Processing Properties
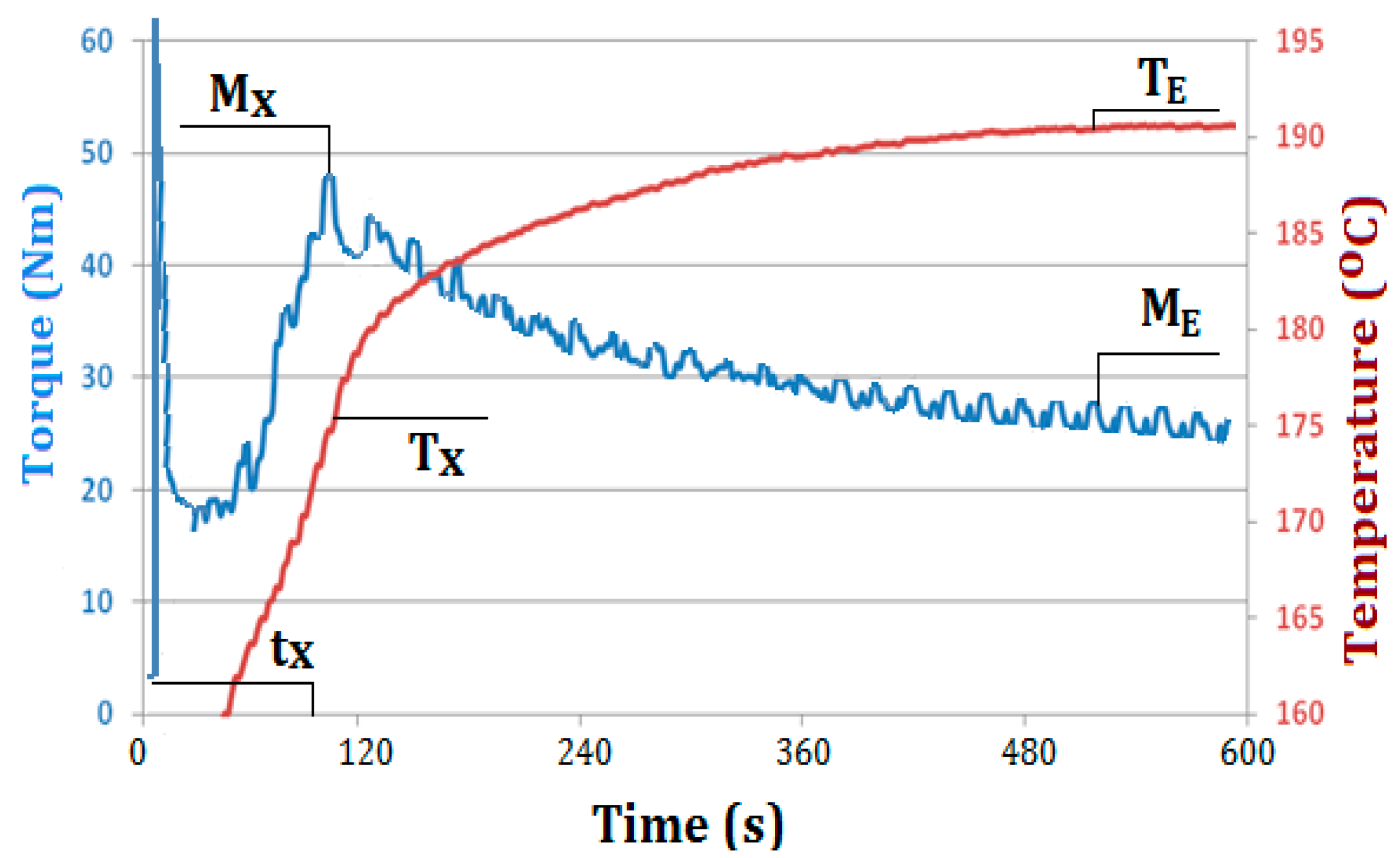
Figure 3 shows an exemplary plastogram of kneading of PVC mixture containing 5 wt % Mg(OH)
2/lignin (PVC/HM2) with marked characteristic values, which were the subject of further analysis. The values of torque (M
X and M
E), temperature (
TX and
TE) and time (
tX), determined from the plastograms, are summarized in
Table 4.
The behavior of the dependency of the torque and the actual charge temperature on kneading time for all produced PVC composites are essentially similar and typical for unplasticized PVC blends (see
Figure 3). The course of the plastograms, in which a distinct maximum of torque occurs, indicates correctly selected kneading conditions, i.e., temperature, the rotor speed and the charge mass, which determine the correct transformation of morphological structure of the PVC grains and the formation of a gelated material at the final kneading stage [
47,
48].
Compared to the kneading of unfilled PVC, the value of the maximum torque (M
X), associated with PVC gelation, is higher and slightly increases along with the increase of the concentration of the hybrid filler in the kneaded blends (see
Table 4). The addition of Mg(OH)
2 to PVC caused a slight increase of the torque values compared to results obtained when the hybrid filler was used. Therefore, the presence of lignin in the hybrid filler slightly decreases the maximum torque of kneaded mixtures. A similar effect was observed during kneading of PVC mixes containing the silica/lignin hybrid filler [
4,
40], although, the reduction of the torque resulting from the presence of lignin was more effective due to the large torque value for the PVC/silica mixtures.
The time of attaining the maximum torque value is significantly shortened with increasing hybrid filler concentration. The shortest gelation time (shorter by approximately 55% compared to PVC) was found in case of mixture containing 10 wt % of the lignin hybrid filler. A lower reduction of the gelation time with increasing filler content of the matrix was observed during kneading of PVC mixtures with Mg(OH)2. The shortest gelation time (shorter by 25% compared to the unfilled PVC) was noted for mixtures containing 10 wt % of the filler.
Similar effects observed during kneading of PVC containing lignin modified with polyacrylate [
49,
50] as well as in our previous studies on PVC composites with the silica/lignin filler [
4,
40] might be caused by a change in densification of the processed PVC compounds, associated with the presence of the hybrid filler. The increase in the filler content contributes to an increase in the compaction of the kneaded PVC compound, an increase in shearing stresses and, as a consequence, an increase in torque and shortening in gelation time. Moreover, the presence of the filler may facilitate the heat transfer between PVC macromolecules and effectively accelerate their mutual penetration, which influences reduction in T
X temperature. The shortening of the gelation time and the decrease of the T
X temperature by 3–5 °C along with increasing hybrid filler content in the PVC matrix is advantageous from the economical point of view and the potential manufacturing of such composites in industrial practice. The analysis plastograms (see
Table 4) also shows that the addition of the hybrid filler to kneaded PVC mixture does not practically cause any changes in the equilibrium-state torque, M
E, and in the final actual temperature of the melted blends, similarly as in case of kneading compounds containing magnesium hydroxide. Therefore, no significant effect of the filler used in the amount of up to 10 wt % on the value of torque of kneaded PVC composites in melted state (M
E) directly related to their viscosity is observed.
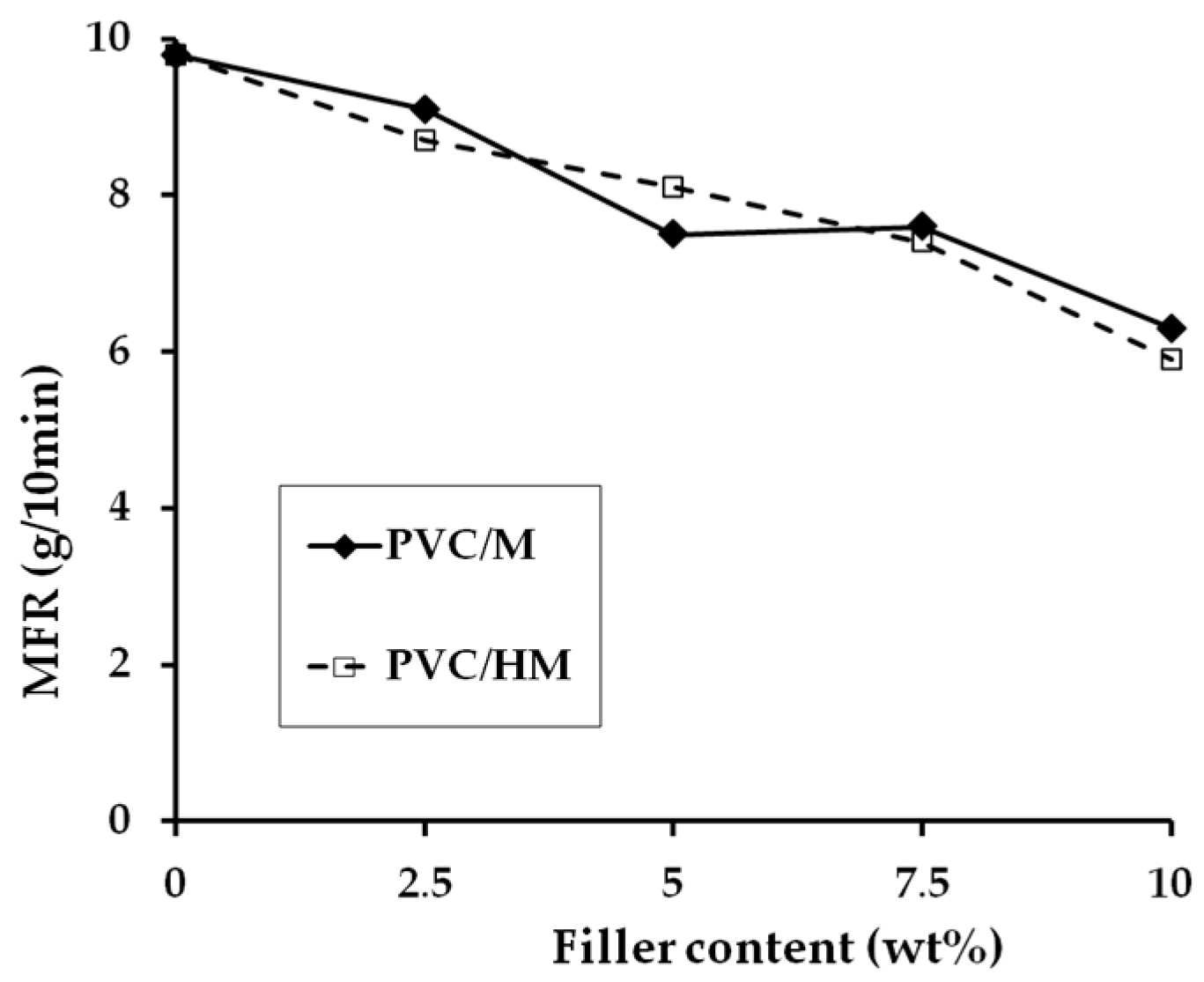
The analysis of data in
Figure 4 shows that the addition of a hybrid filler as well as its components, namely lignin and magnesium hydroxide, one at a time, to the PVC matrix, causes a slight reduction in the value of the composite mass flow rate, compared to unfilled PVC. Introduction of 2.5 wt % hybrid filler results in a decrease of MFR by 18%. Increasing the hybrid filler content in the PVC matrix to 10 wt % causes a further, steady decrease in MFR. The MFR values of PVC composites with Mg(OH)
2 are approximately the same as the MFR values of the composites with hybrid fillers. A similar tendency of lowering the MFR value of the composites with PVC was found when lignin was used as a filler [
4].
The reduction of the MFR value with the increase in the filler content of the PVC matrix is most likely caused by hindering the free movement of PVC macromolecules in the capillary of the plastometer due to the effect of the applied piston pressure. Such a possibility is confirmed by the relationship of the torque in the equilibrium state as a function of the increasing filler content (regardless of its type) shown in
Table 4, although the observed changes of
ME values during kneading in the plastograph chamber are relatively negligible.
The analysis of test results of mechanical properties summarized in
Table 5 indicates that the introduction of the used hybrid filler and, separately, magnesium hydroxide to PVC causes an increase in the modulus of elasticity at tension, the value of which increases along with increasing filler content in the matrix. In each case, the Young’s modulus of the composites is greater than the E
t value for unfilled PVC. During mechanical testing at static tension, it was found that specimens of all materials, i.e., PVC, PVC/M and the PVC/HM composites, indicated a distinct yield point. Regardless of the filler content, the value of the yield stress (
σY) in each case corresponded to the tensile strength (
σM).
The tensile strength of the PVC/HM composites is lower than the σ
M of unmodified PVC and PVC/M composites, and its value decreases with increasing hybrid filler concentration. Furthermore, the modification of PVC with the hybrid magnesium hydroxide/lignin filler leads to a slight decrease of the mechanical strength of the composites, which was also observed in our previous studies, in which both silica/lignin hybrid fillers and a single component in the form of lignin were used [
4,
40]. This is a typical phenomenon for thermoplastic materials filled with both natural fibres and selected fillers with spherical particles and also applies to characteristics, such as impact resistance and relative elongation at break [
51,
52,
53]. The hybrid composites obtained in this study are still characterized by beneficial strength which makes such materials suitable for application as engineering materials, in spite of the reduced σ
M value compared to PVC.
Regardless of their filler content, all PVC/HM and PVC/M composites showed a much lower elongation at break, compared to unfilled PVC, moreover the εB value of the PVC/HM composites is slightly lower in comparison to the composites modified only with magnesium hydroxide.
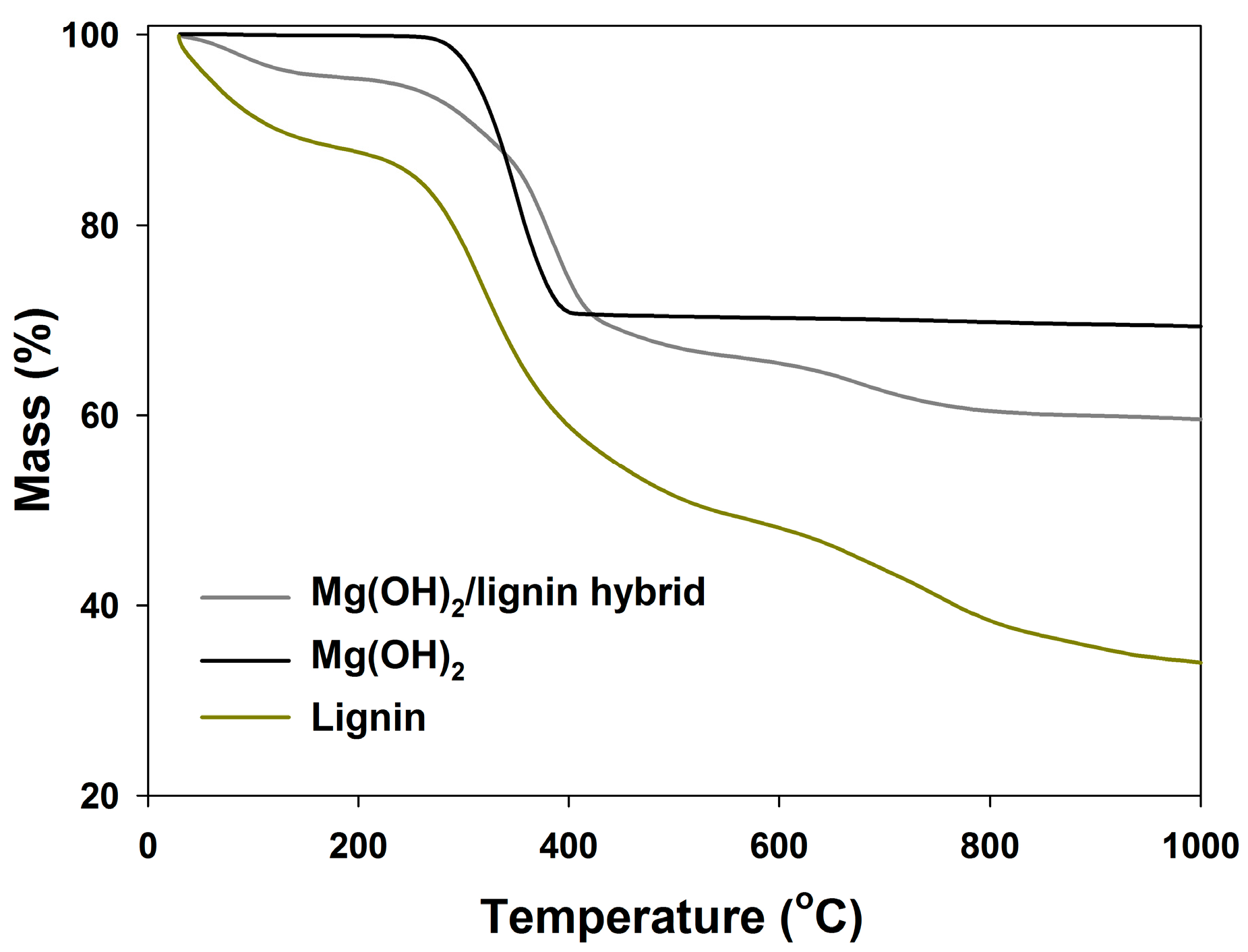
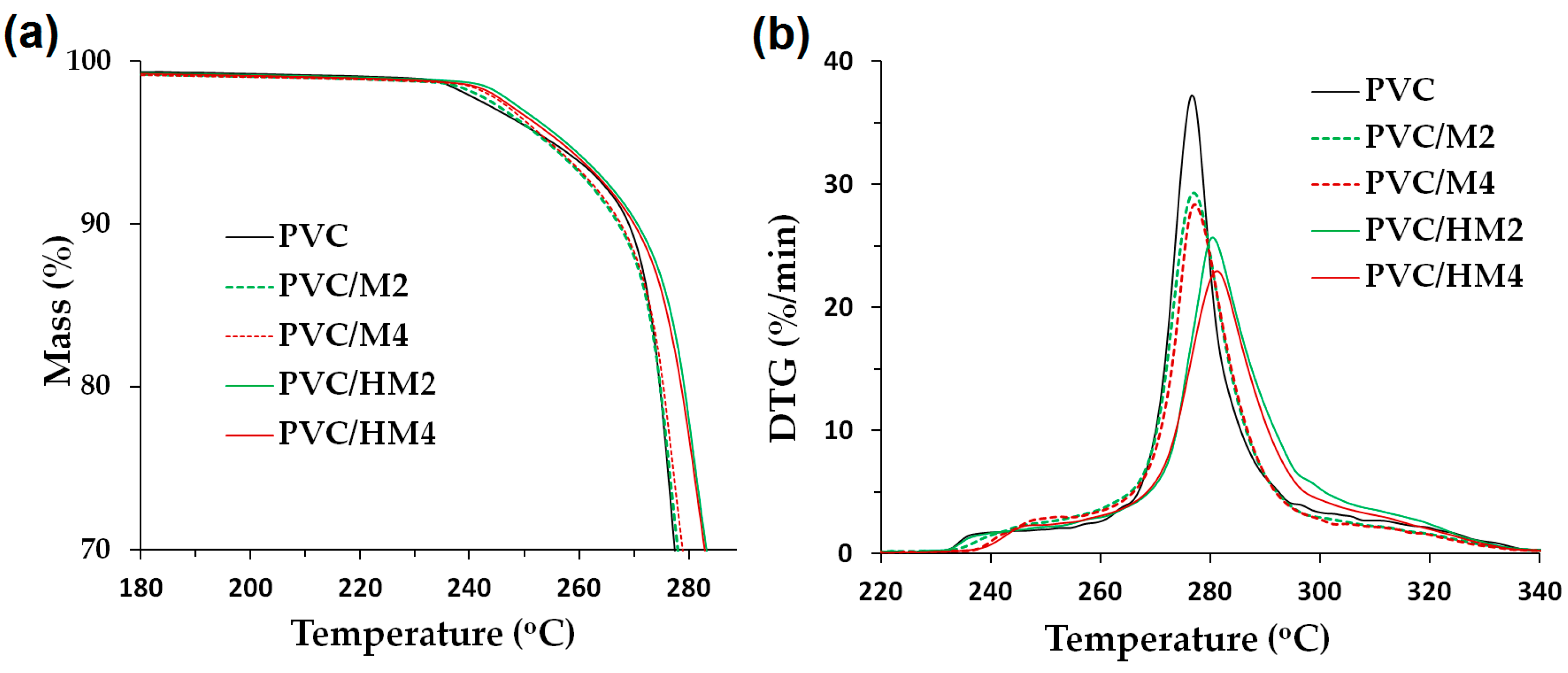
Figure 5 presents TGA thermogram curves for the PVC/HM composites and the reference material, which were used to determine the values of the temperature
T5 and
T50 associated with the mass loss 5% and 50%, respectively, and the value of the temperature T
DTG, at which the decomposition rate is maximal, similar to Blanco et al. [
46].
Figure 6 presents a comparison of selected TGA and DTG thermogram curves for PVC/HM and PVC/M with 5 wt % and with 10 wt % of both types of fillers.
Table 6 also summarizes other thermal properties of the obtained composites, such as thermal stability (determined with the Congo red test) as well as the values of the softening temperature and the oxygen index.
The behavior of the TGA curves for the PVC/HM composites (
Figure 6a) and PVC/M composites is similar and typical for unfilled PVC. The decomposition of PVC proceeds in two stages. During the first stage, at the temperature range of 260–330 °C, the autocatalytic release of hydrogen chloride takes place, with the simultaneous formation of double bonds in the polymer chain. The released HCl additionally catalyzes and accelerates the reactions of destruction of PVC chains. The next stage of PVC decomposition, at the temperature range of 400–460 °C, involves the decomposition of the formed cross-linked polyene structure which results in residual chars [
54].
The PVC composites with the hybrid filler (
Figure 5c) and with magnesium hydroxide (
Figure 6a), regardless of their content, are thermally stable within the processing temperature range, that is up to approximately 200 °C, as evidenced by the constant sample mass within this temperature range. In the case of unfilled PVC, the decomposition of the 5 wt % of the sample mass occurs at the temperature of approximately 255 °C. The addition of the Mg(OH)
2/lignin filler slightly increases this temperature to a maximum value of 260 °C upon introducing 10 wt % of the hybrid to the matrix. In contrast, the value of temperature of the 5% decomposition for PVC/M is higher than for the unmodified PVC and the PVC/HM composites, which is associated with the higher thermal stability of Mg(OH)
2 compared to lignin (see
Figure 2). It should, however, be noted that the 5% mass loss of PVC/HM composites occurs only at the temperature range above 255 °C, i.e., at a much higher temperature than the processing temperature.
The hybrid filler also contributes to the increase of the temperature, at which 50% of the mass is decomposed (by 14 °C for the PVC/HM4 composite in comparison with unfilled PVC). Compared to PVC composites containing a single component of the hybrid, i.e., Mg(OH)
2, PVC/HM composites are characterized by a higher value of temperature
T50 (see
Table 6). It can therefore be presumed that lignin contained in the employed hybrid filler has the effect of increasing the 50% sample mass loss temperature, which has also been found in our previous work [
4].
In the DTG curve (
Figure 5b), two separate endothermic peaks occur, the first of which represents the rate of the release process of HCl. The maximum of this wide peak indicates the value of the
TDTG temperature, at which the dehydrochlorination process takes place with the highest intensity. This temperature was approximately 276 °C for pure PVC, and with the increase of the Mg(OH)
2/lignin filler content in the matrix, it shifts towards higher values, as shown in
Figure 5d, reaching a value of 284 °C in the case of the PVC/HM4 composite. The second, smaller endothermic peak (see
Figure 5b) is associated with further destruction of the material.
Compared to PVC composites modified with magnesium hydroxide, PVC/HM composites are characterized by a higher DTG peak temperature (
Figure 6b) by approximately 3 °C for samples with a 5 wt % filler concentration and approximately 8 °C with a 10 wt % filler concentration.
Moreover, the height of the dehydrochlorination peak on the DTG curve, which indicates the intensity of HCl emission, decreases with the increase of both hybrid filler and Mg(OH)2 content in the PVC matrix. The maximum PVC decomposition rate is approximately 37%/min, introduction of 10 wt % of the magnesium hydroxide reduces this value to 28%/min. Introduction of 10 wt % of the Mg(OH)2/lignin hybrid filler reduces this value to 23%/min. Thus, the Mg(OH)2/lignin hybrid material proposed in this work has an advantageous contribution to reducing the intensity of HCl emission during the decomposition of PVC used as the matrix of PVC/HM composites.
Similar absorption properties of HCl and thus stabilization properties were found in the case of using micrometer and nanometer CaCO
3 as a filler in PVC composites produced by the solution casting method [
25]. The TGA analysis shows that both the decomposition starting temperature and the temperature of maximum degradation rate are higher along with the increase of filler concentration in the matrix. The authors have concluded that the CaCO
3 particles can bind the HCl gas, whereby the self-accelerating effect is avoided when CaCO
3 is used as a filler. Scavenging of HCl does not lead to a complete stop of the decomposition process, though it can significantly reduce the rate of degradation. The stabilizing effect of magnesium hydroxide due to the absorption of hydrogen chloride released during PVC degradation, which has a direct effect of extending the duration of thermal stability, as measured by the Congo red method, is also described [
55]. The effective activity of hydrotalcite (LDH), which includes, inter alia, magnesium hydroxide and aluminum hydroxide, as the modifier of thermal stability and the reducer of smoke emission during PVC flaming, is explained by authors of studies [
26,
56,
57,
58,
59] as the absorption and, consequently, reaction between LDH and gaseous HCl evaporated from PVC, which leads to the formation of metal chlorides.
A potential application of lignocellulose materials as the absorbers of hydrogen chloride released during destruction of polymer medical wastes including PVC was proposed in study [
60]. The mechanism of the reaction of HCl absorbed by lignin with the methoxy groups in phenolic rings, which leads to the formation of chloromethane, was explained by Czégény et al. [
61]. This reaction consumes evaporated HCl which is released during the PVC decomposition.
The filler used in this study contains both of the components described in the literature cited above, i.e., Mg(OH)2 and lignin, hence it can be presumed that the mechanism of the stabilizing effect of this filler on PVC is complex and may result from the simultaneous interactions of both hybrid components with polymer macromolecules.
The favorable effect of the used filler on the static thermal stability is also confirmed by the results of the Congo red method. The analysis of the data presented in
Table 6 shows that introduction of the Mg(OH)
2/lignin filler to the matrix in the amount of 5 wt % and more significantly extends the time after which the change in the indicator’s color is observed, which confirms the destruction of PVC. PVC composites containing 7.5 and 10 wt % are characterized by higher temperature stability compared to unfilled PVC by 29 and 41 min, respectively. The time of the thermal stability of PVC/M composites increases with increasing Mg(OH)
2 content of the matrix, though the stability improvement is not as considerable as in the case of the hybrid filler. It can therefore be inferred that the significantly increased thermal stability of PVC/HM is associated with the presence of the lignin component, as has also been found in work [
4].
It can be presumed that the presence of lignin in the hybrid filler is an important factor preventing the chain reactions of PVC dehydrochlorination, probably due to attaching HCl molecules initiating the chain reactions of decomposition to metoxy groups in phenolic rings, which leads to the formation of chloromethane [
61]. In addition, the participation of the Mg(OH)
2 component reinforces this effect, the HCl released at the initial stage of destruction may be a substrate for the reaction of synthesis from Mg(OH)
2, resulting in MgCl
2 [
19,
20,
62].
The PVC/HM composites are distinguished by a higher softening temperature compared to unfilled PVC and PVC/M composites (see
Table 6); introduction of 2.5 wt % of the hybrid filler to the matrix causes the softening temperature to increase by 5 and 6 °C, respectively. Further increase in VST with growing hybrid filler concentration is less notable. The VST temperature of the composites modified with magnesium hydroxide is similar to that of the unmodified PVC regardless of the concentration of the filler.
A similar effect of improving the functional properties of PVC matrix-based composite materials in terms of widening their temperature applicability range is described for the use of the wood flour filler [
44] and the SiO
2/lignin hybrid filler [
4].
Within the present study, a preliminary investigation was also carried out to determine the usefulness of the Mg(OH)2/lignin filler as an agent enhancing the fire resistance, as determined by the LOI method. The value of the LOI index of unfilled PVC sample is 45.1%, which allows it to be ranked among self-extinguishing materials. It has been found that the PVC/HM composites are characterized by LOI values ranging from 45.8% to 49.6%, which may suggest a potential for using the Mg(OH)2/lignin hybrid as an agent which additionally increases the fire resistance of PVC.
As expected, the LOI value of PVC composites containing 10 wt % of Mg(OH)2 is higher by approximately 20% compared to pristine PVC and by approximately 11% compared to a PVC sample containing the identical hybrid filler fraction.
Within this study, the examination of the LOI of PVC composites with a lignin addition was also carried out. The LOI values of composites are similar and range from 43.6% to 44.0% regardless of the lignin content up to 10 wt %. Thus, the increase in the oxygen index of PVC-based composites is associated with the presence of an inorganic component in the hybrid filler.
Figure 7 shows microscopic images of filler particles and the structure of the PVC/HM1 and PVC/HM4 composites taken in reflected light. In case of the PVC/HM1 composite (
Figure 7b), uniformly dispersed 5–12 µm hybrid filler particles within the PVC matrix were observed. While the content of the filler in composite was at 10 wt %, its homogeneous distribution (
Figure 7c) was also observed, however, isolated 20–50 µm particle aggregates are visible in the picture.
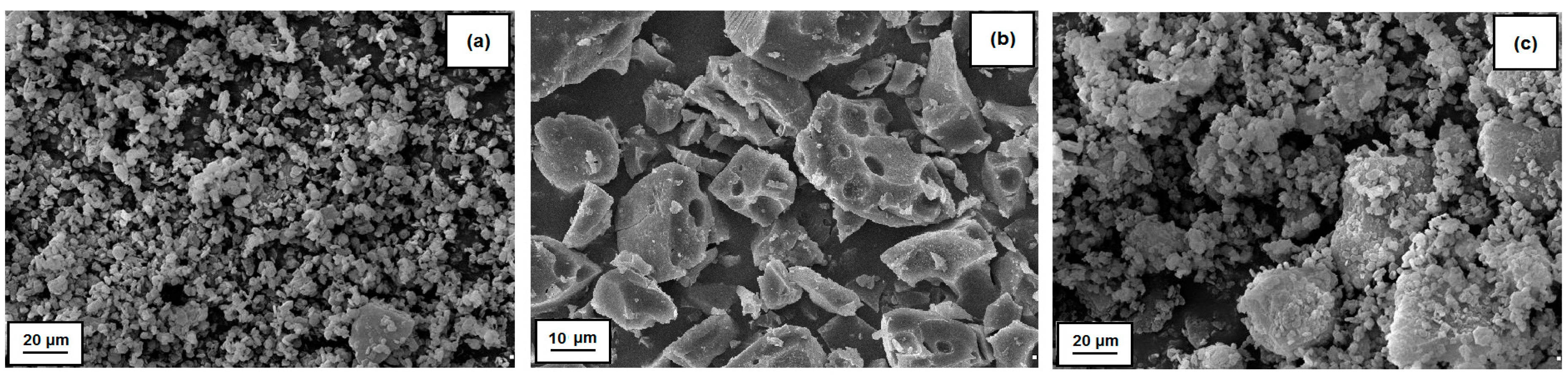
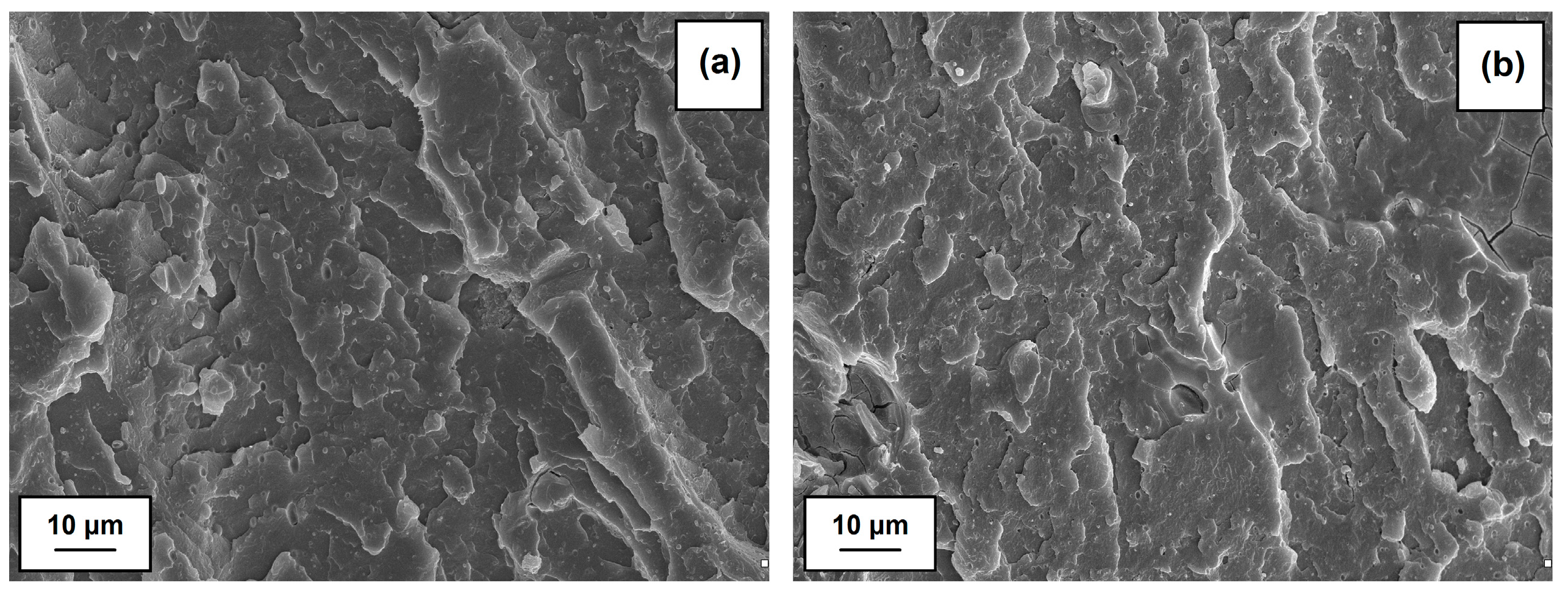
SEM images (see
Figure 8) confirm the homogeneous dispersion of the hybrid filler in the PVC matrix. Moreover, the surface of fractures indicates a layer structure characteristic of gelated PVC, which verifies that the conditions of composites processing were properly chosen. Based on SEM observations, it may be stated that the adhesion between the PVC matrix and hybrid filler is sufficient; the phase separation is not observed. Similar morphology was also noticed for polypropylene composites with the mixture of cellulose, hemp, fax and lignin [
63].