Enhancement of Thermal Diffusivity in Phase-Separated Bismaleimide/Poly(ether imide) Composite Films Containing Needle-Shaped ZnO Particles
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Measurements
3. Results and Discussion
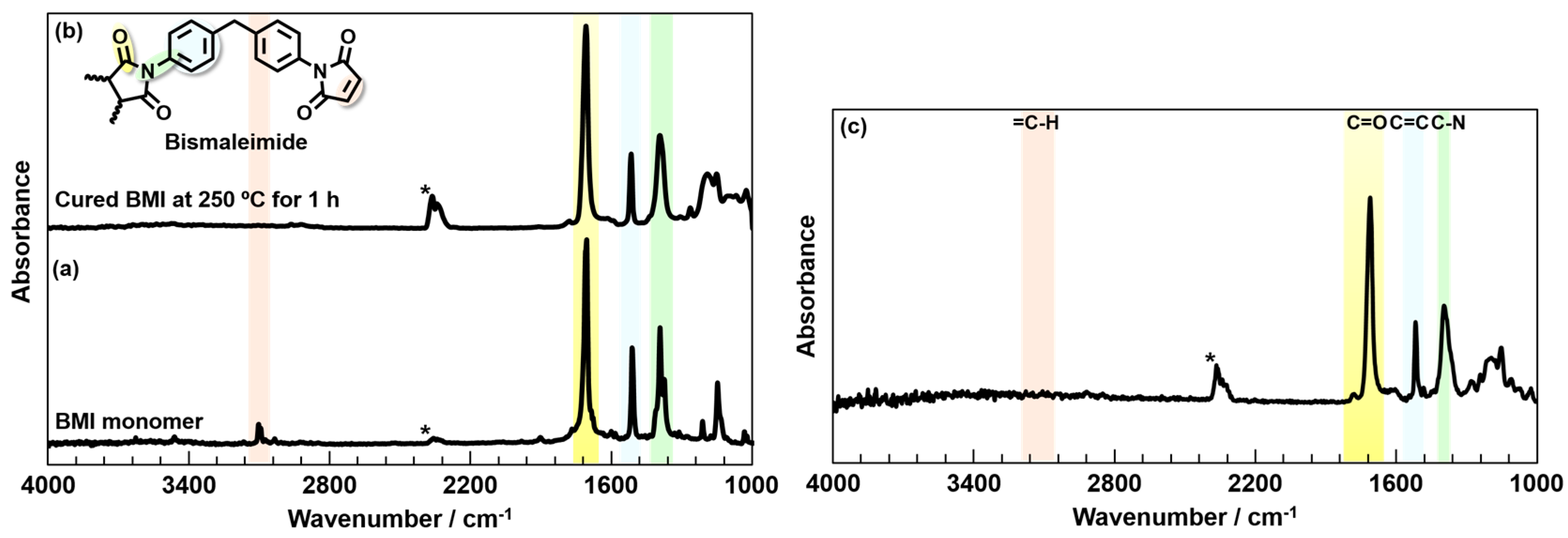
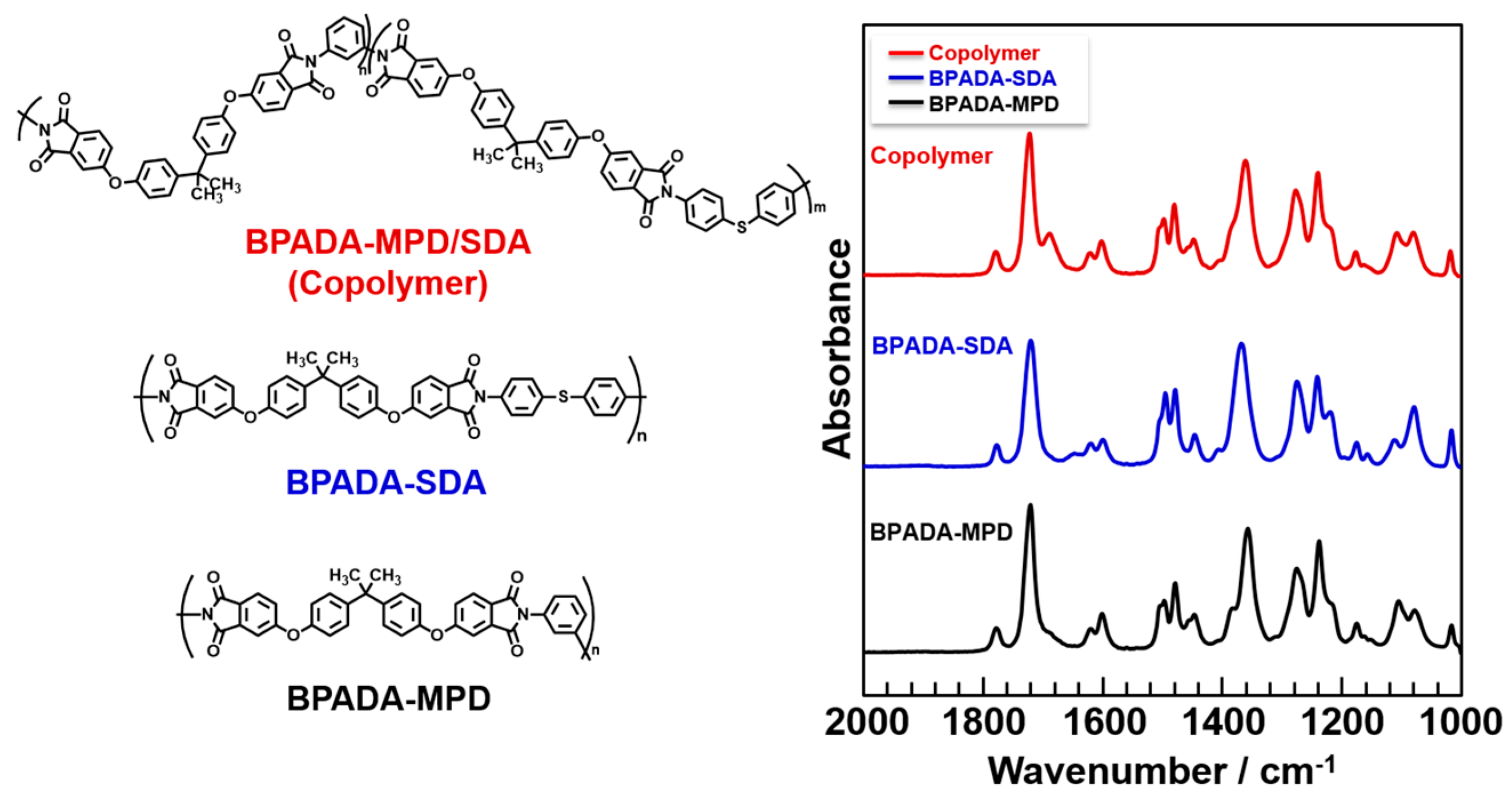
3.1. Characterization of BMI and BPADA-MPD
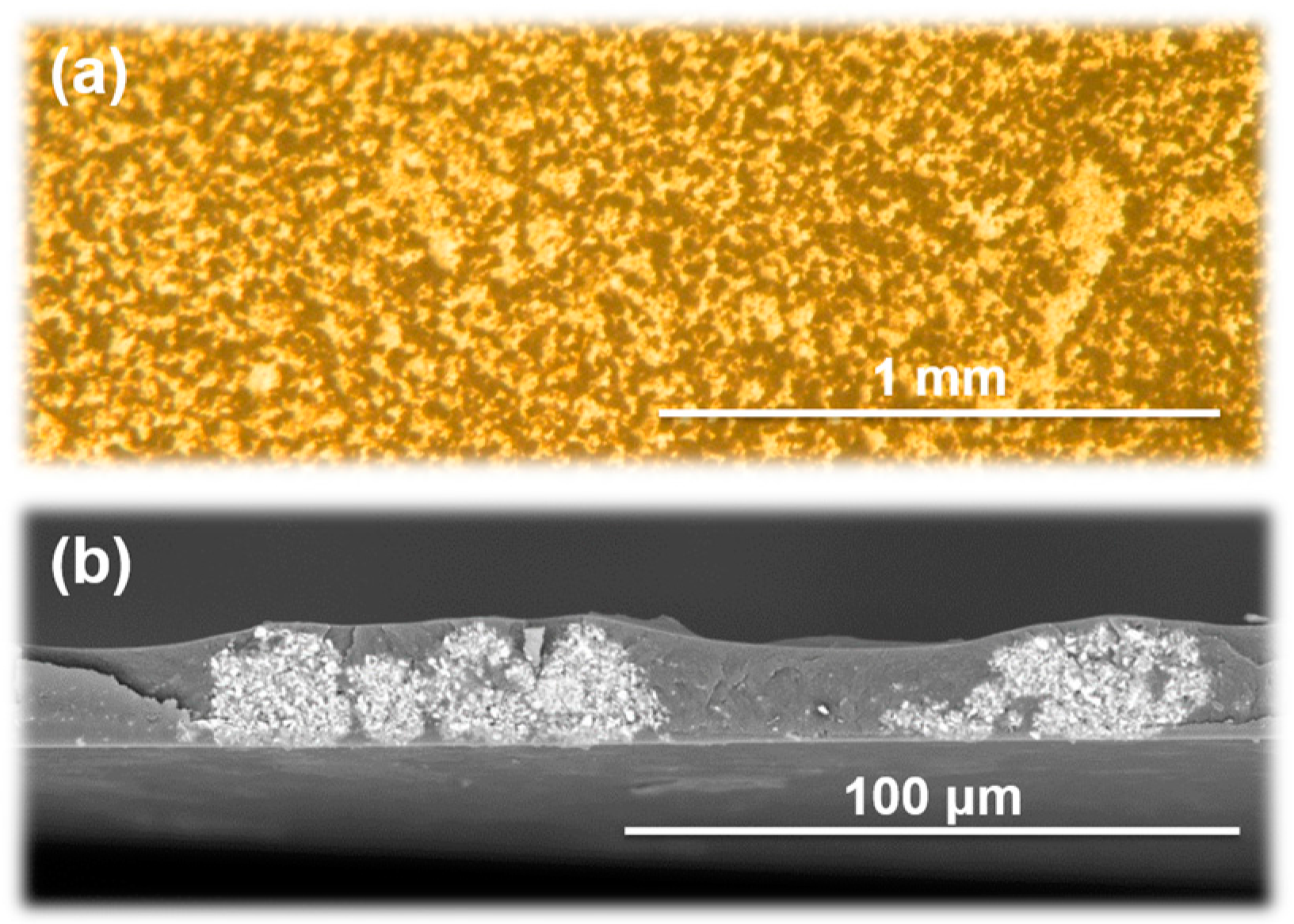
3.2. Characterization and Morphology of BMI/PI Composite Films
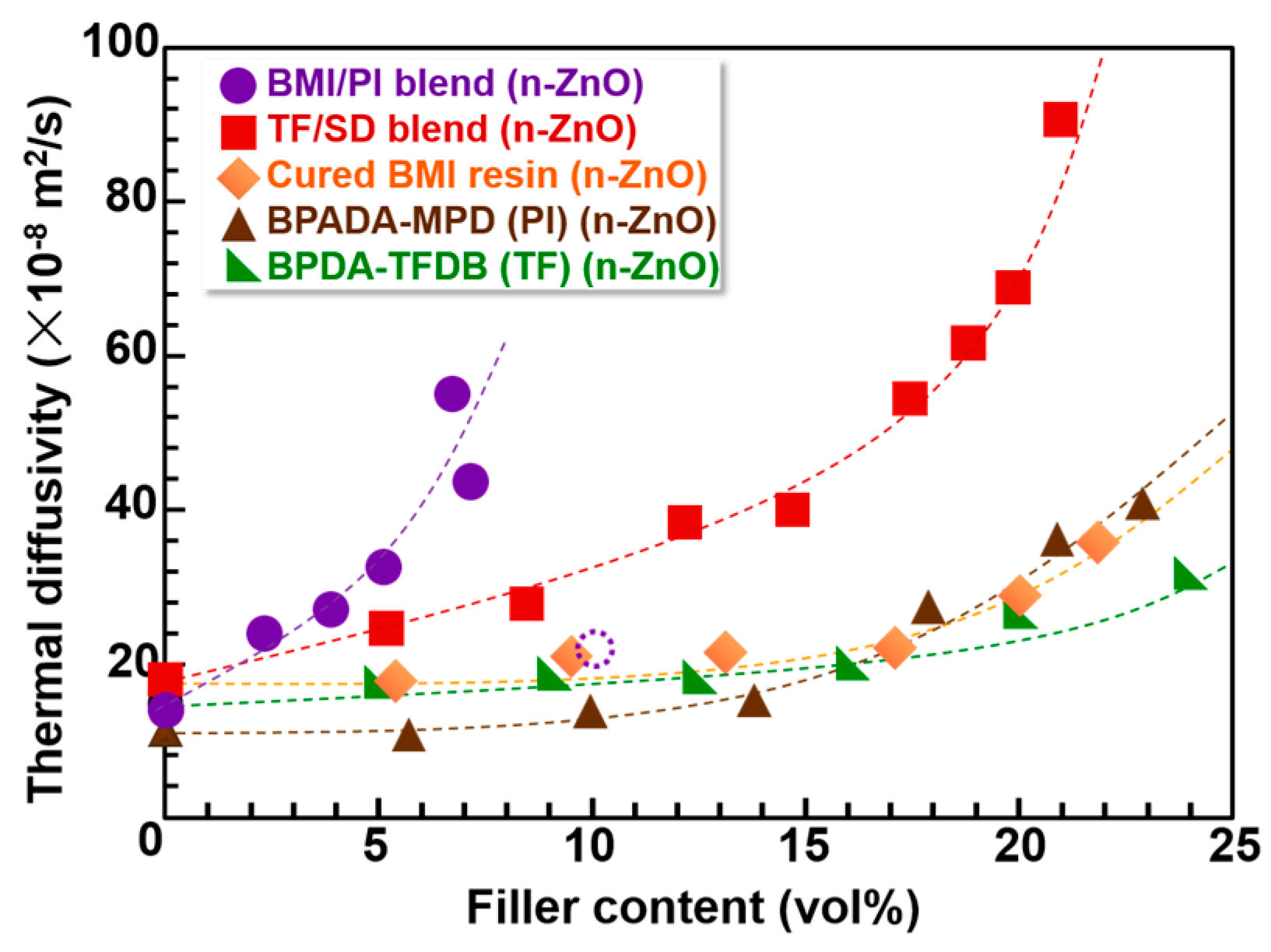
3.3. Out-of-Plane Thermal Diffusivities
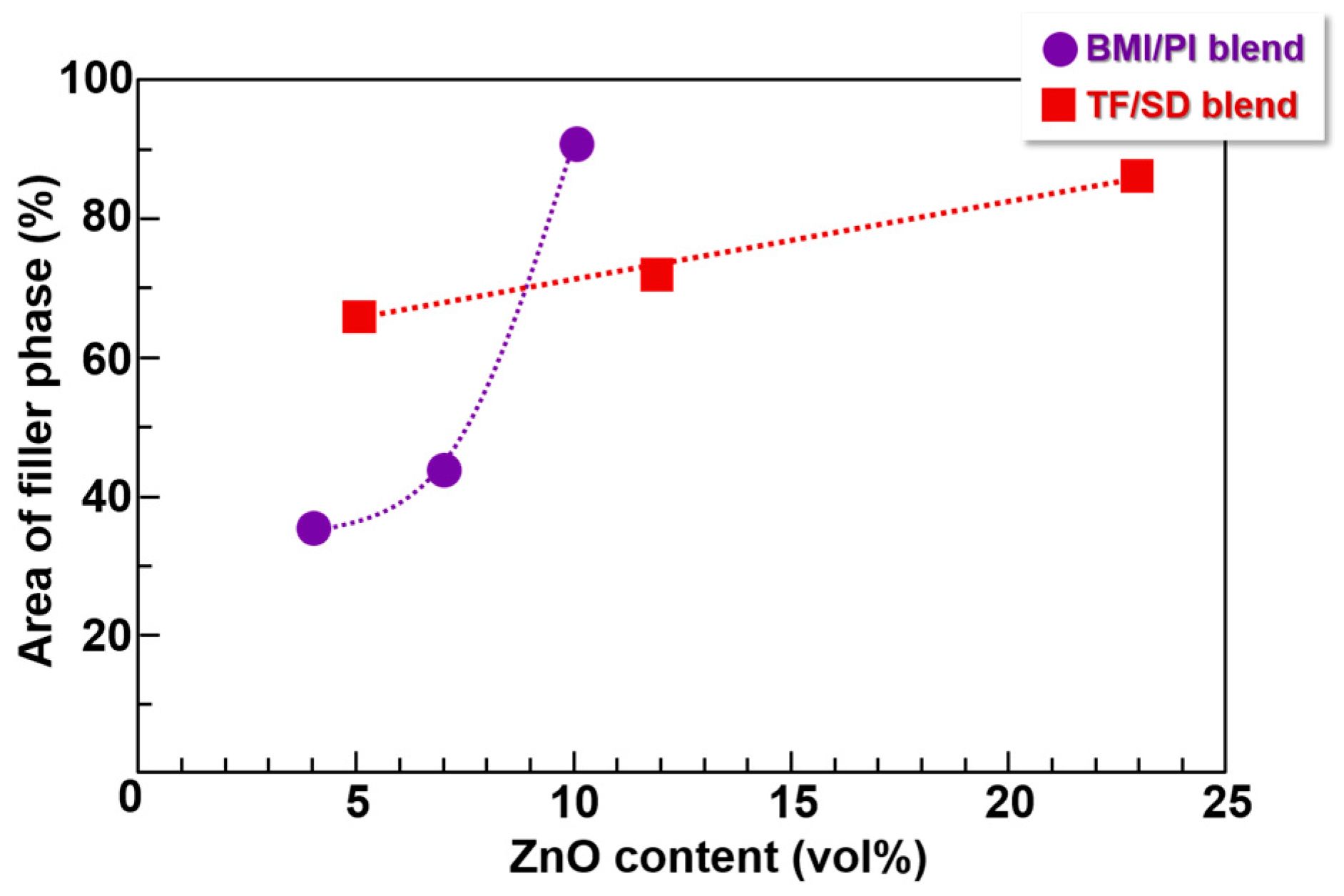
3.4. Image Analysis of Optical Microscope Photographs
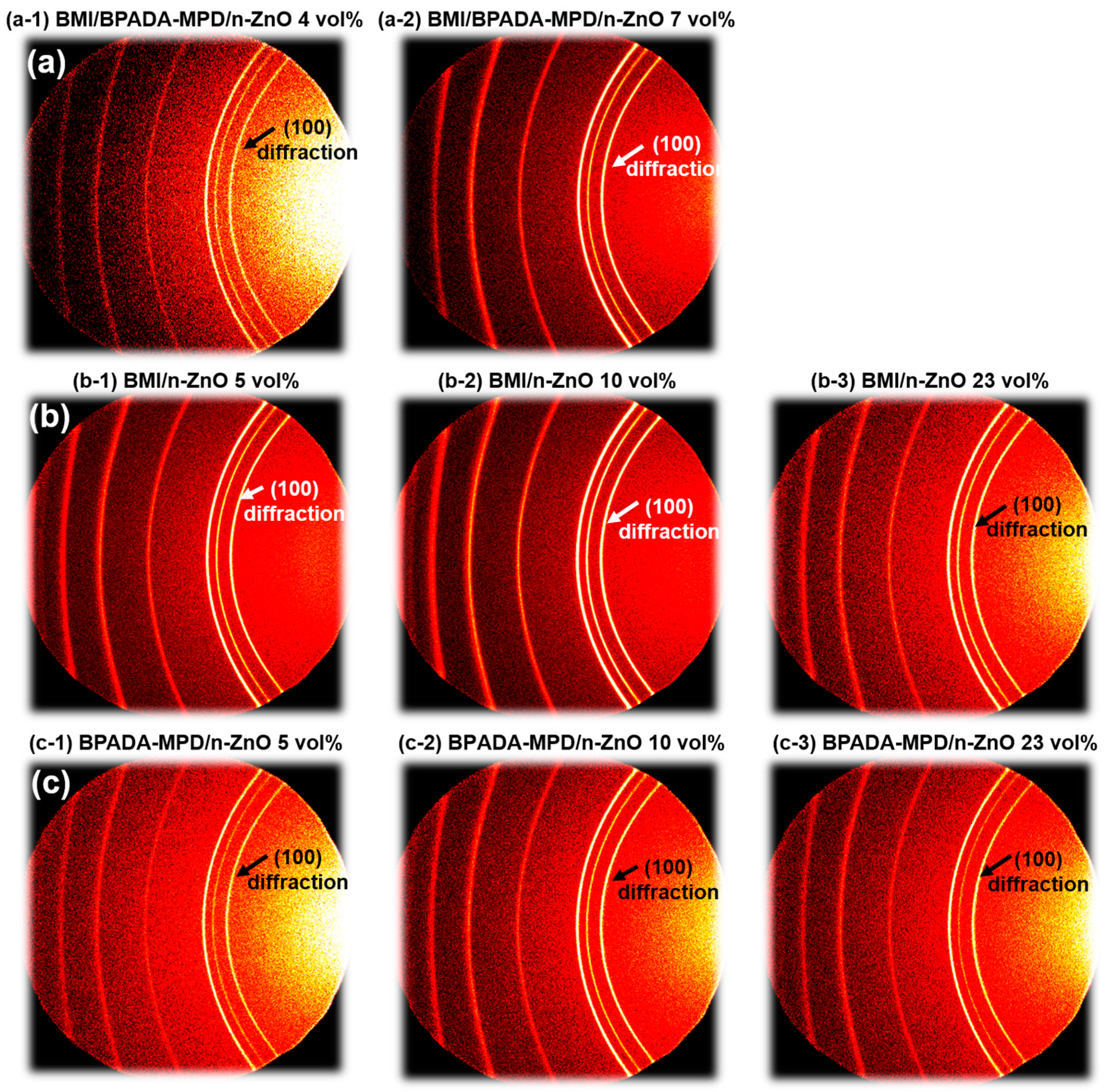
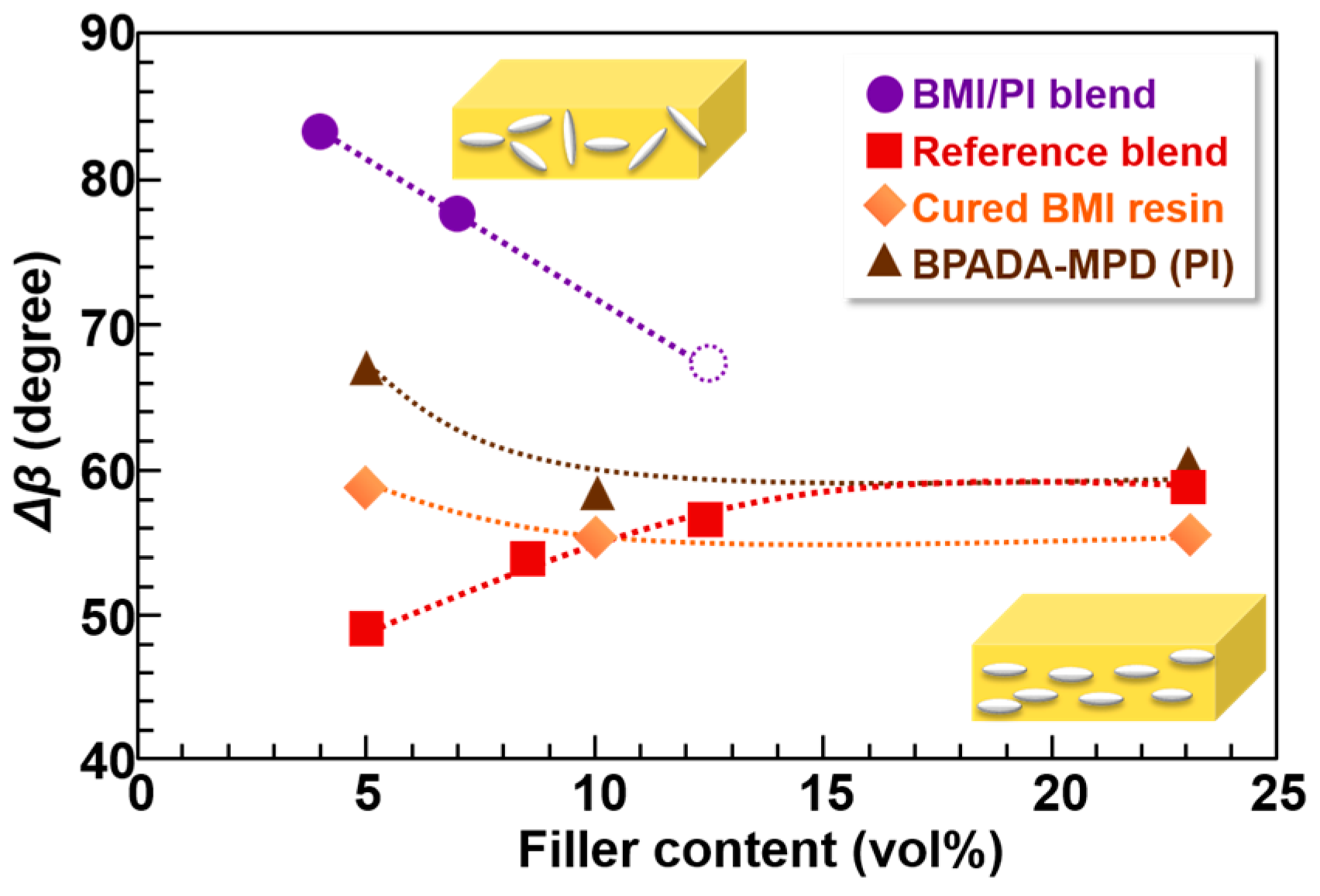
3.5. Orientation Analysis of Composite Films by WAXD
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Note
- Shen, H.; Cai, C.; Guo, J.; Qian, Z.; Zhao, N.; Xu, J. Fabrication of oriented hBN scaffolds for thermal interface materials. RSC Adv. 2016, 6, 16489–16494. [Google Scholar] [CrossRef]
- Tanimoto, M.; Yamagata, T.; Miyata, K.; Ando, S. Anisotropic thermal diffusivity of hexagonal boron nitride-filled polyimide films: Effects of filler particle size, aggregation, orientation, and polymer chain rigidity. ACS Appl. Mater. Interfaces 2013, 5, 4374–4382. [Google Scholar] [CrossRef] [PubMed]
- Uchida, S.; Murakami, T.; Iwamura, T.; Ishige, R.; Ando, S. Enhanced thermal conductivity in immiscible polyimide blend composites with needle-shaped ZnO particles. RSC Adv. 2017, 7, 15492–15499. [Google Scholar] [CrossRef]
- Yorifuji, D.; Ando, S. Enhanced thermal diffusivity by vertical double percolation structures in polyimide blend films containing silver nanoparticles. Macromol. Chem. Phys. 2010, 211, 2118–2124. [Google Scholar] [CrossRef]
- Yorifuji, D.; Ando, S. Enhanced thermal conductivity over percolation threshold in polyimide blend films containing ZnO nano-pyramidal particles: Advantage of vertical double percolation structure. J. Mater. Chem. 2011, 21, 4402–4407. [Google Scholar] [CrossRef]
- Zhao, L.; Li, L.; Than, J.; Zhuang, J.; Li, S. Synthesis and characterization of bismaleimide-polyetherimide-titania hybrid. Compos. Part A 2004, 35, 1217–1224. [Google Scholar] [CrossRef]
- Jiao, Y.; Yuan, L.; Liang, G.; Gu, A. Facile preparation and origin of high-k carbon nanotube/poly(ether imide)/bismaleimide composites through controlling the location and distribution of carbon nanotubes. J. Phys. Chem. C 2014, 118, 24091–24101. [Google Scholar] [CrossRef]
- Iwamura, T.; Goto, S.; Sakaguchi, M.; Chujo, Y. Synthesis of submicrometer zinc oxide particles and zinc oxide nanowires using microwave irradiation. Chem. Lett. 2016, 45, 508–510. [Google Scholar] [CrossRef]
- Fiedot, M.; Maliszewska, I.; Rac, O.; Woźniak, P.S.; Teterycz, H. The relationship between the mechanism of zinc oxide crystallization and its antimicrobial properties for the surface modification of surgical meshes. Materials 2017, 10, 353. [Google Scholar] [CrossRef]
- Kim, K.M.; Choi, M.H.; Lee, J.K.; Jeong, J.; Kim, Y.R.; Kim, M.K.; Paek, S.M.; Oh, J.M. Physicochemical properties of surface charge-modified ZnO nanoparticles with different particle size. Int. J. Nanomed. 2014, 9, 41–56. [Google Scholar] [PubMed]
- Hashimoto, T.; Morikawa, J.; Kurihara, T.; Tsuji, T. Frequency dependent thermal diffusivity of polymers by temperature wave analysis. Thermochim. Acta 1997, 304, 151–156. [Google Scholar] [CrossRef]
- Morikawa, J.; Hashimoto, T. Thermal diffusivity of aromatic polyimide thin films by temperature wave analysis. J. Appl. Phys. 2009, 105, 113506–1–9. [Google Scholar] [CrossRef]
- Tsai, W.H. Moment-preserving thresholding: A new approach. Comput. Vis. Graph. Image Process. 1985, 29, 377–393. [Google Scholar] [CrossRef]
- Takeichi, T.; Saito, Y.; Agag, T.; Muto, H.; Kawauchi, T. High performance polymer alloys of polybenzoxazine and bismaleimide. Polymer 2008, 49, 1173–1179. [Google Scholar] [CrossRef]
- Belana, J.; Cañadas, J.C.; Diego, J.A.; Mudarra, M.; Diaz, R.; Friederichs, S.; Jaims, C.; Sanchis, M. Physical ageing studies in polyetherimide ULTEM 1000. Polym. Int. 1998, 46, 29–32. [Google Scholar] [CrossRef]
- Sumita, M.; Sakata, K.; Asai, S.; Miyasaka, K.; Nakagawa, H. Dispersion of fillers and the electrical conductivity of polymer blends filled with carbon black. Polym. Bull. 1991, 25, 265–271. [Google Scholar] [CrossRef]
- Vandebril, S.; Vermant, J.; Moldenaers, P. Efficiently suppressing coalescence in polymer blends using nanoparticles: Role of interfacial rheology. Soft Matter 2010, 6, 3353–3362. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Park, M.J.; Kim, D.S.; Kong, J.W.; Kim, M.; Kim, W.; Park, I.S. Interfacial adhesion and microfailure modes of electrodeposited carbon fiber/epoxy-PEI composites by microdroplet and surface wettability tests. J. Colloid Interface Sci. 2002, 249, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.W.; Wightman, J.P. Surface characterization and adhesive bonding of toughened bismaleimide composites. Compos. Part A 1996, 27, 419–428. [Google Scholar] [CrossRef]
- Torchnsky, I.; Rosenman, G. Wettability modification of nanomaterials by low-energy electron flux. Nanoscale Res. Lett. 2009, 4, 1209–1217. [Google Scholar] [CrossRef]
- Yorifuji, D.; Ando, S. Molecular structure dependence of out-of-plane thermal diffusivities in polyimide films: A key parameter for estimating thermal conductivity of polymers. Macromolecules 2010, 43, 7583–7593. [Google Scholar] [CrossRef]
- Tanimoto, M.; Ando, S. Effect of chain rigidity/flexibility of polyimides on morphological structures and thermal diffusivity of hBN-filled composites. Compos. Sci. Technol. 2014, 99, 103–108. [Google Scholar] [CrossRef]
- Tanimoto, M.; Ando, S. Prevention of void formation in particulate-filled polymer composites: Effects of thermoplastic matrices and residual solvent. Compos. Sci. Technol. 2016, 123, 268–275. [Google Scholar] [CrossRef]
- Smith, J.V. Index (Inorganic) to the Powder Diffraction File; American Society for Testing and Materials: Philadelphia, PA, USA, 1967. [Google Scholar]
- Znaidi, J.; Illia, G.J.A.A.S.; Benyahia, S.; Sanchez, C.; Kanaev, A.V. Oriented ZnO thin films synthesis by sol-gel process for laser application. Thin solid films 2003, 428, 257–262. [Google Scholar] [CrossRef]

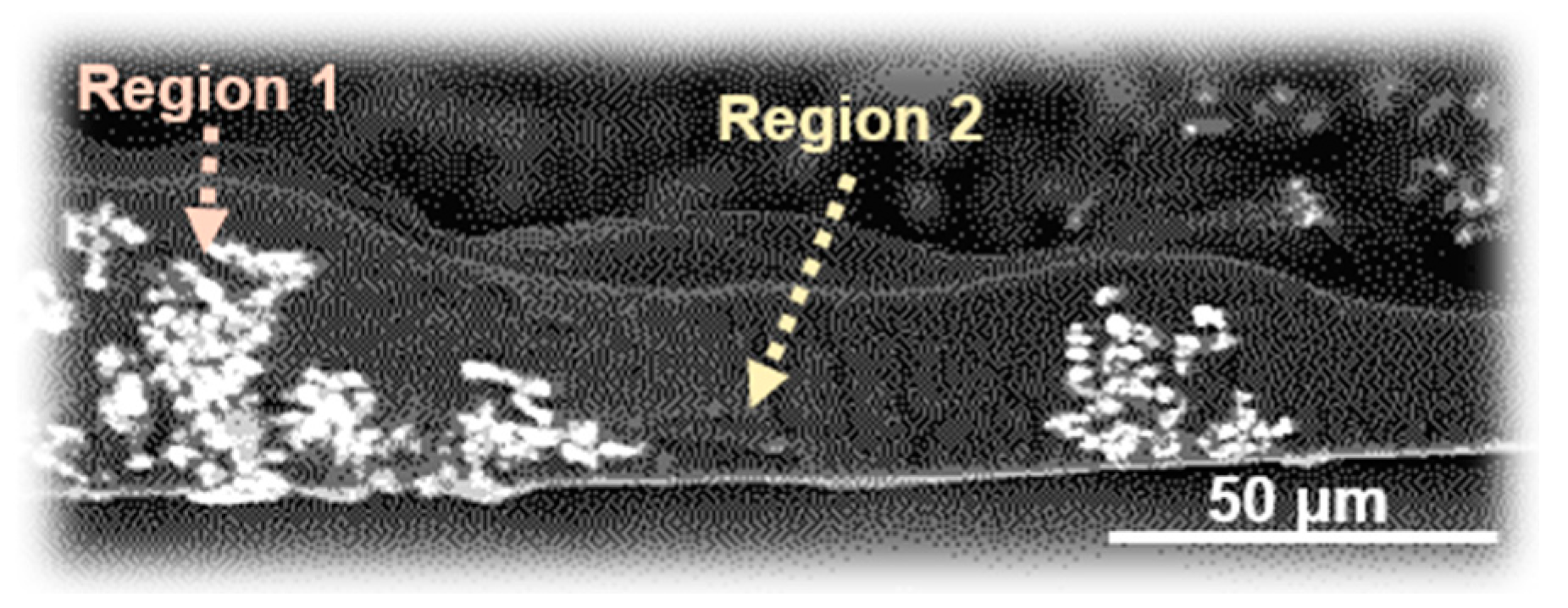
| Region | Carbon (%) | Oxygen (%) | Sulfur (%) | Zinc (%) |
|---|---|---|---|---|
| 1 | 58.85 | 13.29 | 0.09 | 4.01 |
| 2 | 61.25 | 13.84 | 0.53 | 0.33 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchida, S.; Ishige, R.; Ando, S. Enhancement of Thermal Diffusivity in Phase-Separated Bismaleimide/Poly(ether imide) Composite Films Containing Needle-Shaped ZnO Particles. Polymers 2017, 9, 263. https://doi.org/10.3390/polym9070263
Uchida S, Ishige R, Ando S. Enhancement of Thermal Diffusivity in Phase-Separated Bismaleimide/Poly(ether imide) Composite Films Containing Needle-Shaped ZnO Particles. Polymers. 2017; 9(7):263. https://doi.org/10.3390/polym9070263
Chicago/Turabian StyleUchida, Shoya, Ryohei Ishige, and Shinji Ando. 2017. "Enhancement of Thermal Diffusivity in Phase-Separated Bismaleimide/Poly(ether imide) Composite Films Containing Needle-Shaped ZnO Particles" Polymers 9, no. 7: 263. https://doi.org/10.3390/polym9070263





