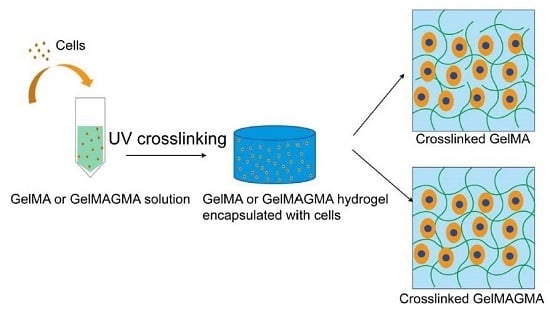
Fabrication of Highly Crosslinked Gelatin Hydrogel and Its Influence on Chondrocyte Proliferation and Phenotype
Abstract
:1. Introduction
2. Materials and Methods
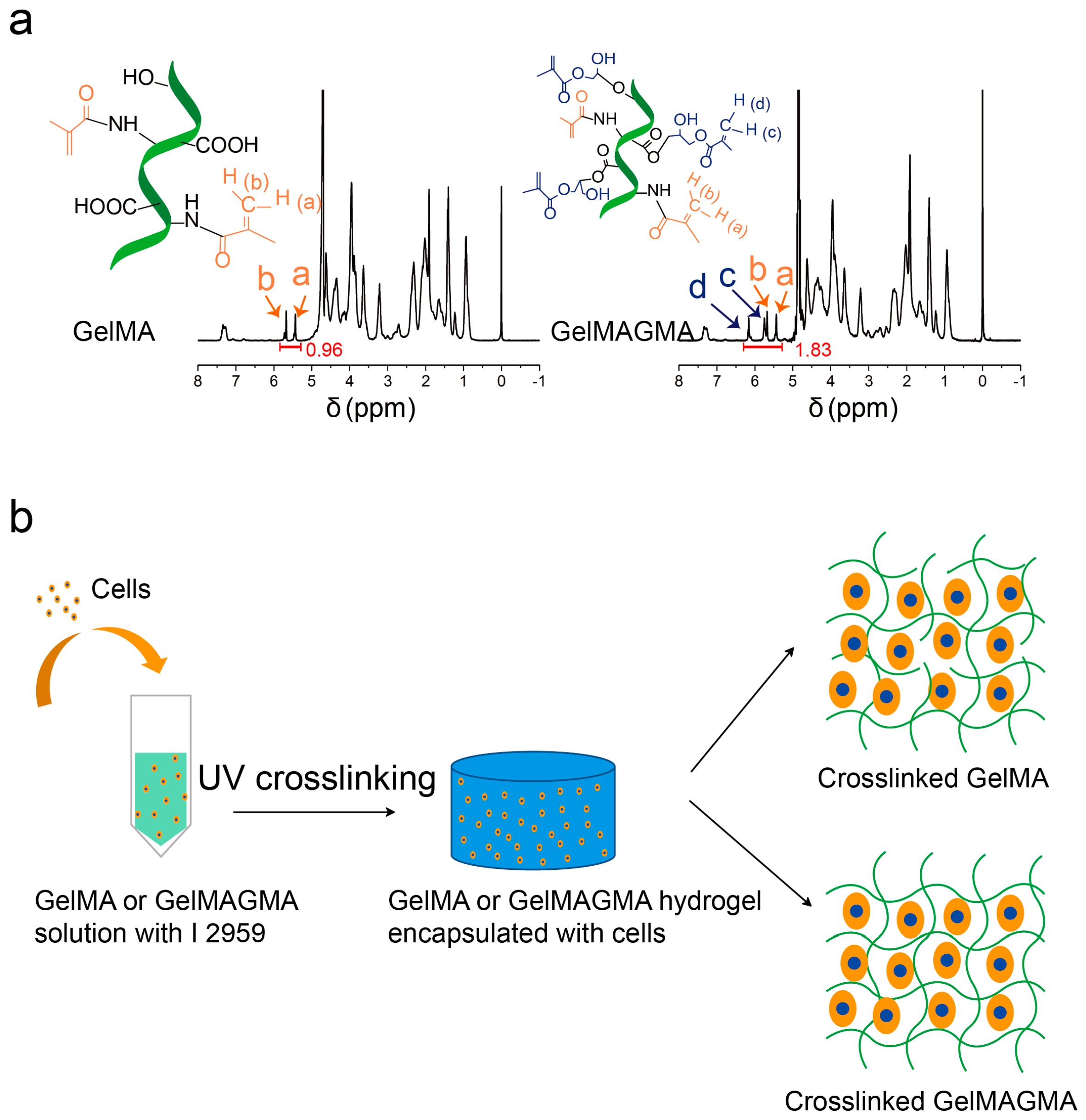
2.1. Synthesis of GelMA and GelMAGMA
2.2. 1H Nuclear Magnetic Resonance (NMR)
2.3. Preparation of GelMA and GelMAGMA Hydrogels
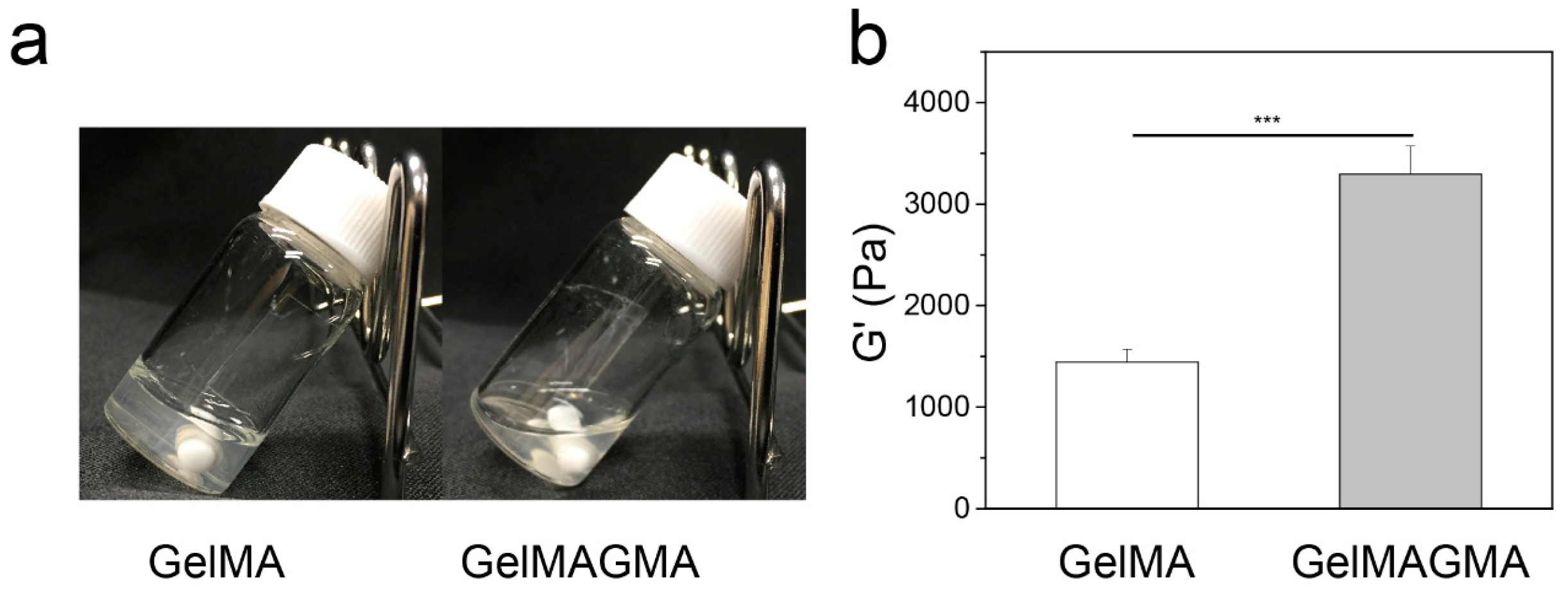
2.4. Rheological Measurements
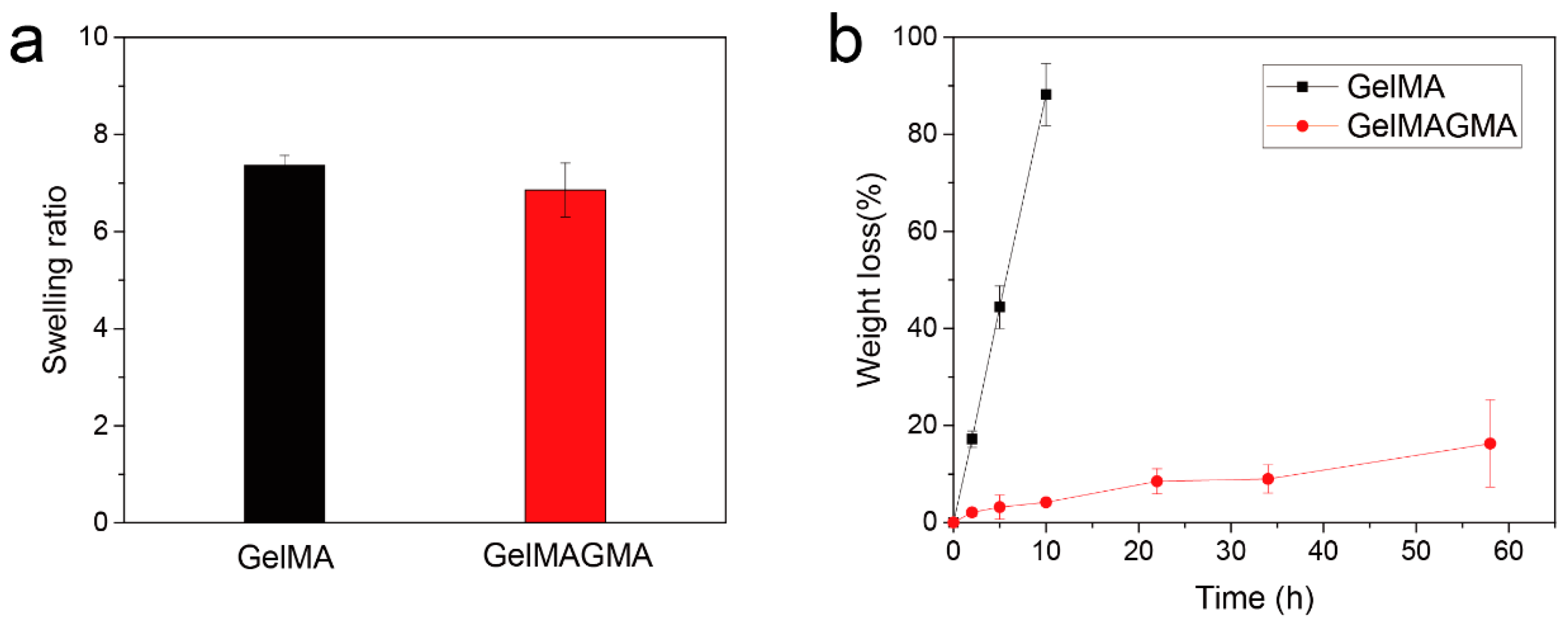
2.5. Swelling Ratio and Enzymatic Degradation of the Hydrogels
2.6. Chondrocytes Isolation and Culture In Vitro
2.7. Cell Viability Assay
2.8. In Vivo Implantation
2.9. Quantification of DNA and Sulfated Glycosaminoglycan (sGAG)
2.10. Histological and Immunohistochemical Staining
2.11. Real-Time Polymerase Chain Reaction (PCR) Analysis
2.12. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and 1H NMR of GelMA and GelMAGMA Macromers
3.2. Rheological Property of GelMA and GelMAGMA Hydrogels
3.3. Swelling Behavior and Enzymatic Degradation
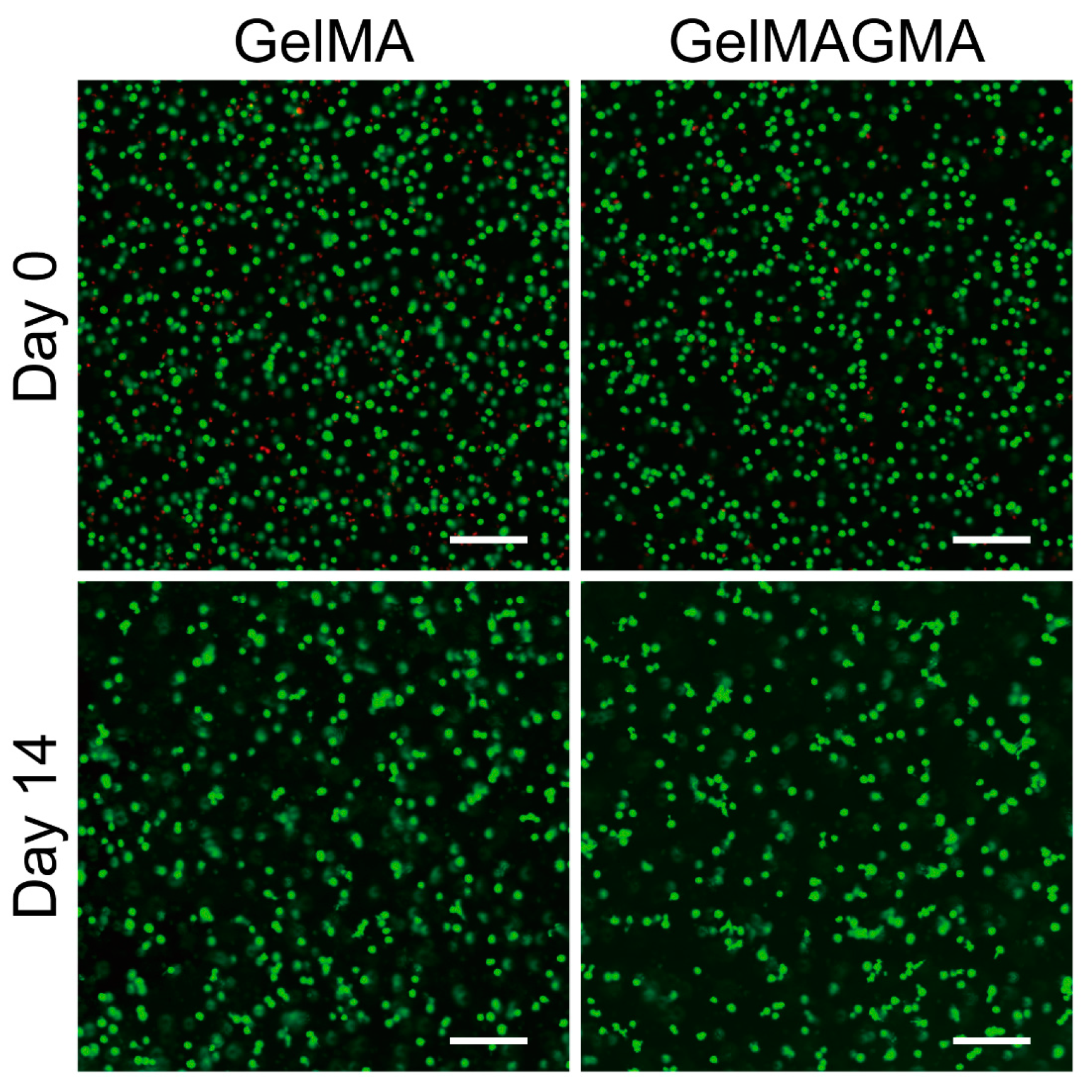
3.4. Cell Viability in the Hydrogels During In Vitro Culture
3.5. DNA and sGAG Quantification
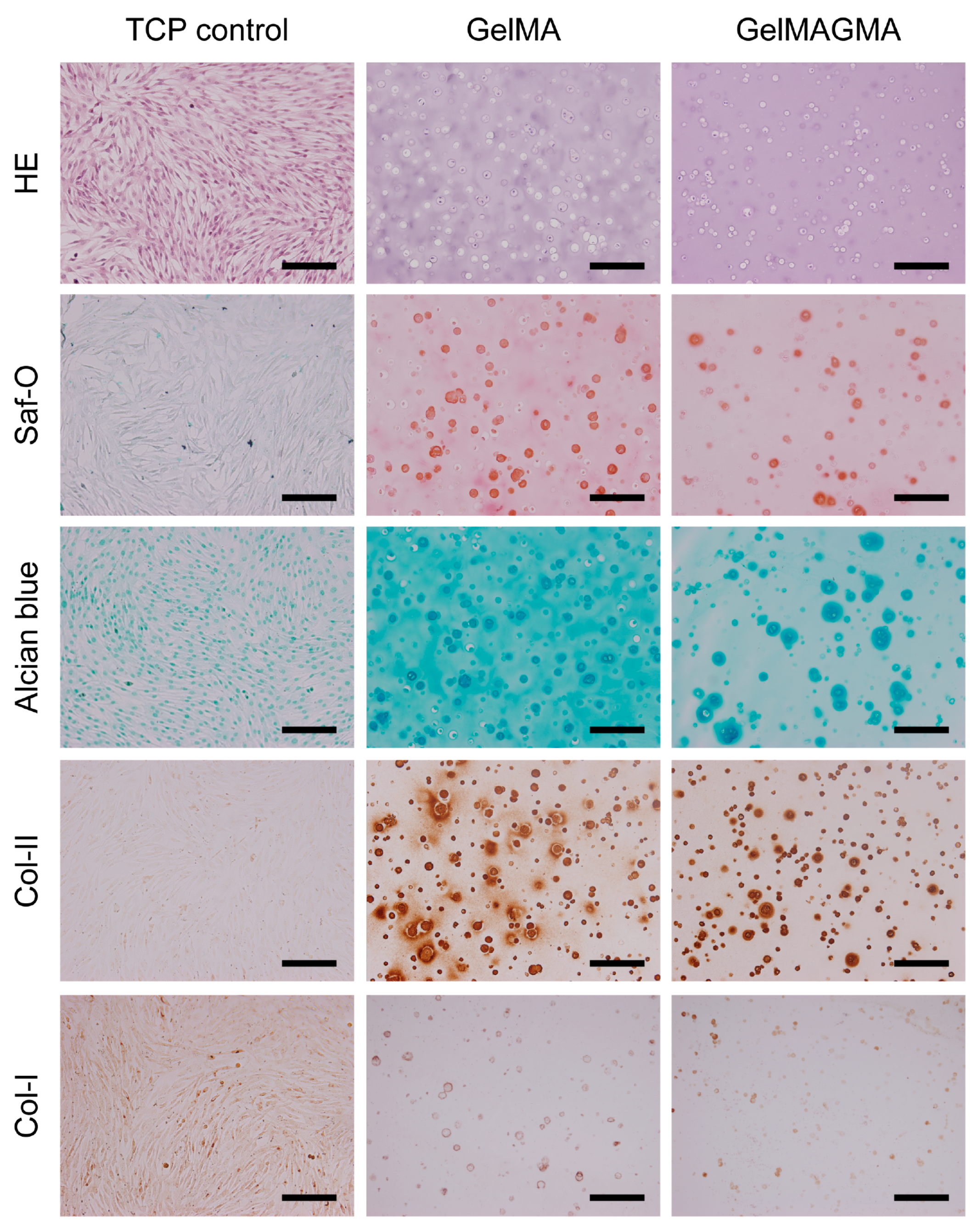
3.6. Histological and Immunohistochemical Stainings
3.7. Cartilaginous Gene Expression
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, G.; Sato, T.; Ushida, T.; Hirochika, R.; Tateishi, T. Redifferentiation of dedifferentiated bovine chondrocytes when cultured in vitro in a PLGA-collagen hybrid mesh. FEBS Lett. 2003, 542, 95–99. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, X.; Zhang, K.; Di, H.; Feng, L.; Li, G.; Fang, J.; Cui, L.; Chen, X.; Yin, J. Injectable in situ forming poly (l-glutamic acid) hydrogels for cartilage tissue engineering. J. Mater. Chem. B 2016, 4, 947–961. [Google Scholar] [CrossRef]
- Deepthi, S.; Gafoor, A.A.A.; Sivashanmugam, A.; Nair, S.V.; Jayakumar, R. Nanostrontium ranelate incorporated injectable hydrogel enhanced matrix production supporting chondrogenesis in vitro. J. Mater. Chem. B 2016, 4, 4092–4103. [Google Scholar] [CrossRef]
- Wang, T.; Lai, J.H.; Han, L.-H.; Tong, X.; Yang, F. Modulating stem cell–chondrocyte interactions for cartilage repair using combinatorial extracellular matrix-containing hydrogels. J. Mater. Chem. B 2016, 4, 7641–7650. [Google Scholar] [CrossRef]
- Serafim, A.; Tucureanu, C.; Petre, D.-G.; Dragusin, D.-M.; Salageanu, A.; Van Vlierberghe, S.; Dubruel, P.; Stancu, I.-C. One-pot synthesis of superabsorbent hybrid hydrogels based on methacrylamide gelatin and polyacrylamide. Effortless control of hydrogel properties through composition design. New J. Chem. 2014, 38, 3112–3126. [Google Scholar] [CrossRef]
- Zeng, L.; Chen, X.; Zhang, Q.; Yu, F.; Li, Y.; Yao, Y. Redifferentiation of dedifferentiated chondrocytes in a novel three-dimensional microcavitary hydrogel. J. Biomed. Mater. Res. A 2015, 103, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, S.; Li, J.; Wang, X.; Zhang, J.; Kawazoe, N.; Chen, G. 3D culture of chondrocytes in gelatin hydrogels with different stiffness. Polymers 2016, 8, 269. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Y.; Chen, Y.; Liu, X.; Guo, S.; Zhu, L.; Wang, Y. An in situ phototriggered-imine-crosslink composite hydrogel for bone defect repair. J. Mater. Chem. B 2016, 4, 973–981. [Google Scholar] [CrossRef]
- Yu, Y.; Guo, L.; Wang, W.; Wu, J.; Yuan, Z. Dual-peptide-modified alginate hydrogels for the promotion of angiogenesis. Sci. China Chem. 2015, 58, 1866–1874. [Google Scholar] [CrossRef]
- Han, L.; Xu, J.; Lu, X.; Gan, D.; Wang, Z.; Wang, K.; Zhang, H.; Yuan, H.; Weng, J. Biohybrid methacrylated gelatin/polyacrylamide hydrogels for cartilage repair. J. Mater. Chem. B 2017, 5, 731–741. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, L.; Liao, J.; Tan, Y.; Ouyang, K.; Ning, C.; Ni, G.; Tan, G. Cell-laden photocrosslinked GelMA–DexMA copolymer hydrogels with tunable mechanical properties for tissue engineering. J. Mater. Sci. Mater. Med. 2014, 25, 2173–2183. [Google Scholar] [CrossRef] [PubMed]
- Bryant, S.J.; Anseth, K.S. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly (ethylene glycol) hydrogels. J. Biomed. Mater. Res. A 2002, 59, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Metters, A.; Anseth, K.; Bowman, C. Fundamental studies of a novel, biodegradable PEG-b-PLA hydrogel. Polymer 2000, 41, 3993–4004. [Google Scholar] [CrossRef]
- Wang, L.-S.; Du, C.; Toh, W.S.; Wan, A.C.; Gao, S.J.; Kurisawa, M. Modulation of chondrocyte functions and stiffness-dependent cartilage repair using an injectable enzymatically crosslinked hydrogel with tunable mechanical properties. Biomaterials 2014, 35, 2207–2217. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Hou, C.; Tous, E.; Rai, R.; Mauck, R.L.; Burdick, J.A. The influence of hyaluronic acid hydrogel crosslinking density and macromolecular diffusivity on human MSC chondrogenesis and hypertrophy. Biomaterials 2013, 34, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Bartnikowski, M.; Bartnikowski, N.; Woodruff, M.; Schrobback, K.; Klein, T. Protective effects of reactive functional groups on chondrocytes in photocrosslinkable hydrogel systems. Acta Biomater. 2015, 27, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, Y.; Kawazoe, N.; Chen, G. Influence of microporous gelatin hydrogels on chondrocyte functions. J. Mater. Chem. B 2017. [Google Scholar] [CrossRef]
- Reis, A.V.; Fajardo, A.R.; Schuquel, I.T.; Guilherme, M.R.; Vidotti, G.J.; Rubira, A.F.; Muniz, E.C. Reaction of glycidyl methacrylate at the hydroxyl and carboxylic groups of poly (vinyl alcohol) and poly (acrylic acid): Is this reaction mechanism still unclear? J. Org. Chem. 2009, 74, 3750–3757. [Google Scholar] [CrossRef] [PubMed]
- Hoch, E.; Hirth, T.; Tovar, G.E.; Borchers, K. Chemical tailoring of gelatin to adjust its chemical and physical properties for functional bioprinting. J. Mater. Chem. B 2013, 1, 5675–5685. [Google Scholar] [CrossRef]
- Li, X.; Li, B.; Ma, J.; Wang, X.; Zhang, S. Development of a silk fibroin/HTCC/PVA sponge for chronic wound dressing. J. Bioact. Comp. Polym. 2014, 29, 398–411. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Q.; Nakamoto, T.; Kawazoe, N.; Chen, G. Gelatin scaffolds with controlled pore structure and mechanical property for cartilage tissue engineering. Tissue Eng. C 2016, 22, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Nakamoto, T.; Kawazoe, N.; Chen, G. Influence of stepwise chondrogenesis-mimicking 3D extracellular matrix on chondrogenic differentiation of mesenchymal stem cells. Biomaterials 2015, 52, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Ko, Y.-G.; Kawazoe, N.; Chen, G. Cartilage tissue engineering using funnel-like collagen sponges prepared with embossing ice particulate templates. Biomaterials 2010, 31, 5825–5835. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Lin, R.Z.; Qi, H.; Yang, Y.; Bae, H.; Melero-Martin, J.M.; Khademhosseini, A. Functional human vascular network generated in photocrosslinkable gelatin methacrylate hydrogels. Adv. Funct. Mater. 2012, 22, 2027–2039. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, Q.; Kawazoe, N.; Chen, G. Effect of high molecular weight hyaluronic acid on chondrocytes cultured in collagen/hyaluronic acid porous scaffolds. RSC Adv. 2015, 5, 94405–94410. [Google Scholar] [CrossRef]
- Fan, C.; Wang, D.A. Effects of permeability and living space on cell fate and neo-tissue development in hydrogel-based scaffolds: A study with cartilaginous model. Macromol. Biosci. 2015, 15, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.L.; Khetan, S.; Baker, B.M.; Chen, C.S.; Burdick, J.A. Fibrous hyaluronic acid hydrogels that direct MSC chondrogenesis through mechanical and adhesive cues. Biomaterials 2013, 34, 5571–5580. [Google Scholar] [CrossRef] [PubMed]
- Moreira Teixeira, L.; Leijten, J.; Sobral, J.; Jin, R.; Apeldoorn, A.; Feijen, J.; Blitterswijk, C.; Dijkstra, P.; Karperien, H. High throughput generated micro-aggregates of chondrocytes stimulate cartilage formation in vitro and in vivo. Eur. Cells Mater. 2012, 23, 387–399. [Google Scholar] [CrossRef]
- Fang, J.; Yong, Q.; Zhang, K.; Sun, W.; Yan, S.; Cui, L.; Yin, J. Novel injectable porous poly (γ-benzyl-l-glutamate) microspheres for cartilage tissue engineering: Preparation and evaluation. J. Mater. Chem. B 2015, 3, 1020–1031. [Google Scholar] [CrossRef]
- Bryant, S.J.; Durand, K.L.; Anseth, K.S. Manipulations in hydrogel chemistry control photoencapsulated chondrocyte behavior and their extracellular matrix production. J. Biomed. Mater. Res. A 2003, 67, 1430–1436. [Google Scholar] [CrossRef] [PubMed]
- Gosset, M.; Berenbaum, F.; Thirion, S.; Jacques, C. Primary culture and phenotyping of murine chondrocytes. Nat. Protoc. 2008, 3, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.C.; Saunders, K.M.; Burton-Wurster, N.; Macleod, J.N. Phenotypic stability of articular chondrocytes in vitro: The effects of culture models, bone morphogenetic protein 2, and serum supplementation. J. Bone Miner. Res. 2000, 15, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Sung, L.-Y.; Lo, W.-H.; Chiu, H.-Y.; Chen, H.-C.; Chung, C.-K.; Lee, H.-P.; Hu, Y.-C. Modulation of chondrocyte phenotype via baculovirus-mediated growth factor expression. Biomaterials 2007, 28, 3437–3447. [Google Scholar] [CrossRef] [PubMed]
- De Crombrugghe, B.; Lefebvre, V.; Behringer, R.R.; Bi, W.; Murakami, S.; Huang, W. Transcriptional mechanisms of chondrocyte differentiation. Matrix Biol. 2000, 19, 389–394. [Google Scholar] [CrossRef]
- Lefrebvre, V.; de Crombrugghe, B. Toward understanding SOX9 function in chondrocyte differentiation. Matrix Biol. 1998, 16, 529–540. [Google Scholar] [CrossRef]
- Shin, S.R.; Aghaei-Ghareh-Bolagh, B.; Dang, T.T.; Topkaya, S.N.; Gao, X.; Yang, S.Y.; Jung, S.M.; Oh, J.H.; Dokmeci, M.R.; Tang, X.S. Cell-laden microengineered and mechanically tunable hybrid hydrogels of gelatin and graphene oxide. Adv. Mater. 2013, 25, 6385–6391. [Google Scholar] [CrossRef] [PubMed]
- Miao, T.; Miller, E.J.; McKenzie, C.; Oldinski, R.A. Physically crosslinked polyvinyl alcohol and gelatin interpenetrating polymer network theta-gels for cartilage regeneration. J. Mater. Chem. B 2015, 3, 9242–9249. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Chen, S.; Kawazoe, N.; Chen, G. Preparation of gelatin/Fe3O4 composite scaffolds for enhanced and repeatable cancer cell ablation. J. Mater. Chem. B 2016, 4, 5664–5672. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Kawazoe, N.; Chen, G. Composite scaffolds of gelatin and gold nanoparticles with tunable size and shape for photothermal cancer therapy. J. Mater. Chem. B 2017, 5, 245–253. [Google Scholar] [CrossRef]
- Truong, V.X.; Hun, M.L.; Li, F.; Chidgey, A.P.; Forsythe, J.S. In situ-forming click-crosslinked gelatin based hydrogels for 3D culture of thymic epithelial cells. Biomater. Sci. 2016, 4, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Wissink, M.; Beernink, R.; Pieper, J.; Poot, A.A.; Engbers, G.; Beugeling, T.; Van Aken, W.; Feijen, J. Immobilization of heparin to EDC/NHS-crosslinked collagen. Characterization and in vitro evaluation. Biomaterials 2001, 22, 151–163. [Google Scholar] [CrossRef]
- Martucci, J.; Accareddu, A.; Ruseckaite, R. Preparation and characterization of plasticized gelatin films cross-linked with low concentrations of glutaraldehyde. J. Mater. Sci. 2012, 47, 3282–3292. [Google Scholar] [CrossRef]
- Yan, L.P.; Wang, Y.J.; Ren, L.; Wu, G.; Caridade, S.G.; Fan, J.B.; Wang, L.Y.; Ji, P.H.; Oliveira, J.M.; Oliveira, J.T. Genipin-cross-linked collagen/chitosan biomimetic scaffolds for articular cartilage tissue engineering applications. J. Biomed. Mater. Res. A 2010, 95, 465–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Xu, L.; Huang, X.; Wei, S.; Zhai, M. Structural study and preliminary biological evaluation on the collagen hydrogel crosslinked by γ-irradiation. J. Biomed. Mater. Res. A 2012, 100, 2960–2969. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, A.; Ling, Q.-D.; Kumar, S.S.; Chang, Y.; Alarfaj, A.A.; Munusamy, M.A.; Murugan, K.; Hsu, S.-T.; Umezawa, A. Physical cues of cell culture materials lead the direction of differentiation lineages of pluripotent stem cells. J. Mater. Chem. B 2015, 3, 8032–8058. [Google Scholar] [CrossRef]
- Wang, X.; Nakamoto, T.; Dulińska-Molak, I.; Kawazoe, N.; Chen, G. Regulating the stemness of mesenchymal stem cells by tuning micropattern features. J. Mater. Chem. B 2016, 4, 37–45. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhang, J.; Kawazoe, N.; Chen, G. Fabrication of Highly Crosslinked Gelatin Hydrogel and Its Influence on Chondrocyte Proliferation and Phenotype. Polymers 2017, 9, 309. https://doi.org/10.3390/polym9080309
Li X, Zhang J, Kawazoe N, Chen G. Fabrication of Highly Crosslinked Gelatin Hydrogel and Its Influence on Chondrocyte Proliferation and Phenotype. Polymers. 2017; 9(8):309. https://doi.org/10.3390/polym9080309
Chicago/Turabian StyleLi, Xiaomeng, Jing Zhang, Naoki Kawazoe, and Guoping Chen. 2017. "Fabrication of Highly Crosslinked Gelatin Hydrogel and Its Influence on Chondrocyte Proliferation and Phenotype" Polymers 9, no. 8: 309. https://doi.org/10.3390/polym9080309