Effect of Preparation Methods on the Tensile, Morphology and Solar Energy Conversion Efficiency of RGO/PMMA Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation of Graphene Oxide (GO)
2.3. Preparation of Reduced Graphene Oxide (RGO)
2.4. Preparation of RGO-PMMA Composite
2.4.1. In Situ Polymerization of RGO-PMMA Nanocomposite
2.4.2. Solution Blending of RGO-PMMA Nanocomposite
2.5. Characterization of Nanofillers and Nanocomposites
2.6. Solar Energy Conversion Efficiency
2.6.1. Specific Heat Capacity Measurement
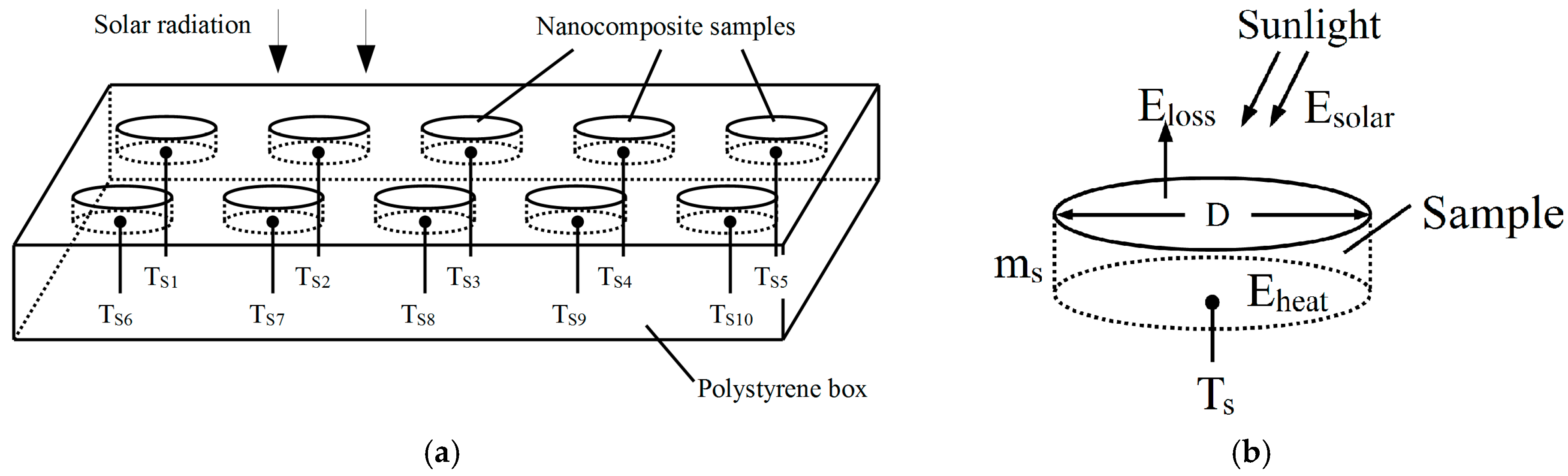
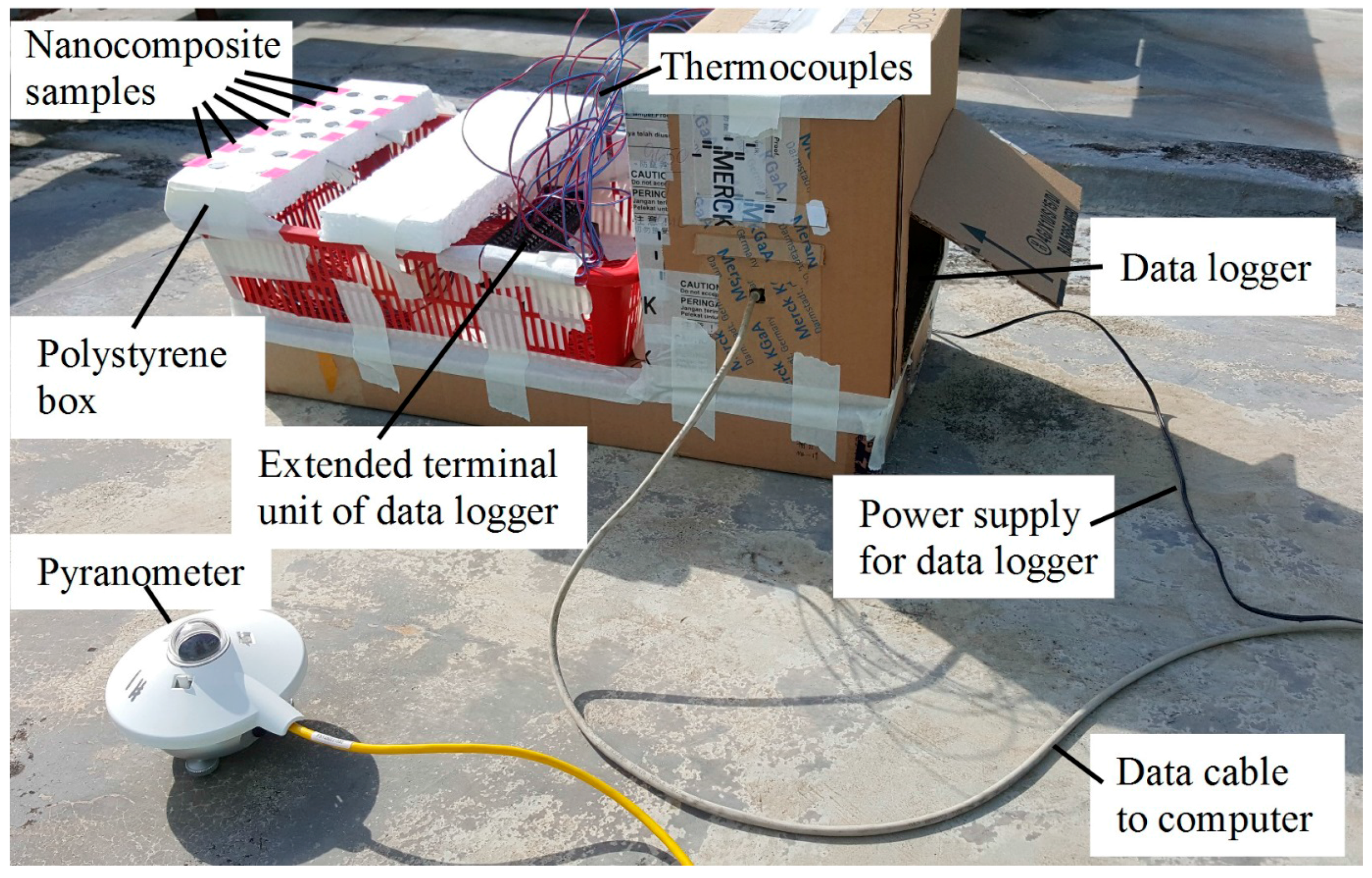
2.6.2. Outdoor Solar Energy Conversion Efficiency Measurement
3. Results and Discussion
3.1. Characterization of the Nanofiller and Nanocomposites
3.1.1. Raman Spectroscopy
3.1.2. X-ray Photoelectron Spectroscopy (XPS)
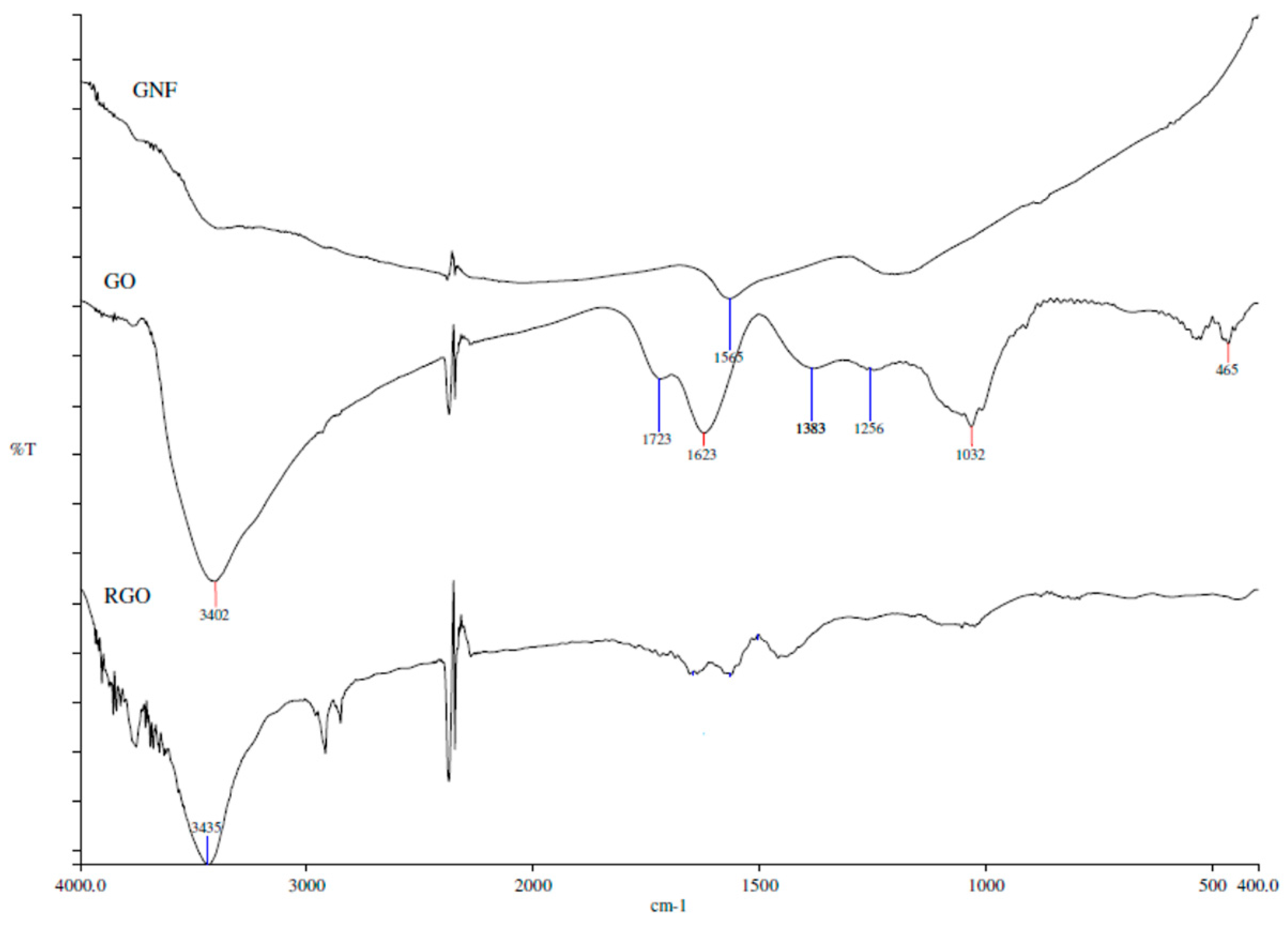
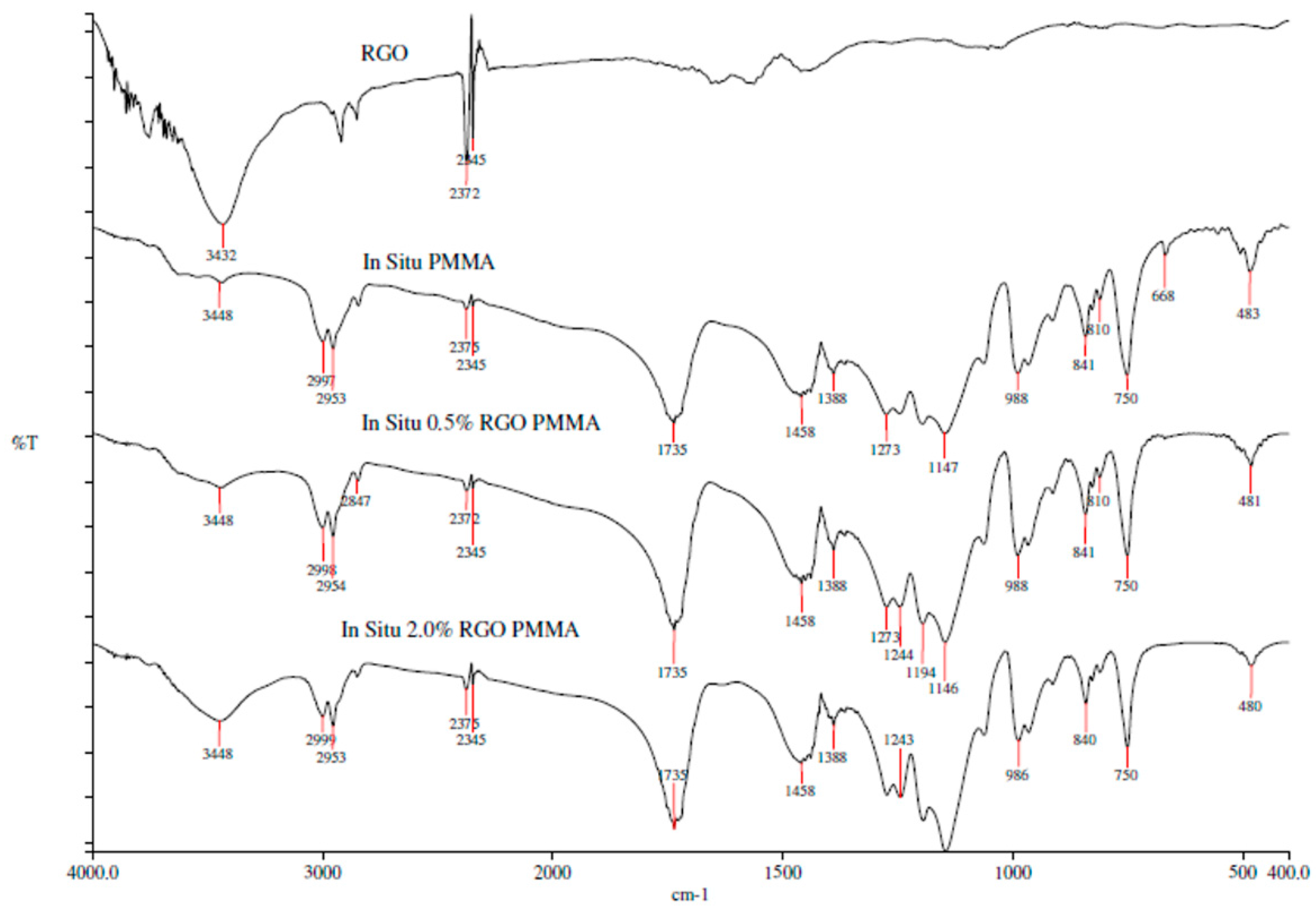
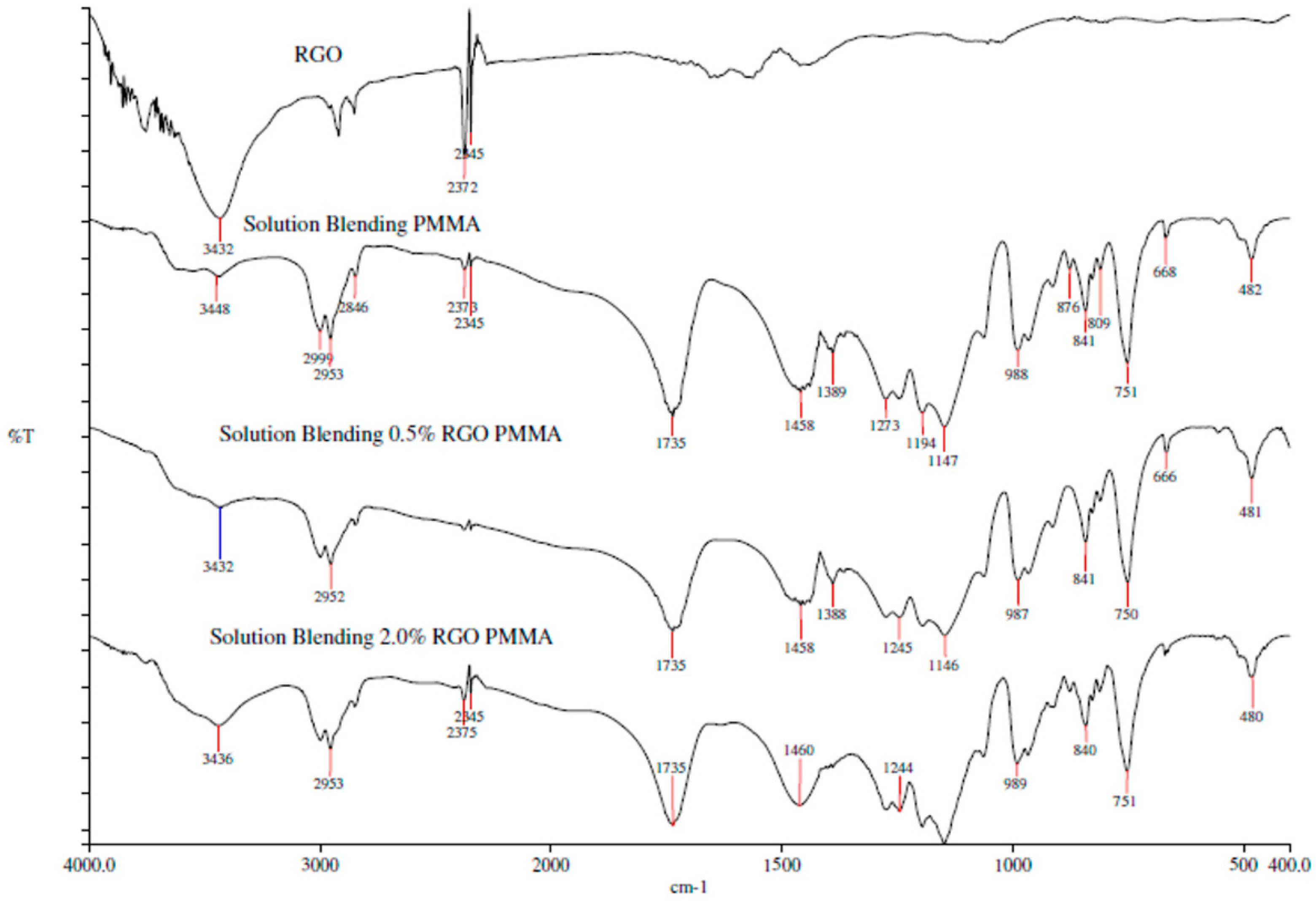
3.1.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.2. Mechanical Properties
3.3. Morphology Properties
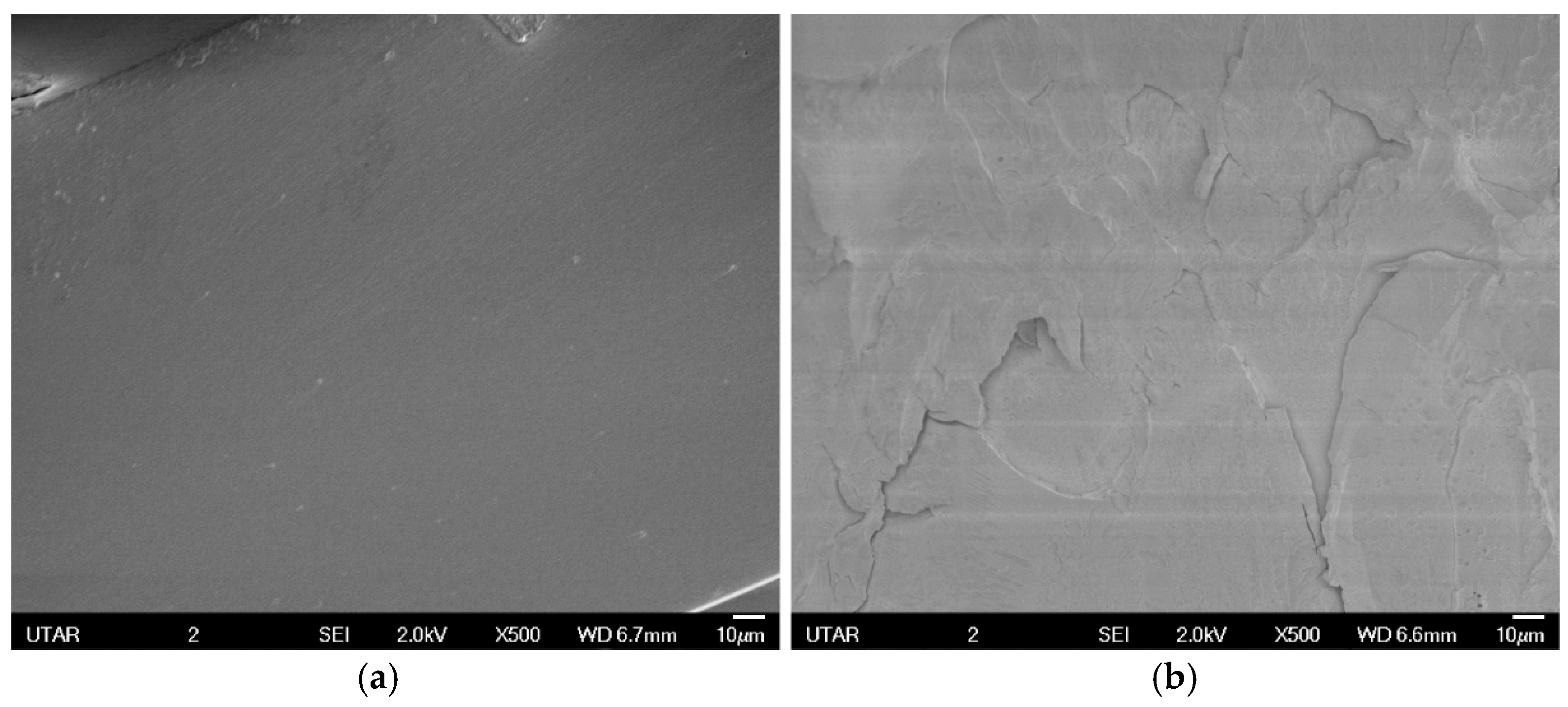
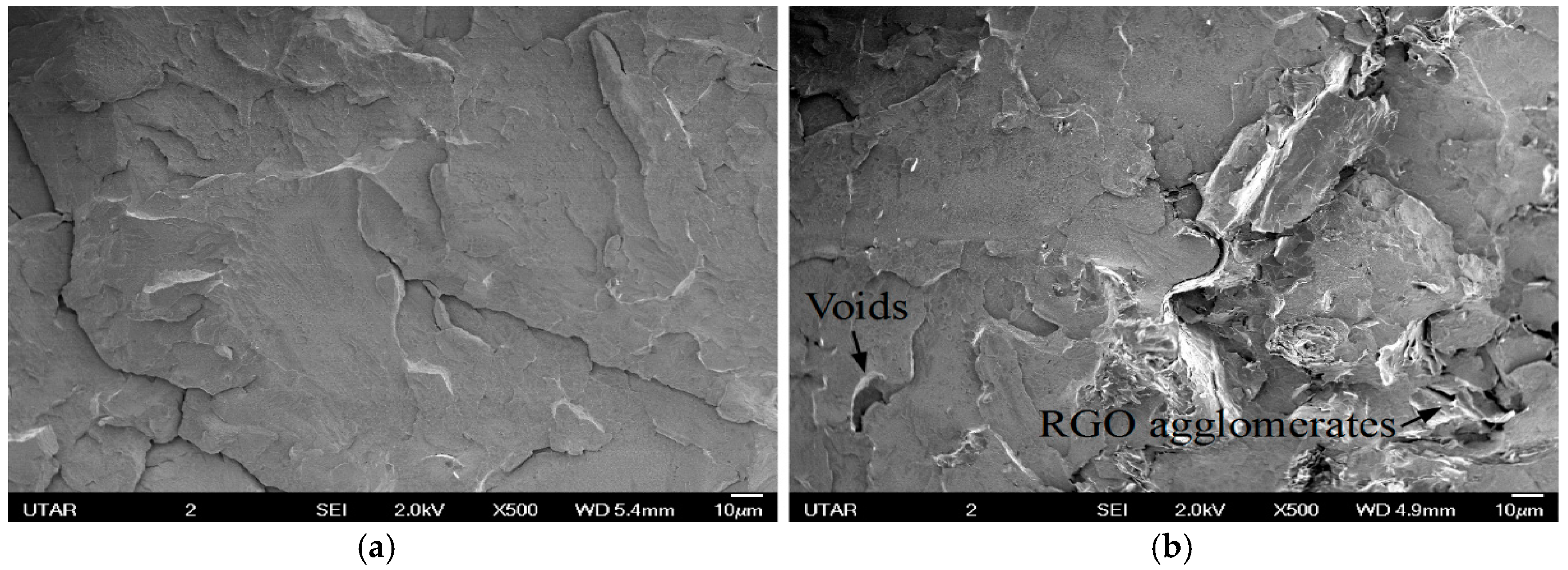
3.3.1. Scanning Electron Microscope (SEM)
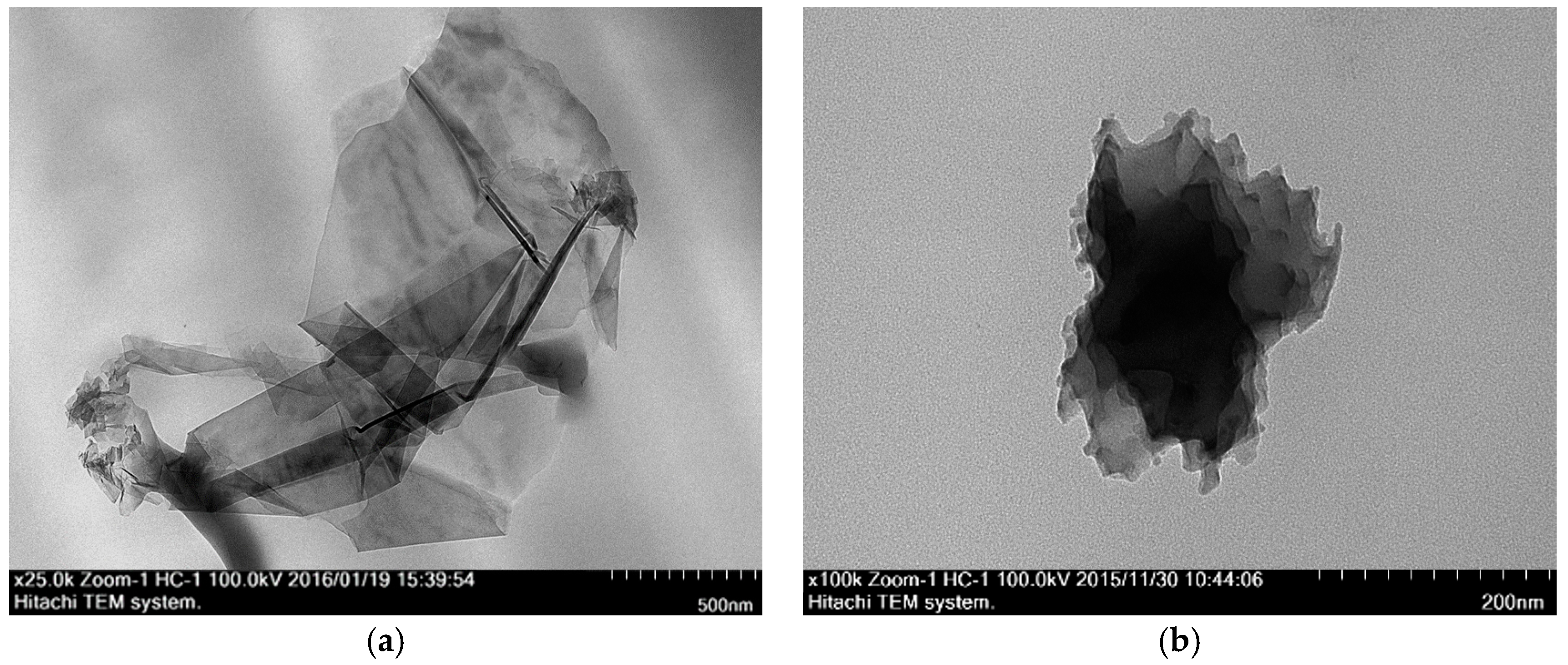
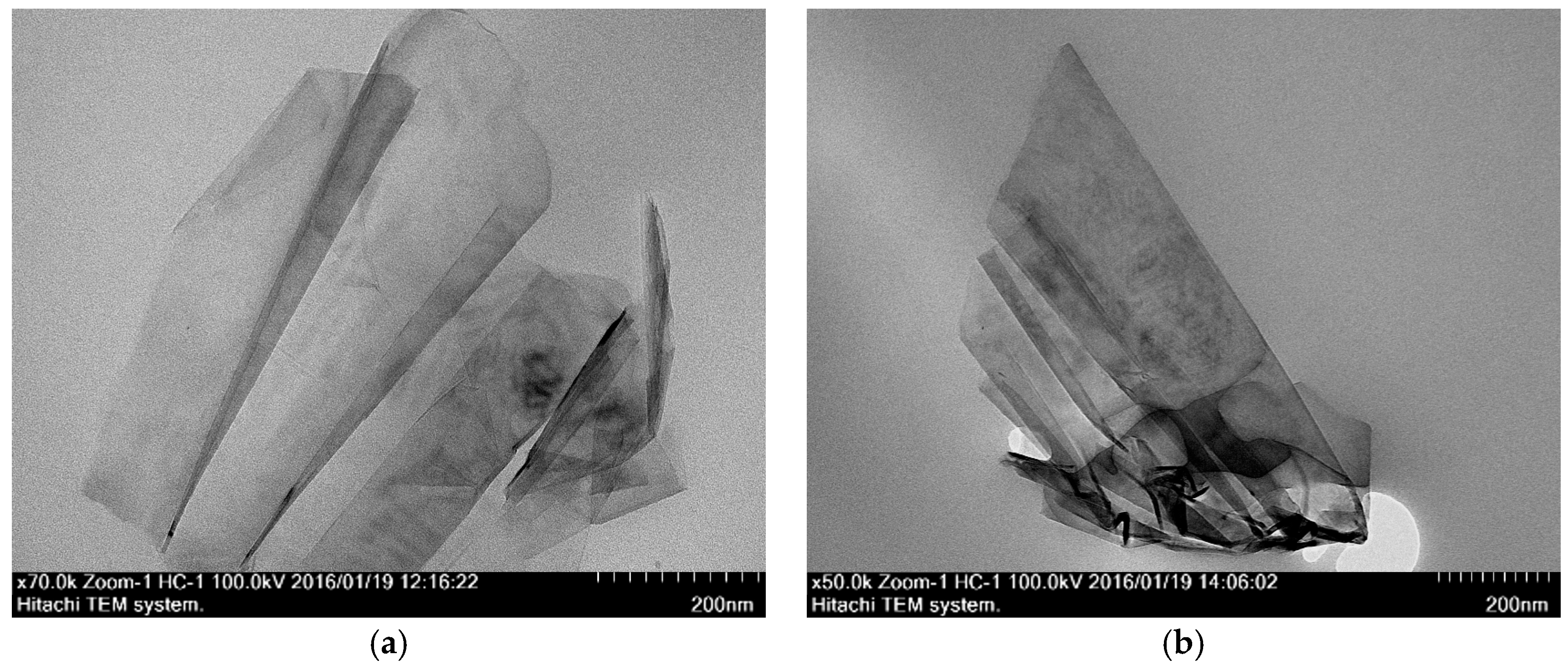
3.3.2. Transmission Electron Microscope (TEM)
3.4. Solar Energy Conversion Efficiency
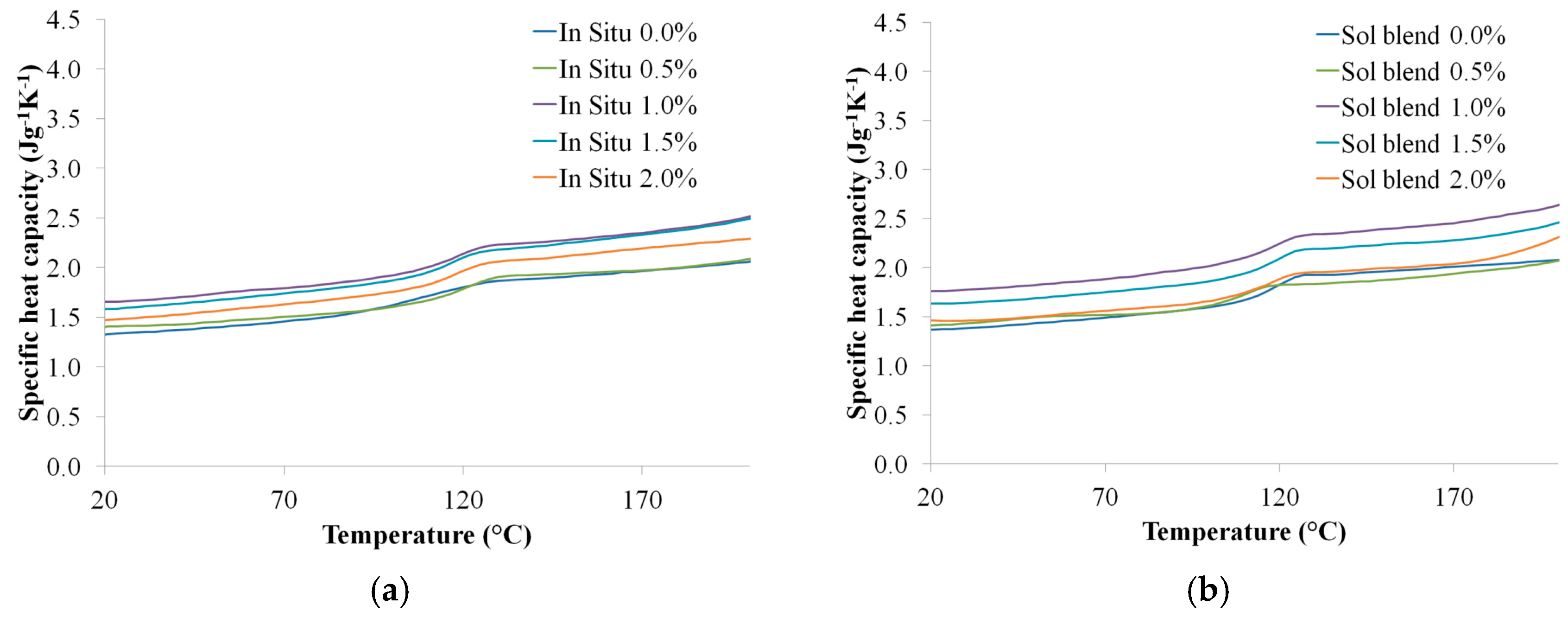
3.4.1. Specific Heat Capacity
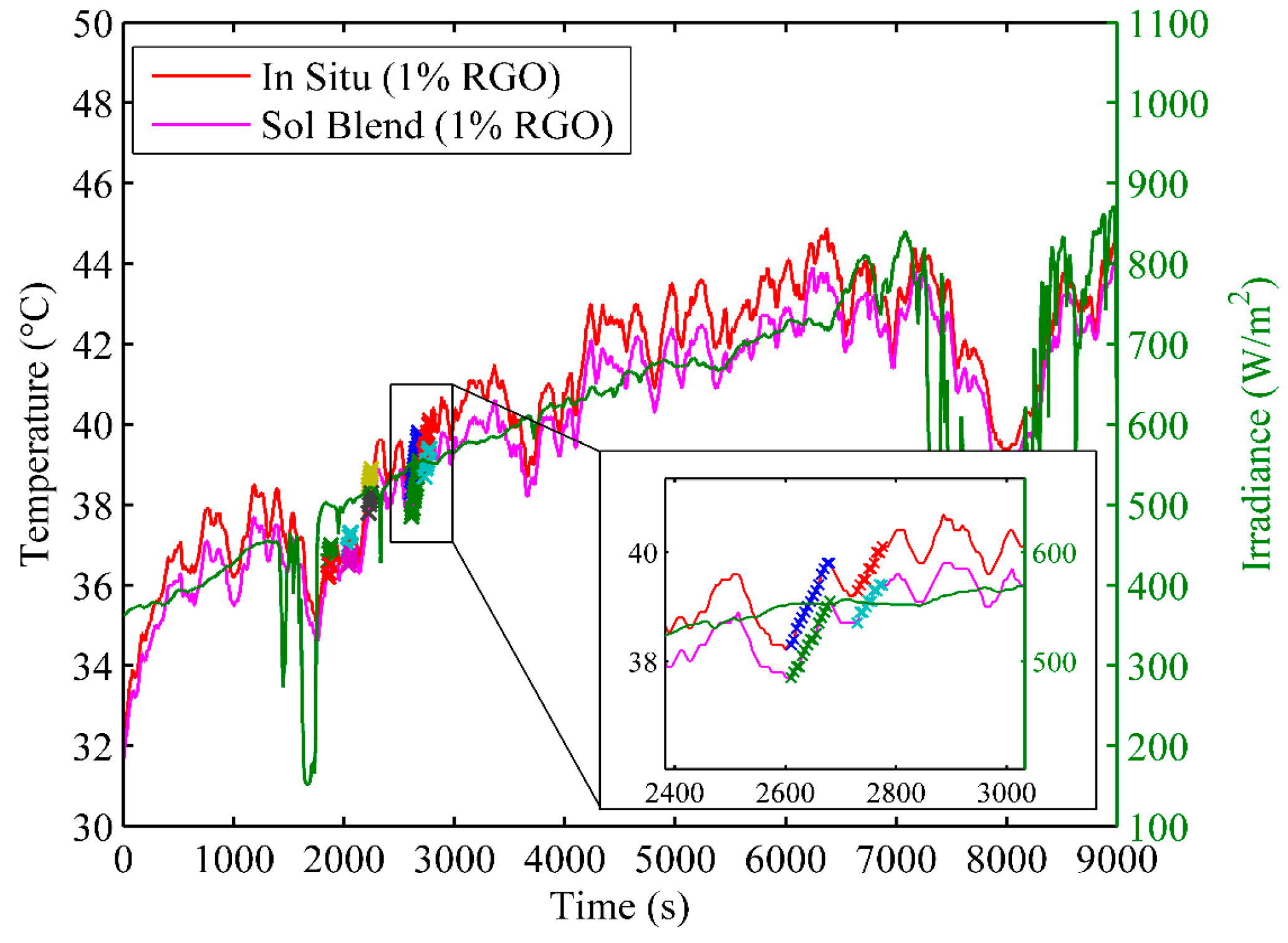
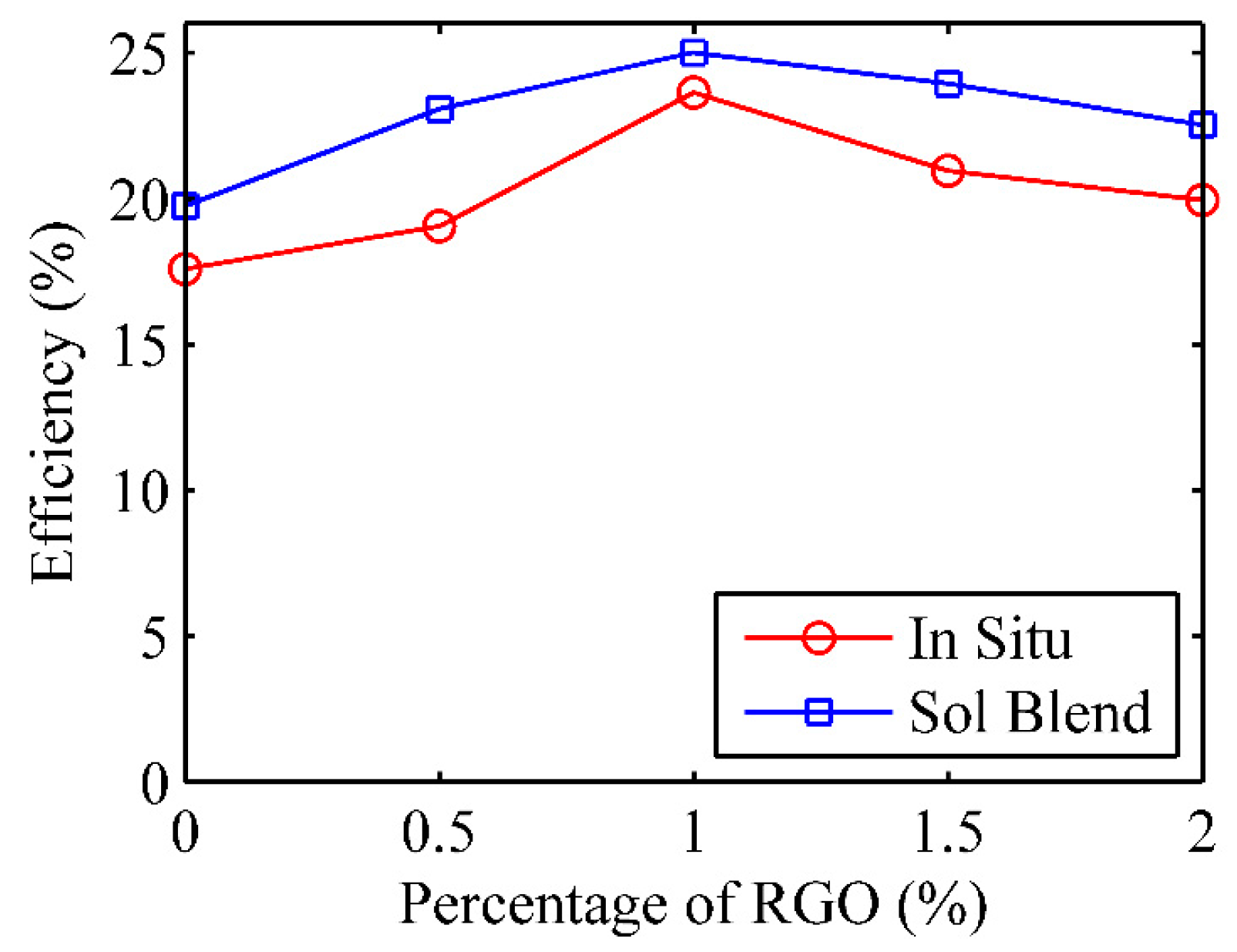
3.4.2. Solar Energy Conversion Efficiency
- (1)
- The starting temperature ranged from 30 to 40 °C;
- (2)
- The solar irradiance ranged from 500 to 1000 W/m2;
- (3)
- The temperatures of all nanocomposites increased for more than 20 s;
- (4)
- The changes in solar irradiance were less than 1% during the period of time.
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, W.S.; Moon, S.Y.; Koyanagi, J.; Ogasawara, T. Three-dimensional porous graphene-based ultra-lightweight aerofoam exhibiting good thermal insulation. Adv. Compos. Mater. 2016, 25, 105–113. [Google Scholar] [CrossRef]
- Berean, K.J.; Ou, J.Z.; Nour, M.; Field, M.R.; Alsaif, M.M.; Wang, Y.; Ramanathan, R.; Bansal, V.; Kentish, S.; Doherty, C.M.; et al. Enhanced gas permeation through graphene nanocomposites. J. Phys. Chem. C 2015, 119, 13700–13712. [Google Scholar] [CrossRef]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef] [PubMed]
- Grandthyll, S.; Gsell, S.; Weinl, M.; Schreck, M.; Hüfner, S.; Müller, F. Epitaxial growth of graphene on transition metal surfaces: Chemical vapor deposition versus liquid phase deposition. J. Phys. Condens. Matter 2012, 24, 314204. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Pan, Y.; Huang, L.; Hu, H.; Zhang, L.Z.; Guo, H.M.; Du, S.X.; Gao, H.-J. Epitaxial growth and structural property of graphene on Pt(111). Appl. Phys. Lett. 2011, 98, 033101. [Google Scholar] [CrossRef]
- Chrissafis, K.; Bikiaris, D. Can nanoparticles really enhance thermal stability of polymers? Part I: An overview on thermal decomposition of addition polymers. Thermochim. Acta 2011, 523, 1–24. [Google Scholar] [CrossRef]
- Berean, K.J.; Ou, J.Z.; Daeneke, T.; Carey, B.J.; Nguyen, E.P.; Wang, Y.; Russo, S.P.; Kaner, R.B.; Kalantar-zadeh, K. 2D MoS2 PDMS Nanocomposites for NO2 Separation. Small 2015, 11, 5035–5040. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Shao, Y.; Ellis, M.W.; Moore, R.B.; Yi, B. Graphene-based electrochemical energy conversion and storage: Fuel cells, supercapacitors and lithium ion batteries. Phys. Chem. Chem. Phys. 2011, 13, 15384–15402. [Google Scholar] [CrossRef] [PubMed]
- Atif, R.; Shyha, I.; Inam, F. Mechanical, thermal, and electrical properties of graphene-epoxy nanocomposites—A Review. Polymers 2016, 8, 281. [Google Scholar] [CrossRef]
- Ghaemi, F.; Abdullah, L.C.; Tahir, P. Core/shell structure of Ni/NiO encapsulated in carbon nanosphere coated with few-and multi-layered graphene: Synthesis, mechanism and application. Polymers 2016, 8, 381. [Google Scholar] [CrossRef]
- Cai, D.; Song, M. Recent advance in functionalized graphene/polymer nanocomposites. J. Mater. Chem. 2010, 20, 7906–7915. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, C.; Hu, H.; Wan, L.; Chen, R.; Zheng, H.; Liu, F.; Zhang, M.; Shang, X.; Wang, X. Synthesis, mechanical, and barrier properties of LDPE/graphene nanocomposites using vinyl triethoxysilane as a coupling agent. J. Nanopart. Res. 2010, 13, 869–878. [Google Scholar] [CrossRef]
- Ahmadi-Moghadam, B.; Sharafimasooleh, M.; Shadlou, S.; Taheri, F. Effect of functionalization of graphene nanoplatelets on the mechanical response of graphene/epoxy composites. Mater. Des. 2015, 66 Pt A, 142–149. [Google Scholar] [CrossRef]
- Araby, S.; Saber, N.; Ma, X.; Kawashima, N.; Kang, H.; Shen, H.; Zhang, L.; Xu, J.; Majewski, P.; Ma, J. Implication of multi-walled carbon nanotubes on polymer/graphene composites. Mater. Des. 2015, 65, 690–699. [Google Scholar] [CrossRef]
- Al-Hartomy, O.A.; Al-Ghamdi, A.; Al-Salamy, F.; Dishovsky, N.; Shtarkova, R.; Iliev, V.; El-Tantawy, F. Effect of carbon nanotubes and graphene nanoplatelets on the dielectric and microwave properties of natural rubber composites. Adv. Compos. Mater. 2013, 22, 361–376. [Google Scholar] [CrossRef]
- Gaska, K.; Xu, X.; Gubanski, S.; Kádár, R. Electrical, Mechanical, and Thermal Properties of LDPE Graphene Nanoplatelets Composites Produced by Means of Melt Extrusion Process. Polymers 2017, 9, 11. [Google Scholar] [CrossRef]
- Yi, P.; Awang, R.A.; Rowe, W.S.; Kalantar-zadeh, K.; Khoshmanesh, K. PDMS nanocomposites for heat transfer enhancement in microfluidic platforms. Lab Chip 2014, 14, 3419–3426. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.Q.; Liu, Z.; Gao, J.G.; Chen, C.M.; Zhang, Q.; Zhou, G.M.; Tao, Z.C.; Zhang, X.H.; Wang, M.Z.; Li, F.; et al. Hierarchical graphene-carbon fiber composite paper as a flexible lateral heat spreader. Adv. Funct. Mater. 2014, 24, 4222–4228. [Google Scholar] [CrossRef]
- Pham, V.H.; Dang, T.T.; Hur, S.H.; Kim, E.J.; Chung, J.S. Highly Conductive Poly(methyl methacrylate) (PMMA)-Reduced Graphene Oxide Composite Prepared by Self-Assembly of PMMA Latex and Graphene Oxide through Electrostatic Interaction. ACS Appl. Mater. Interfaces 2012, 4, 2630–2636. [Google Scholar] [CrossRef] [PubMed]
- Shahil, K.M.; Balandin, A.A. Graphene–Multilayer Graphene Nanocomposites as Highly Efficient Thermal Interface Materials. Nano Lett. 2012, 12, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.F.; Ju, L.; Wang, F.; Heinz, T.F. Optical spectroscopy of graphene: From the far infrared to the ultraviolet. Solid State Commun. 2012, 152, 1341–1349. [Google Scholar] [CrossRef]
- Choi, J.O.; Moore, J.A.; Corelli, J.C.; Silverman, J.P.; Bakhru, H. Degradation of poly(methylmethacrylate) by deep ultraviolet, X-ray, electron beam, and proton beam irradiations. J. Vac. Sci. Technol. B Microelectron. Process. Phenom. 1988, 6, 2286–2289. [Google Scholar] [CrossRef]
- Villar-Rodil, S.; Paredes, J.I.; Martínez-Alonso, A.; Tascón, J.M. Preparation of graphene dispersions and graphene-polymer composites in organic media. J. Mater. Chem. 2009, 19, 3591–3593. [Google Scholar] [CrossRef]
- Lightcap, I.V.; Kamat, P.V. Graphitic design: Prospects of graphene-based nanocomposites for solar energy conversion, storage, and sensing. Acc. Chem. Res. 2013, 46, 2235–2243. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, N.; Zhang, C.; Yin, H.; Hou, Y. Graphene-based nanocomposites for energy storage and conversion in lithium batteries, supercapacitors and fuel cells. J. Mater. Chem. A 2014, 2, 15–32. [Google Scholar] [CrossRef]
- Qu, L.; Zhao, Y.; Cui, L.; Gao, J.; Xu, T. Polymer/graphene hybrids for advanced energy conversion and storage materials. Chem. Asian J. 2016, 11, 1151–1168. [Google Scholar]
- Rouaramadan, E.; Hasan, A.A. Study of the optical constants of the PVC/PMMA blends. Int. J. Appl. Innov. Eng. Manag. 2013, 2, 240–245. [Google Scholar]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Childres, I.; Jauregui, L.A.; Park, W.; Cao, H.; Chen, Y.P. Raman spectroscopy of graphene and related materials. In New Developments Photon and Materials Research; Nova Science Publishers: New York, NY, USA, 2013; pp. 978–981. [Google Scholar]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Yang, D.; Velamakanni, A.; Bozoklu, G.; Park, S.; Stoller, M.; Piner, R.D.; Stankovich, S.; Jung, I.; Field, D.A.; Ventrice, C.A., Jr.; et al. Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy. Carbon 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Ren, P.-G.; Yan, D.-X.; Ji, X.; Chen, T.; Li, Z.-M. Temperature dependence of graphene oxide reduced by hydrazine hydrate. Nanotechnology 2011, 22, 055705. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, K.; Joshi, G.M. Thermo-mechanical properties of poly (vinyl chloride)/graphene oxide as high performance nanocomposites. Polym. Test. 2014, 34, 211–219. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Hussein, M.Z. Poly(lactic acid)/Poly(ethylene glycol) Polymer Nanocomposites: Effects of Graphene Nanoplatelets. Polymers 2013, 6, 93–104. [Google Scholar] [CrossRef]
- Tripathi, S.N.; Saini, P.; Gupta, D.; Choudhary, V. Electrical and mechanical properties of PMMA/reduced graphene oxide nanocomposites prepared via in situ polymerization. J. Mater. Sci. 2013, 48, 6223–6232. [Google Scholar] [CrossRef]
- Kuila, T.; Acharya, H.; Srivastava, S.K.; Bhowmick, A.K. Effect of vinyl acetate content on the mechanical and thermal properties of ethylene vinyl acetate/MgAl layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2008, 108, 1329–1335. [Google Scholar] [CrossRef]







| Method | RGO loading (wt %) | Young’s modulus (MPa) | Tensile strength (MPa) | Elongation at break (%) |
|---|---|---|---|---|
| In situ | 0.0 | 362.28 ± 9.79 | 47.73 ± 1.75 | 29.96 ± 2.77 |
| 0.5 | 428.51 ± 32.32 | 44.10 ± 3.02 | 13.50 ± 1.13 | |
| 2.0 | 288.72 ± 48.96 | 29.68 ± 1.25 | 13.95 ± 1.76 | |
| Solution Blending | 0.0 | 330.47 ± 26.00 | 35.89 ± 1.88 | 15.10 ± 2.61 |
| 0.5 | 463.02 ± 47.66 | 36.64 ± 1.18 | 10.02 ± 1.10 | |
| 2.0 | 470.72 ± 33.34 | 25.51 ± 6.01 | 6.94 ± 1.13 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kee, S.Y.; Munusamy, Y.; Ong, K.S.; Lai, K.C. Effect of Preparation Methods on the Tensile, Morphology and Solar Energy Conversion Efficiency of RGO/PMMA Nanocomposites. Polymers 2017, 9, 230. https://doi.org/10.3390/polym9060230
Kee SY, Munusamy Y, Ong KS, Lai KC. Effect of Preparation Methods on the Tensile, Morphology and Solar Energy Conversion Efficiency of RGO/PMMA Nanocomposites. Polymers. 2017; 9(6):230. https://doi.org/10.3390/polym9060230
Chicago/Turabian StyleKee, Shin Yiing, Yamuna Munusamy, Kok Seng Ong, and Koon Chun Lai. 2017. "Effect of Preparation Methods on the Tensile, Morphology and Solar Energy Conversion Efficiency of RGO/PMMA Nanocomposites" Polymers 9, no. 6: 230. https://doi.org/10.3390/polym9060230






