Polymerizable Ionic Liquid Crystals Comprising Polyoxometalate Clusters toward Inorganic-Organic Hybrid Solid Electrolytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Genaral Methods for Characterization
2.3. Synthesis
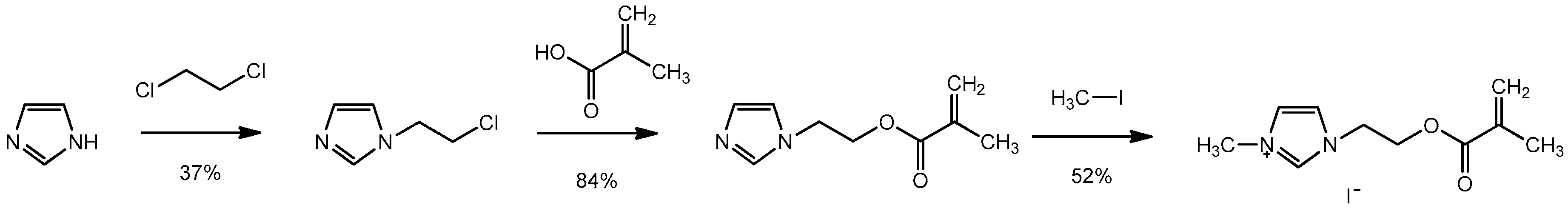
2.3.1. Synthesis of MAImC1-I
Synthesis of 1-(2-Chloroethyl)-1H-imidazole
Synthesis of 2-(1H-Imidazol-1-yl) Ethyl Methacrylate
Synthesis of 1-(2-(Methacryloyloxy) Ethyl)-3-Methyl-1H-Imidazol-3-ium Iodide (MAImC1-I)
Synthesis of 1-(2-(Methacryloyloxy) Ethyl)-3-Octyl-1H-Imidazol-3-ium Bromide (MAImC8-Br)
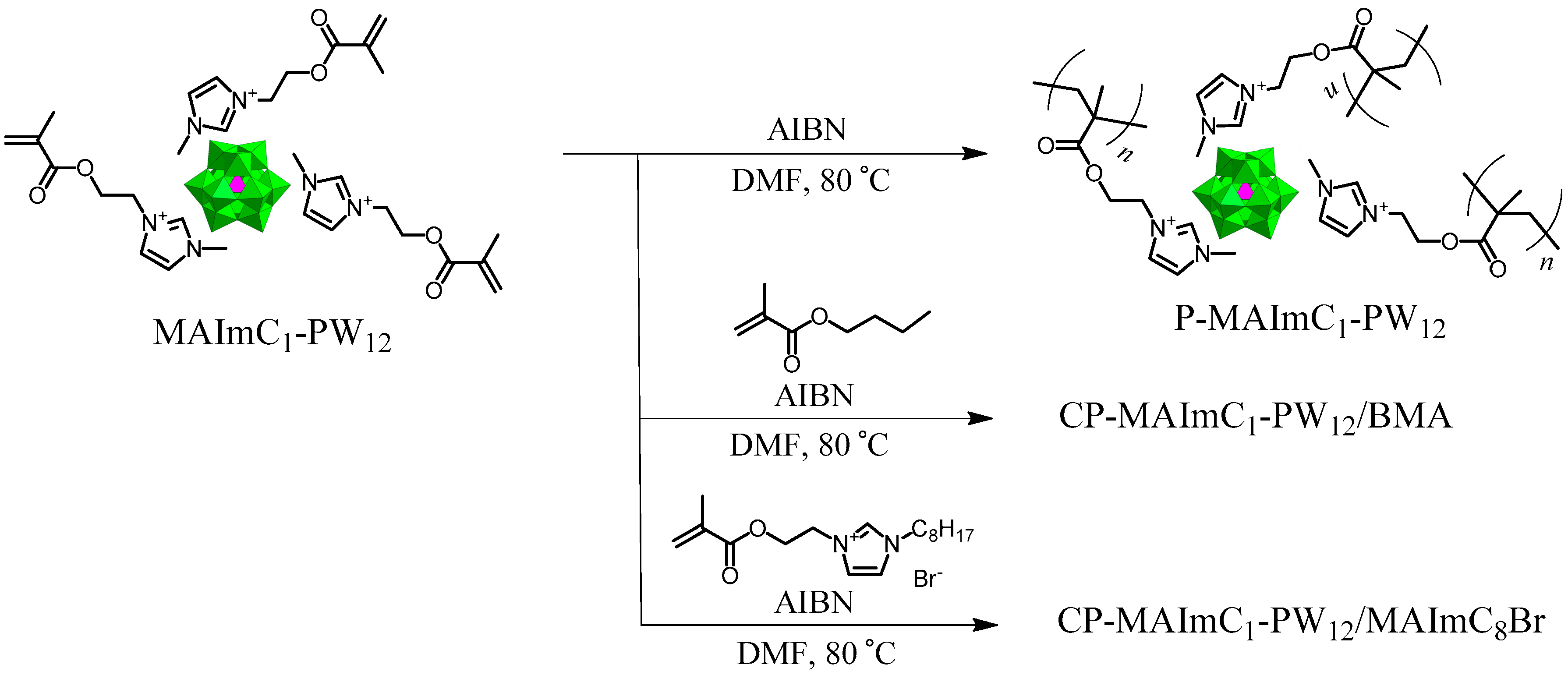
2.3.2. Synthesis of MAImC1-PW12 Hybrid Monomer
2.3.3. Syntheses of P-MAImC1-PW12 Hybrid Homopolymers
2.3.4. Syntheses of CP-MAImC1-PW12/BMA Hybrid Copolymers
2.3.5. Syntheses of CP-MAImC1-PW12/MAImC8Br Hybrid Copolymers
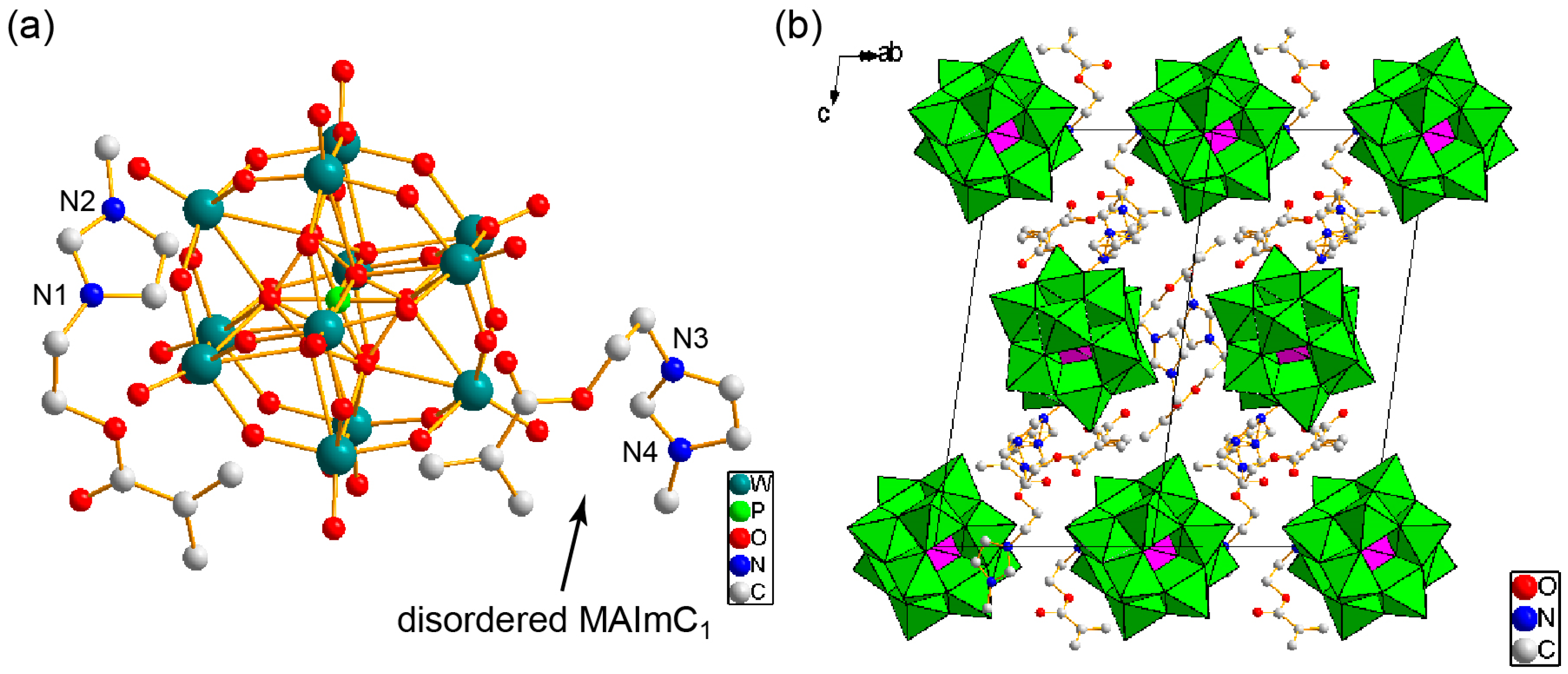
2.4. X-ray Crystallography
3. Results
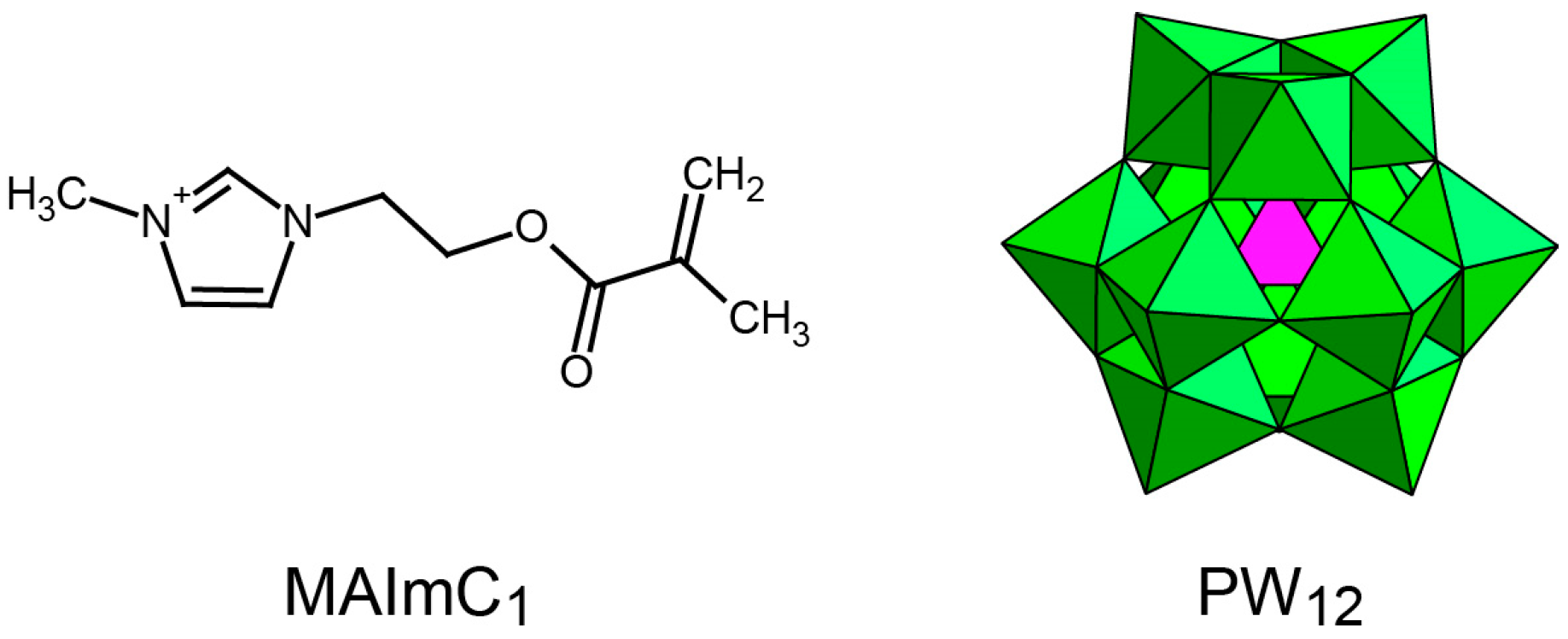
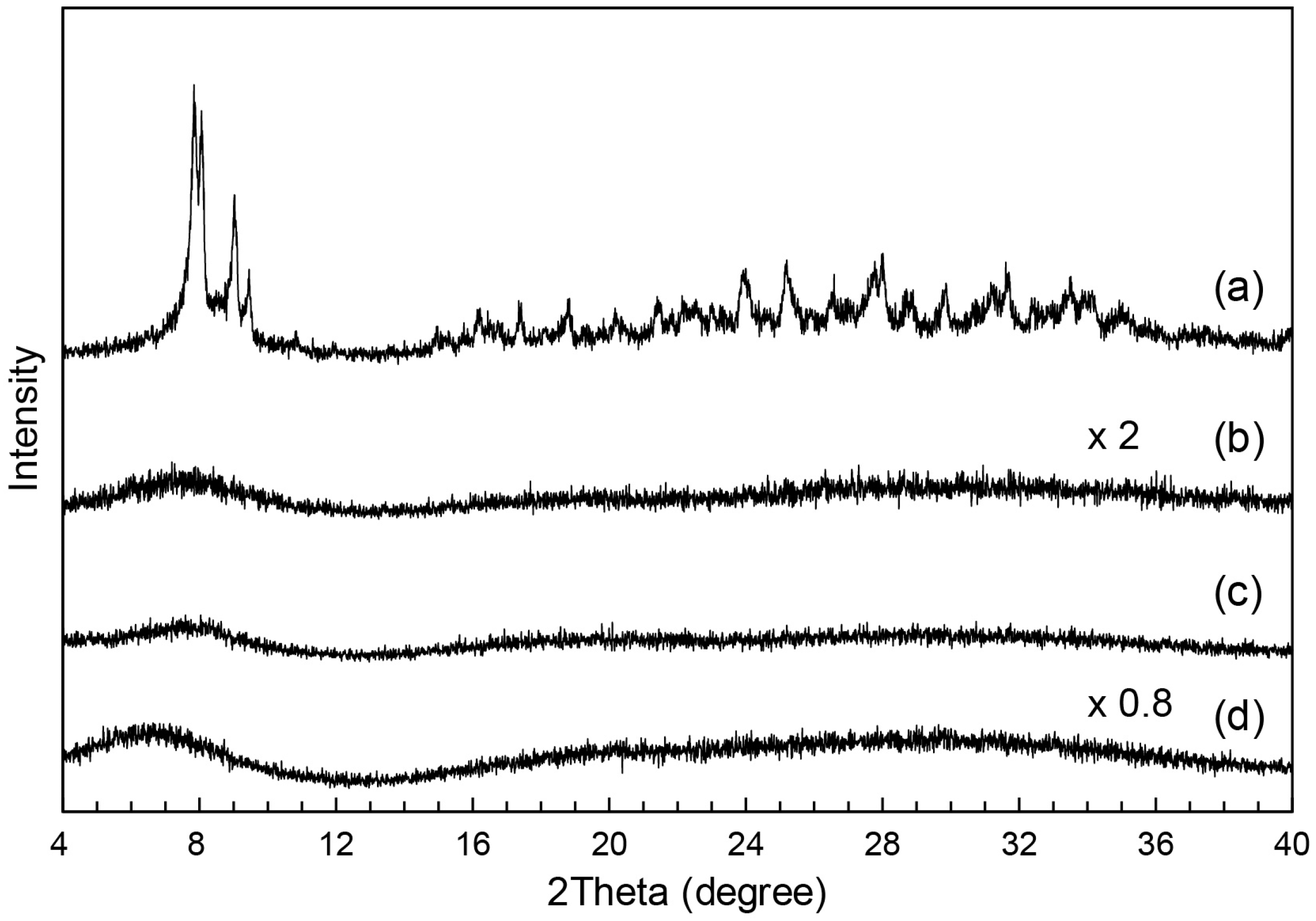
3.1. Synthesis and Structure of MAImC1-PW12 Hybrid Monomer
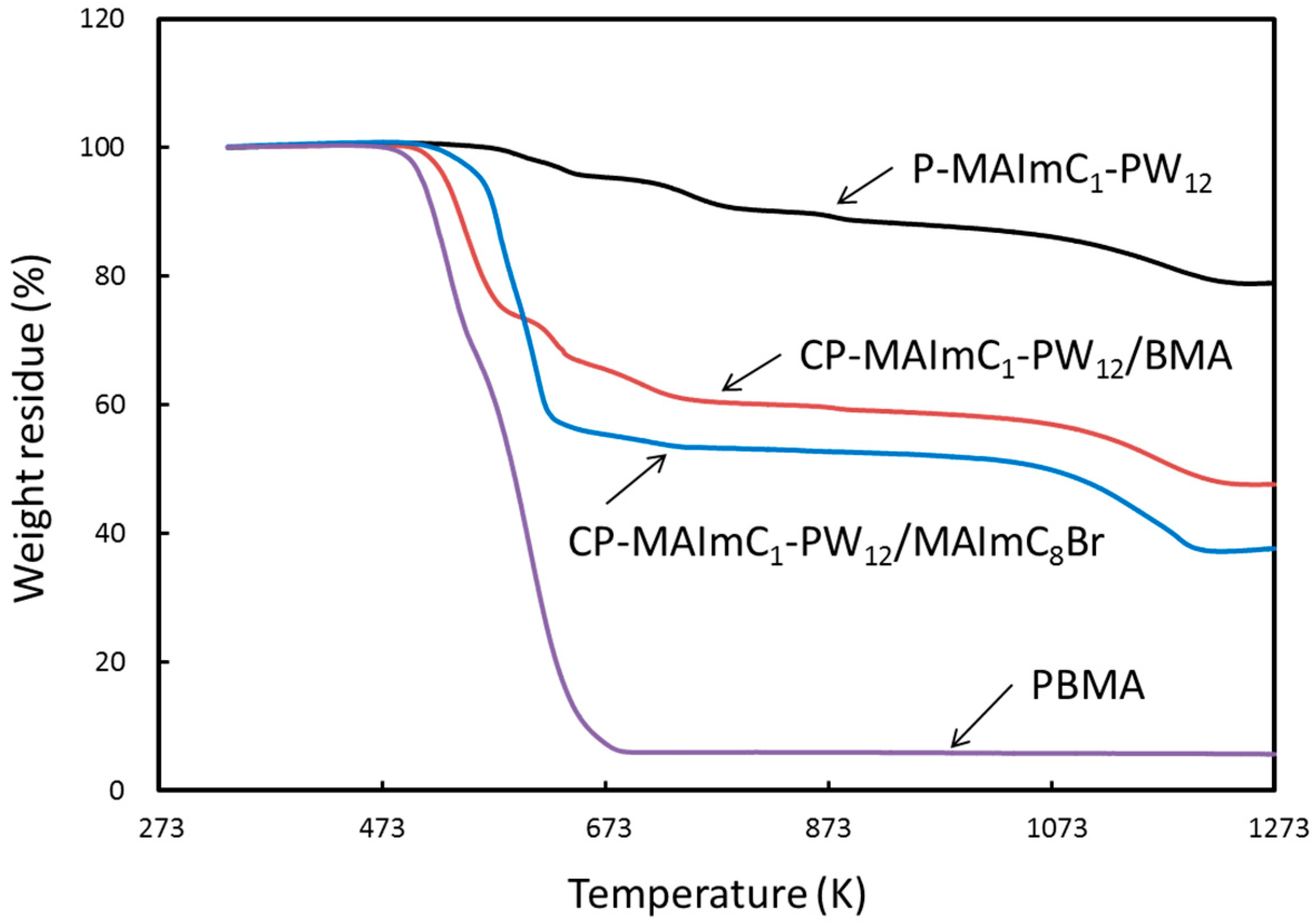
3.2. Synthesis of MAImC1-PW12 Hybrid Homo- and Copolymers
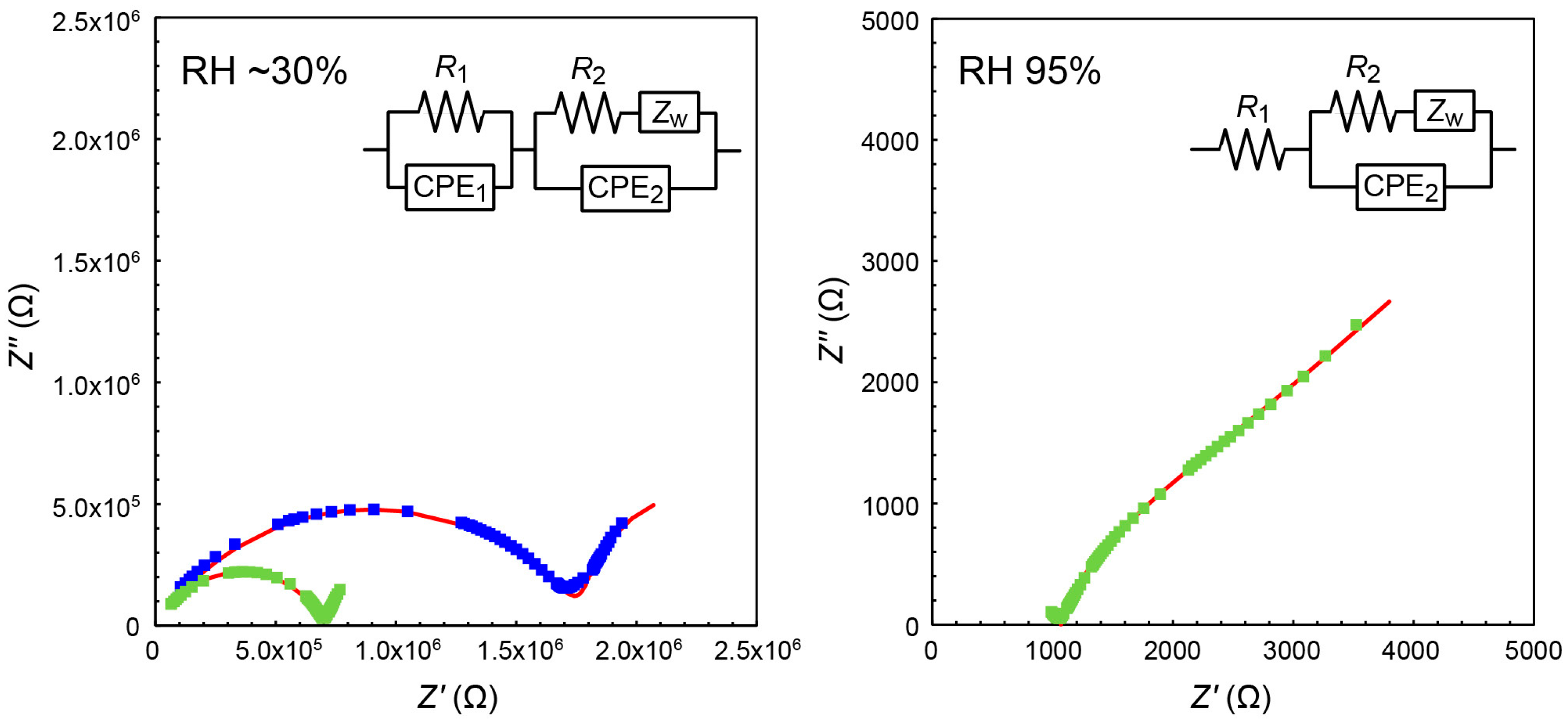
3.3. Conductivity of MAImC1-PW12 Hybrid Monomer and Polymers
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Whittingham, M.S. Lithium batteries and cathode materials. Chem. Rev. 2004, 104, 4271–4301. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Park, K.-S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.; Suh, K.; Natarajan, S.; Kim, K. Proton conduction in metal-organic frameworks and related modularly built porous solids. Angew. Chem. Int. Ed. 2013, 52, 2688–2700. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E.; Gómez-García, C.J. Polyoxometalate-based molecular materials. Chem. Rev. 1998, 98, 273–296. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E.; Giménez-Saiz, C.; Gómez-García, C.J. Recent advances in polyoxometalate-containing molecular conductors. Coord. Chem. Rev. 2005, 249, 1776–1796. [Google Scholar] [CrossRef]
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2083. [Google Scholar] [CrossRef] [PubMed]
- Wasserscheid, P.; Keim, W. Ionic liquids-new “solutions” for transition maetal catalysis. Angew. Chem. Int. Ed. 2000, 39, 3772–3789. [Google Scholar] [CrossRef]
- Haumann, M.; Riisager, A. Hydroformylation in room temperature ionic liquids (RTILs): Catalyst and process developments. Chem. Rev. 2008, 108, 1474–1497. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Mizoshita, N.; Kishimoto, K. Functional liquid-crystalline assemblies: Self-organized soft materials. Angew. Chem. Int. Ed. 2006, 45, 38–68. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, N.; Ohno, H. 15th anniversary of polymerised ionic liquids. Polymer 2014, 55, 3289–3297. [Google Scholar] [CrossRef]
- Bureekaew, S.; Horike, S.; Higuchi, M.; Mizuno, M.; Kawamura, T.; Tanaka, D.; Yanai, N.; Kitagawa, S. One-dimensional imidazole aggregate in aluminium porous coordination polymers with high proton conductivity. Nat. Mater. 2009, 8, 831–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, M.; Thomas, M.L.; Zhang, S.; Ueno, K.; Yasuda, T.; Dokko, K. Application of ionic liquids to energy storage and conversion materials and devices. Chem. Rev. 2017, 117, 7190–7239. [Google Scholar] [CrossRef] [PubMed]
- Long, D.-L.; Burkholder, E.; Cronin, L. Polyoxometalate clusters, nanostructures and materials: From self assembly to designer materials and devices. Chem. Soc. Rev. 2007, 36, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Nyman, M. Polyoxoniobate chemistry in the 21st century. Dalton Trans. 2011, 40, 8049–8058. [Google Scholar] [CrossRef] [PubMed]
- Proust, A.; Matt, B.; Villanneau, R.; Guillemot, G.; Gouzerh, P.; Izzet, G. Functionalization and post-functionalization: A step towards polyoxometalate-based materials. Chem. Soc. Rev. 2012, 41, 7605–7622. [Google Scholar] [CrossRef] [PubMed]
- Okuhara, T.; Mizuno, N.; Misono, M. Catalytic chemistry of heteropoly compounds. Adv. Catal. 1996, 41, 113–252. [Google Scholar]
- Yamase, T. Photo- and electrochromism of polyoxometalates and related materials. Chem. Rev. 1998, 98, 307–325. [Google Scholar] [CrossRef] [PubMed]
- Sadakane, M.; Steckhan, E. Electrochemical properties of polyoxometalates as electrocatalysts. Chem. Rev. 1998, 98, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, O.; Kodama, T.; Ogino, I.; Miyake, Y. High-conductivity solid proton conductors: Dodecamolybdophosphoric acid and dodecatungstophosphoric acid crystals. Chem. Lett. 1979, 8, 17–18. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Raman, K.; Herrera, R.; Zhang, Q.; Archer, L.A.; Giannelis, E.P. A liquid derivative of 12-tungstophosphoric acid with unusually high conductivity. J. Am. Chem. Soc. 2004, 126, 15358–15359. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Tong, X.; Wu, Q.; Ding, H.; Yan, W. Reversible phase transformation-type electrolyte based on layered shape polyoxometalate. J. Mater. Chem. A 2014, 2, 5780–5784. [Google Scholar] [CrossRef]
- Honma, I.; Yamada, M. Bio-inspired membranes for advanced polymer electrolyte fuel cells. Anhydrous proton-conducting membrane via molecular self-assembly. Bull. Chem. Soc. Jpn. 2007, 80, 2110–2123. [Google Scholar] [CrossRef]
- Oh, S.-Y.; Yoshida, T.; Kawamura, G.; Muto, H.; Sakai, M.; Matsuda, A. Inorganic-organic composite electrolytes consisting of polybenzimidazole and Cs-substituted heteropoly acids and their application for medium temperature fuel cells. J. Mater. Chem. 2010, 20, 6359–6366. [Google Scholar] [CrossRef]
- Judeinstein, P. Synthesis and properties of polyoxometalates based inorganic-organic polymers. Chem. Mater. 1992, 4, 4–7. [Google Scholar] [CrossRef]
- Mayer, C.R.; Thouvenot, R.; Lalot, T. New hybrid covalent networks based on polyoxometalates: Part 1. Hybrid networks based on poly(ethyl methacrylate) chains covalently cross-linked by heteropolyanions: Synthesis and swelling properties. Chem. Mater. 2000, 12, 257–260. [Google Scholar] [CrossRef]
- Mayer, C.R.; Thouvenot, R.; Lalot, T. Hybrid hydrogels obtained by the copolymerization of acrylamide with aggregates of methacryloyl derivatives of polyoxotungstates. A comparison with polyacrylamide hydrogels with trapped aggregates. Macromolecules 2000, 33, 4433–4437. [Google Scholar] [CrossRef]
- Moore, A.R.; Kwen, H.; Beatty, A.M.; Eric, A.; Maatta, E.A. Organoimido-polyoxometalates as polymer pendants. Chem. Commun. 2000, 1793–1794. [Google Scholar] [CrossRef]
- Lu, M.; Xie, B.; Kang, J.; Chen, F.-C.; Yang, Y.; Peng, Z. Synthesis of main-chain polyoxometalate-containing hybrid polymers and their applications in photovoltaic cells. Chem. Mater. 2005, 17, 402–408. [Google Scholar] [CrossRef]
- Xu, B.; Lu, M.; Kang, J.; Wang, D.; Brown, J.; Peng, Z. Synthesis and optical properties of conjugated polymers containing polyoxometalate clusters as side-chain pendants. Chem. Mater. 2005, 17, 2841–2851. [Google Scholar] [CrossRef]
- Hasegawa, T.; Shimizu, K.; Seki, H.; Murakami, H.; Yoshida, S.; Yoza, K.; Nomiya, K. Polymerizable inorganic-organic hybrid: Syntheses and structures of mono-lacunary Dawson polyoxometalate-based olefin-containing organosilyl derivatives. Inorg. Chem. Commun. 2007, 10, 1140–1144. [Google Scholar] [CrossRef]
- Hasegawa, T.; Murakami, H.; Shimizu, K.; Kasahara, Y.; Yoshida, S.; Kurashina, T.; Seki, H.; Nomiya, K. Formation of inorganic protonic-acid polymer via inorganic-organic hybridization: Synthesis and characterization of polymerizable olefinic organosilyl derivatives of mono-lacunary Dawson polyoxometalate. Inorg. Chim. Acta 2008, 361, 1385–1394. [Google Scholar] [CrossRef]
- Horan, J.L.; Genupur, A.; Ren, H.; Sikora, B.J.; Kuo, M.-C.; Meng, F.; Dec, S.F.; Haugen, G.M.; Yandrasits, M.A.; Hamrock, S.J.; et al. Copolymerization of divinylsilyl-11-silicotungstic acid with butyl acrylate and hexanediol diacrylate: Synthesis of a highly proton-conductive membrane for fuel-cell applications. ChemSusChem 2009, 2, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xiao, Y.; Zhang, Z.; Liu, B.; Zheng, P.; He, S.; Wang, W. Synthesis of polyoxometalate-polymer hybrid polymers and their hybrid vesicular assembly. Macromolecules 2009, 42, 6543–6548. [Google Scholar] [CrossRef]
- Miao, W.-K.; Yan, Y.-K.; Wang, X.-L.; Xiao, Y.; Ren, L.-J.; Zheng, P.; Wang, C.-H.; Ren, L.-X.; Wang, W. Incorporation of polyoxometalates into polymers to create linear poly(polyoxometalate)s with catalytic function. ACS Macro Lett. 2014, 3, 211–215. [Google Scholar] [CrossRef]
- Macdonell, A.; Johnson, N.A.B.; Surman, A.J.; Cronin, L. Configurable nanosized metal oxide oligomers via precise “click” coupling control of hybrid polyoxometalates. J. Am. Chem. Soc. 2015, 137, 5662–5665. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qi, W.; Li, W.; Sun, H.; Bu, W.; Wu, L. A highly transparent and luminescent hybrid based on the copolymerization of surfactant-encapsulated polyoxometalate and methyl methacrylate. Adv. Mater. 2005, 17, 2688–2692. [Google Scholar] [CrossRef]
- Qi, W.; Wu, L. Polyoxometalate/polymer hybrid materials: Fabrication and properties. Polym. Int. 2009, 58, 1217–1225. [Google Scholar] [CrossRef]
- Otwinowski, Z.; Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997, 276, 307–326. [Google Scholar] [PubMed]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Ide, R.; Kosaka, K.; Hasegawa, S.; Mikurube, K.; Taira, M.; Naruke, H.; Koguchi, S. Polyoxomolybdate-surfactant layered crystals derived from long-tailed alkylamine and ionic-liquid. Chem. Lett. 2013, 42, 1400–1402. [Google Scholar] [CrossRef]
- Ito, T.; Mikurube, K.; Hasegawa, K.; Matsumoto, T.; Kosaka, K.; Naruke, H.; Koguchi, S. Structural variation in polyoxomolybdate hybrid crystals comprising ionic-liquid surfactants. Crystals 2014, 4, 42–52. [Google Scholar] [CrossRef]
- Kobayashi, J.; Kawahara, R.; Uchida, S.; Koguchi, S.; Ito, T. Conductive hybrid crystal composed from polyoxomolybdate and deprotonatable ionic-liquid surfactant. Int. J. Mol. Sci. 2016, 17, 994. [Google Scholar] [CrossRef] [PubMed]
- Rocchiccioli-Deltcheff, C.; Fournier, M.; Franck, R.; Thouvenot, R. Vibrational investigations of polyoxometalates. 2. Evidence for anion-anion interactions in molybdenum(v1) and tungsten(v1) compounds related to the Keggin structure. Inorg. Chem. 1983, 22, 207–216. [Google Scholar] [CrossRef]
- Evans, H.T., Jr.; Pope, M.T. Reinterpretation of five recent crystal structures of heteropoly and isopoly complexes: Divanadodecamolybdopbosphate, trivanadoenneamolybdopbospbate, “γ-dodecatungstophospbate”, the dodecamolybdate-dodecamolybdomolybdate blue complex, and dihydrogen decavanadate. Inorg. Chem. 1984, 23, 501–504. [Google Scholar]
- Dong, J.; Hu, J.; Chi, Y.; Lin, Z.; Zou, B.; Yang, S.; Hill, C.L.; Hu, C. A polyoxoniobate–polyoxovanadate double-anion catalyst for simultaneous oxidative and hydrolytic decontamination of chemical warfare agent simulants. Angew. Chem. Int. Ed. 2017, 56, 4473–4477. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, S.; Ritchie, C.; Streb, C. Polyoxometalate—Conductive polymer composites for energy conversion, energy storage and nanostructured sensors. Dalton Trans. 2015, 44, 7092–7104. [Google Scholar] [CrossRef] [PubMed]


| Compound | MAImC1-PW12 |
|---|---|
| Chemical formula | C30H45N6PW12O46 |
| Formula weight | 3462.87 |
| Crystal system | Monoclinic |
| Space group | C2/c (No. 15) |
| a (Å) | 18.9467(2) |
| b (Å) | 14.6994(2) |
| c (Å) | 22.9023(4) |
| α (°) | 90.0000 |
| β (°) | 103.390(1) |
| γ (°) | 90.0000 |
| V (Å3) | 6205.02(15) |
| Z | 4 |
| ρcalcd (g cm−3) | 3.707 |
| T (K) | 100(2) |
| Wavelength (Å) | 0.70000 |
| μ (mm−1) | 20.615 |
| No. of reflections measured | 29,677 |
| No. of independent reflections | 8833 |
| Rint | 0.0525 |
| No. of parameters | 505 |
| R1 (I > 2σ(I)) | 0.0641 |
| wR2 (all data) | 0.1651 |
| Code | Yield (%) | Mn 1 × 10−3 | Mw 1 × 10−3 | Mw/Mn |
|---|---|---|---|---|
| P-MAImC1-PW12 | 85 | 7.47 (a) | 7.96 (a) | 1.07 (a) |
| CP-MAImC1-PW12/BMA (1:1) | 67 | 35.4 (a) | 45.4 (a) | 1.28 (a) |
| CP-MAImC1-PW12/BMA (1:3) | 68 | 9.88 (b) | 12.1 (b) | 1.17 (b) |
| PBMA homopolymer | 71 | 20.1 (b) | 28.8 (b) | 1.44 (b) |
| Code | Without Additional Humidity (Measured at 298 K) (S·cm−1) | Without Additional Humidity (Measured Temperature) (S·cm−1) | With Additional Humidity of 95% RH (Measured Temperature) (S·cm−1) |
|---|---|---|---|
| MAImC1-PW12 | 1.2 × 10−8 | 3.0 × 10−8 (327 K) | 3.0 × 10−7 (327 K) |
| P-MAImC1-PW12 | 8.4 × 10−10 | 2.0 × 10−7 (373 K) | 7.4 × 10−6 (373 K) |
| CP-MAImC1-PW12/BMA (1:3) | 8.6 × 10−10 | 5.2 × 10−8 (373 K) | 1.5 × 10−9 (373 K) |
| CP-MAImC1-PW12/MAImC8Br (1:1) | 3.3 × 10−7 | 8.4 × 10−7 (313 K) | 5.7 × 10−4 (313 K) |
| CP-MAImC1-PW12/MAImC8Br (3:1) | 6.0 × 10−8 | 7.2 × 10−8 (313 K) | 3.5 × 10−6 (313 K) |
| P-MAImC8Br | 1.8 × 10−6 | 9.2 × 10−6 (313 K) | 3.2 × 10−3 (313 K) 1 |
| Circuit Parameter | Without Additional Humidity at 298 K | Without Additional Humidity at 313 K | With Additional Humidity of 95% RH at 313 K |
|---|---|---|---|
| R1 (Ω) | 1.78 × 106 | 7.06 × 105 | 1.09 × 103 |
| CPE1-P 1 | 2.70 × 10−10 | 1.23 × 10−10 | - |
| CPE1-n 1 | 0.627 | 0.714 | - |
| R2 (Ω) | 2.77 × 107 | 4.98 × 109 | 2.98 × 103 |
| CPE2-P 1 | 4.79 × 10−8 | 1.51 × 10−7 | 3.26 × 10−6 |
| CPE2-n 1 | 0.757 | 0.759 | 0.76 |
| σ 2 | 2.90 × 104 | 2.90 × 104 | 2.90 × 104 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, T.; Otobe, S.; Oda, T.; Kojima, T.; Ono, S.; Watanabe, M.; Kiyota, Y.; Misawa, T.; Koguchi, S.; Higuchi, M.; et al. Polymerizable Ionic Liquid Crystals Comprising Polyoxometalate Clusters toward Inorganic-Organic Hybrid Solid Electrolytes. Polymers 2017, 9, 290. https://doi.org/10.3390/polym9070290
Ito T, Otobe S, Oda T, Kojima T, Ono S, Watanabe M, Kiyota Y, Misawa T, Koguchi S, Higuchi M, et al. Polymerizable Ionic Liquid Crystals Comprising Polyoxometalate Clusters toward Inorganic-Organic Hybrid Solid Electrolytes. Polymers. 2017; 9(7):290. https://doi.org/10.3390/polym9070290
Chicago/Turabian StyleIto, Takeru, Saki Otobe, Tatsuma Oda, Tatsuhiro Kojima, Seiji Ono, Masayuki Watanabe, Yoshiki Kiyota, Toshiyuki Misawa, Shinichi Koguchi, Masashi Higuchi, and et al. 2017. "Polymerizable Ionic Liquid Crystals Comprising Polyoxometalate Clusters toward Inorganic-Organic Hybrid Solid Electrolytes" Polymers 9, no. 7: 290. https://doi.org/10.3390/polym9070290






