Biphasic Calcium Phosphate (BCP)-Immobilized Porous Poly (d,l-Lactic-co-Glycolic Acid) Microspheres Enhance Osteogenic Activities of Osteoblasts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
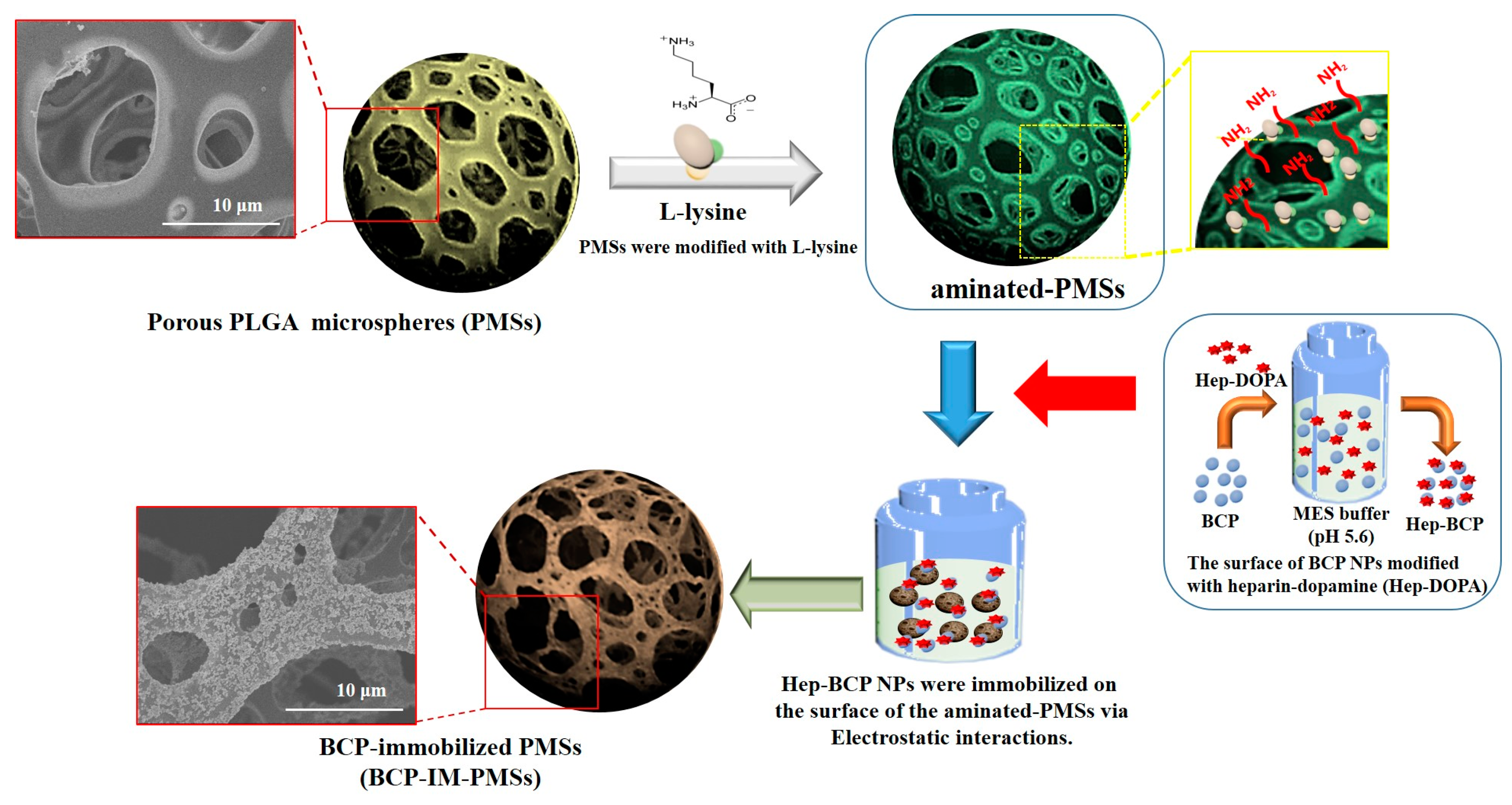
2.2. Fabrication of Porous PLGA Microspheres (PMSs), BCP-Mixed PMSs, and BCP-Immobilized PMSs
2.3. Characterization of the PMSs, Aminated-PMSs, BCP-M-PMSs, and BCP-IM-PMSs
2.4. Cell Proliferation
2.5. Alkaline Phosphatase (ALP) Activity
2.6. Calcium Deposition
2.7. Gene Expression
2.8. Statistical Analysis
3. Results
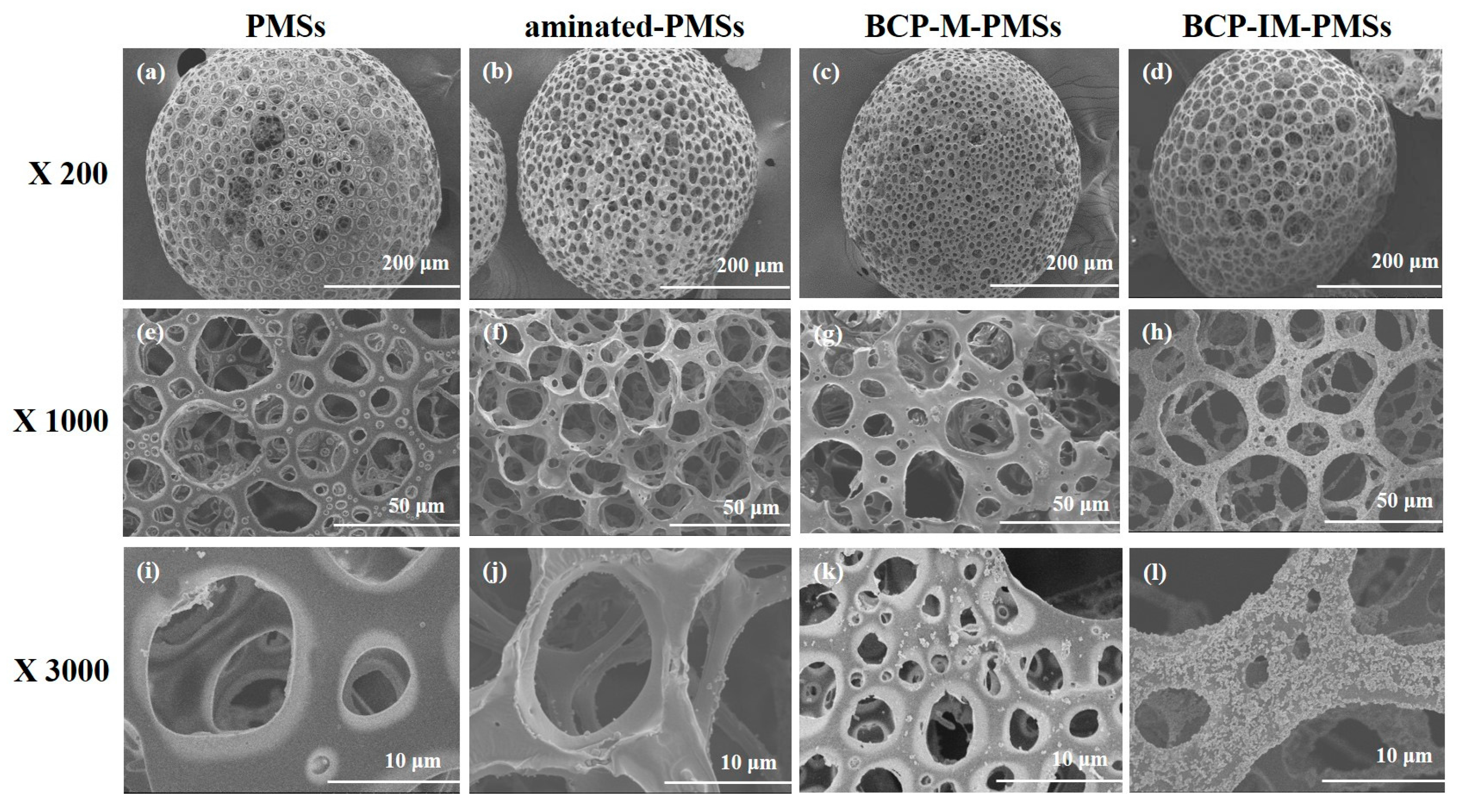
3.1. Characterization of PMSs, aminated–PMSs, BCP-M-PMSs, and BCP-IM-PMSs
3.2. Cell Proliferaiton
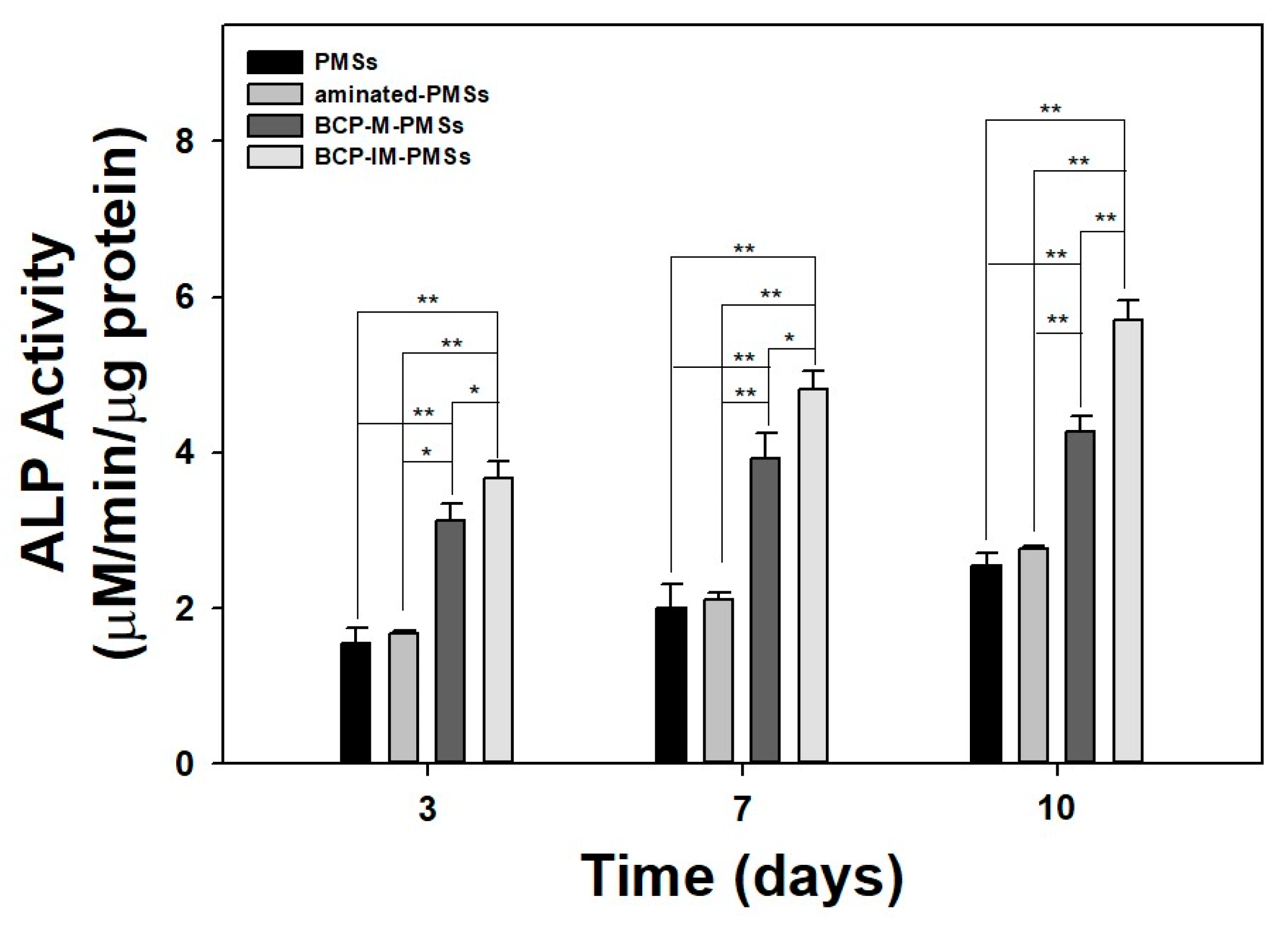
3.3. ALP Activity
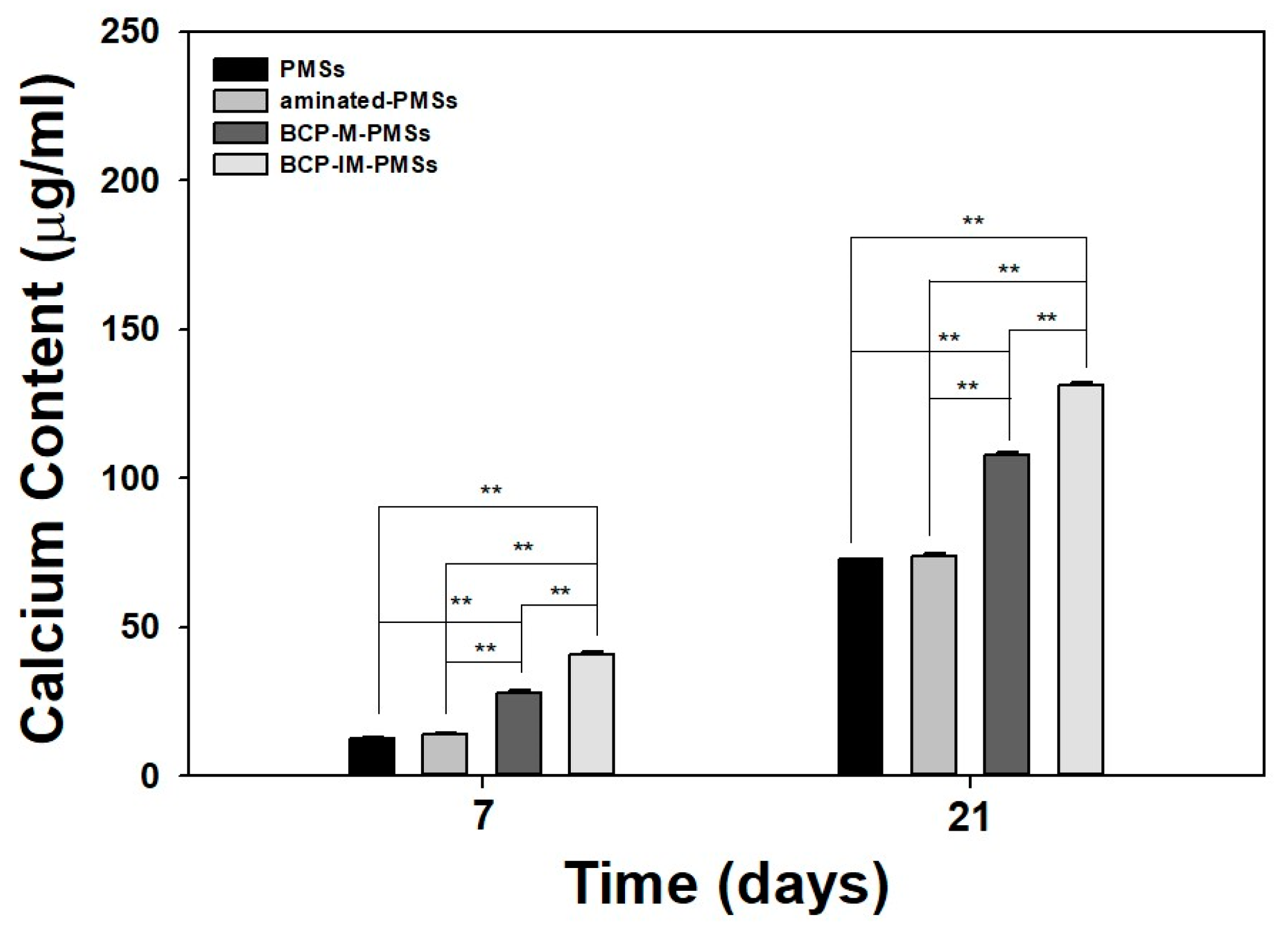
3.4. Calcium Deposition
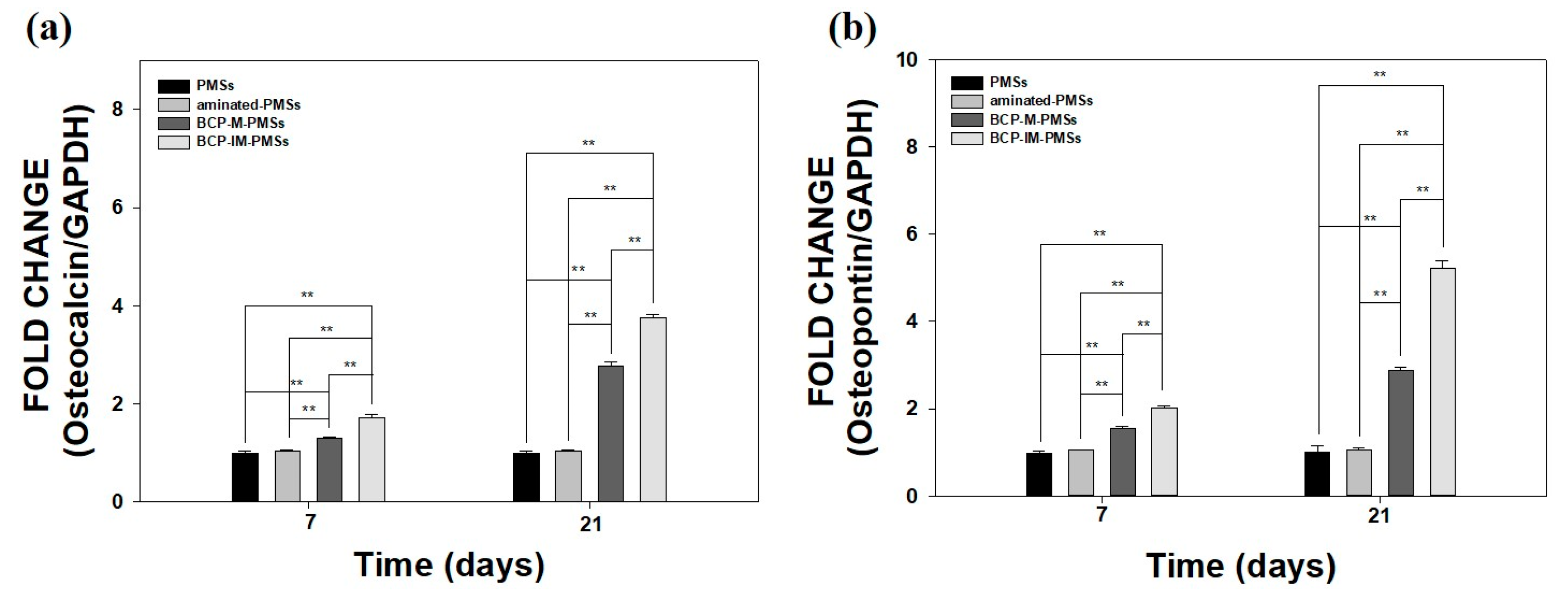
3.5. Gene Expression
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.K.; Roy, S.; Mukherjee, P.; Kundu, B.; De, D.K.; Basu, D. Orthopaedic applications of bone graft & graft substitutes: A review. Indian J. Med. Res. 2010, 132, 15–30. [Google Scholar] [PubMed]
- Athanasiou, V.T.; Papachristou, D.J.; Panagopoulos, A.; Saridis, A.; Scopa, C.D.; Megas, P. Histological comparison of autograft, allograft-dbm, xenograft, and synthetic grafts in a trabecular bone defect: An experimental study in rabbits. Med. Sci. Monit. 2010, 16, BR24–BR31. [Google Scholar] [PubMed]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed]
- Antonov, E.N.; Bagratashvili, V.N.; Whitaker, M.J.; Barry, J.J.; Shakesheff, K.M.; Konovalov, A.N.; Popov, V.K.; Howdle, S.M. Three-dimensional bioactive and biodegradable scaffolds fabricated by surface-selective laser sintering. Adv. Mater. 2004, 17, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Bettinger, C.J.; Weinberg, E.J.; Kulig, K.M.; Vacanti, J.P.; Wang, Y.D.; Borenstein, J.T.; Langer, R. Three-dimensional microfluidic tissue-engineering scaffolds using a flexible biodegradable polymer. Adv. Mater. 2006, 18, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Dreifke, M.B.; Ebraheim, N.A.; Jayasuriya, A.C. Investigation of potential injectable polymeric biomaterials for bone regeneration. J. Biomed. Mater. Res. A 2013, 101, 2436–2447. [Google Scholar] [CrossRef] [PubMed]
- Munarin, F.; Petrini, P.; Bozzini, S.; Tanzi, M.C. New perspectives in cell delivery systems for tissue regeneration: Natural-derived injectable hydrogels. J. Appl. Biomater. Funct. Mater. 2012, 10, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Freiberg, S.; Zhu, X.X. Polymer microspheres for controlled drug release. Int. J. Pharm. 2004, 282, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Biondi, M.; Ungaro, F.; Quaglia, F.; Netti, P.A. Controlled drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2008, 60, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.A.; Leeuwenburgh, S.C.G.; Li, Y.B.; Jansen, J.A. The use of micro- and nanospheres as functional components for bone tissue regeneration. Tissue Eng. B 2012, 18, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Yun, Y.P.; Shim, K.S.; Park, K.; Choi, S.W.; Suh, D.H. Effect of lactoferrin-impregnated porous poly(lactide-co-glycolide) (plga) microspheres on osteogenic differentiation of rabbit adipose-derived stem cells (radscs). Colloids Surf. B 2014, 122, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Yun, Y.P.; Shim, K.S.; Park, K.; Choi, S.W.; Shin, D.H.; Suh, D.H. Fabrication of a bmp-2-immobilized porous microsphere modified by heparin for bone tissue engineering. Colloids Surf. B 2015, 134, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, S.F.; Young, F.A.; Mathews, R.S.; Klawitter, J.J.; Talbert, C.D.; Stelling, F.H. Potential of ceramic materials as permanently implantable skeletal prostheses. J. Biomed. Mater. Res. 1970, 4, 433–456. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z.; Lin, S.; Rohanizadeh, R.; Mijares, D.; LeGeros, J.P. Biphasic calcium phosphate bioceramics: Preparation, properties and applications. J. Mater. Sci. Mater. Med. 2003, 14, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Suneelkumar, C.; Datta, K.; Srinivasan, M.R.; Kumar, S.T. Biphasic calcium phosphate in periapical surgery. J. Conserv. Dent. 2008, 11, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; van Blitterswijk, C.A.; de Groot, K.; de Bruijn, J.D. Cross-species comparison of ectopic bone formation in biphasic calcium phosphate (BCP) and hydroxyapatite (HA) scaffolds. Tissue Eng. 2006, 12, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Stahli, C.; Bohner, M.; Bashoor-Zadeh, M.; Doebelin, N.; Baroud, G. Aqueous impregnation of porous beta-tricalcium phosphate scaffolds. Acta Biomater. 2010, 6, 2760–2772. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Chen, D.; Suo, J.; Zou, P.; Feng, S.; Yang, Q.; Yang, S.; Ye, S. Physicochemical characterization and biocompatibility in vitro of biphasic calcium phosphate/polyvinyl alcohol scaffolds prepared by freeze-drying method for bone tissue engineering applications. Colloids Surf. B 2012, 100, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yang, H.L. Calcium phosphate scaffolds combined with bone morphogenetic proteins or mesenchymal stem cells in bone tissue engineering. Chin. Med. J. (Engl.) 2015, 128, 1121–1127. [Google Scholar] [PubMed]
- Nie, L.; Suo, J.P.; Zou, P.; Feng, S.B. Preparation and properties of biphasic calcium phosphate scaffolds multiply coated with ha/plla nanocomposites for bone tissue engineering applications. J. Nanomater. 2012, 2012, 213549. [Google Scholar] [CrossRef]
- Kang, S.W.; Yang, H.S.; Seo, S.W.; Han, D.K.; Kim, B.S. Apatite-coated poly(lactic-co-glycolic acid) microspheres as an injectable scaffold for bone tissue engineering. J. Biomed. Mater. Res. A. 2008, 85, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, S.S.; Lee, S.H.; Eun Ahn, S.; Gwak, S.J.; Song, J.H.; Kim, B.S.; Chung, H.M. In vivo bone formation from human embryonic stem cell-derived osteogenic cells in poly(d,l-lactic-co-glycolic acid)/hydroxyapatite composite scaffolds. Biomaterials 2008, 29, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Hong, Z.; Yu, T.; Chen, X.; Jing, X. In vivo mineralization and osteogenesis of nanocomposite scaffold of poly(lactide-co-glycolide) and hydroxyapatite surface-grafted with poly(l-lactide). Biomaterials 2009, 30, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Luangphakdy, V.; Walker, E.; Shinohara, K.; Pan, H.; Hefferan, T.; Bauer, T.W.; Stockdale, L.; Saini, S.; Dadsetan, M.; Runge, M.B.; et al. Evaluation of osteoconductive scaffolds in the canine femoral multi-defect model. Tissue Eng. A 2013, 19, 634–648. [Google Scholar] [CrossRef] [PubMed]
- Mekala, N.K.; Baadhe, R.R.; Parcha, S.R.; Yalavarthy, P.D. Physical and degradation properties of PLGA scaffolds fabricated by salt fusion technique. J. Biomed. Res. 2013, 27, 318–325. [Google Scholar] [PubMed]
- Maquet, V.; Boccaccini, A.R.; Pravata, L.; Notingher, I.; Jerome, R. Porous poly(α-hydroxyacid)/bioglass composite scaffolds for bone tissue engineering. I: Preparation and in vitro characterisation. Biomaterials 2004, 25, 4185–4194. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Park, M.S.; Gwak, S.J.; Choi, C.Y.; Kim, B.S. Accelerated bonelike apatite growth on porous polymer/ceramic composite scaffolds in vitro. Tissue Eng. 2006, 12, 2997–3006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.Y.; Ma, P.X. Porous poly(l-lactic acid)/apatite composites created by biomimetic process. J. Biomed. Mater. Res. 1999, 45, 285–293. [Google Scholar] [CrossRef]
- Kim, S.E.; Choi, H.W.; Lee, H.J.; Chang, J.H.; Choi, J.; Kim, K.J.; Lim, H.J.; Jun, Y.J.; Lee, S.C. Designing a highly bioactive 3D bone-regenerative scaffold by surface immobilization of nano-hydroxyapatite. J. Mater. Chem. 2008, 18, 4994–5001. [Google Scholar] [CrossRef]
- Kim, S.E.; Lee, D.W.; Yun, Y.P.; Shim, K.S.; Jeon, D.I.; Rhee, J.K.; Kim, H.J.; Park, K. Heparin-immobilized hydroxyapatite nanoparticles as a lactoferrin delivery system for improving osteogenic differentiation of adipose-derived stem cells. Biomed. Mater. 2016, 11, 025004. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, S.E.; Yun, Y.P.; Choi, S.W.; Jeon, D.I.; Kim, H.J.; Park, K.; Song, H.R. Osteogenesis and new bone formation of alendronate-immobilized porous plga microspheres in a rat calvarial defect model. J. Ind. Eng. Chem. 2017, 52, 277–286. [Google Scholar] [CrossRef]
- Cai, Y.P.; Chen, Y.H.; Hong, X.Y.; Liu, Z.G.; Yuan, W.E. Porous microsphere and its applications. Int. J. Nanomed. 2013, 8, 1111–1120. [Google Scholar]
- Lee, T.J.; Kang, S.W.; Bhang, S.H.; Kang, J.M.; Kim, B.S. Apatite-coated porous poly(lactic-co-glycolic acid) microspheres as an injectable bone substitute. J. Biomater. Sci. Polym. E 2010, 21, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.J.; Kim, I.K.; Kim, T.G.; Park, T.G. Highly open porous biodegradable microcarriers: In vitro cultivation of chondrocytes for injectable delivery. Tissue Eng. A 2008, 14, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Li, J.S.; Chen, Y.; Mak, A.F.T.; Tuan, R.S.; Li, L.; Li, Y. A one-step method to fabricate plla scaffolds with deposition of bioactive hydroxyapatite and collagen using ice-based microporogens. Acta Biomater. 2010, 6, 2013–2019. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Yun, Y.P.; Lee, J.Y.; Park, K.; Suh, D.H. Osteoblast activity of mg-63 cells is enhanced by growth on a lactoferrin-immobilized titanium substrate. Colloid Surf. B 2014, 123, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Yun, Y.P.; Song, H.R.; Chun, H.J.; Yang, D.H.; Park, K.; Kim, S.E. The effect of titanium with heparin/bmp-2 complex for improving osteoblast activity. Carbohydr. Polym. 2013, 98, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.C.; Zheng, L.; Zhao, H.S.; Miao, J.Y.; Sun, C.H.; Ren, N.; Wang, J.Y.; Liu, H.; Tao, X.T. In vitro assessment of the differentiation potential of bone marrow-derived mesenchymal stem cells on genipin-chitosan conjugation scaffold with surface hydroxyapatite nanostructure for bone tissue engineering. Tissue Eng. A 2011, 17, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Le Nihouannen, D.; Saffarzadeh, A.; Gauthier, O.; Moreau, F.; Pilet, P.; Spaethe, R.; Layrolle, P.; Daculsi, G. Bone tissue formation in sheep muscles induced by a biphasic calcium phosphate ceramic and fibrin glue composite. J. Mater. Sci. Mater. Med. 2008, 19, 667–675. [Google Scholar] [CrossRef] [PubMed]

| Samples | C (%) | O (%) | N (%) | P (%) | Ca (%) | Total (%) |
|---|---|---|---|---|---|---|
| PMSs | 57.31 | 42.69 | - | - | - | 100 |
| aminated-PMSs | 42.36 | 30.44 | 27.20 | - | - | 100 |
| BCP-M-PMSs | 54.14 | 43.51 | - | 0.99 | 1.36 | 100 |
| BCP-IM-PMSs | 44.52 | 38.96 | 11.84 | 1.90 | 2.78 | 100 |
| Samples | C1s (%) | O1s (%) | N1s (%) | P2p (%) | Ca2p (%) | Total (%) |
|---|---|---|---|---|---|---|
| PMSs | 73.96 | 26.04 | - | - | - | 100 |
| aminated-PMSs | 56.48 | 34.80 | 8.72 | - | - | 100 |
| BCP-M-PMSs | 63.54 | 33.68 | - | 0.87 | 1.91 | 100 |
| BCP-IM-PMSs | 61.12 | 28.55 | 5.44 | 1.02 | 3.87 | 100 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shim, K.-S.; Kim, S.E.; Yun, Y.-P.; Choi, S.; Kim, H.-J.; Park, K.; Song, H.-R. Biphasic Calcium Phosphate (BCP)-Immobilized Porous Poly (d,l-Lactic-co-Glycolic Acid) Microspheres Enhance Osteogenic Activities of Osteoblasts. Polymers 2017, 9, 297. https://doi.org/10.3390/polym9070297
Shim K-S, Kim SE, Yun Y-P, Choi S, Kim H-J, Park K, Song H-R. Biphasic Calcium Phosphate (BCP)-Immobilized Porous Poly (d,l-Lactic-co-Glycolic Acid) Microspheres Enhance Osteogenic Activities of Osteoblasts. Polymers. 2017; 9(7):297. https://doi.org/10.3390/polym9070297
Chicago/Turabian StyleShim, Kyu-Sik, Sung Eun Kim, Young-Pil Yun, Somang Choi, Hak-Jun Kim, Kyeongsoon Park, and Hae-Ryong Song. 2017. "Biphasic Calcium Phosphate (BCP)-Immobilized Porous Poly (d,l-Lactic-co-Glycolic Acid) Microspheres Enhance Osteogenic Activities of Osteoblasts" Polymers 9, no. 7: 297. https://doi.org/10.3390/polym9070297






