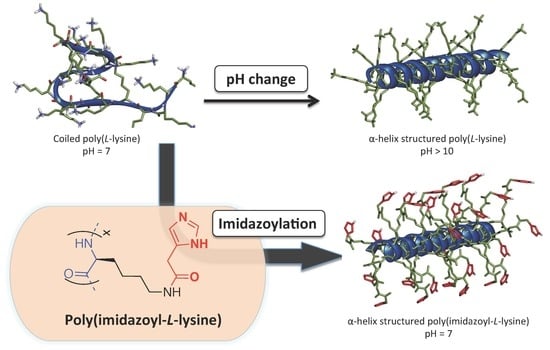
Smart Poly(imidazoyl-l-lysine): Synthesis and Reversible Helix-to-Coil Transition at Neutral pH
Abstract
:1. Introduction
2. Materials and Methods
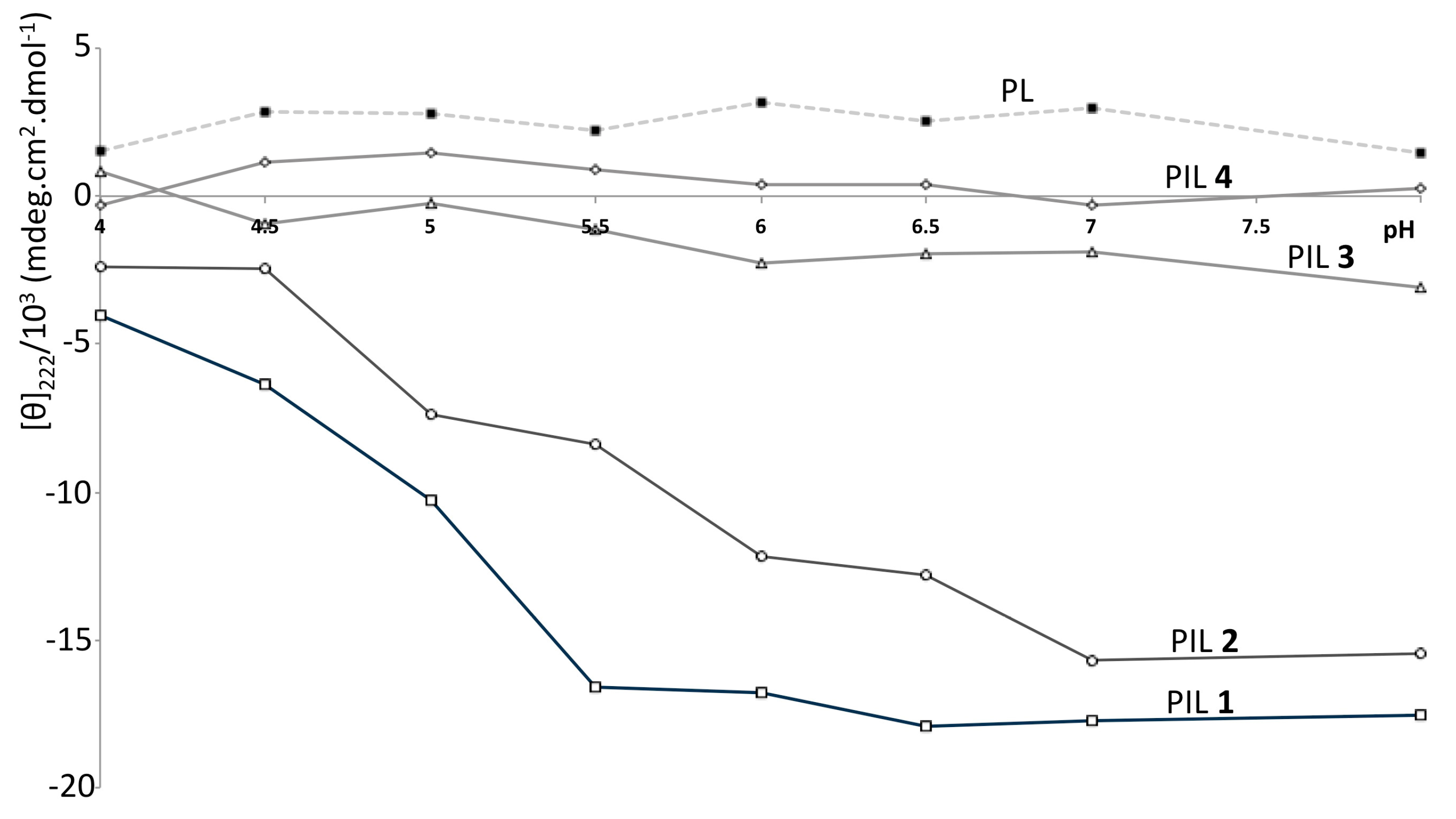
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huang, J.; Heise, A. Stimuli responsive synthetic polypeptides derived from N-carboxyanhydride (NCA) polymerisation. Chem. Soc. Rev. 2013, 42, 7373–7390. [Google Scholar] [CrossRef] [PubMed]
- Quadir, M.A.; Martin, M.; Hammond, P.T. Clickable synthetic polypeptides—Routes to new highly adaptive biomaterials. Chem. Mater. 2014, 26, 461–476. [Google Scholar] [CrossRef]
- Deming, T.J. Synthesis of side-chain modified polypeptides. Chem. Rev. 2015, 116, 786–808. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Muller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Fasman, G.D. Poly-α-Amino Acids; Decker: New York, NY, USA, 1967. [Google Scholar]
- Carlsen, A.; Lecommandoux, S. Self-assembly of polypeptide-based block copolymer amphiphiles. Curr. Opin. Colloid Interface Sci. 2009, 14, 329–339. [Google Scholar] [CrossRef]
- Rinaudo, M.; Domard, A. Circular dichroism studies on α-l-glutamic acid oligomers in solution. J. Am. Chem. Soc. 1976, 98, 6360–6364. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, M.; Holtzer, A. The helix-coil transition in solutions of polyglutamic acid. J. Am. Chem. Soc. 1964, 86, 538–543. [Google Scholar] [CrossRef]
- Parker, R.C.; Applegate, K.; Slutsky, L.J. Ultrasonic study of the Helix—Coil transition in Poly-l-lysine. J. Phys. Chem. 1966, 70, 3018–3019. [Google Scholar] [CrossRef]
- Vacogne, C.D.; Brosnan, S.M.; Masic, A.; Schlaad, H. Fibrillar gels via the self-assembly of poly(l-glutamate)-based statistical copolymers. Polym. Chem. 2015, 6, 5040–5052. [Google Scholar] [CrossRef]
- Krannig, K.-S.; Sun, J.; Schlaad, H. Stimuli-responsivity of secondary structures of glycopolypeptides derived from poly(l-glutamate–co–allylglycine). Biomacromolecules 2014, 15, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Mildner, R.; Menzel, H. Hydrophobic spacers enhance the helicity and lectin binding of synthetic, pH-responsive glycopolypeptides. Biomacromolecules 2014, 15, 4528–4533. [Google Scholar] [CrossRef] [PubMed]
- Krannig, K.-S.; Schlaad, H.; Krannig, K.S.; Schlaad, H. pH-responsive bioactive glycopolypeptides with enhanced helicity and solubility in aqueous solution. J. Am. Chem. Soc. 2012, 134, 18542–18545. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.R.; Deming, T.J. Multimodal switching of conformation and solubility in homocysteine derived polypeptides. J. Am. Chem. Soc. 2014, 136, 5547–5550. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.R.; Deming, T.J. Glycopolypeptides with a redox-triggered helix-to-coil transition. J. Am. Chem. Soc. 2012, 134, 4112–4115. [Google Scholar] [CrossRef] [PubMed]
- Bonduelle, C.; Makni, F.; Severac, L.; Piedra-Arroni, E.; Serpentini, C.-L.; Lecommandoux, S.; Pratviel, G. Smart metallopoly(l-glutamic acid) polymers: Reversible helix-to-coil transition at neutral pH. RSC Adv. 2016, 6, 84694–84697. [Google Scholar] [CrossRef]
- Nguyen, M.; Stigliani, J.-L.; Pratviel, G.; Bonduelle, C. Nucleopolypeptides with DNA-triggered α helix-to-β sheet transition. Chem. Commun. 2017, 53, 7501–7504. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.P.; John, J.V.; Kim, I. Poly(l-histidine)-containing polymer bioconjugate hybrid materials as stimuli-responsive theranostic systems. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Truong, N.P.; Gu, W.; Prasadam, I.; Jia, Z.; Crawford, R.; Xiao, Y.; Monteiro, M.J. An influenza virus-inspired polymer system for the timed release of siRNA. Nat. Commun. 2013, 4, 1902. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Kun, N.; Bae, Y.H. Polymeric micelle for tumor pH and folate-mediated targeting. J. Controll. Releas. 2003, 91, 103–113. [Google Scholar] [CrossRef]
- Lee, E.S.; Na, K.; Bae, Y.H. Super pH-sensitive multifunctional polymeric micelle. Nano Lett. 2005, 5, 325. [Google Scholar] [CrossRef] [PubMed]
- Mavrogiorgis, D.; Bilalis, P.; Karatzas, A.; Skoulas, D.; Fotinogiannopoulou, G.; Iatrou, H. Controlled polymerization of histidine and synthesis of well-defined stimuli responsive polymers. Elucidation of the structure-aggregation relationship of this highly multifunctional material. Polym. Chem. 2014, 5, 6256–6278. [Google Scholar] [CrossRef]
- Bikram, M.; Ahn, C.H.; Chae, S.Y.; Lee, M.; Yockma, J.W.; Kim, S.W. Biodegradable Poly(ethylene glycol)–co–poly(l-lysine)–g–histidine Multiblock Copolymers for Nonviral Gene Delivery. Macromolecules 2004, 37, 1903–1916. [Google Scholar] [CrossRef]
- Kim, E.K.; Yang, J.; Kim, H.O.; An, Y.; Lim, E.K.; Lee, G.; Kwon, T.; Cheong, J.H.; Suh, J.S.; Huh, Y.M.; et al. Hyaluronic acid receptor-targetable imidazolized nanovectors for induction of gastric cancer cell death by RNA interference. Biomaterials 2013, 4327–4338. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.C.; Lee, H.-I.; Hammond, P.T. Highly efficient “grafting onto” a polypeptide backbone using click chemistry. Angew. Chem. Int. Ed. 2009, 48, 9334–9338. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Nguyen, L.; Song, Z.; Peng, S.; Lee, J.; Zheng, N.; Kapoor, I.; Hagler, L.D.; Cai, K.; Cheng, J.; et al. Integrating display and delivery functionality with a cell penetrating peptide mimic as a scaffold for intracellular multivalent multitargeting. J. Am. Chemi. Soc. 2016, 138, 9498–9507. [Google Scholar] [CrossRef] [PubMed]
- Masujima, T. Conformational Changes of the Poly (α-l-glutamic acid)–Cu (II) Macromolecular Complexes in the pH Range of 4–7. A Light Scattering Study with Emphasis on Aggregation and Helix-coil Transitions. Bull. Chem. Soc. Jpn. 1983, 56, 838–845. [Google Scholar] [CrossRef]
- Maeda, H.; Hiramatsu, T.; Ikeda, S. Effects of Bivalent Metal Cations on the Conformation and Aggregation of Poly(l-glutamic acid). Bull. Chem. Soc. Jpn. 1986, 59, 587–589. [Google Scholar] [CrossRef]
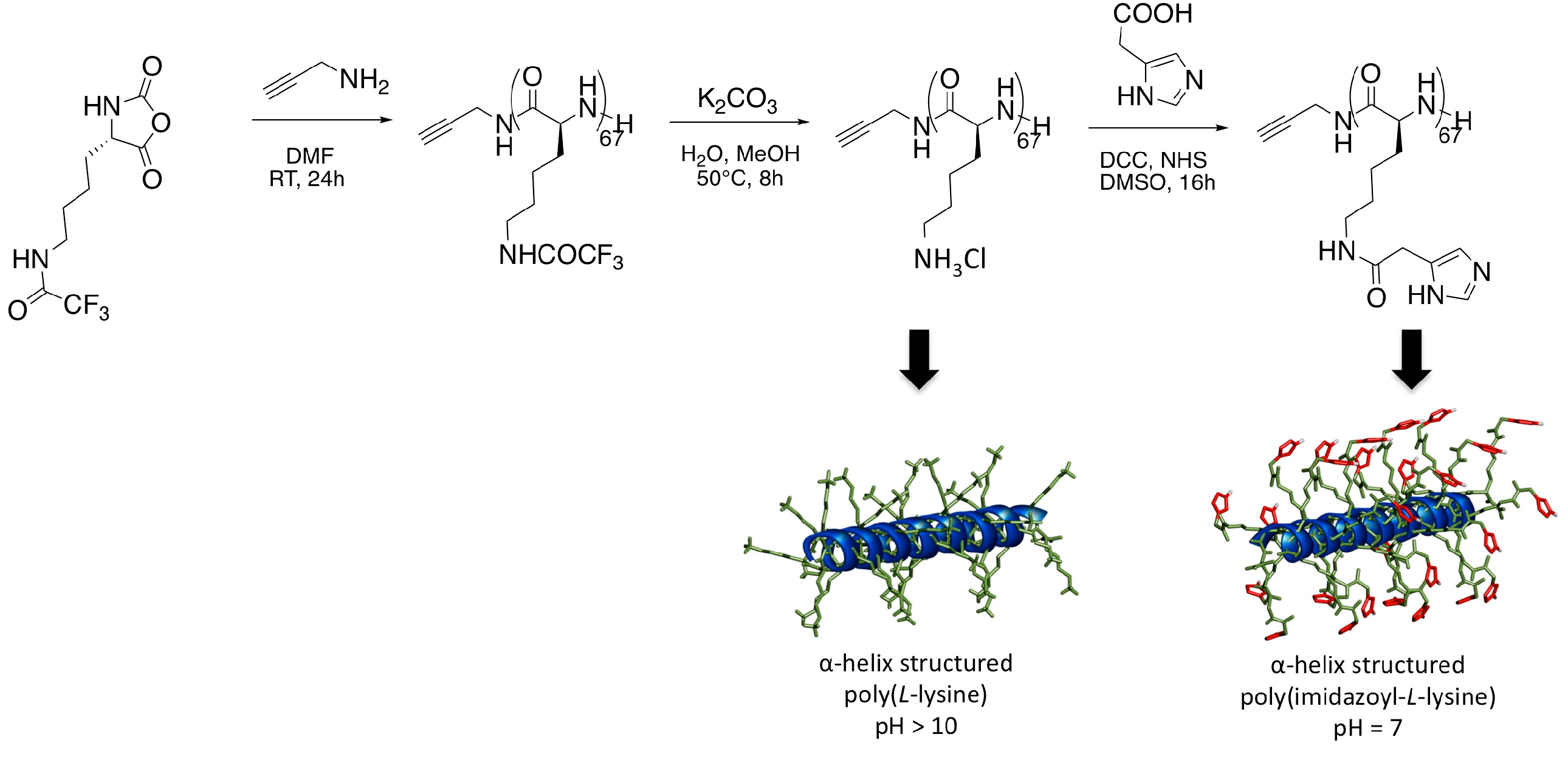
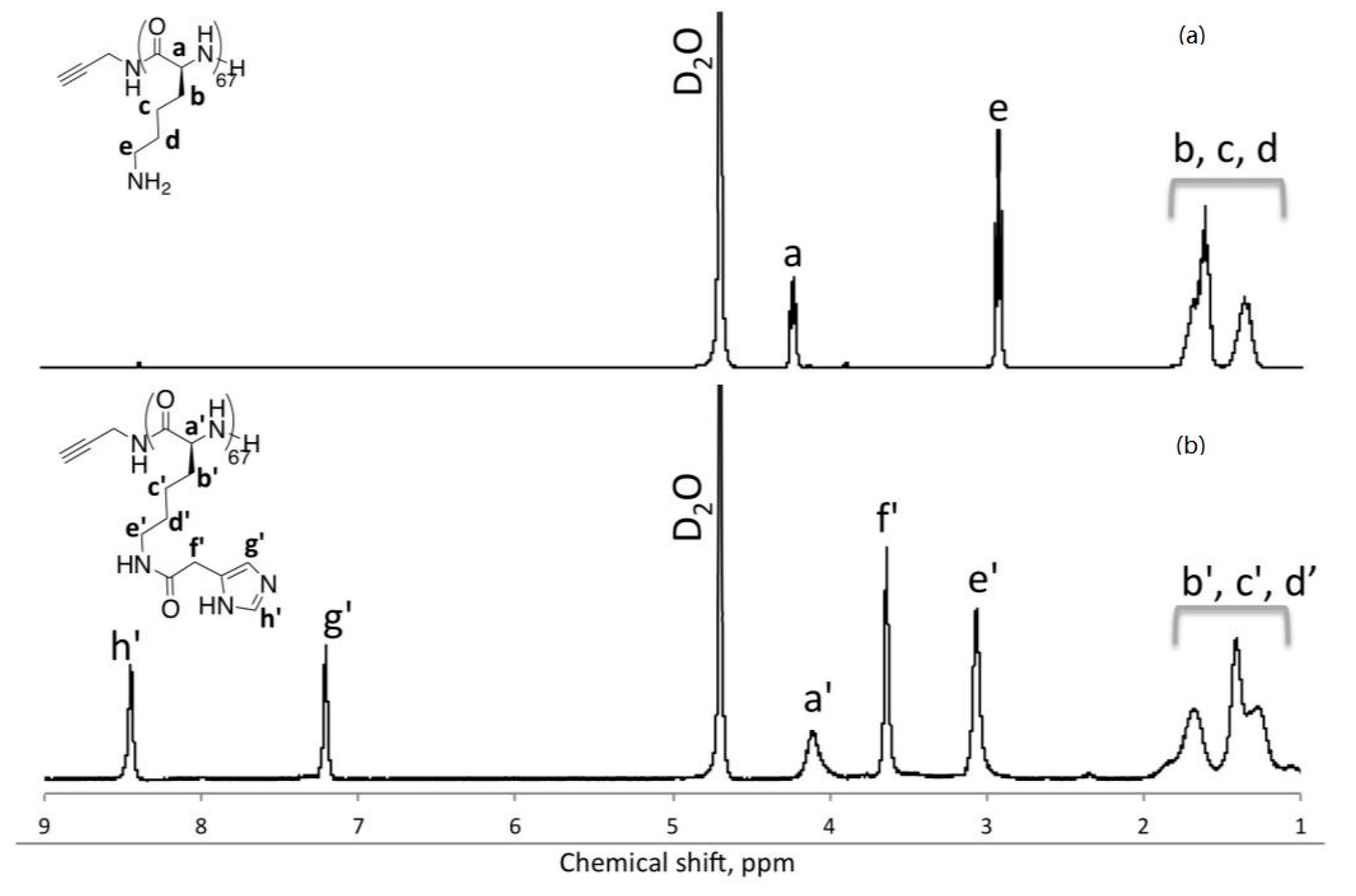
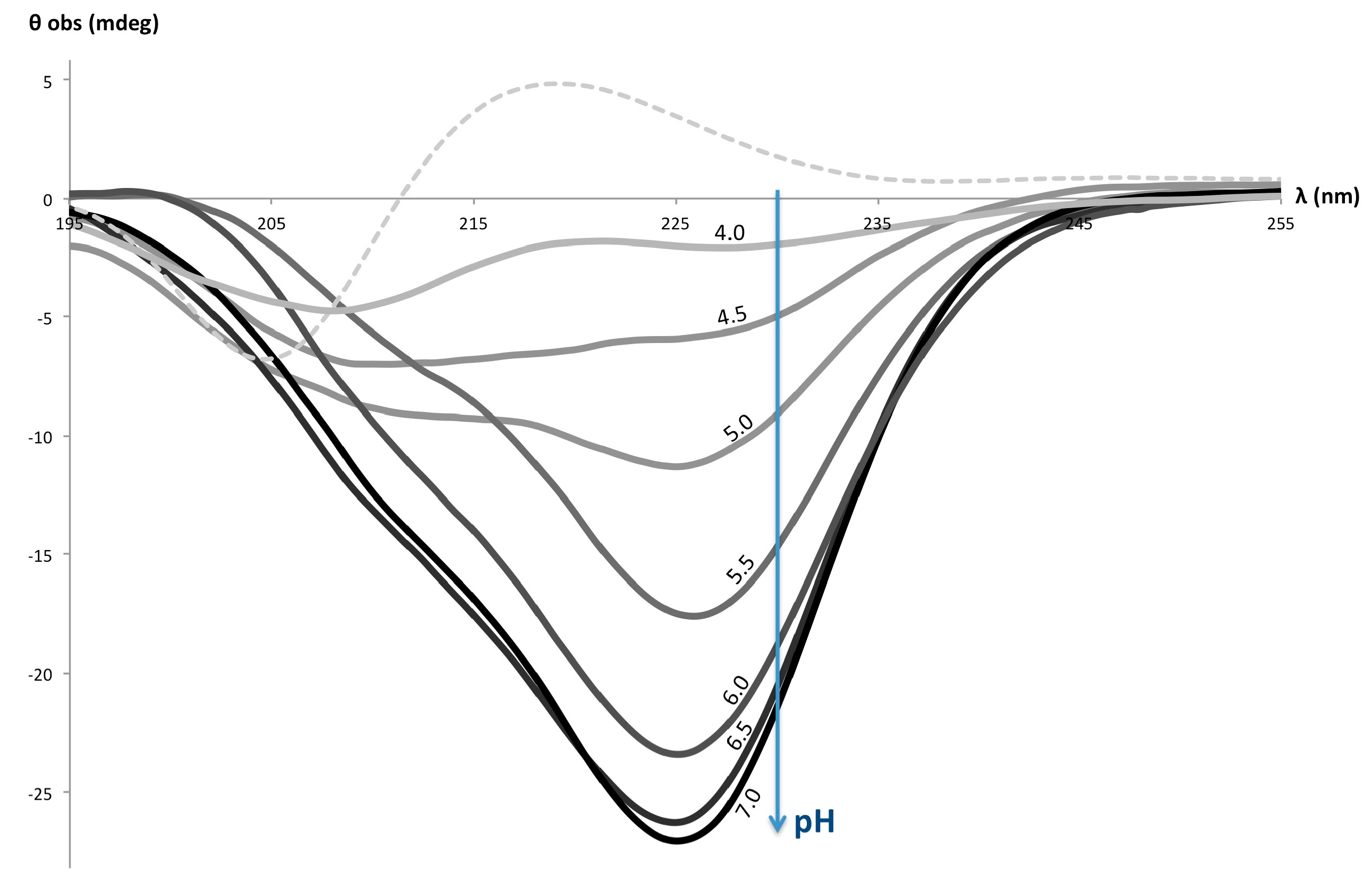

| Polymer | IAA Used for Coupling a | % Imidazole Grafting b | Yield of the Coupling Step | Mn d (PDI) |
|---|---|---|---|---|
| PL | - | 0 | - | 21,500 (1.15) |
| PIL 1 | 2 eq. | >99 c | 85% | 38,400 (1.17) |
| PIL 2 | 1 eq. | 75 | 84% | nd e |
| PIL 3 | 0.6 eq. | 47 | 86% | nd e |
| PIL 4 | 0.3 eq. | 31 | 89% | nd e |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piedra-Arroni, E.; Makni, F.; Severac, L.; Stigliani, J.-L.; Pratviel, G.; Bonduelle, C. Smart Poly(imidazoyl-l-lysine): Synthesis and Reversible Helix-to-Coil Transition at Neutral pH. Polymers 2017, 9, 276. https://doi.org/10.3390/polym9070276
Piedra-Arroni E, Makni F, Severac L, Stigliani J-L, Pratviel G, Bonduelle C. Smart Poly(imidazoyl-l-lysine): Synthesis and Reversible Helix-to-Coil Transition at Neutral pH. Polymers. 2017; 9(7):276. https://doi.org/10.3390/polym9070276
Chicago/Turabian StylePiedra-Arroni, Estefania, Fatma Makni, Laura Severac, Jean-Luc Stigliani, Geneviève Pratviel, and Colin Bonduelle. 2017. "Smart Poly(imidazoyl-l-lysine): Synthesis and Reversible Helix-to-Coil Transition at Neutral pH" Polymers 9, no. 7: 276. https://doi.org/10.3390/polym9070276