Effective Removal of Chromium(III) from Low Concentration Aqueous Solution Using a Novel Diazene/Methoxy-Laced Coordination Polymer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and General Methods
2.2. Preparation of [CdL2(H2O)0.5]n (1)
2.3. X-ray Data Collection and Structure Determination
2.4. Adsorption Test
3. Results and Discussion
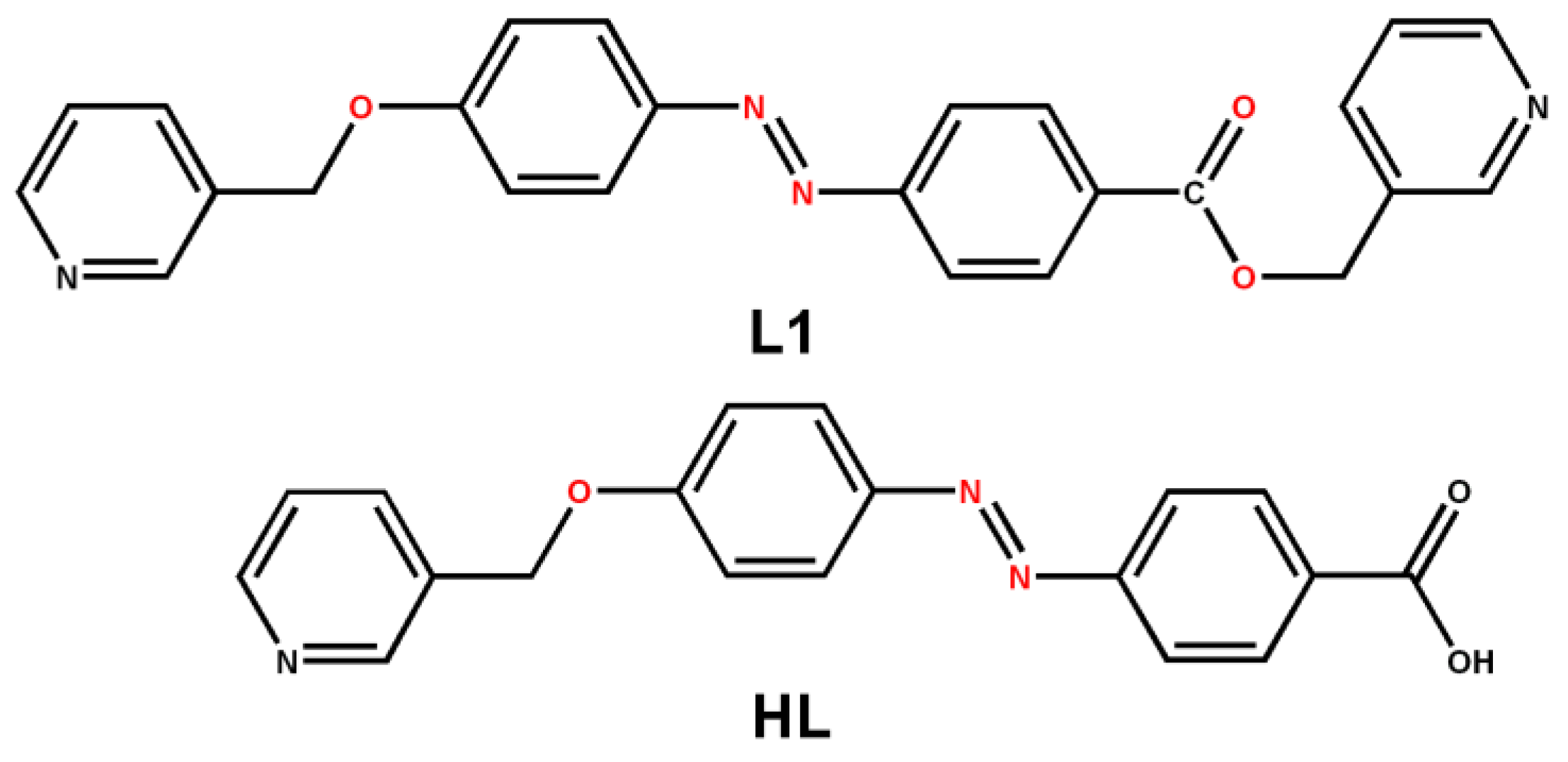
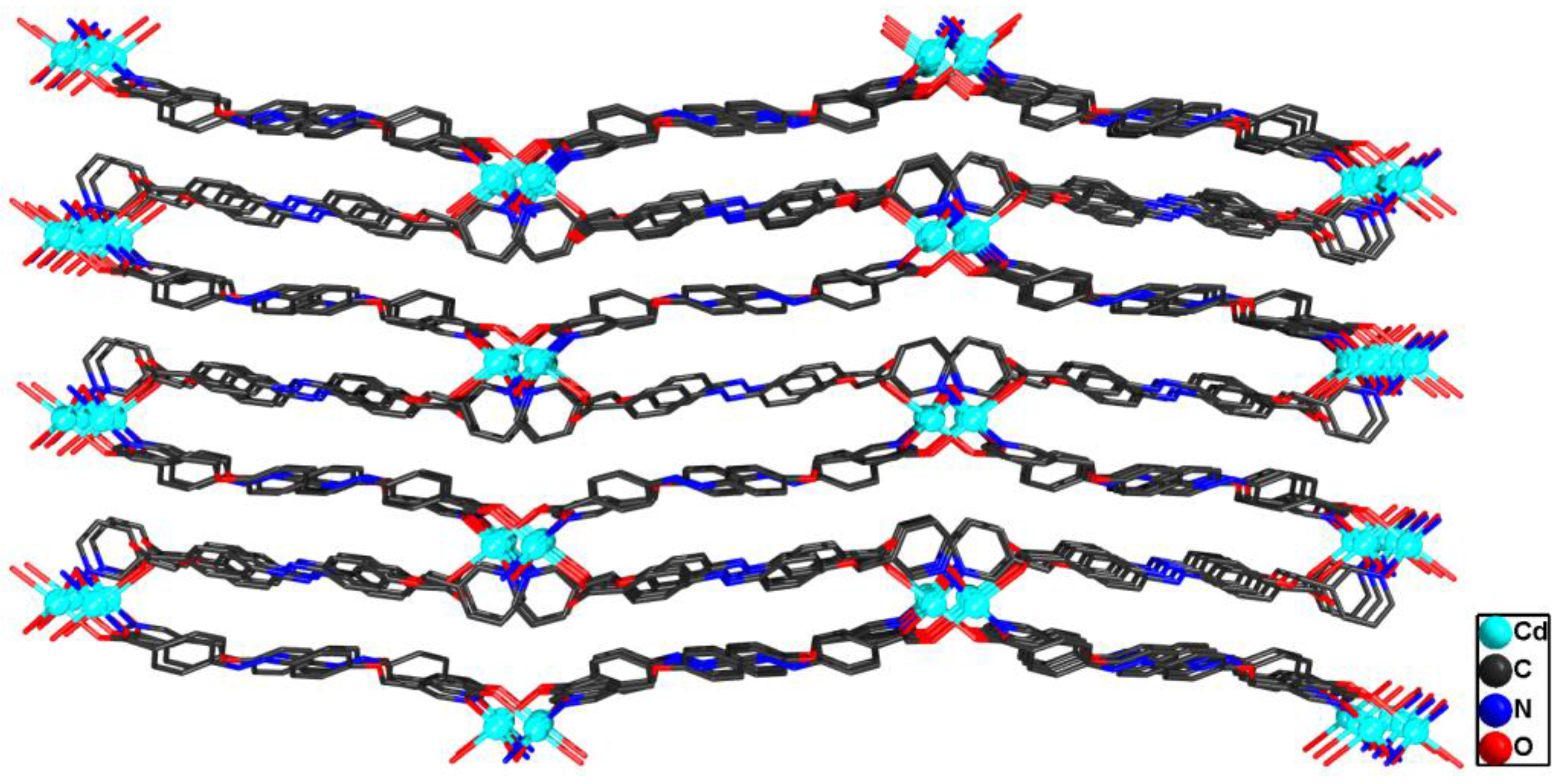
3.1. Synthetic and Structural Characterization
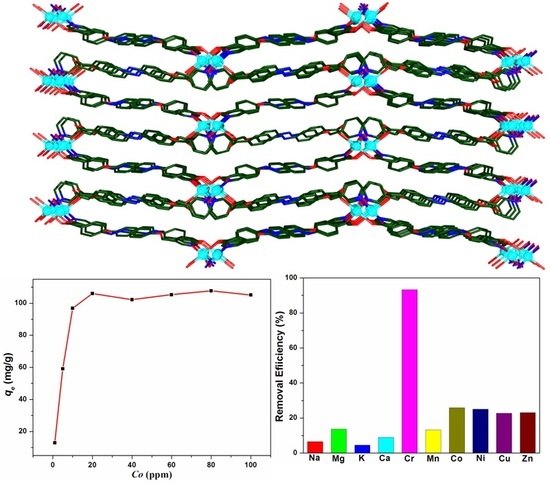
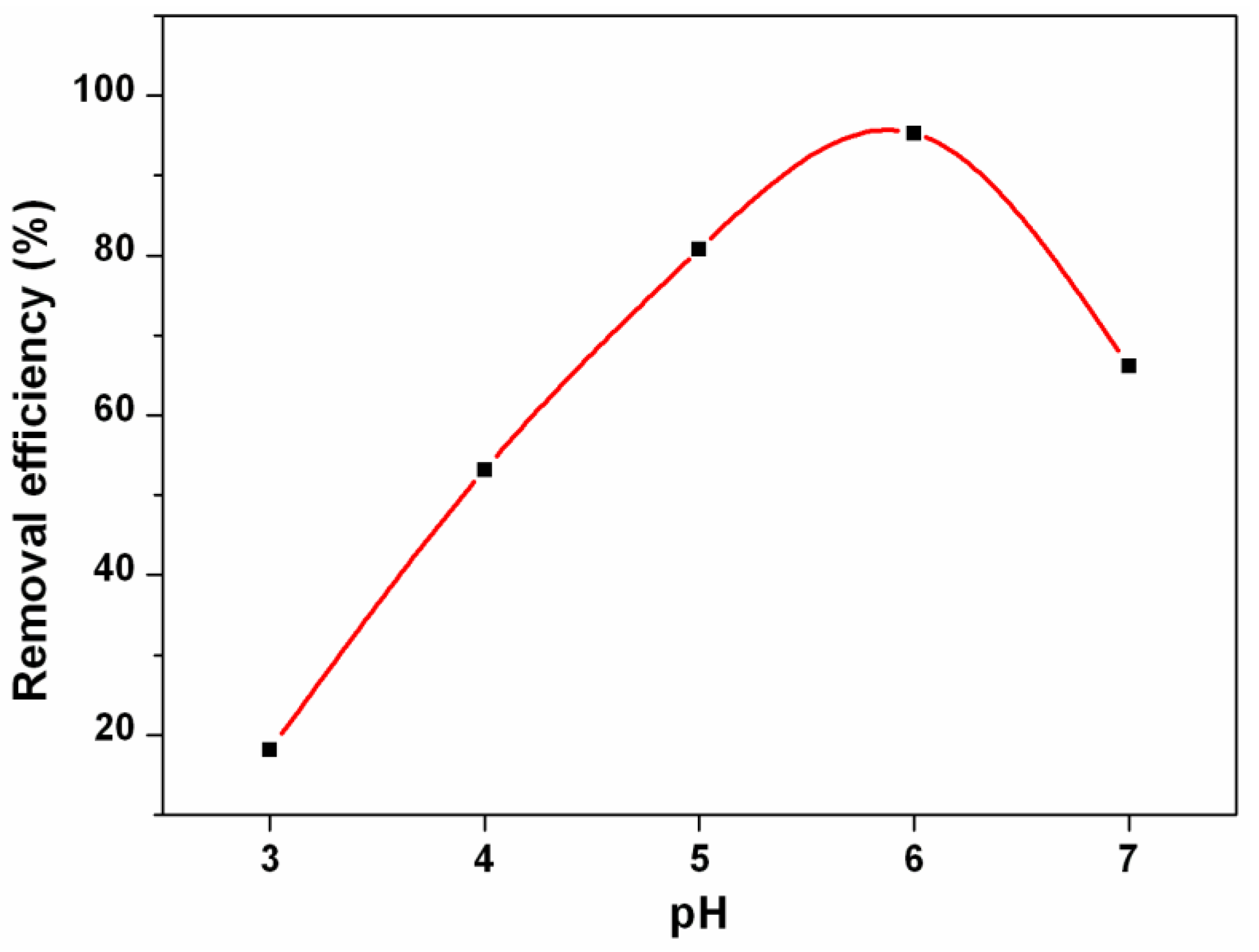
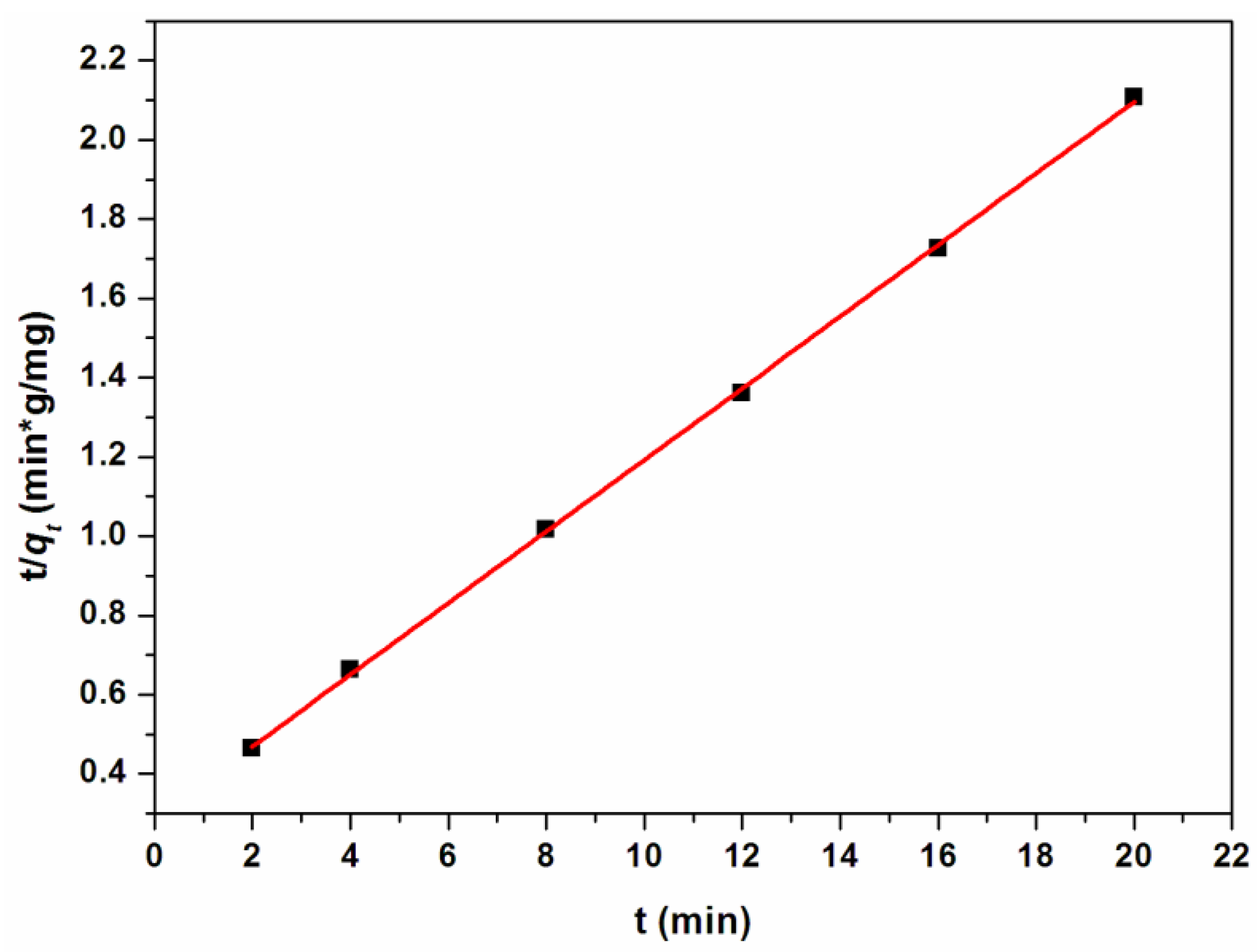
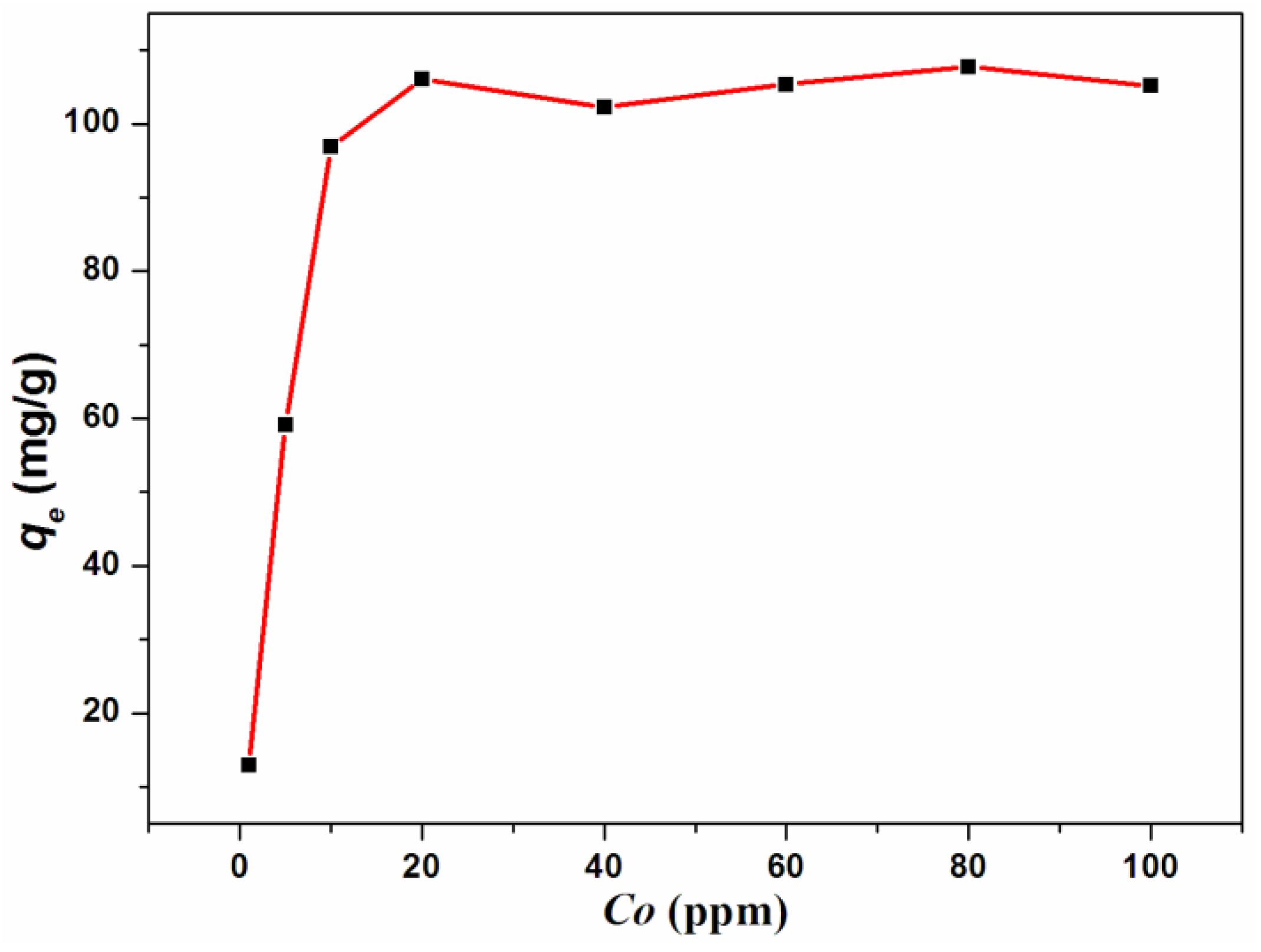
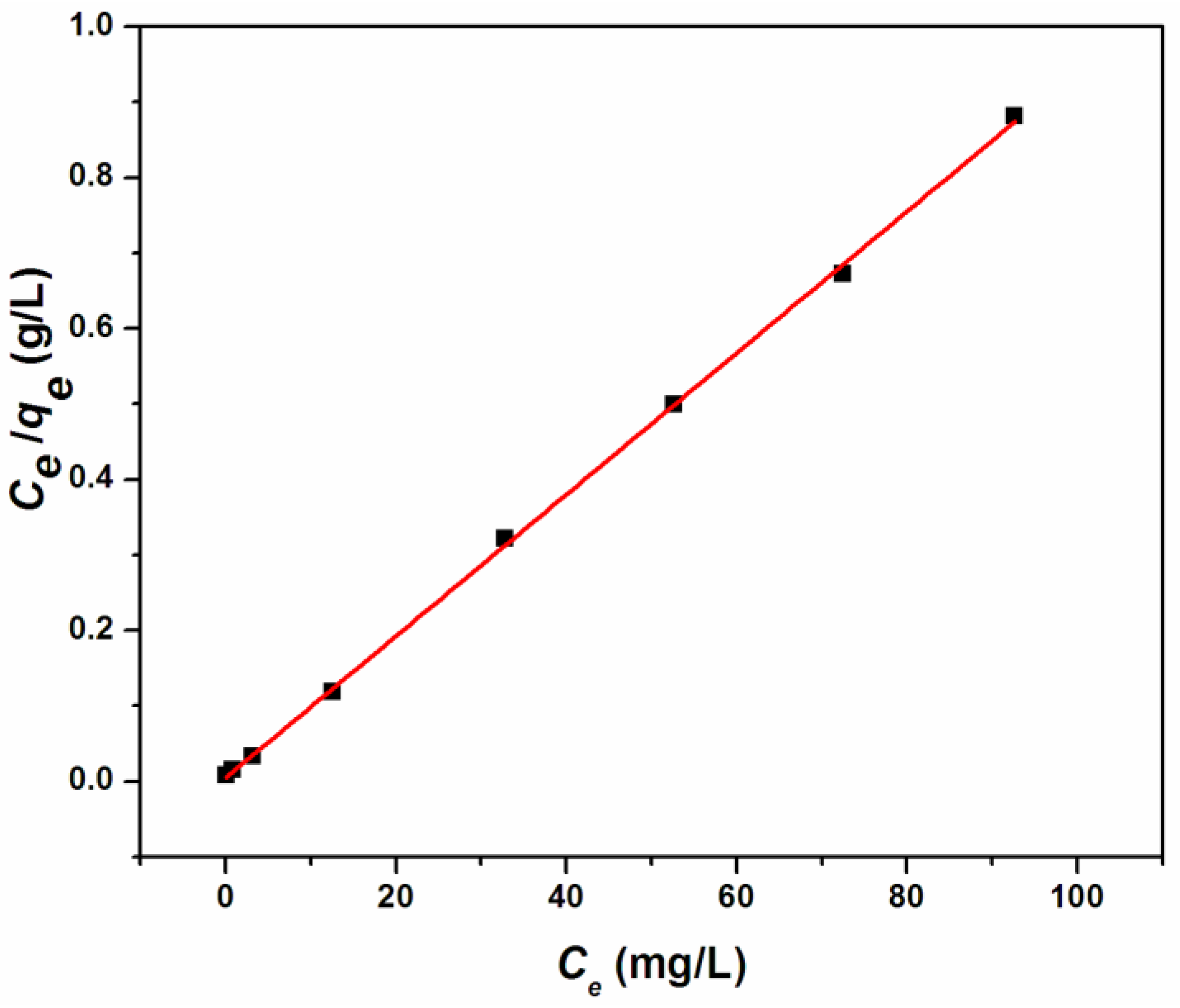
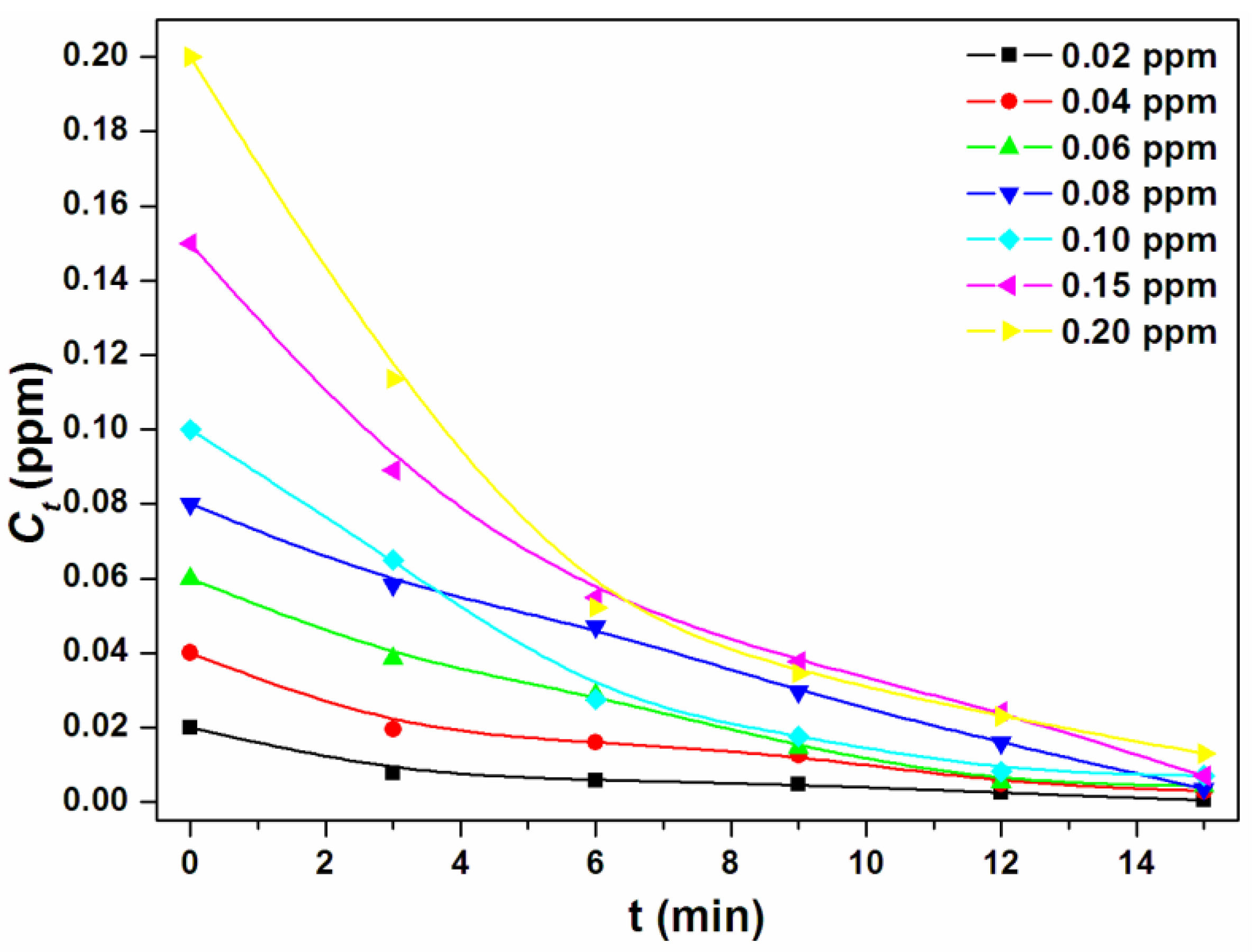
3.2. Cr(III) Adsorption Studies
3.3. The Stability of 1
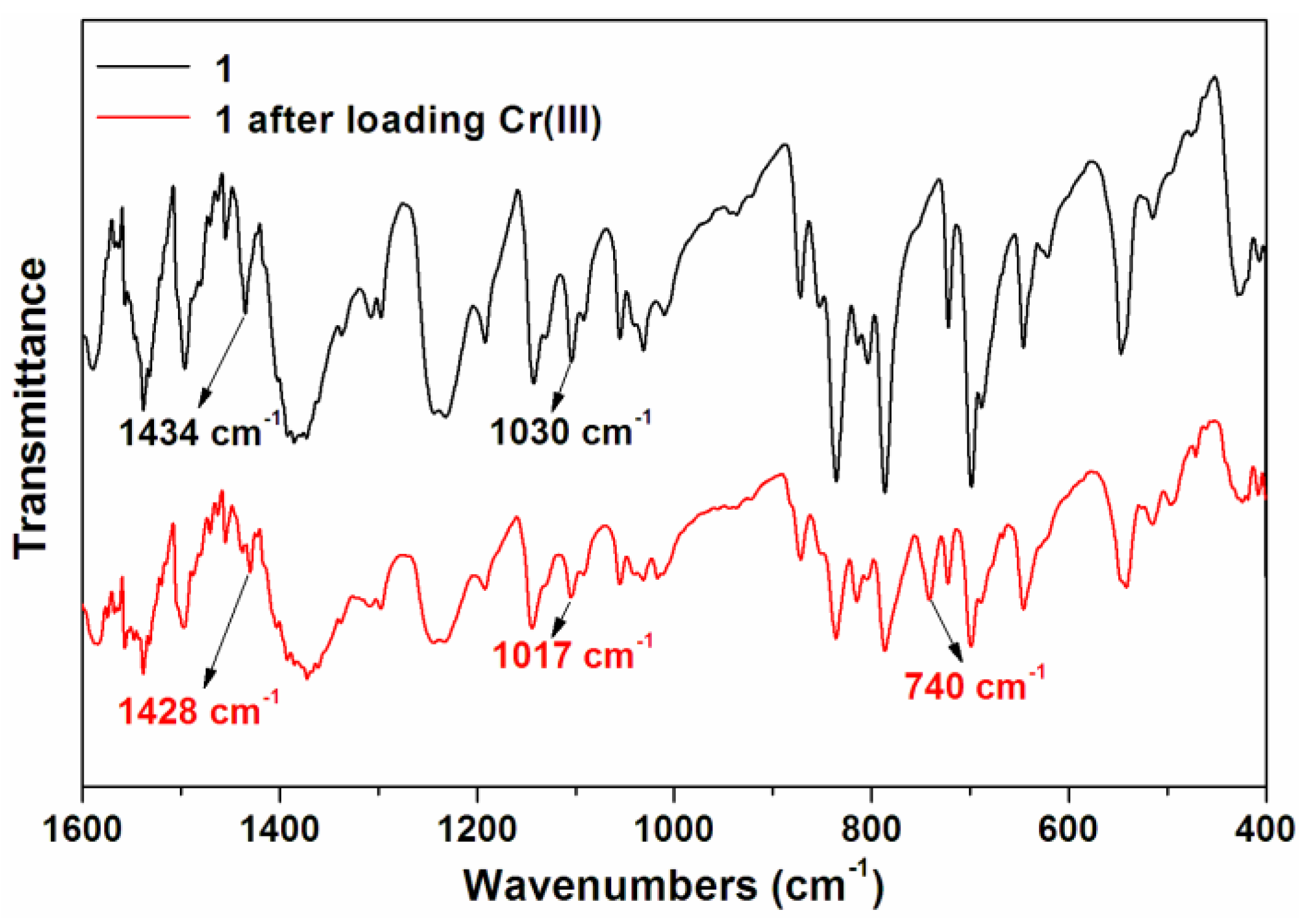
3.4. The Mechanism for Removal of Cr(III)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Feng, Z.; Zhu, S.; Martins de Godoi, D.R.; Samia, A.C.S.; Scherson, D. Adsorption of Cd2+ on carboxyl-terminated superparamagnetic iron oxide nanoparticles. Anal. Chem. 2012, 84, 3764–3770. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Hadi, P.; Chen, G.; McKay, G.J. Removal of cadmium ions from wastewater using innovative electronic waste-derived material. J. Hazard. Mater. 2014, 273, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Miretzkya, P.; Cirelli, A.F. Cr(VI) and Cr(III) removal from aqueous solution by raw and modified lignocellulosic materials: A review. J. Hazard. Mater. 2010, 180, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, R.; Upreti, R.K.; Seth, P.K.; Chaturvedi, U.C. Effects of chromium on the immune system. FEMS Immunol. Med. Microbiol. 2002, 34, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.G.; Yu, M.X.; Yang, H.; Huang, K.W.; Li, F.Y.; Yi, T.; Huang, C.H. FRET-based sensor for imaging chromium(III) in living cells. Chem. Commun. 2008, 3387–3389. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, K.; Saha, R.; Ghosh, A.; Saha, B. Chromium removal technologies. Res. Chem. Intermed. 2013, 39, 2267–2286. [Google Scholar] [CrossRef]
- Thomas, D.W.; Merian, E. Metals and Their Compounds in the Environment; VCH: Weinheim, Germany, 1991. [Google Scholar]
- Vignati, D.A.L.; Dominik, J.; Beye, M.L.; Pettine, M.; Ferrari, B.J.D. Chromium(VI) is more toxic than chromium(III) to freshwater algae: A paradigm to revise? Ecotoxicol. Environ. Saf. 2010, 73, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Kaszycki, P.; Fedorovych, D.; Ksheminska, H.; Babyak, L.; Wójcik, D.; Koloczek, H. Chromium accumulation by living yeast at various environmental conditions. Microbiol. Res. 2004, 159, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Suwalsky, M.; Castro, R.; Villena, F.; Sotomayor, C.P. Cr(III) exerts stronger structural effects than Cr(VI) on the human erythrocyte membrane and molecular models. J. Inorg. Biochem. 2008, 102, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Eastmond, D.A.; MacGregor, J.T.; Slesinski, R.S. Trivalent chromium: Assessing the genotoxic risk of an essential trace element and widely used human and animal nutritional supplement. Crit. Rev. Toxicol. 2008, 38, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Figgitt, M.; Newson, R.; Leslie, I.J.; Fisher, J.; Ingham, E.; Case, C.P. The genotoxicity of physiological concentrations of chromium (Cr(III) and Cr(VI)) and cobalt (Co(II)): An in vitro study. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2010, 688, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.N.; Zhang, Y.; Zhou, H.J.; Geng, Z.G.; Liu, G.; Zhang, Y.X.; Zhao, H.J.; Wang, G.Z. Enhanced removal of trace Cr(VI) from neutral and alkaline aqueous solution by FeCo bimetallic nanoparticles. J. Colloid. Interf. Sci. 2016, 472, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yu, J.; Cai, J.; Zhang, L.; Cui, Y.; Yang, Y.; Chen, B.; Qian, G. A porous Zr-cluster-based cationic metal–organic framework for highly efficient Cr2O72− removal from water. Chem. Commun. 2015, 51, 14732–14734. [Google Scholar] [CrossRef] [PubMed]
- Melak, F.; Laing, G.D.; Ambelu, A.; Alemayehu, E. Application of freeze desalination for chromium(VI) removal from water. Desalination 2016, 377, 23–27. [Google Scholar] [CrossRef]
- Jyothi, M.S.; Nayak, V.; Padaki, M.; Balakrishna, R.G.; Soontarapa, K. Aminated polysulfone/TiO2 composite membranes for an effective removal of Cr(VI). Chem. Eng. J. 2016, 283, 1494–1505. [Google Scholar] [CrossRef]
- Qiu, W.M.; Yang, D.; Xu, J.C.; Hong, B.; Jin, H.X.; Jin, D.F.; Peng, X.L.; Li, J.; Ge, H.L.; Wang, X.Q. Efficient removal of Cr(VI) by magnetically separable CoFe2O4/activated carbon composite. J. Alloys Compd. 2016, 678, 179–184. [Google Scholar] [CrossRef]
- Gheju, M.; Balcu, I.; Mosoarca, G. Removal of Cr(VI) from aqueous solutions by adsorption on MnO2. J. Hazard. Mater. 2016, 310, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Saleem, H.; Rafique, U.; Davies, R.P. Investigations on post-synthetically modified UiO-66-NH2 for the adsorptive removal of heavy metal ions from aqueous solution. Microporous Mesoporoius Mater. 2016, 221, 238–244. [Google Scholar] [CrossRef]
- Chen, Z.P.; Li, Y.R.; Guo, M.; Xu, F.Y.; Wang, P.; Du, Y.; Na, P. One-pot synthesis of Mn-doped TiO2 grown on graphene and the mechanism for removal of Cr(VI) and Cr(III). J. Hazard Mater. 2016, 310, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, T.; Borthwick, A.G.L.; Wang, Y.Q.; Yin, X.C.; Li, X.Z.; Ni, J.R. Adsorption of Pb2+, Cd2+, Cu2+ and Cr3+ onto titanate nanotubes: Competition and effect of inorganic ions. Sci. Total. Environ. 2013, 456–457, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, A.R.; Bhatti, H.N.; Hanif, M.A.; Nadeem, R. Kinetic and thermodynamic aspects of Cu(II) and Cr(III) removal from aqueous solutions using rose waste biomass. J. Hazard Mater. 2009, 161, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Suleiman, J.S.; He, M.; Hu, B. Chromium(III)-imprinted silica gel for speciation analysis of chromium in environmental water samples with ICP-MS detection. Talanta 2008, 75, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Tang, S.S.; Chen, M.L.; Wang, J.H. Iron phosphate as a novel sorbent for selective adsorption of chromium(III) and chromium speciation with detection by ETAAS. J. Anal. At. Spectrom. 2012, 27, 466–472. [Google Scholar] [CrossRef]
- Babazadeh, M.; Hosseinzadeh-Khanmiri, R.; Abolhasani, J.; Ghorbani-Kalhor, E.; Hassanpour, A. Solid phase extraction of heavy metal ions from agricultural samples with the aid of a novel functionalized magnetic metal–organic framework. RSC Adv. 2015, 5, 19884–19892. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, H.; Zeng, H.; Liang, M.; Lu, R. Adsorption of chromium(VI) from aqueous solution by the iron(III)-impregnated sorbent prepared from sugarcane bagasse. Int. J. Environ. Sci. Technol. 2012, 9, 463–472. [Google Scholar] [CrossRef]
- WHO (World Health Organization); Geneva, Switzerland. 1996.
- Tanabe, K.K.; Cohen, S.M. Postsynthetic modification of metal–organic frameworks-a progress report. Chem. Soc. Rev. 2011, 40, 498–519. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Yang, S.H.; Blake, A.J.; Schröder, M. Studies on metal–organic frameworks of Cu(II) with isophthalate linkers for hydrogen storage. Acc. Chem. Res. 2014, 47, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Kosaka, W.; Matsuda, R.; Hori, A.; Hijikata, Y.; Belosludov, R.V.; Sakaki, S.; Takata, M.; Kitagawa, S. Self-accelerating CO sorption in a soft nanoporous crystal. Science 2014, 343, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.Q.; Wang, Q.; Li, H.J.; Meng, W.; Han, Y.; Hou, H.W.; Fan, Y.T. Self-assembly of unprecedented [8+12] Cu-metallamacrocycle-based 3D metal–organic frameworks. Chem. Commun. 2012, 48, 5736–5738. [Google Scholar] [CrossRef] [PubMed]
- Li, H.X.; Zhao, W.; Li, H.Y.; Xu, Z.L.; Wang, W.X.; Lang, J.P. [Cu30I16(mtpmt)12(μ10-S4)]: An unusual 30-membered copper(I) cluster derived from the C–S bond cleavage and its use in heterogeneous catalysis. Chem. Commun. 2013, 49, 4259–4261. [Google Scholar] [CrossRef] [PubMed]
- Heine, J.; Müller-Buschbaum, K. Engineering metal-based luminescence in coordination polymers and metal–organic frameworks. Chem. Soc. Rev. 2013, 49, 9232–9242. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.L.; Yu, C.X.; Zhou, W.; Zhang, Q.G.; Liu, S.M.; Shi, Y.F. Construction of four Zn(II) coordination polymers used as catalysts for the photodegradation of organic dyes in water. Polymers 2016, 8, 3. [Google Scholar] [CrossRef]
- Zhang, S.R.; Li, J.; Du, D.Y.; Qin, J.S.; Li, S.L.; He, W.W.; Su, Z.M.; Lan, Y.Q. A multifunctional microporous anionic metal–organic framework for column-chromatographic dye separation and selective detection and adsorption of Cr3+. J. Mater. Chem. A 2015, 3, 23426–23434. [Google Scholar] [CrossRef]
- Yao, Z.J.; Jin, G.X. Transition metal complexes based on carboranyl ligands containing N, P, and S donors: Synthesis, reactivity and applications. Coord. Chem. Rev. 2013, 257, 2522–2535. [Google Scholar] [CrossRef]
- Ma, L.F.; Wang, X.N.; Deng, D.S.; Luo, F.; Ji, B.M.; Zhang, J. Five porous zinc(II) coordination polymers functionalized with amide groups: Cooperative and size-selective catalysis. J. Mater. Chem. A 2015, 3, 20210–20217. [Google Scholar] [CrossRef]
- Li, B.Y.; Zhang, Y.M.; Ma, D.X.; Shi, Z.; Ma, S.Q. Mercury nano-trap for effective and efficient removal of mercury(II) from aqueous solution. Nat. Commun. 2014, 5, 5537–5543. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.Q.; Tu, B.B.; Ning, E.L.; Li, Q.W.; Zhao, D.Y. Distinct packings of supramolecular building blocks in metal–organic frameworks based on imidazoledicarboxylic acid. Inorg. Chem. 2015, 54, 9678–9680. [Google Scholar] [CrossRef] [PubMed]
- Abney, C.W.; Gilhula, J.C.; Lu, K.; Lin, W. Metal-organic framework templated inorganic sorbents for rapid and efficient extraction of heavy metals. Adv. Mater. 2014, 26, 7993–7997. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ye, G.Q.; Chen, H.H.; Hu, X.Y.; Niu, Z.; Ma, S.Q. Functionalized metal-organic framework as a new platform for efficient and selective removal of cadmium(II) from aqueous solution. J. Mater. Chem. A 2015, 3, 15292–15298. [Google Scholar] [CrossRef]
- Roosen, J.; Spooren, J.; Binnemans, K. Adsorption performance of functionalized chitosan–silica hybrid materials toward rare earths. J. Mater. Chem. A 2014, 2, 19415–19426. [Google Scholar] [CrossRef]
- Fang, Q.R.; Yuan, D.Q.; Sculley, J.L.; Li, J.R.; Han, Z.B.; Zhou, H.C. Functional mesoporous metal–organic frameworks for the capture of heavy metal ions and size-selective catalysis. Inorg. Chem. 2010, 49, 11637–11642. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.J.; He, M.; Chen, B.B.; Hu, B. A designable magnetic MOF composite and facile coordination-based post-synthetic strategy for the enhanced removal of Hg2+ from water. J. Mater. Chem. A 2015, 3, 11587–11595. [Google Scholar] [CrossRef]
- Luo, X.; Liu, L.; Deng, F.; Luo, S. Novel ion-imprinted polymer using crown ether as a functional monomer for selective removal of Pb(II) ions in real environmental water samples. J. Mater. Chem. A 2013, 1, 8280–8286. [Google Scholar] [CrossRef]
- Liu, T.; Che, J.X.; Hu, Y.Z.; Dong, X.W.; Liu, X.Y.; Che, C.M. Alkenyl/thiol-derived metal–organic frameworks (MOFs) by means of postsynthetic modification for effective mercury adsorption. Chem. Eur. J. 2014, 20, 14090–14095. [Google Scholar] [CrossRef] [PubMed]
- Zha, M.Q.; Liu, J.; Wong, Y.L.; Xu, Z.T. Extraction of palladium from nuclear waste-like acidic solutions by a metal–organic framework with sulfur and alkene functions. J. Mater. Chem. A 2015, 3, 3928–3934. [Google Scholar] [CrossRef]
- Ricco, R.; Konstas, K.; Styles, M.J.; Richardson, J.J.; Babarao, R.; Suzuki, K.; Scopece, P.; Falcaro, P. Lead(II) uptake by aluminium based magnetic framework composites (MFCs) in water. J. Mater. Chem. A 2015, 3, 19822–19831. [Google Scholar] [CrossRef]
- He, J.; Yee, K.K.; Xu, Z.T.; Zeller, M.; Hunter, A.D.; Chui, S.S.Y.; Che, C.M. Thioether side chains improve the stability, fluorescence, and metal uptake of a metal–organic framework. Chem. Mater. 2011, 23, 2940–2947. [Google Scholar] [CrossRef]
- Yee, K.K.; Reimer, N.; Liu, J.; Cheng, S.Y.; Yiu, S.M.; Weber, J.; Stock, N.; Xu, Z.T. Effective mercury sorption by thiol-laced metal–organic frameworks: In strong acid and the vapor phase. J. Am. Chem. Soc. 2013, 135, 7795–7798. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Chen, J.L.; Dang, L.L.; Zhou, W.N.; Lin, H.L.; Li, J.Q.; Liu, S.J.; Luo, M.B. High-performance Hg2+ removal from ultra-low-concentration aqueous solution using both acylamide-and hydroxyl-functionalized metal–organic framework. J. Mater. Chem. A 2015, 3, 9616–9620. [Google Scholar] [CrossRef]
- Wang, L.L.; Luo, F.; Dang, L.L.; Li, J.Q.; Wu, X.L.; Liu, S.J.; Luo, M.B. Ultrafast high-performance extraction of uranium from seawater without pretreatment using an acylamide-and carboxyl-functionalized metal-organic framework. J. Mater. Chem. A 2015, 3, 13724–13730. [Google Scholar] [CrossRef]
- Tahmasebi, E.; Masoomi, M.Y.; Yamini, Y.; Morsali, A. Application of mechanosynthesized azine-decorated Zinc(II) metal–organic frameworks for highly efficient removal and extraction of some heavy-metal Ions from aqueous samples: A comparative study. Inorg. Chem. 2015, 54, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.; Chen, K.T.; Li, Y.S.; Cheng, P.W.; Chen, T.R.; Chen, J.D. Crystal-to-crystal transformations and photoluminescence changes in the Cu(I) coordination networks based on a formamidinate ligand. CrystEngComm 2014, 16, 10640–10648. [Google Scholar] [CrossRef]
- Liu, L.L.; Yu, C.X.; Du, J.M.; Liu, S.M.; Cao, J.S.; Ma, L.F. Construction of five Zn(II)/Cd(II) coordination polymers derived from a new linear carboxylate/pyridyl ligand: Design, synthesis, and photocatalytic properties. Dalton Trans. 2016, 45, 12352–12361. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXS-97 and SHELXL-97, Program for X-ray Crystal Structure Solution; University of Göettingen: Göttingen, Germany, 1997. [Google Scholar]
- Rai, D.; Sass, B.M.; Moore, D.A. Chromium(III) hydrolysis constants and solubility of chromium(III) hydroxide. Inorg. Chem. 1987, 26, 345–349. [Google Scholar] [CrossRef]
- Kotaś, J.; Stasicka, Z. Chromium occurrence in the environment and methods of its speciation. Environ. Pollut. 2000, 107, 263–283. [Google Scholar] [CrossRef]
- Godea, F.; Pehlivan, E. Removal of chromium(III) from aqueous solutions using Lewatit S 100: The effect of pH, time, metal concentration and temperature. J. Hazard. Mater. 2006, 136, 330–337. [Google Scholar] [CrossRef] [PubMed]
- El-Bayaa, A.A.; Badawy, N.A.; Alkhalik, E.A. Effect of ionic strength on the adsorption of copper and chromium ions by vermiculite pure clay mineral. J. Hazard. Mater. 2009, 170, 1204–1209. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.J.; Wang, Q.; Islam, S.M.; Liu, Y.C.; Ma, S.L.; Kanatzidis, M.G. Highly selective and efficient removal of heavy metals by layered double hydroxide intercalated with the MoS42− ion. J. Am. Chem. Soc. 2016, 138, 2858–2866. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhu, L.Y.; Shan, G.Q. Removal of Cd2+ from contaminated water by nano-sized aragonite mollusk shell and the competition of coexisting metal ions. J. Colloid. Interf. Sci. 2012, 367, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Chertihin, G.V.; Bare, W.D.; Andrews, L. Reactions of laser-ablated chromium atoms with dioxygen. Infrared spectra of CrO, OCrO, CrOO, CrO3, Cr(OO)2, Cr2O2, Cr2O3 and Cr2O4 in solid argon. J. Chem. Phys. 1997, 107, 2798–2806. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Gu, J.L.; Wang, Y.; Li, B.; Li, Y.S.; Zhao, W.R.; Shi, J.L. Inherent anchorages in UiO-66 nanoparticles for efficient capture of alendronate and its mediated release. Chem. Commun. 2014, 50, 8779–8782. [Google Scholar] [CrossRef] [PubMed]
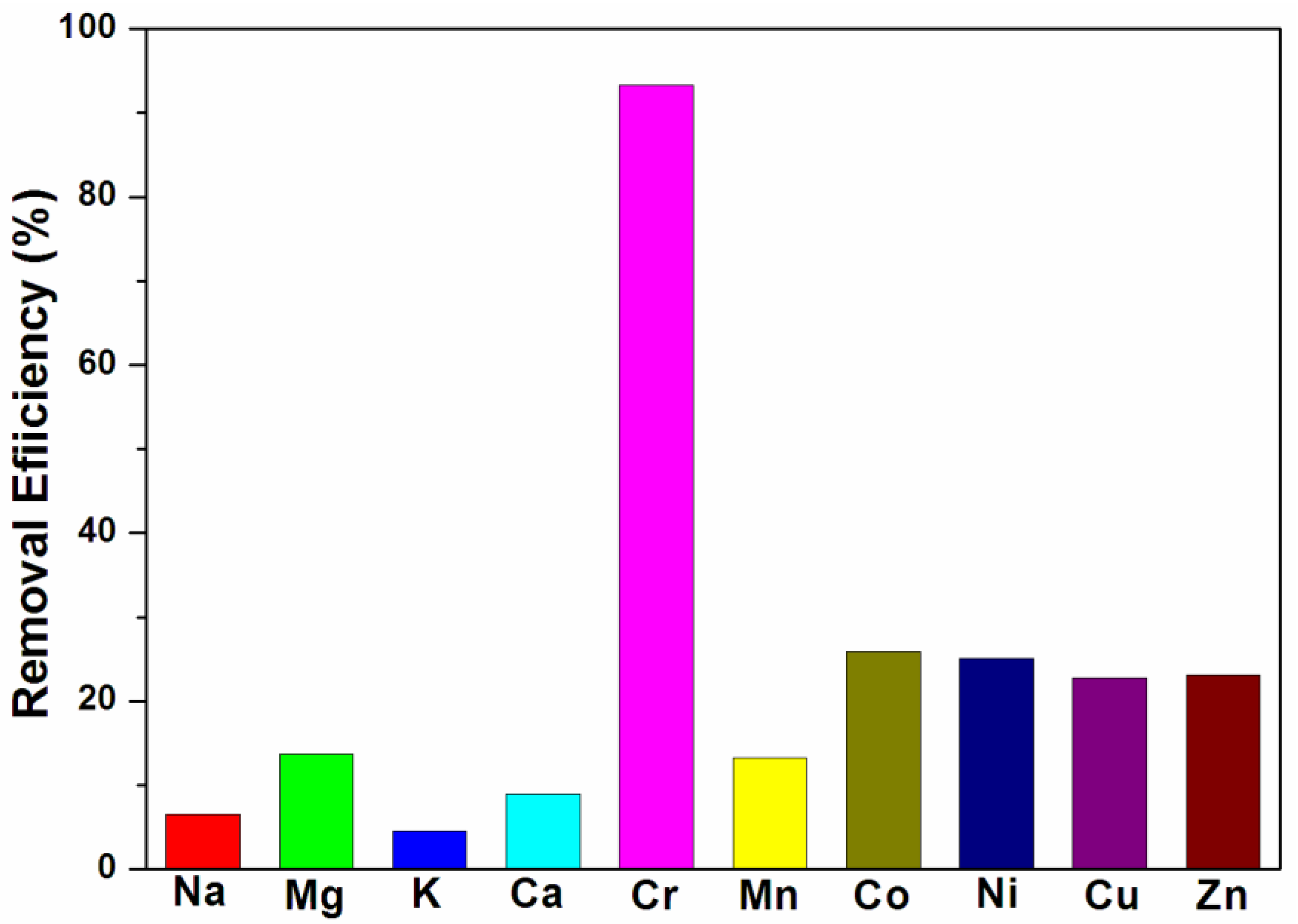


| Metal ions | Cr3+ | Na+ | Mg2+ | K+ | Ca2+ | Mn2+ | Co2+ | Ni2+ | Cu2+ | Zn2+ |
|---|---|---|---|---|---|---|---|---|---|---|
| Kd (mL·g−1) | 199,253 | 990 | 2262 | 673 | 1401 | 2172 | 4980 | 4777 | 4202 | 4294 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.-L.; Xing, Y.; Yu, H.-Y.; Zhang, C.-W.; Ye, M.-Q.; Miao, M.-Z.; Yu, C.-X. Effective Removal of Chromium(III) from Low Concentration Aqueous Solution Using a Novel Diazene/Methoxy-Laced Coordination Polymer. Polymers 2017, 9, 273. https://doi.org/10.3390/polym9070273
Liu L-L, Xing Y, Yu H-Y, Zhang C-W, Ye M-Q, Miao M-Z, Yu C-X. Effective Removal of Chromium(III) from Low Concentration Aqueous Solution Using a Novel Diazene/Methoxy-Laced Coordination Polymer. Polymers. 2017; 9(7):273. https://doi.org/10.3390/polym9070273
Chicago/Turabian StyleLiu, Lei-Lei, Yun Xing, Hui-Ying Yu, Cai-Wen Zhang, Meng-Qi Ye, Ming-Zhen Miao, and Cai-Xia Yu. 2017. "Effective Removal of Chromium(III) from Low Concentration Aqueous Solution Using a Novel Diazene/Methoxy-Laced Coordination Polymer" Polymers 9, no. 7: 273. https://doi.org/10.3390/polym9070273