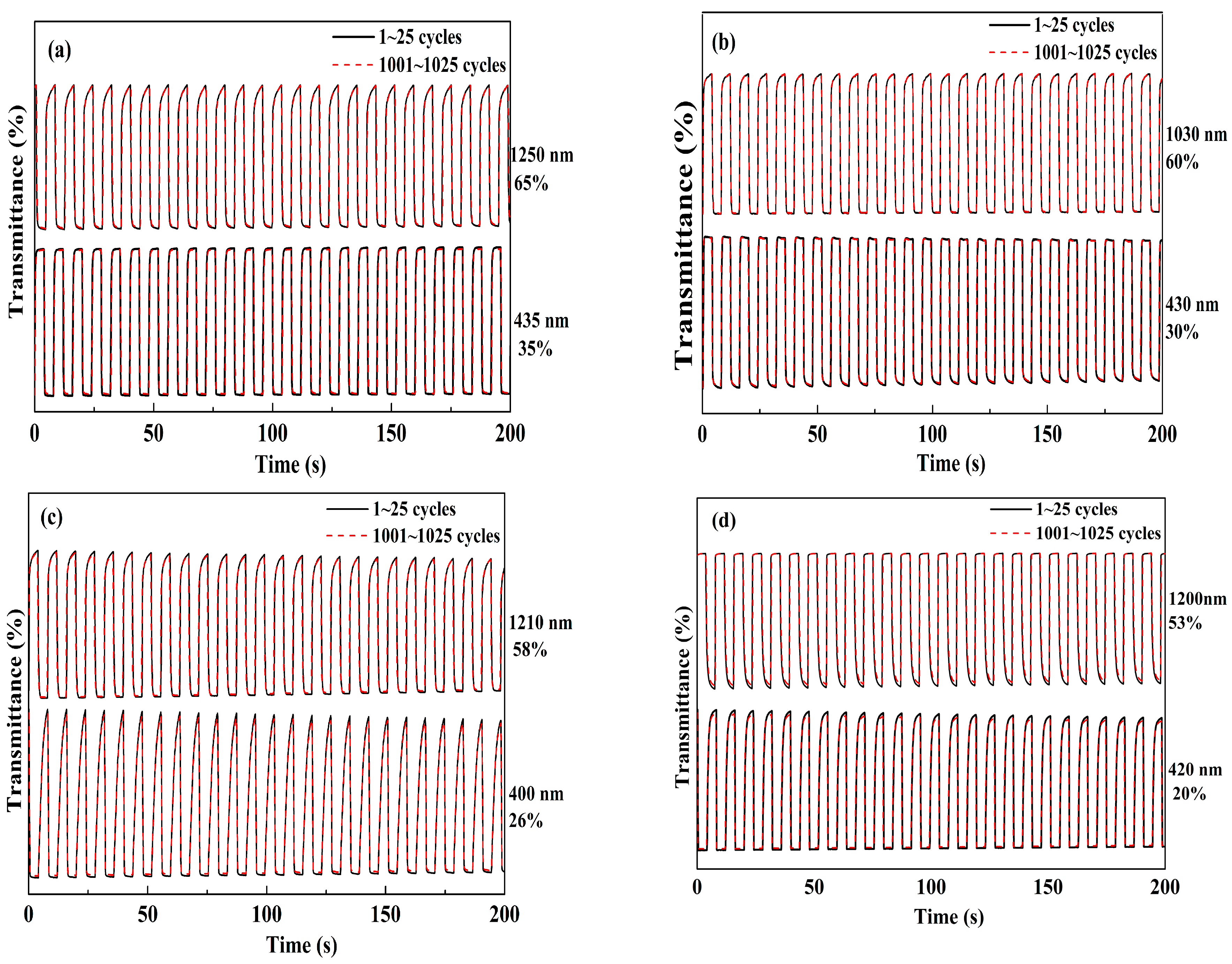
3.6. Switching Properties of the Polymer Films
4. Conclusion
Supplementary Materials
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Liou, G.-S.; Hsiao, S.-H.; Su, T.-H. Synthesis, luminescence and electrochromism of aromatic poly (amine-amide)s with pendent triphenylamine moieties. J. Mater. Chem. 2005, 15, 1812–1820. [Google Scholar] [CrossRef]
- Kuorosawa, T.; Chueh, C.-C.; Liu, C.-L.; Higashihara, T.; Ueda, M.; Chen, W.-C. High performance volatile polymeric memory devices based on novel triphenylamine-based polyimides containing mono-or dual-mediated phenoxy linkages. Macromolecules 2010, 43, 1236–1244. [Google Scholar] [CrossRef]
- Kim, J.; You, J.; Kim, E. Flexible conductive polymer patterns from vapor polymerizable and photo-cross-linkable EDOT. Macromolecules 2010, 43, 2322–2327. [Google Scholar] [CrossRef]
- Wang, J.; Fei, F.; Luo, Q.; Nie, S.H.; Wu, N.; Chen, X.L.; Su, W.M.; Li, Y.J.; Ma, C.Q. Modification of highly conductive PEDOT: PSS layer for use in silver nanogrid electrodes for flexible inverted polymer solar cells. ACS Appl. Mater. Interface 2017, 9, 7834–7842. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.S.; Lin, H.-Y.; Yen, H.-J. Synthesis and characterization of electroactive hyperbranched aromatic polyamides based on A2B-type triphenylamine moieties. J. Mater. Chem. 2009, 19, 7666–7673. [Google Scholar] [CrossRef]
- Liu, J.; Wu, J.; Shao, S.Y.; Deng, Y.F.; Meng, B.; Xie, Z.Y.; Geng, Y.H.; Wang, L.X.; Zhang, F.L. Printable highly conductive conjugated polymer sensitized ZnO NCs as cathode interfacial layer for efficient polymer solar cells. ACS. Appl. Mater. Interfaces 2014, 6, 8237–8245. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.C.; Lee, J.; Huang, D.; Subramanian, V.; Murphy, A.R.; Fréchet, J.M.J. Film morphology and thin film transistor performance of solution-processed oligothiophenes. Chem. Mater. 2004, 16, 4783–4789. [Google Scholar] [CrossRef]
- Yen, H.J.; Liou, G.-S. Enhanced near-infrared electrochromism in triphenylamine-based aramides bearing phenothiazine redox centers. J. Mater. Chem. 2010, 20, 9886–9894. [Google Scholar] [CrossRef]
- Vohra, V.; Giovanella, U.; Tubino, R.; Murata, H.; Botta, C. Electroluminescence from conjugated polymer electrospum nanofibers in solution processable organic light-emitting diodes. ACS Nano 2011, 5, 5572–5578. [Google Scholar] [CrossRef] [PubMed]
- Cingil, H.E.; Storm, I.M.; Yorulmaz, Y.; te Brake, D.W.; de Vries, R.; Stuart, M.A.C.; Sprakel, J. Monitoring protein capsid assembly with a conjugated polymer strain sensor. J. Am. Chem. Soc. 2015, 137, 9800–9803. [Google Scholar] [CrossRef] [PubMed]
- Amb, C.M.; Dyer, A.L.; Reynolds, J.R. Navigating the color palette of solution-processable electrochromic polymers. Chem. Mater. 2011, 23, 397–415. [Google Scholar] [CrossRef]
- Chen, X.M.; Xu, Z.P.; Mi, S.; Zheng, J.M.; Xu, C.Y. Spray-processable red-to-transmissive electrochromic polymers towards fast switching time for display applications. New J. Chem. 2015, 39, 5389–5394. [Google Scholar] [CrossRef]
- Cho, C.M.; Ye, Q.; Neo, W.T.; Lin, T.T.; Lu, X.H.; Xu, J.W. Ultrahigh electro-deficient pyrrolo-acenaphtho-pyridazine-dione based donor-acceptor conjugated polymers for electrochromic applications. Poly. Chem. 2015, 6, 7570–7579. [Google Scholar] [CrossRef]
- Koyuncu, F.B.; Koyuncu, S.; Ozdemir, E. A novel donor-acceptor polymeric electrochromic material containing carbazole and 1,8-naphtalimide as subunit. Electrochim. Acta 2010, 55, 4935–4941. [Google Scholar] [CrossRef]
- Wang, P.I.; Shie, W.R.; Jiang, J.C.; Li, L.J.; Liaw, D.J. Novel poly(triphenylamine-alt-fluorene) with asymmetric hexaphenylbenzene and pyrene moieties: synthesis, flurescence, flexible near-infrared electrochromic devices and theoretical investigation. Polym. Chem. 2016, 7, 1505–1516. [Google Scholar] [CrossRef]
- Koyuncu, S.; Usluer, O.; Can, M.; Demic, S.; Icli, S.; Sariciftci, N.S. Electrochromic and electroluminescent devices based on a novel branched quai-dendric fluorene-carbazole-2,5-bis(2-thienyl)-1H-pyrrole system. J. Mater. Chem. 2011, 21, 2684–2693. [Google Scholar] [CrossRef]
- Wang, H.M.; Hsiao, S.-H. Enhancement of redox stability and electrochromic performance of aromatic polyamides by incorporation of (3,6-dimethoxycarbazol-9-yl)-triphenylamide units. J. Polym. Sci. Pol. Chem. 2014, 52, 272–286. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Chou, Y.T. Synthesis and Electrochromic Properties of Aromatic Polyamides with Pendent Triphenylamine Units. Macromol. Chem. Phys. 2014, 215, 958–970. [Google Scholar] [CrossRef]
- Jin, H.Y.; Tian, J.H.; Wang, S.; Tan, T.F.; Xiao, Y.; Li, X.G. Novel photochromic and electrochromic diarylethenes bearing triphenylamine units. RSC Adv. 2014, 4, 16839–16848. [Google Scholar] [CrossRef]
- Lin, L.C.; Yen, H.J.; Kung, Y.R.; Leu, C.M.; Lee, T.M.; Liou, G.-S. Novel near-infrared and multi-colored electrochromic polybenzoxazines with electroactive triarylamine moieties. J. Mater. Chem. C. 2014, 2, 7796–7803. [Google Scholar] [CrossRef]
- Karabiyik, E.; Sefer, E.; Koyuncu, F.B.; Tonga, M.; Özdemir, E.; Koyuncu, S. Toward purple-to-green-to-transmissive-to-black color switching in polymeric electrochrome. Macromolecules 2014, 47, 8578–8584. [Google Scholar] [CrossRef]
- Algı, F.; Cihaner, A. An ambipolar neutral state green polymeric electrochromic. Org. Electron. 2009, 10, 704–710. [Google Scholar] [CrossRef]
- Yiğit, D.; Hacioglu, S.O.; Güllü, M.; Toppare, L. Synthesis and spectroelectrochemical characterization of multi-colored novel poly(3,6-dithienylcarbazole)derivatives containing azobenzene and coumarin chromophore units. Electrochim. Acta 2016, 196, 140–152. [Google Scholar] [CrossRef]
- Hu, Y.J.; Hu, D.F.; Ming, S.L.; Duan, X.M.; Zhao, F.; Hou, J.; Xu, J.K.; Jiang, F.X. Synthesis of polyether-bridged bithiophenes and their electrochemical polymerization to electrochromic property. Electrochim. Acta 2016, 189, 64–73. [Google Scholar] [CrossRef]
- Cai, S.W.; Wen, H.L.; Wang, S.Z.; Niu, H.J.; Wang, C.; Jiang, X.K.; Bai, X.D.; Wang, W. Electrochromic polymers electrochemically polymerized from 2,5-dithienylpyrrole (DTP) with different triarylamine units: synthesis, characterization and optoelectrochemical properties. Electrochim. Acta 2017, 228, 332–342. [Google Scholar] [CrossRef]
- Cheng, X.F.; Zhao, J.S.; Cui, C.S.; Fu, Y.Z.; Zhang, X.X. Star-shaped conjugated system derived from thienyl-derivatized poly(triphenylamine)s as active materials for electrochromic devices. J. Electroanal. Chem. 2012, 677, 24–30. [Google Scholar] [CrossRef]
- Xu, C.X.; Zhao, J.S.; Cui, C.S.; Wang, M.; Kong, Y.; Zhang, X.X. Triphenylamine-based multielectrochromic material and its neutral green electrochromic devices. J. Electroanal. Chem. 2012, 682, 29–36. [Google Scholar] [CrossRef]
- Zhao, J.S.; Yang, X.J.; Wang, S.H.; Yu, J.S. Two new near-infrared switchable electrochromic bithiophenes derivatives based on 4-methoxytriphenylamine unit and their application for electrochromic devices. ECS. J. Solid. State. SC. 2014, 3, R121–R130. [Google Scholar] [CrossRef]
- Yang, X.J.; Wang, M.; Zhao, J.S.; Cui, C.S.; Wang, S.H.; Liu, J.F. Multichromic polymers containing alternating bithiophenes derivatives and 4-cyanotriphenylamine unit and their application for electrochromic devices. J. Electroanal. Chem. 2014, 714–715, 1–10. [Google Scholar] [CrossRef]
- Hou, Y.F.; Kong, L.Q.; Ju, X.P.; Liu, X.L.; Zhao, J.S.; Niu, Q.S. Multichromic polymers containing alternating bi(3-methoxythiophene) and triphenylamine based units with para-protective substituents. Materials 2016, 9, 779–795. [Google Scholar] [CrossRef]
- Doyranlı, C.; Çolak, B.; Lacinel, G.; Can, M.; Koyuncu, F.B.; Koyuncu, S. Effect of the planar center moiety for a donor-acceptor polymeric electrochrome. Polymer 2017, 108, 423–431. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Liou, G.-S. Poly(triphenylamine)s derived from oxidative coupling reaction: substituent effects on the polymerization, electrochemical, and electro-optical properties. J. Polym. Sci. Pol. Chem. 2009, 47, 285–294. [Google Scholar] [CrossRef]
- Gunbas, G.E.; Durmus, A.; Toppare, L. Could green be greener? Novel donor-acceptor-type electrochromic polymers: towards excellent neutral green materials with exceptional transmissive oxidized states for completion of RGB color space? Adv. Mater. 2008, 20, 691–695. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, J.S.; Cui, C.S.; Wang, M.; Wang, Z.; He, Q.P. Electrochemical synthesis, characterization and electrochromic properties of a copolymer based on 1,4-bis(2-thienyl)naphthalene and pyrene. Opt. Mater. 2012, 34, 1095–1101. [Google Scholar] [CrossRef]
- Ming, S.L.; Zhen, S.J.; Liu, X.M.; Lin, K.W.; Liu, H.T.; Zhao, Y.; Lu, B.Y.; Xu, J.K. Chalcogenodiazolo[3,4-c]pyridine based donor-acceptor-donor polymers for green and near-infrared electrochromics. Polym. Chem. 2016, 6, 8248–8258. [Google Scholar] [CrossRef]
- Liu, Y.X.; Wang, M.; Zhao, J.S.; Cui, C.S.; Liu, J.F. Effects of alkyl or alkoxy side chains on the electrochromic properties of four ambipolar donor-acceptor type polymers. RSC Adv. 2014, 4, 52712–52726. [Google Scholar] [CrossRef]
- Dai, Y.Y.; Li, W.J.; Qu, X.X.; Liu, J.; Yan, S.M.; Ouyang, M.; Lv, X.J.; Zhang, C. Electrochemistry, electrochromic and color memory properties of polymer/copolymer based on novel dithienylpyrrole structure. Electrochim. Acta 2017, 229, 271–280. [Google Scholar] [CrossRef]
- Bulloch, R.H.; Reynolds, J.R. Photostability in dioxyheterocycle electrochromic polymers. Polym. Chem. 2015, 6, 8248–8258. [Google Scholar] [CrossRef]
- Marks, Z.D.; Glugla, D.; Friedlein, J.T.; Shaheen, S.E.; McLeod, R.R.; Kahook, M.Y.; Nair, D.P. Switchable diffractive optics using patterned PEDOT:PSS based electrochromic thin-films. Org. Electron. 2016, 37, 271–279. [Google Scholar] [CrossRef]
- Ju, X.P.; Kong, L.Q.; Zhao, J.S.; Bai, G.Y. Synthesis and electrochemical capacitive performance of thieno[3,4-b] pyrazine-based donor-acceptor type copolymers used as supercapacitor electrode material. Electrochim. Acta 2017, 238, 36–48. [Google Scholar] [CrossRef]
- Sato, K.; Mizukami, R.; Mizuma, T.; Nishide, H.; Oyaizu, K. Synthesis of dimethyl-substituted polyviologen and control of charge transport in electrodes for high-resolution electrochromic displays. Polymers 2017. [Google Scholar] [CrossRef]
- Wu, T.Y.; Chung, H.H. Application of tris(4-(thiophen-2-yl)phenyl)amine and dithienylpyrrole-based conjugated copolymers in high-contrast electrochromic devices. Polymers 2016. [Google Scholar] [CrossRef]
- Kuo, C.W.; Wu, T.L.; Lin, Y.C.; Chang, J.K.; Chen, H.R.; Wu, T.Y. Copolymers based on 1,3-bis(carbazol-9-yl)benzene and three 3,4-ethylenedioxythiophene derivatives as potential anodically coloring copolymers in high-contrast electrochromic devices. Polymers 2016. [Google Scholar] [CrossRef]


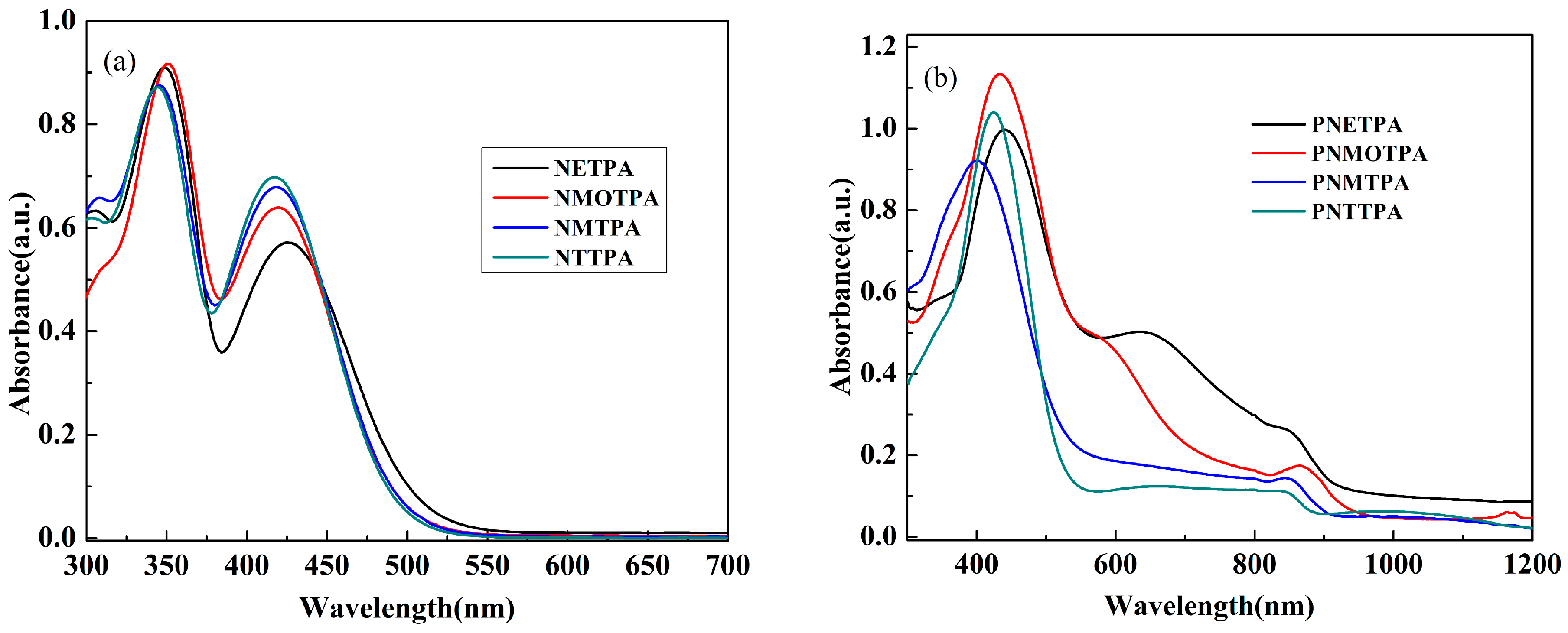

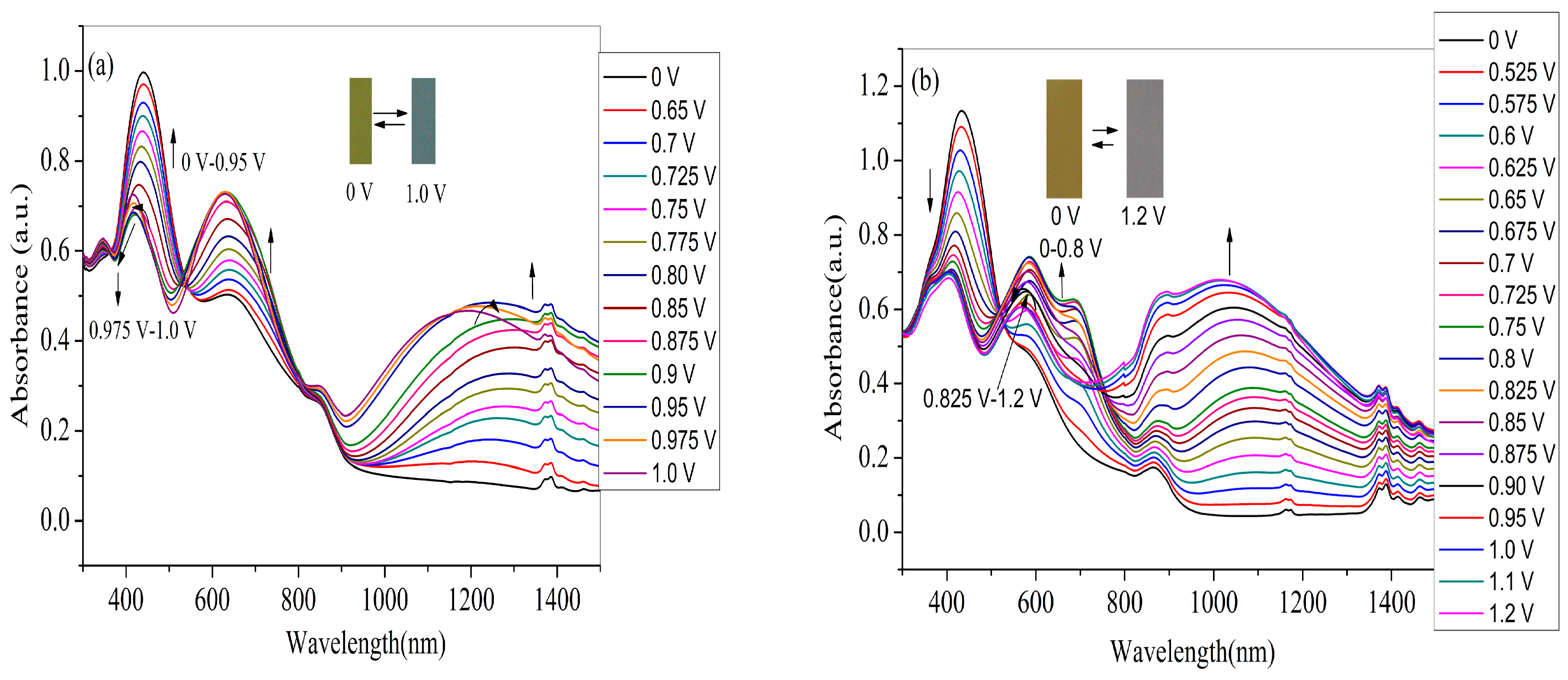
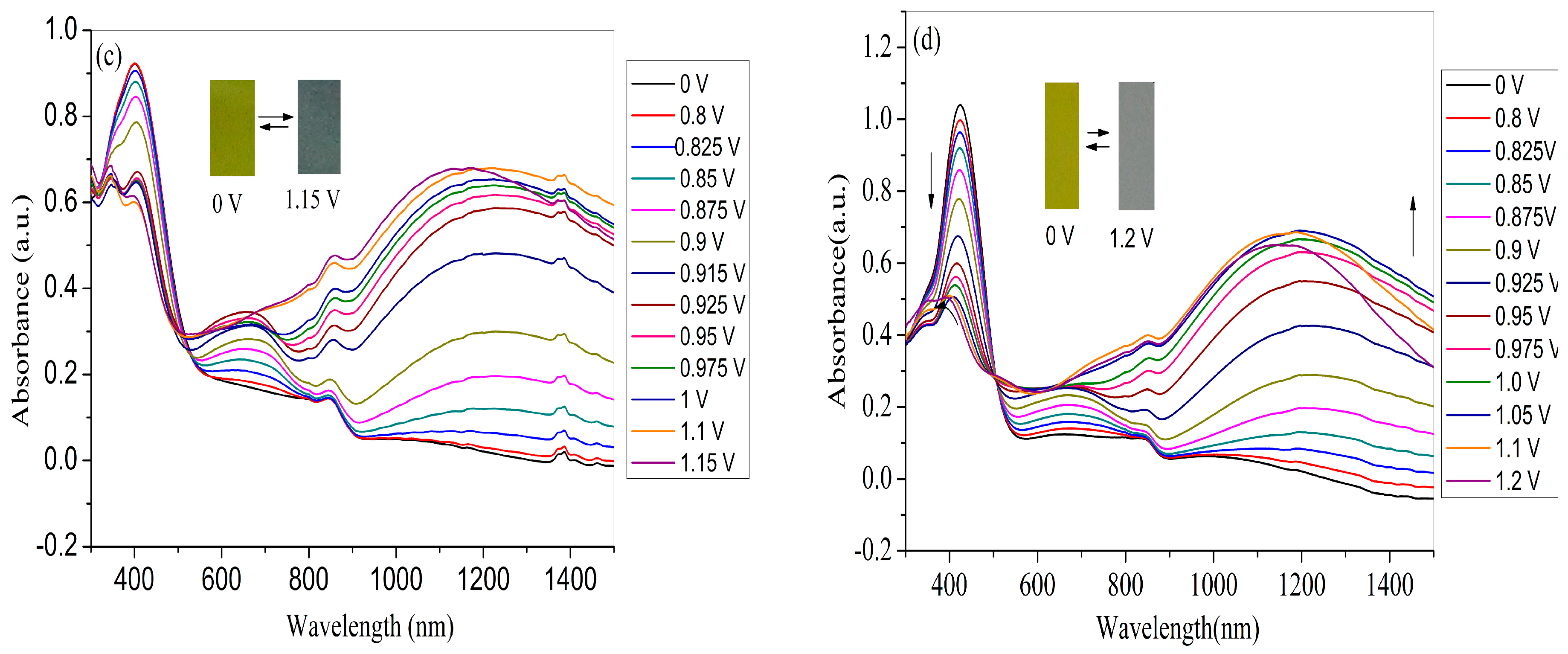
| Compounds | Eonset, vs. (Ag-wire) (V) | λmax (nm) /λonset (nm) | Eg a (eV) | HOMO b (eV) | LUMO c (eV) | ΔE d (eV) | HOMO d (eV) | LUMO d (eV) |
|---|---|---|---|---|---|---|---|---|
| NETPA | 0.64 | 348,426/512 | 2.42 | −5.24 | −2.82 | 2.68 | −5.39 | −2.71 |
| NMOTPA | 0.92 | 350,420/497 | 2.50 | −5.52 | −3.02 | 2.75 | −5.59 | −2.84 |
| NMTPA | 0.94 | 346,418/493 | 2.52 | −5.54 | −3.02 | 2.77 | −5.58 | −2.81 |
| NTTPA | 0.97 | 344,416/489 | 2.54 | −5.57 | −3.03 | 2.79 | −5.65 | −2.86 |
| PNETPA | 0.42 | 440,634/926 | 1.34 | −5.02 | −3.68 | - | - | - |
| PNMOTPA | 0.58 | 434/780 | 1.59 | −5.18 | −3.59 | - | - | - |
| PNMTPA | 0.85 | 400/550 | 2.26 | −5.45 | −3.19 | - | - | - |
| PNTTPA | 0.77 | 424/530 | 2.34 | −5.37 | −3.03 | - | - | - |
| PMTTPA e | 0.18 | 435/522 | 2.37 | −4.58 | −2.21 | - | - | - |
| PMETPA e | 0.11 | 454/533 | 2.32 | −4.51 | −1.29 | - | - | - |
| 705/955 | 1.29 | −4.51 | −3.22 | - | - | - | ||
| P1 f | 0.60 | 416/502 | 2.47 | −5.00 | −2.53 | - | - | - |
| P2 f | 0.20 | 441/517 | 2.39 | −4.60 | −2.21 | - | - | - |
| 642/900 | 1.37 | −4.60 | −3.23 | - | - | - |
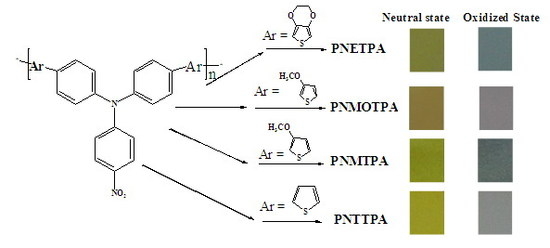
| Polymers | E vs. (Ag wire) (V) | L | a | b | Colors |
|---|---|---|---|---|---|
| PNETPA | 0 | 64.30 | −4.07 | 40.65 | green |
| 1.05 | 64.73 | −5.88 | 1.51 | steel gray | |
| PNMOTPA | 0 | 72.69 | −0.45 | 17.42 | Pale brown |
| 1.2 | 71.18 | 4.07 | −4.95 | light grey | |
| PNMTPA | 0 | 76.77 | −8.60 | 47.10 | yellow green |
| 1.15 | 68.82 | −5.88 | 6.24 | dark gray | |
| PNTTPA | 0 | 93.55 | −13.12 | 51.40 | bright yellow |
| 1.2 | 86.67 | −3.17 | 6.24 | light grey |
| Compounds | λ (nm) | Optical Contrast (ΔT%) | Response Time (s) | Coloration Efficiency (CE, cm2 C−1) | Retained Optical Activity (after 1000 Cycles, ΔT% ) |
|---|---|---|---|---|---|
| PNETPA | 435 | 35.0 | 0.7 | 322.5 | 98.6 |
| 1250 | 65.0 | 0.6 | 358.3 | 99.0 | |
| PNMOTPA | 430 | 30.0 | 0.4 | 207.8 | 97.7 |
| 1030 | 60.0 | 0.6 | 288.6 | 98.2 | |
| PNMTPA | 400 | 26.0 | 1.6 | 247.3 | 95.3 |
| 1210 | 58.0 | 0.7 | 334.6 | 97.0 | |
| PNTTPA | 420 | 20.0 | 1.8 | 280.3 | 94.2 |
| 1200 | 53.0 | 1.7 | 360.6 | 96.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Liu, G.; Ju, X.; Zhang, Y.; Zhao, J. Synthesis, Characterization and Application of Four Novel Electrochromic Materials Employing Nitrotriphenylamine Unit as the Acceptor and Different Thiophene Derivatives as the Donor. Polymers 2017, 9, 173. https://doi.org/10.3390/polym9050173
Li S, Liu G, Ju X, Zhang Y, Zhao J. Synthesis, Characterization and Application of Four Novel Electrochromic Materials Employing Nitrotriphenylamine Unit as the Acceptor and Different Thiophene Derivatives as the Donor. Polymers. 2017; 9(5):173. https://doi.org/10.3390/polym9050173
Chicago/Turabian StyleLi, Shuai, Guoliang Liu, Xiuping Ju, Yan Zhang, and Jinsheng Zhao. 2017. "Synthesis, Characterization and Application of Four Novel Electrochromic Materials Employing Nitrotriphenylamine Unit as the Acceptor and Different Thiophene Derivatives as the Donor" Polymers 9, no. 5: 173. https://doi.org/10.3390/polym9050173