In Vitro Response of Human Peripheral Blood Mononuclear Cells (PBMC) to Collagen Films Treated with Cold Plasma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collagen Film Preparation and Plasma Treatment
2.2. In Vitro Experiment with Peripheral Blood Mononuclear Cell (PBMC)
2.2.1. PBMC Isolation
2.2.2 PBMC Culture on Collagen Biomaterials
2.3. Characterization of Materials Surface
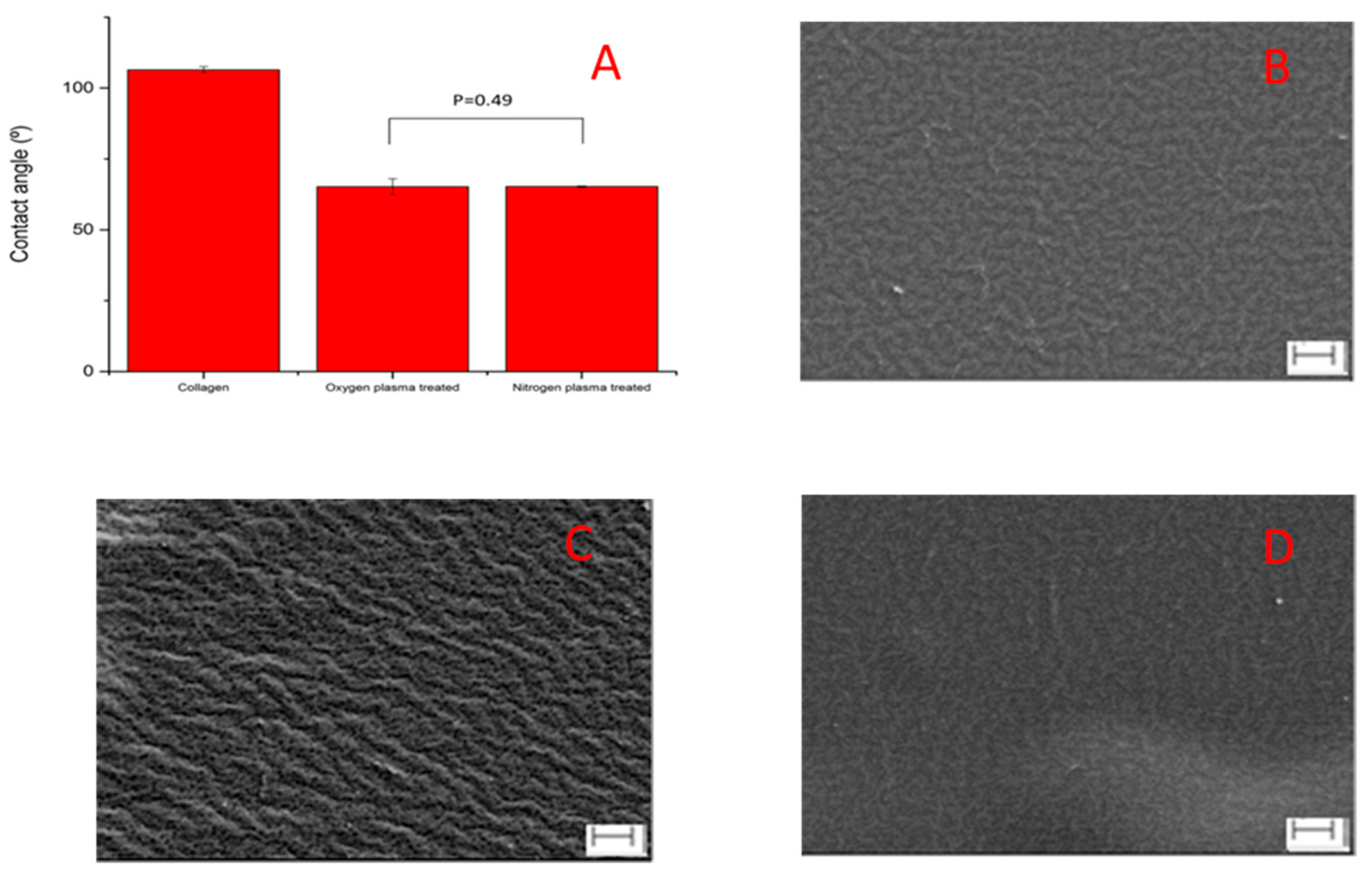
2.3.1. Advancing Contact Angle
2.3.2. Scanning Electron Microscopy (SEM)
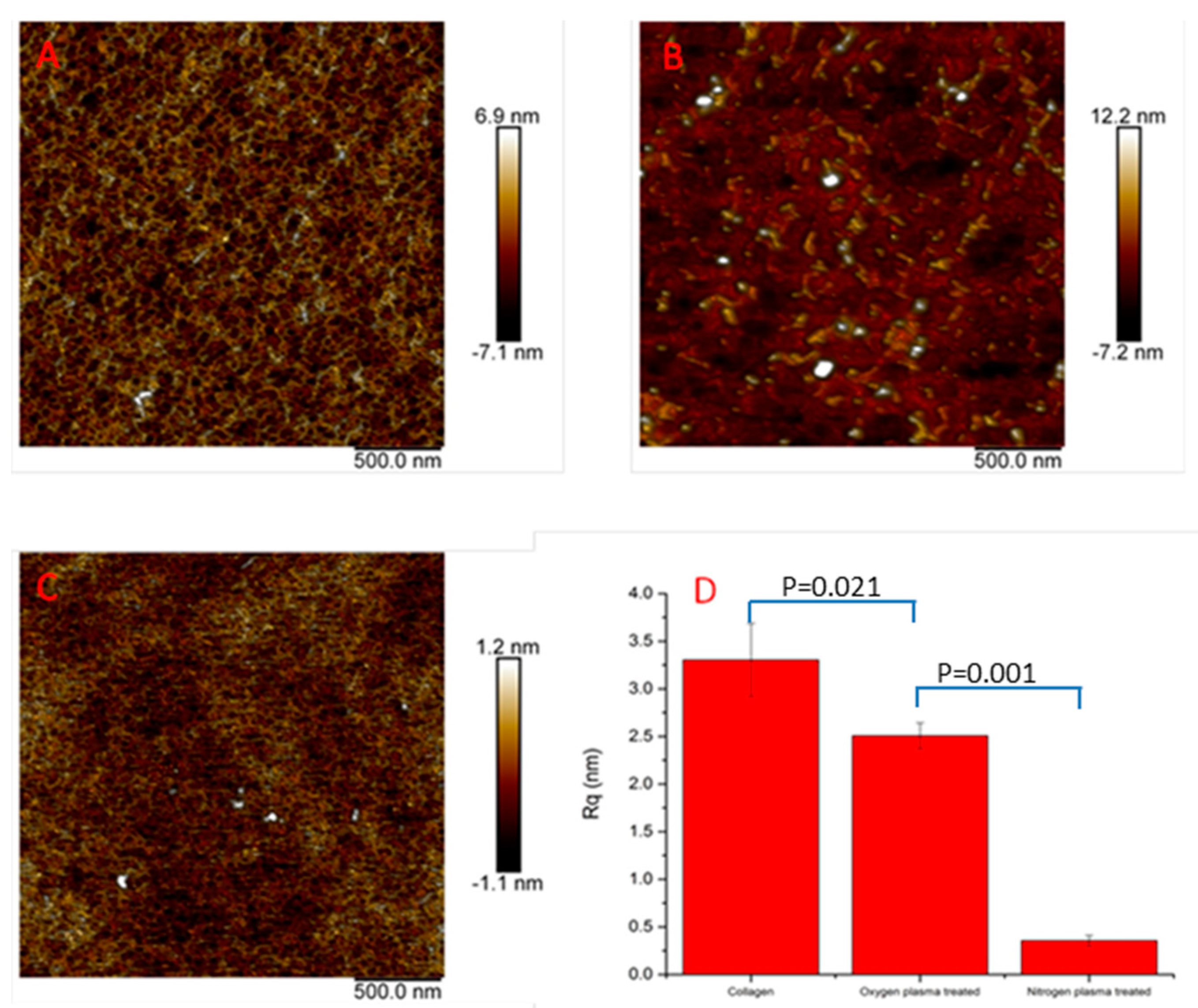
2.3.3. Atomic Force Microscopy (AFM)
2.4. Analysis of PBMCs Cultured on Collagen Biomaterials
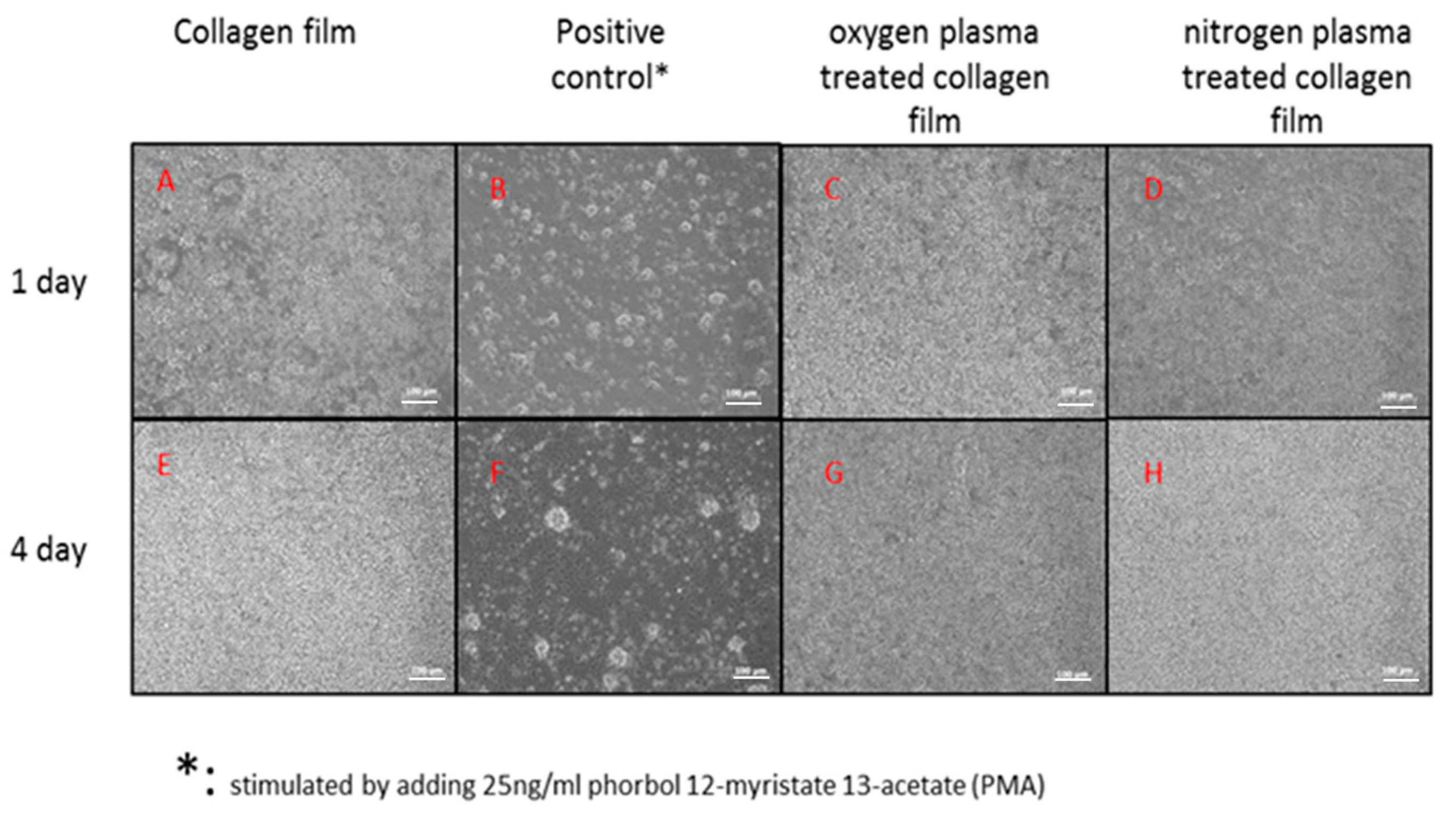
2.4.1. Cell Morphology and Cell Proliferation
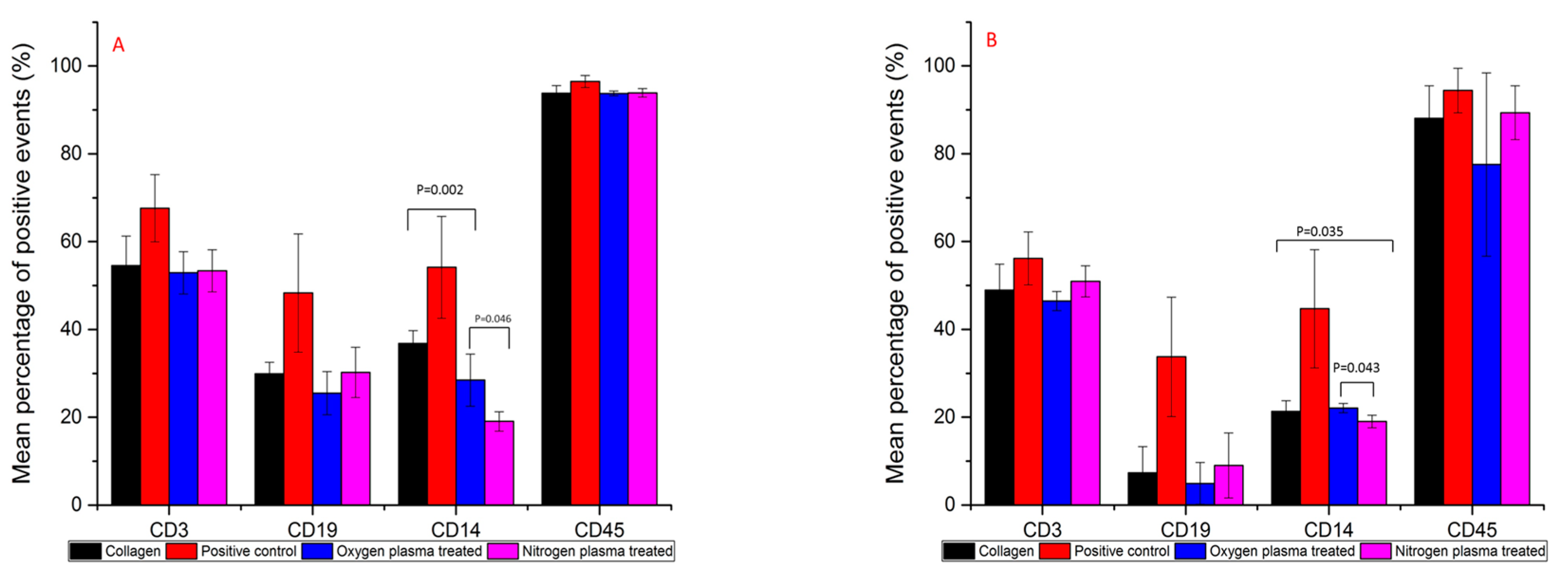
2.4.2. Immunofluorescence Flow Cytometry
2.4.3. Pro/anti-Inflammatory Cytokine Release
3. Results
3.1. Surface Characterization
3.2. PBMCs Attachment on the Collagen Surface
3.3. Immunofluorescence Flow Cytometry Results
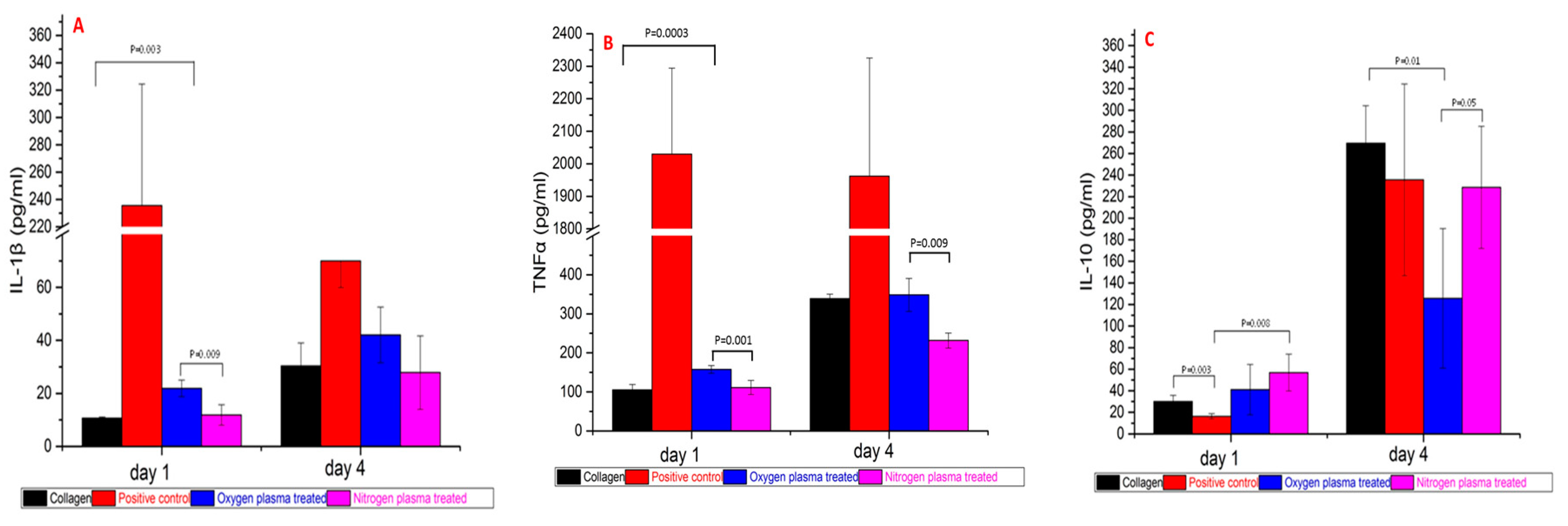
3.4. Pro/anti-Inflammatory Cytokine Release after 1 Day and 4 Days PBMC Culture
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Funt, D.; Pavicic, T. Dermal fillers in aesthetics: An overview of adverse events and treatment approaches. Clin. Cosmet. Investig. Dermatol. 2013, 6, 295–316. [Google Scholar] [CrossRef] [PubMed]
- Dreifke, M.B.; Jayasuriya, A.A.; Jayasuriya, A.C. Current wound healing procedures and potential care. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 48, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Gabay, M.; Boucher, B.A. An essential primer for understanding the role of topical hemostats, surgical sealants, and adhesives for maintaining hemostasis. Pharmacotherapy 2013, 33, 935–955. [Google Scholar] [CrossRef] [PubMed]
- Mutter, D.; Jamali, F.R.; Moody, D.L.; Rodeheaver, G.T.; Therin, M.; Maresaux, J. The concept of protected mesh to minimize adhesion formation in intraperitoneal abdominal wall reinforcement. Preclinical evaluation of a new composite mesh. Hernia 2000, 4, s3–s9. [Google Scholar] [CrossRef]
- Solouk, A.; Mirzadeh, H.; Shokrgozar, M.A.; Solati-Hashjin, M.; Najarian, S.; Seifalian, A.M. The study of collagen immobilization on a novel nanocomposite to enhance cell adhesion and growth. Iran. Biomed. J. 2011, 15, 6–14. [Google Scholar] [PubMed]
- Marín-Pareja, N.; Salvagni, E.; Guillem-Marti, J.; Aparicio, C.; Ginebra, M.P. Collagen-functionalised titanium surfaces for biological sealing of dental implants: Effect of immobilisation process on fibroblasts response. Colloid Surf. B 2014, 122, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Pu, F.; Rhodes, N.P.; Bayon, Y.; Hunt, J.A. In vitro cellular response to oxidized collagen-PLLA hybrid scaffolds designed for the repair of muscular tissue defects and complex incisional hernias. J. Tissue Eng. Regen. Med. 2016, 10, E454–E466. [Google Scholar] [CrossRef] [PubMed]
- Raghavendran, H.R.B.; Mohan, S.; Genasan, K.; Murali, M.R.; Naveen, S.V.; Talebian, S.; McKean, R.; Kamarul, T. Synergistic interaction of platelet derived growth factor (PDGF) with the surface of PLLA/Col/HA and PLLA/HA scaffolds produces rapid osteogenic differentiation. Colloid Surf. B 2016, 139, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Li, W.; Lv, X.; Lei, Z.; Bian, Y.; Deng, H.; Wang, H.; Li, J.; Li, X. Biomimetic LBL structured nanofibrous matrices assembled by chitosan/collagen for promoting wound healing. Biomaterials 2015, 53, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Curran, J.M.; Hunt, J.A. Leukocyte-Biomaterial Interaction In Vitro. In Comprehensive Biomaterials; Ducheyne, P., Healy, K.E., Hutmacher, D.W., Grainger, D.W., Kirkpatrick, C.J., Eds.; Elsevier: Oxford, UK, 2011; Volume 4, pp. 49–62. [Google Scholar]
- Williams, D.F. A model for biocompatibility and its evaluation. J. Biomed. Eng. 1989, 11, 185–191. [Google Scholar] [CrossRef]
- Browne, S.; Pandit, A. Biomaterial-mediated modification of the local inflammatory environment. Front. Bioeng. Biotechnol. 2015, 3, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Brodbeck, W.G.; Nakayama, Y.; Matsuda, T.; Colton, E.; Ziats, N.P.; Anderson, J.M. Biomaterial surface chemistry dictates adherent monocyte/macrophage cytokine expression in vitro. Cytokine 2002, 18, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Stio, M.; Martinesi, M.; Treves, C.; Borgioli, F. In vitro response of human peripheral blood mononuclear cells to AISI 316L austenitic stainless steel subjected to nitriding and collagen coating treatments. J. Mater. Sci. Mater. Med. 2015, 26, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Murray, D.W.; Rae, T.; Rushton, N. The influence of the surface energy and roughness of implants on bone resorption. J. Bone Jt. Surg. B 1989, 71, 632–637. [Google Scholar]
- Refai, A.K.; Textor, M.; Brunette, D.M.; Waterfield, J.D. Effect of titanium surface topography on macrophage activation and secretion of proinflammatory cytokines and chemokines. J. Biomed. Mater. Res. A 2004, 70, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.S. Effects of biomaterial-induced inflammation on fibrosis and rejection. Semin. Immunol. 2008, 20, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Risbud, M.V.; Hambir, S.; Jog, J.; Bhonde, R. Biocompatibility assessment of polytetrafluoroethylene/wollastonite composites using endothelial cells and macrophages. J. Biomater. Sci. Polym. Ed. 2001, 12, 1177–1189. [Google Scholar] [CrossRef] [PubMed]
- Duque, G.A.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491–502. [Google Scholar] [CrossRef]
- Tanaka, T.; Shigeta, M.; Yamakawa, N.; Usui, M. Cell adhesion to acrylic intraocular lens associated with lens surface properties. J. Cataract. Refract. Surg. 2005, 31, 1648–1651. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, T.; Shandas, R. A survey of surface modification techniques for next-generation shape memory polymer stent devices. Polymers 2014, 6, 2309–2331. [Google Scholar] [CrossRef]
- Siow, K.S.; Britcher, L.; Kumar, S.; Griesser, H.J. Plasma methods for the generation of chemically reactive surfaces for biomolecule immobilization and cell colonization—A review. Plasma Process. Polym. 2006, 3, 392–418. [Google Scholar] [CrossRef]
- Girard-Lauriault, P.L.; Unger, W.E.S.; Dietrich, P.M. Innovative and Established Strategies for the Surface Analysis of Nitrogen and Oxygen-Rich Plasma Polymer Films by XPS: An Introductory Guide. Plasma Process. Polym. 2015, 12, 953–967. [Google Scholar] [CrossRef]
- Esposito, A.R.; Kamikawa, C.M.; Lucchesi, C.; Ferreira, B.M.P.; de Rezende Duek, E.A. Benefits of oxygen and nitrogen plasma treatment in Vero cell affinity to poly(lactide-co-glycolide acid). Mater. Res. 2013, 16, 695–702. [Google Scholar] [CrossRef]
- López-García, J.; Asadinezhad, A.; Pacherník, J.; lehocký, M.; Junkar, I.; Humpolíček, P.; Sáha, P.; Valášek, P. Cell proliferation of HaCaT keratinocytes on collagen films modified by argon plasma treatment. Molecules 2010, 15, 2845–2856. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Bayon, Y.; Hunt, J.A. Preliminary study on the effects of ageing cold oxygen plasma treated PET/PP with respect to protein adsorption. Colloid Surf. B 2012, 96, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Pu, F.R.; Williams, R.L.; Markkula, T.K.; Hunt, J.A. Expression of leukocyte-endothelial cell adhesion molecules on monocyte adhesion to human endothelial cells on plasma treated PET and PTFE in vitro. Biomaterials 2002, 23, 4705–4718. [Google Scholar] [CrossRef]
- Markkula, T.K.; Hunt, J.A.; Pu, F.R.; Williams, R.L. Surface chemical derivatization of plasma-treated PET and PTFE. Surf. Interface Anal. 2002, 34, 583–587. [Google Scholar] [CrossRef]
- Sysolyatina, E.; Vasiliev, M.; Kurnaeva, M.; Kornienko, I.; Petrov, O.; Fortov, V.; Gintsburg, A.; Petersen, E.; Ermolaeva, S. Frequency of cell treatment with cold microwave argon plasma is important for the final outcome. J. Phys. D Appl. Phys. 2016, 49, 294002. [Google Scholar] [CrossRef]
- Klämpfl, T.G.; Isbary, G.; Shimizu, T.; Li, Y.F.; Zimmermann, J.L.; Stolz, W.; Schlegel, J.; Morfill, G.E.; Schmidt, H.U. Cold atmospheric air plasma sterilization against spores and other microorganisms of clinical interest. Appl. Environ. Microbiol. 2012, 78, 5077–5082. [Google Scholar] [CrossRef] [PubMed]
- Curran, J.M.; Gallagher, J.A.; Hunt, J.A. The inflammatory potential of biphasic calcium phosphate granules in osteoblast/macrophage co-culture. Biomaterials 2005, 26, 5313–5320. [Google Scholar] [CrossRef] [PubMed]
- Cachinho, S.C.P.; Pu, F.; Hunt, J.A. Cytokine secretion from human peripheral blood mononuclear cells cultured in vitro with metal particles. J. Biomed. Mater. Res. Part A 2013, 101, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Hirata, I.; Hioki, Y.; Toda, M.; Kitazawa, T.; Murakami, Y.; Kitano, E.; Kitamura, H.; Ikada, Y.; Iwata, H. Deposition of complement protein C3b on mixed self-assembled monolayers carrying surface hydroxyl and methyl groups studied by surface plasmon resonance. J. Biomed. Mater. Res. A 2003, 66, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Labarre, D.; Laurent, A.; Lautier, A.; Bouhni, S.; Kerbellec, L.; Lewest, J.M.; Tersinet, N. Complement activation by substituted polyacrylamide hydrogels for embolisation and implantation. Biomaterials 2002, 23, 2319–2327. [Google Scholar] [CrossRef]
- Toda, M.; Kitazawa, T.; Hirata, I.; Hirano, Y.; Iwata, H. Complement activation on surfaces carrying amino groups. Biomaterials 2008, 29, 407–417. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Curran, J.; Pu, F.; Zhuola, Z.; Bayon, Y.; Hunt, J.A. In Vitro Response of Human Peripheral Blood Mononuclear Cells (PBMC) to Collagen Films Treated with Cold Plasma. Polymers 2017, 9, 254. https://doi.org/10.3390/polym9070254
Chen R, Curran J, Pu F, Zhuola Z, Bayon Y, Hunt JA. In Vitro Response of Human Peripheral Blood Mononuclear Cells (PBMC) to Collagen Films Treated with Cold Plasma. Polymers. 2017; 9(7):254. https://doi.org/10.3390/polym9070254
Chicago/Turabian StyleChen, Rui, Jude Curran, Fanrong Pu, Zhuola Zhuola, Yves Bayon, and John A. Hunt. 2017. "In Vitro Response of Human Peripheral Blood Mononuclear Cells (PBMC) to Collagen Films Treated with Cold Plasma" Polymers 9, no. 7: 254. https://doi.org/10.3390/polym9070254





