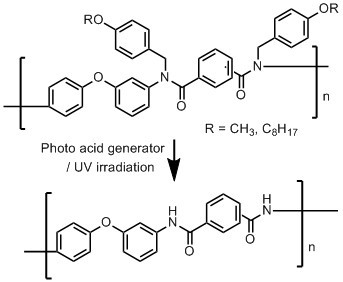
Photodeprotectable N-Alkoxybenzyl Aromatic Polyamides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Measurement
2.2. Materials
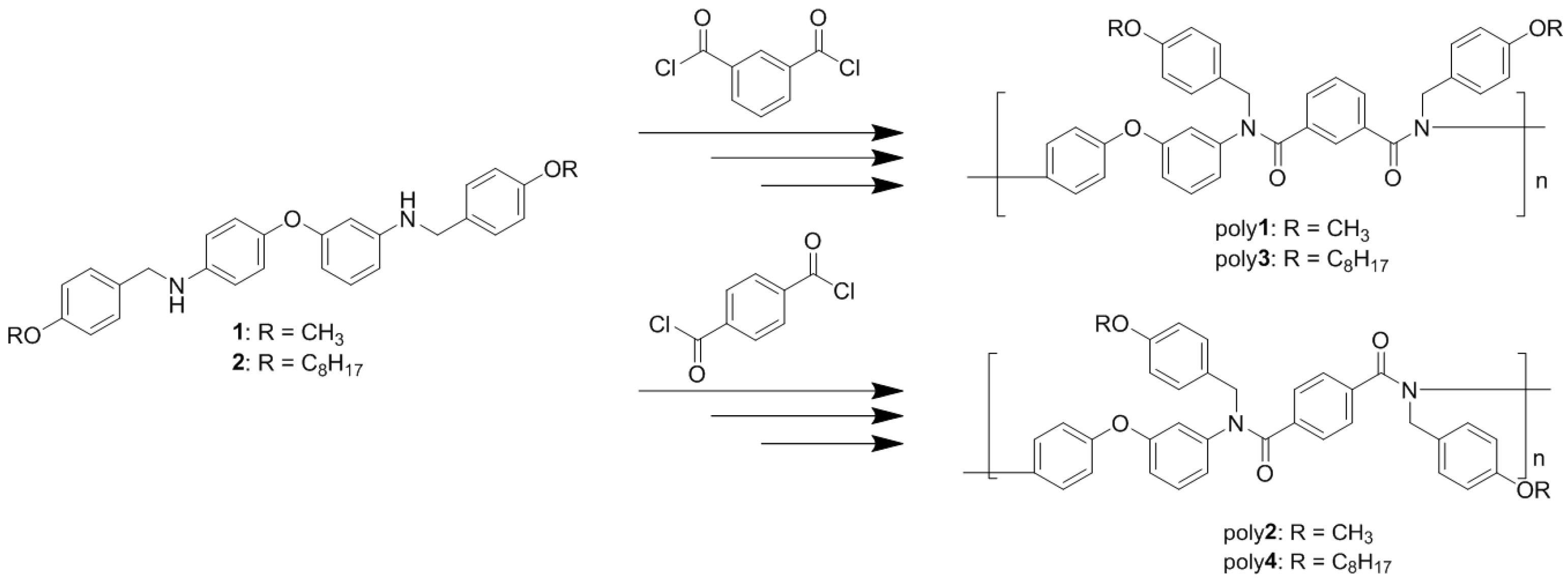
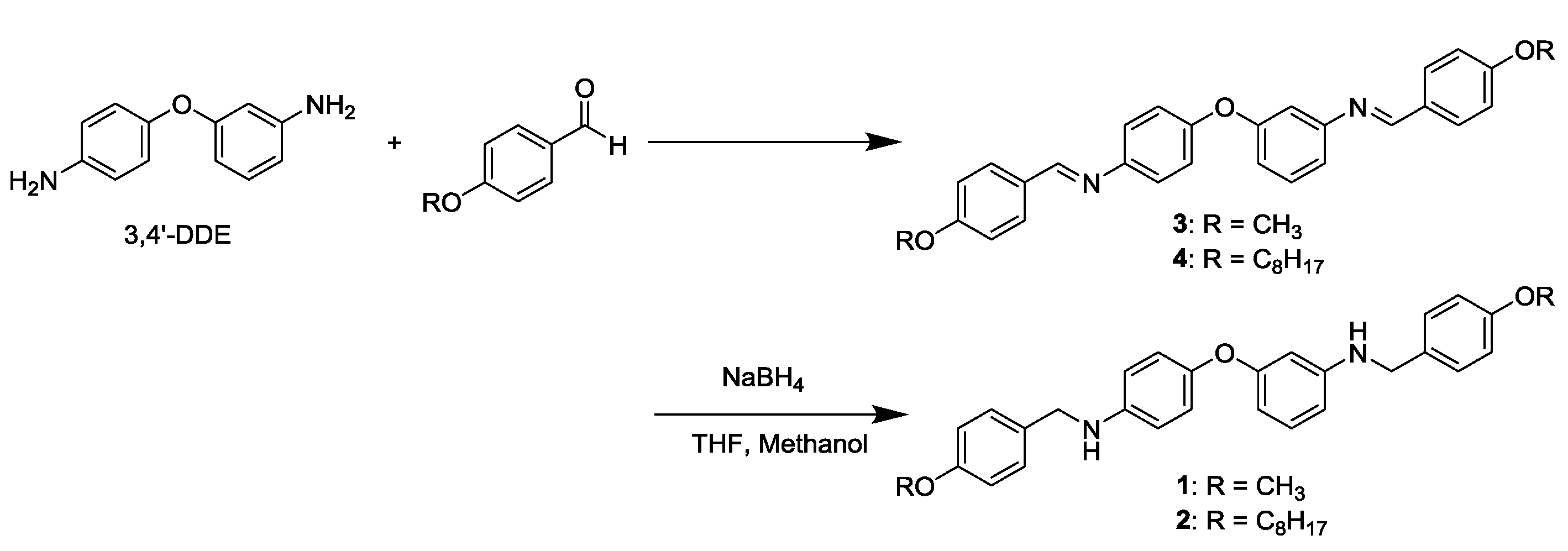
2.3. Synthesis of N-Alkoxybenzyl Aromatic Diamines 1 and 2
2.3.1. Diimine 3
2.3.2. N,N′-bis(4-methoxybenzyl)diamine 1
2.3.3. Diimine 4
2.3.4. N,N′-Bis(4-octyloxybenzyl)diamine 2
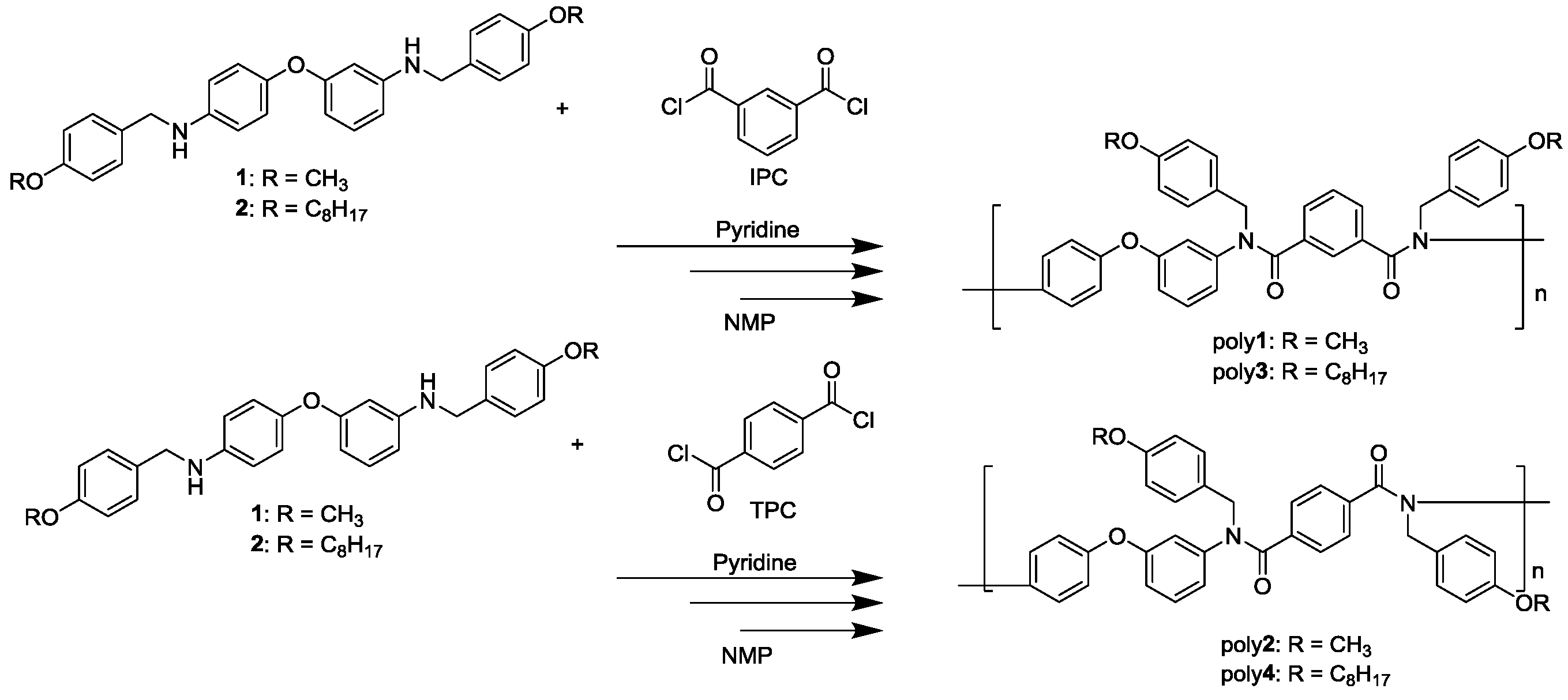
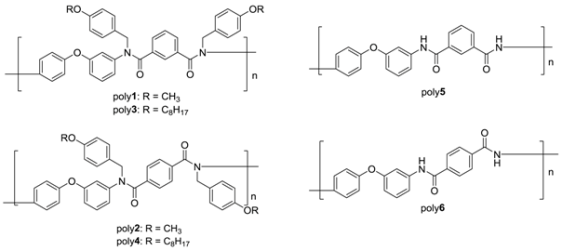
2.4. Synthesis of N-Alkoxybenzyl Aromatic Polyamide
2.4.1. Synthesis of Poly1
2.4.2. Synthesis of Poly2
2.4.3. Synthesis of Poly3
2.4.4. Synthesis of Poly4
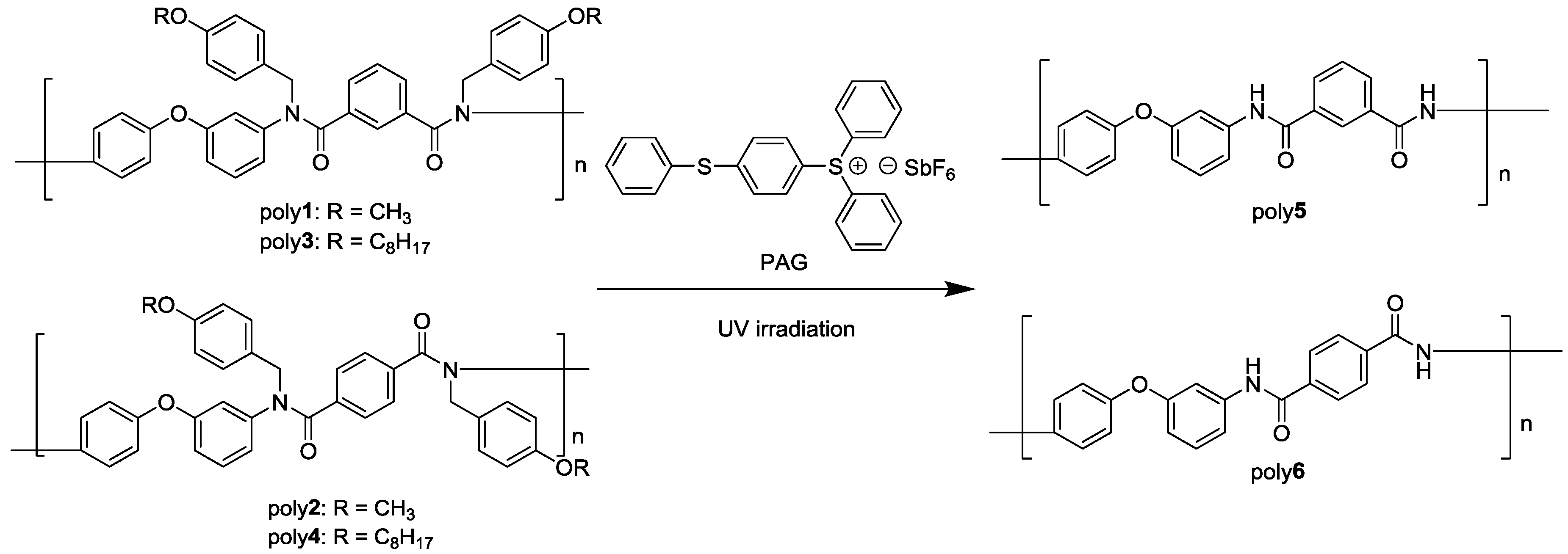
2.5. Synthesis of N-H Aromatic Polyamide
2.5.1. Synthesis of Poly5
2.5.2. Synthesis of Poly6
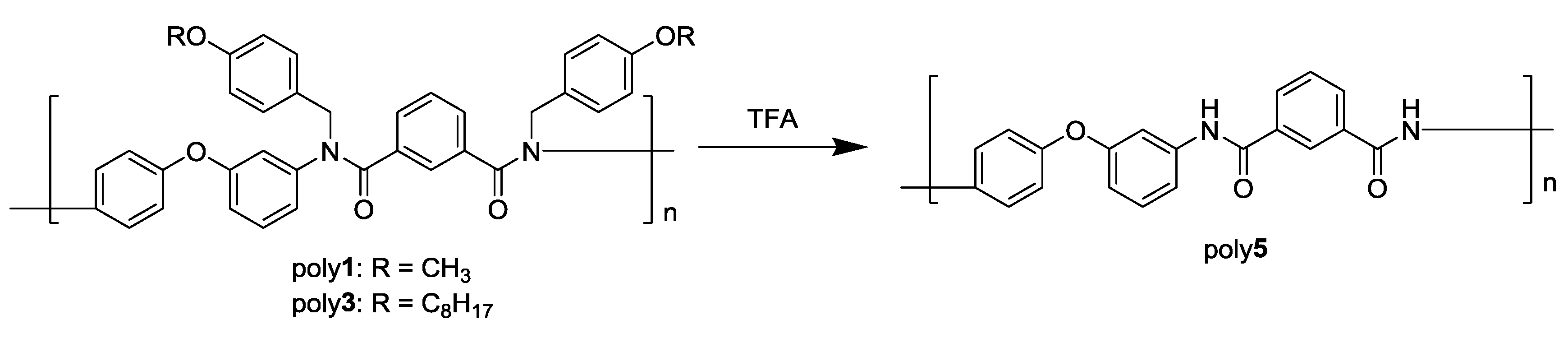
2.6. Deprotection of N-Alkoxybenzyl Aromatic Polyamide
2.7. Evaluation of Photosensitivity
2.7.1. Preparation of Photosensitive Film
2.7.2. Evaluation of Photo-Deprotection
3. Results and Discussion
3.1. Synthesis of N-Alkoxybenzyl Aromatic Diamine
3.2. Synthesis of N-Alkoxybenzyl Aromatic Polyamide
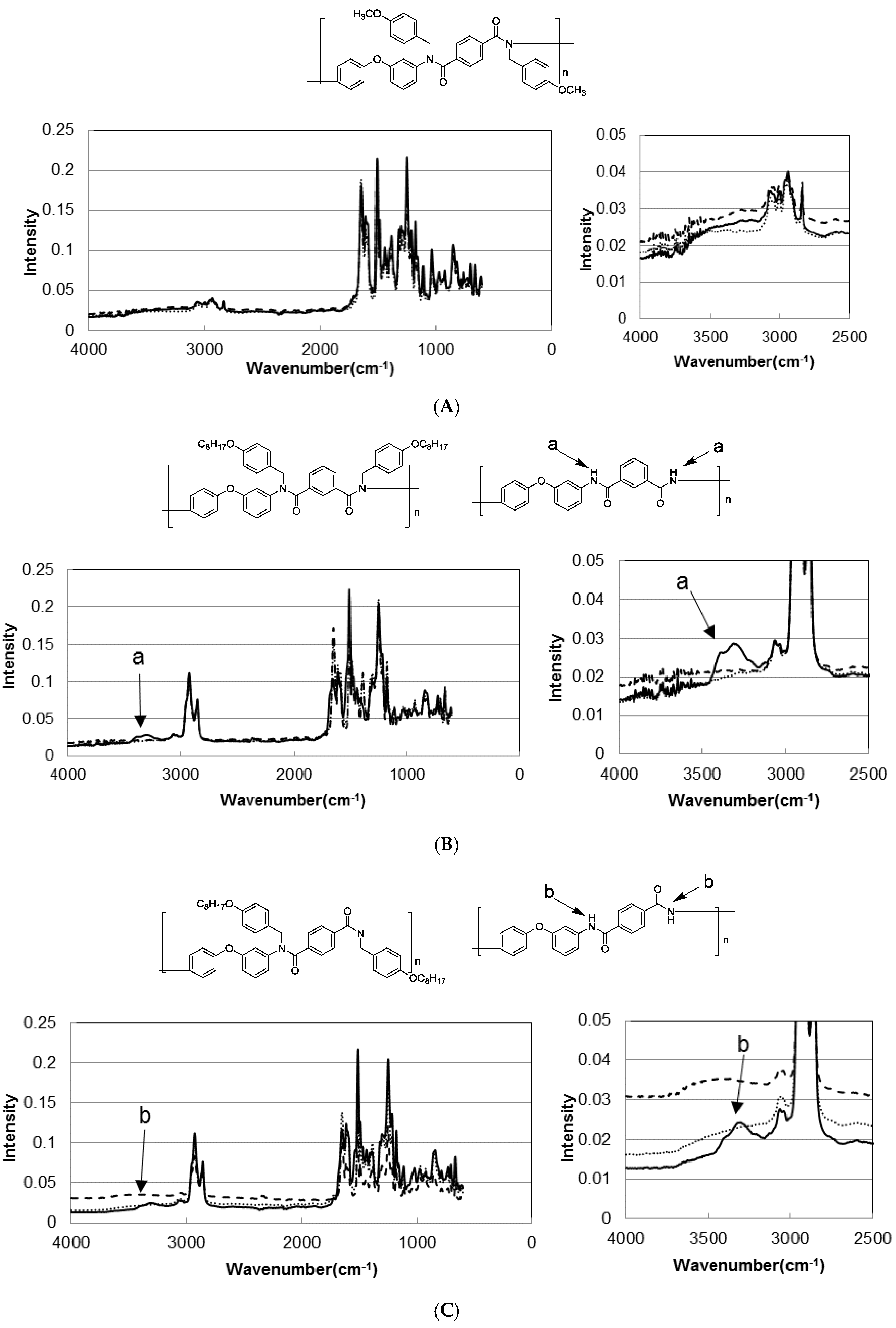
3.3. Photodeprotection of N-Alkoxybenzyl Aromatic Polyamide
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kanki, T.; Ikeda, J.; Kobayashi, Y.; Suda, S.; Nakata, Y.; Nakamura, T. Development of Highly Reliable Cu Wiring of l/s = 1/1mm for Chip to Chip Interconnection. In Proceedings of the 2012 IEEE International Interconnect Technology Conference, San Jose, CA, USA, 4–6 June 2012; pp. 1–3. [Google Scholar]
- Hu, D.-C.; Lin, P.; Chen, Y.H. Fine trace substrate with 2 mm fine line for advanced package. Trans. Jpn. Inst. Electron. Packag. 2015, 8, 18–22. [Google Scholar] [CrossRef]
- Huemoeller, R.; Rusli, S.; Chiang, S.; Chen, T.Y.; Baron, D.; Brandt, L.; Roelfs, B. Unveiling the Next generation in substrate technology. In Proceedings of the Pan Pacific Conference, Bangkok, Thailand, 17–19 October 2007. [Google Scholar]
- Ghosh, M. Polyimides: Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Rubner, R. Photoreactive polymers for electronics. Adv. Mater. 1990, 2, 452–457. [Google Scholar] [CrossRef]
- Iwashita, K.; Katoh, T.; Nakamura, A.; Murakami, Y.; Iwasaki, T.; Sugimasa, Y.; Nunoshige, J.; Nakano, H. Study of adhesion properties of Cu on photosensitive insulation film for next generation packaging. J. Photopolym. Sci. Technol. 2015, 28, 93–97. [Google Scholar] [CrossRef]
- Mark, H.F.; Atlas, S.M.; Ogata, N. Aromatic polyamide. J. Polym. Sci. 1962, 61, S49–S53. [Google Scholar] [CrossRef]
- Dine-Hart, R.A.; Moore, B.J.C.; Wright, W.W. Aromatic polyamides. J. Polym. Sci. B 1964, 2, 369–373. [Google Scholar] [CrossRef]
- Harschnitz, R.; Heather, P.; Derks, W.; van Leeuwendal, R. Polyamide 46—A design material for demanding engineering applications. Kunstst. Ger. Plast. 1990, 80, 1271–1276. [Google Scholar]
- Gijsman, P.; Tummers, D.; Janssen, K. Differences and similarities in the thermooxidative degradation of polyamide 46 and 66. Polym. Degrad. Stab. 1995, 49, 121–125. [Google Scholar] [CrossRef]
- Janssen, K.; Gijsman, P.; Tummers, D. Mechanistic aspects of the stabilization of polyamides by combinations of metal and halogen salts. Polym. Degrad. Stab. 1995, 49, 127–133. [Google Scholar] [CrossRef]
- Arai, T.; Ueno, T.; Kajiya, T.; Ishikawa, T.; Takeda, K. Study on thermal stability and chemical structure of polyamide blended with small amount of Cu. Kobunshi Ronbunshu 2007, 64, 380–386. [Google Scholar] [CrossRef]
- Ohtsuka, K.; Matsumoto, A.; Kimura, H.; Saito, M.; Yamano, K. Improvement of adhesive property of diallyl phthalate resin modified with dimeric acid polyamide. J. Netw. Polym. Jpn. 2008, 29, 86–93. [Google Scholar]
- Yokozawa, T.; Ogawa, M.; Sekino, A.; Sugi, R.; Yokoyama, A. Chain-growth polycondensation for well-defined aramide. Synthesis of unprecedented block copolymer containing aramide with low polydispersity. J. Am. Chem. Soc. 2002, 124, 15158–15159. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, T.; Sugi, R.; Yokoyama, A.; Yokozawa, T. A variety of poly(m-benzamide)s with low polydispersities from inductive effect-assisted chain-growth polycondensation. J. Polym. Sci. A 2006, 44, 4990–5003. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Schwarz, G. Cyclic polymers by kinetically controlled step-growth polymerization. Macromol. Rapid Commun. 2003, 24, 359–381. [Google Scholar] [CrossRef]
- Kricheldorf, H.R. Simultaneous chain-growth and step-growth polymerization—A new route to cyclic polymers. Macromol. Rapid Commun. 2009, 30, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, Y. Surface wettability controllable polyimides by uv light irradiation for printed electronics. J. Photopolym. Sci. Technol. 2016, 29, 383–390. [Google Scholar] [CrossRef]



| Entry | N-Alkoxybenzyl Aromatic Diamine | Dicarboxylic Acid Chloride | N-Alkoxybenzyl Aromatic Polyamide | Yield (%) | Mn b | Mw b | Mw/Mn b |
|---|---|---|---|---|---|---|---|
| 1 | 1 | IPC | poly1 | 94 | 8630 | 13,500 | 1.57 |
| 2 | 1 | TPC | poly2 | 91 | 5200 | 7070 | 1.36 |
| 3 | 2 | IPC | poly3 | 96 | 14,900 | 23,250 | 1.56 |
| 4 | 2 | TPC | poly4 | 77 | 7250 | 9750 | 1.30 |
| Solvent | poly1 | poly2 | poly3 | poly4 | poly5 | poly6 |
|---|---|---|---|---|---|---|
| Hexane | − | − | − | − | − | − |
| Acetone | + | + | + | + | − | − |
| Ethyl acetate | + | + | + | + | − | − |
| CH2Cl2 | + | + | + | + | − | − |
| Chloroform | + | + | + | + | − | − |
| Methanol | − | − | − | − | − | − |
| DMF | + | + | + | + | + | + |
| NMP | + | + | + | + | + | + |
| DMSO | + | + | + | + | + | + |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwashita, K.; Katoh, H.; Ohta, Y.; Yokozawa, T. Photodeprotectable N-Alkoxybenzyl Aromatic Polyamides. Polymers 2017, 9, 246. https://doi.org/10.3390/polym9070246
Iwashita K, Katoh H, Ohta Y, Yokozawa T. Photodeprotectable N-Alkoxybenzyl Aromatic Polyamides. Polymers. 2017; 9(7):246. https://doi.org/10.3390/polym9070246
Chicago/Turabian StyleIwashita, Kenichi, Hironobu Katoh, Yoshihiro Ohta, and Tsutomu Yokozawa. 2017. "Photodeprotectable N-Alkoxybenzyl Aromatic Polyamides" Polymers 9, no. 7: 246. https://doi.org/10.3390/polym9070246