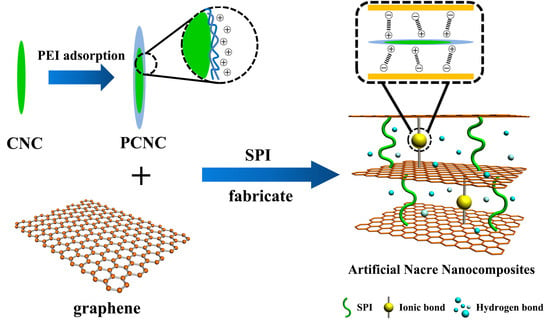
Improvement in Functional Properties of Soy Protein Isolate-Based Film by Cellulose Nanocrystal–Graphene Artificial Nacre Nanocomposite
Abstract
:1. Introduction
2. Materials and Methods
2.1 Materials
2.2 Preparation of Aqueous Graphene Dispersion
2.3 Fabrication of CNC and PEI-Modified CNC Colloidal Suspensions
2.4 Preparation of Graphene/CNC Modified SPI-Based Films
2.5. Characterization
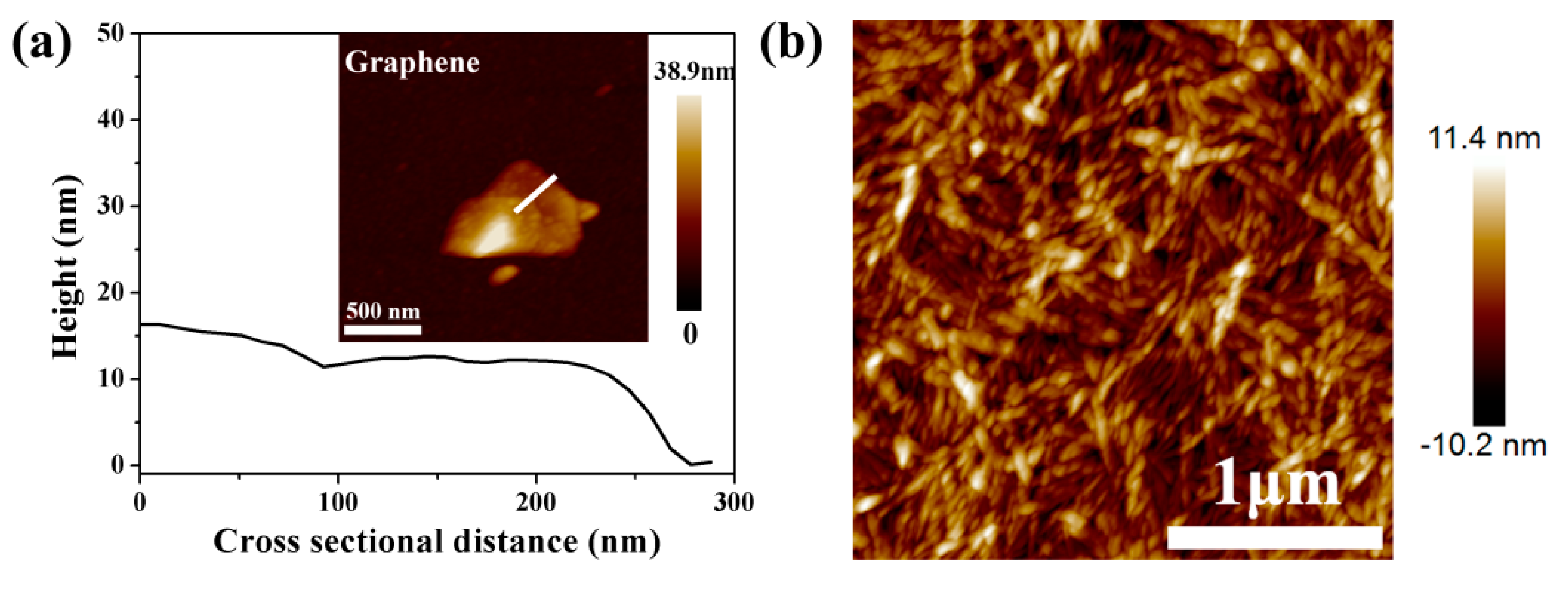
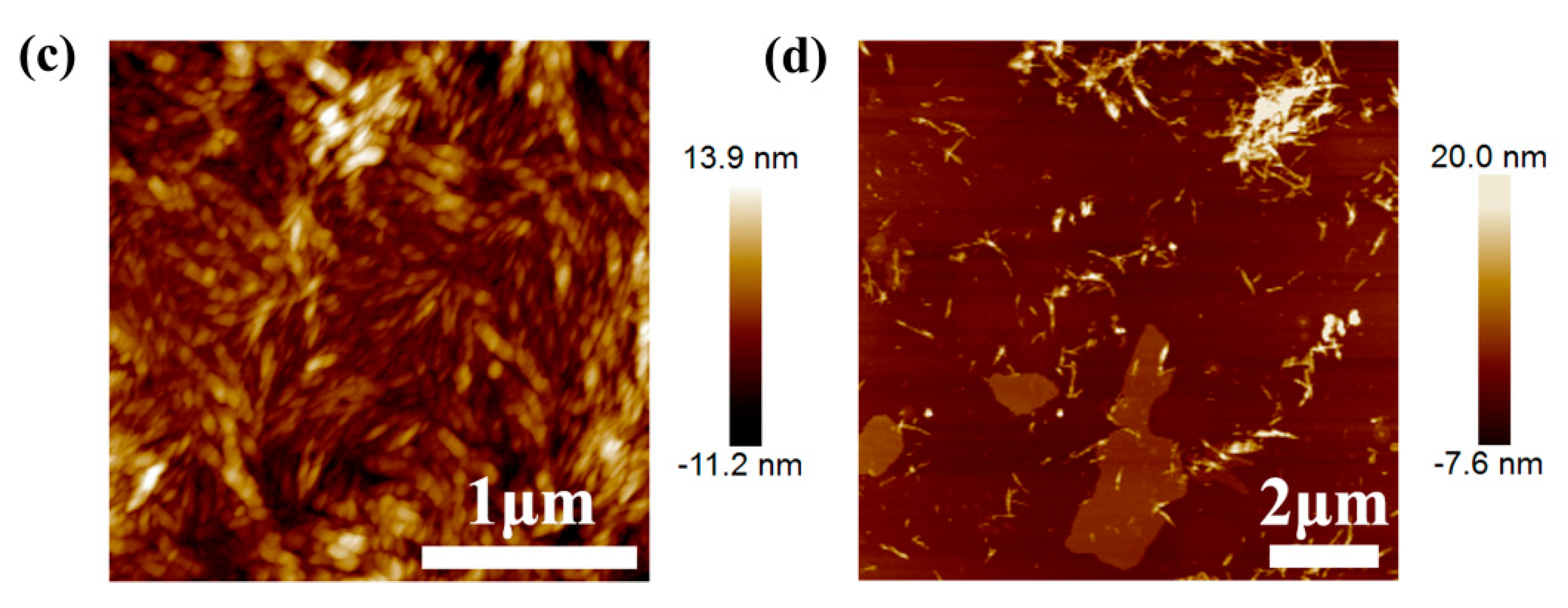
2.5.1. Atomic Force Microscopy
2.5.2. Zeta Potential Measurements
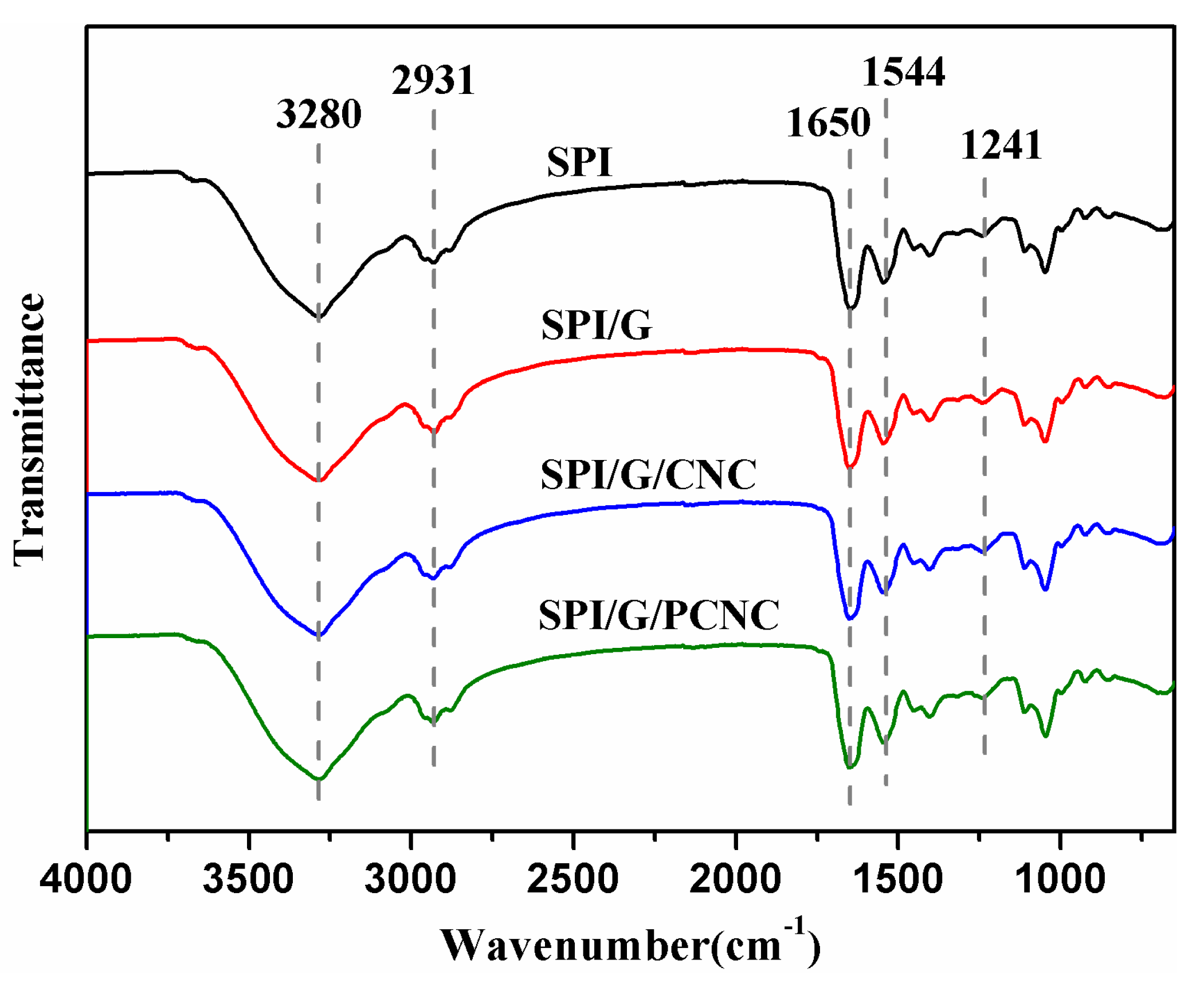
2.5.3. Structural Analysis
2.5.4. Surface Morphology Analysis
2.5.5. Opacity Analysis
2.5.6. Mechanical Properties
2.5.7. Contact Angle Measurements
2.5.8. Water v Vapor Permeability
2.5.9. Moisture Content (MC), Total Soluble Matter (TSM) and Water Uptake (WU) Tests
2.5.10. Thermo-Gravimetric Analysis
3. Results and Discussion
3.1. Characterization of Graphene, CNC and PEI-Modified CNC
3.2. Zeta Potential Measurements
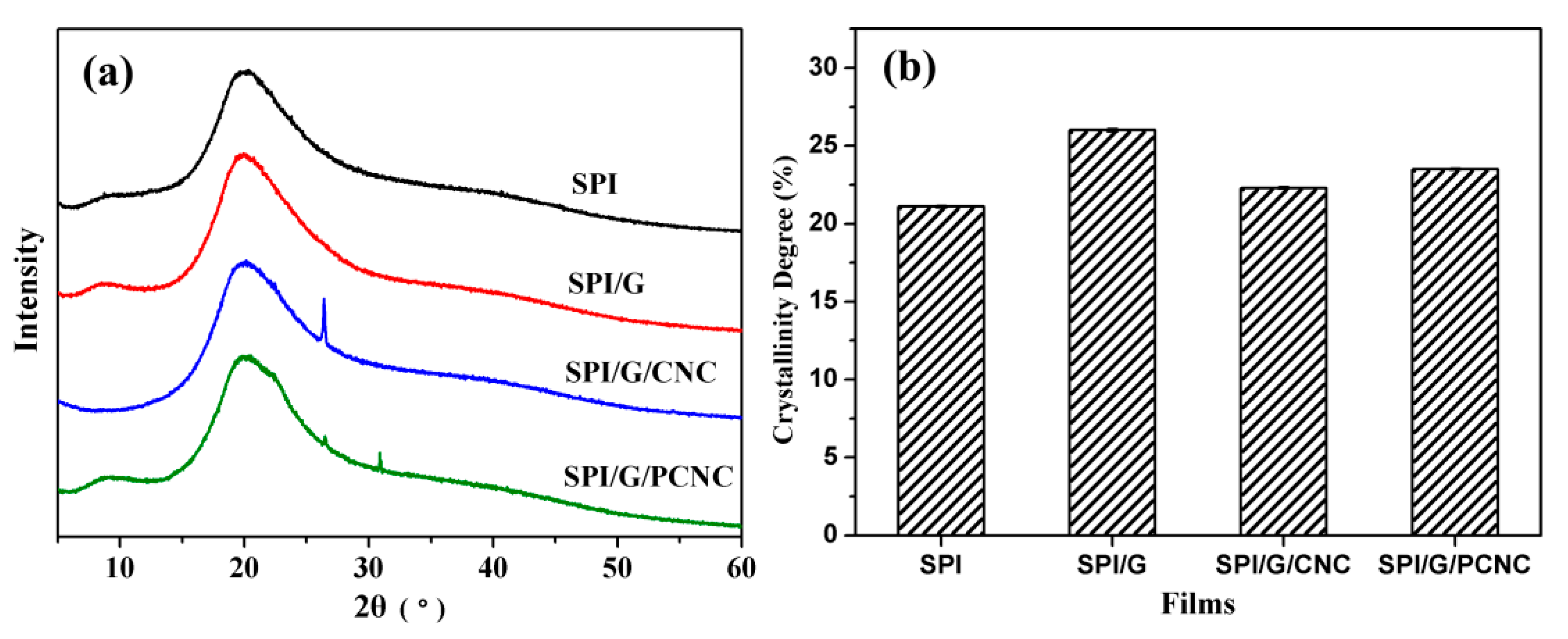
3.3. Structural Analysis
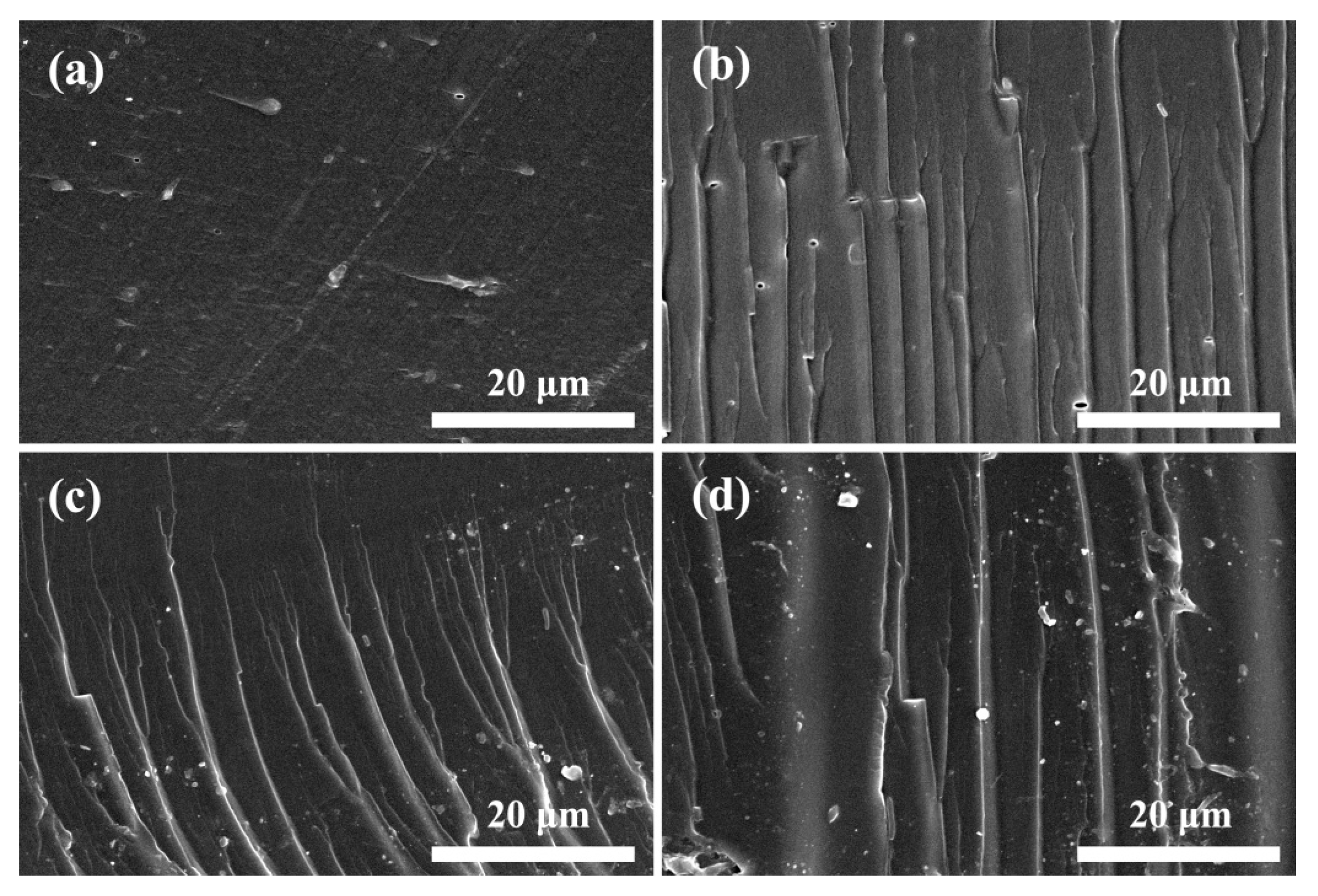
3.4. Micromorphology of SPI-Based Nanocomposite Films
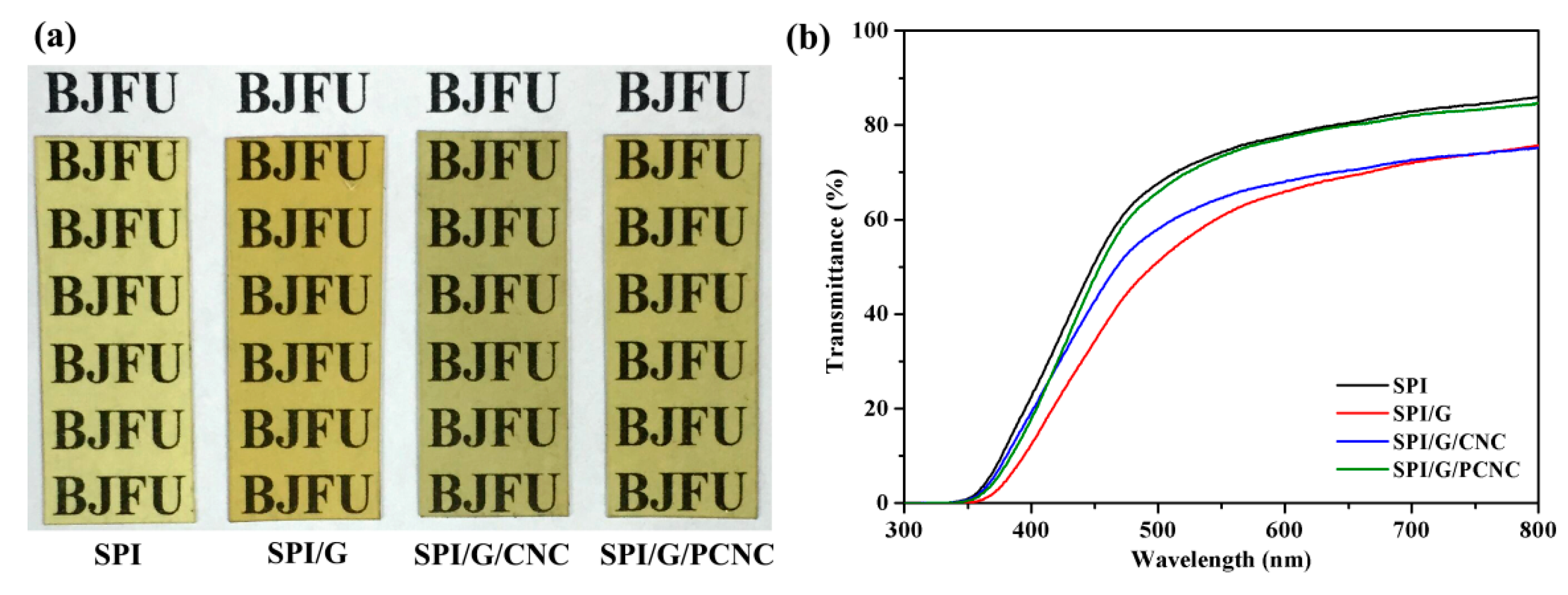
3.5. Opacity Analysis
3.6. Mechanical Properties of SPI-Based Nanocomposite Film
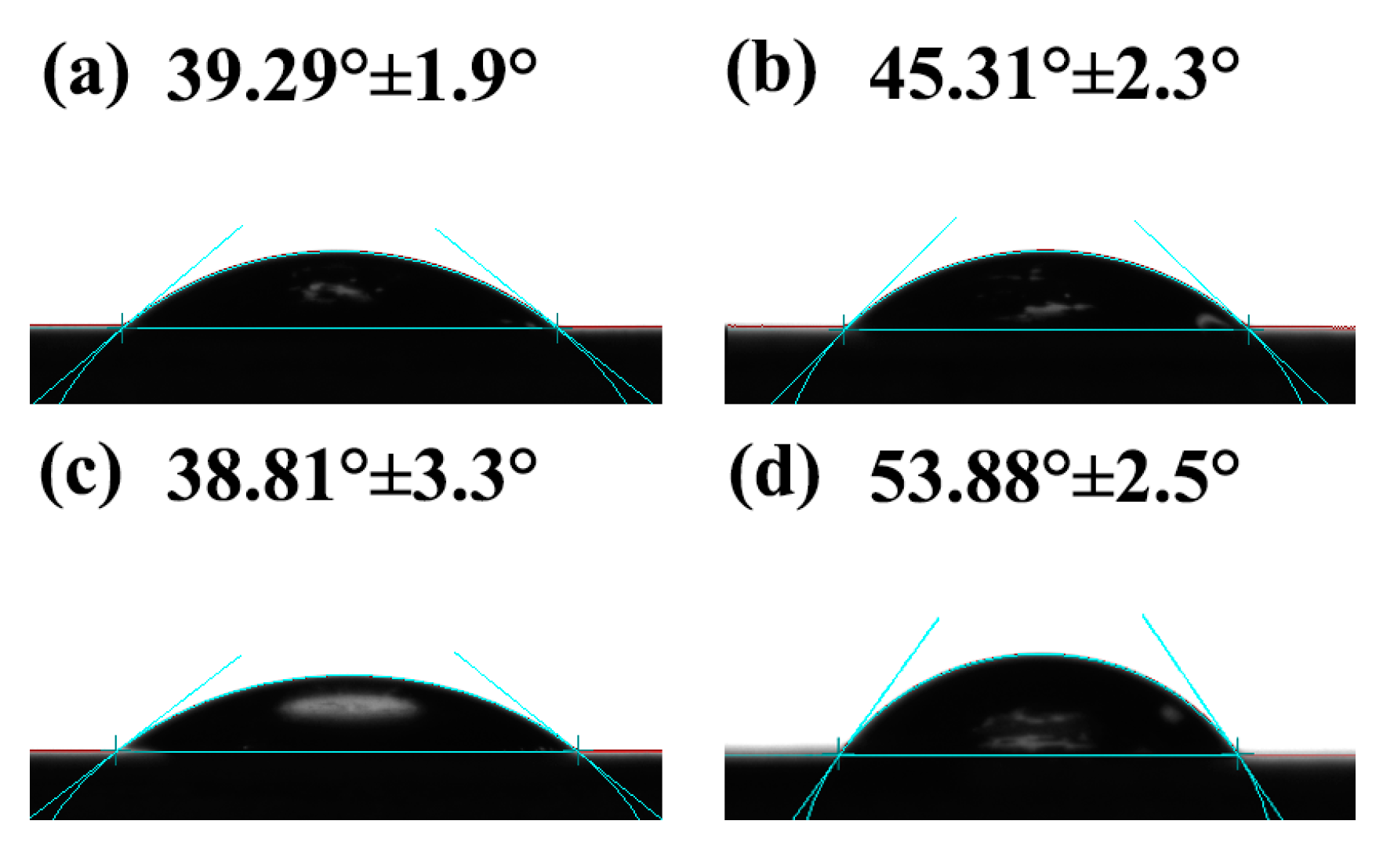
3.7. Surface Hydrophilicity and Water Resistance of SPI-Based Nanocomposite Films
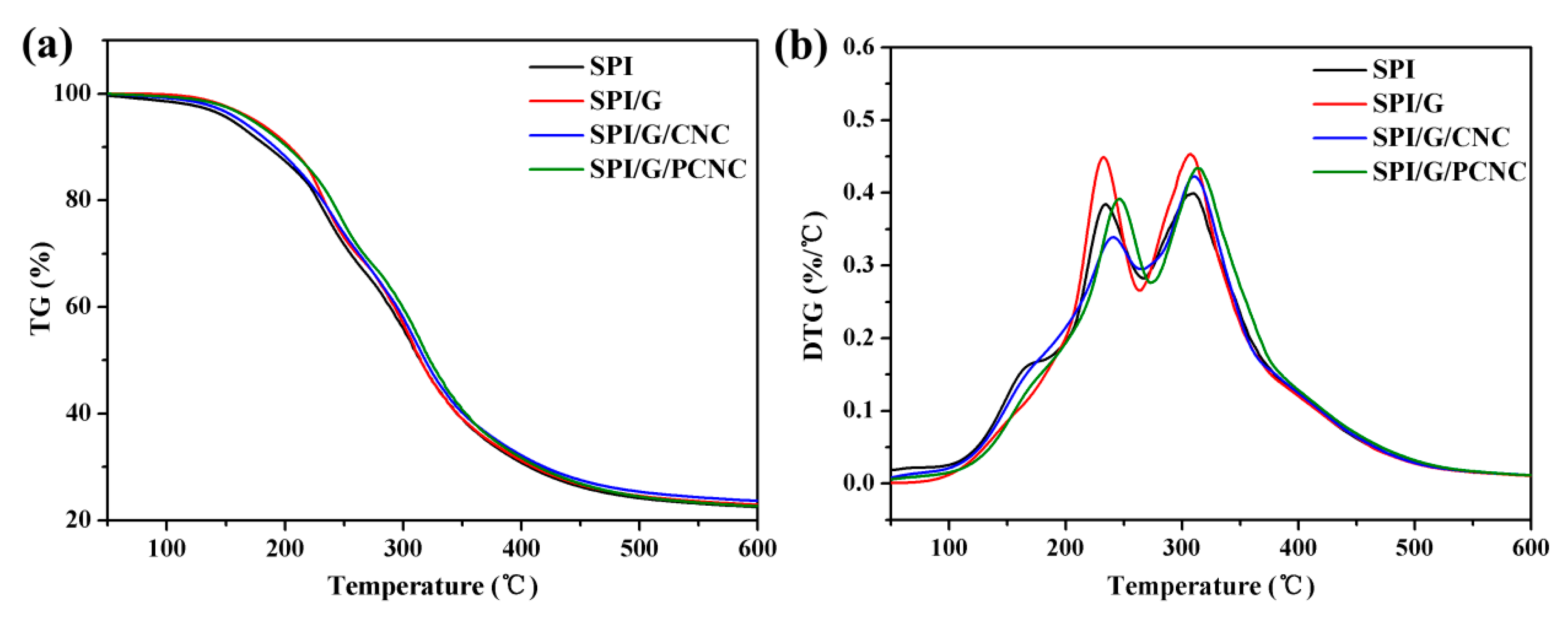
3.8. Thermal Stabilities of SPI-Based Nanocomposite Films
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wihodo, M.; Moraru, C.I. Physical and chemical methods used to enhance the structure and mechanical properties of protein films: A review. J. Food Eng. 2013, 114, 292–302. [Google Scholar] [CrossRef]
- Arora, A.; Padua, G.W. Review: Nanocomposites in food packaging. J. Food Sci. 2010, 75, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Muller, K.; Bugnicourt, E.; Latorre, M.; Jorda, M.; EchegoyenSanz, Y.; Lagaron, J.M.; Miesbauer, O.; Bianchin, A.; Hankin, S.; Bolz, U.; et al. Review on the processing and properties of polymer nanocomposites and nanocoatings and their applications in the packaging, automotive and solar energy fields. Nanomaterials 2017, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Hammann, F.; Schmid, M. Determination and quantification of molecular interactions in protein films: A review. Materials 2014, 7, 7975–7996. [Google Scholar] [CrossRef]
- Li, K.; Jin, S.; Liu, X.; Chen, H.; He, J.; Li, J. Preparation and characterization of chitosan/soyprotein isolate nanocomposite film reinforced by Cu nanoclusters. Polymers 2017, 9, 247. [Google Scholar] [CrossRef]
- Liu, X.; Kang, H.; Wang, Z.; Zhang, W.; Li, J.; Zhang, S. Simultaneously toughening and strengthening soy protein isolate-based composites via carboxymethylated chitosan and halloysite nanotube hybridization. Materials 2017, 10, 653. [Google Scholar] [CrossRef]
- Chen, H.; Lin, L.; Li, H.; Li, J.; Lin, J.M. Aggregation-induced structure transition of protein-stabilized zinc/copper nanoclusters for amplified chemiluminescence. ACS Nano 2015, 9, 2173–2183. [Google Scholar] [CrossRef] [PubMed]
- Coltelli, M.B.; Wild, F.; Bugnicourt, E.; Cinelli, P.; Lindner, M.; Schmid, M.; Weckel, V.; Müller, K.; Rodriguez, P.; Staebler, A.; et al. State of the art in the development and properties of protein-based films and coatings and their applicability to cellulose based products: An extensive review. Coatings 2016, 6, 1. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Dong, Y.; Li, K.; Li, L.; Li, J. Carbon nanoparticles/soy protein isolate bio-films with excellent mechanical and water barrier properties. Ind. Crops Prod. 2016, 82, 133–140. [Google Scholar] [CrossRef]
- Li, K.; Jin, S.; Chen, H.; He, J.; Li, J. A high-performance soy protein isolate-based nanocomposite film modified with microcrystalline cellulose and Cu and Zn nanoclusters. Polymers 2017, 9, 167. [Google Scholar] [CrossRef]
- Fang, Q.; Zhu, M.; Yu, S.; Sui, G.; Yang, X. Studies on soy protein isolate/polyvinyl alcohol hybrid nanofiber membranes as multi-functional eco-friendly filtration materials. Mater. Sci. Eng. B 2016, 214, 1–10. [Google Scholar] [CrossRef]
- Wang, S.; Marcone, M.F.; Barbut, S.; Lim, L.-T. Fortification of dietary biopolymers-based packaging material with bioactive plant extracts. Food Res. Int. 2012, 49, 80–91. [Google Scholar] [CrossRef]
- Chien, K.B.; Shah, R.N. Novel soy protein scaffolds for tissue regeneration: Material characterization and interaction with human mesenchymal stem cells. Acta. Biomater. 2012, 8, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Klüver, E.; Meyer, M. Thermoplastic processing, rheology, and extrudate properties of wheat, soy, and pea proteins. Polym. Eng. Sci. 2015, 55, 1912–1919. [Google Scholar] [CrossRef]
- Ma, L.; Yang, Y.; Yao, J.; Shao, Z.; Chen, X. Robust soy protein films obtained by slight chemical modification of polypeptide chains. Polym. Chem. 2013, 4, 5425. [Google Scholar] [CrossRef]
- Iman, M.; Bania, K.K.; Maji, T.K. Green jute-based cross-linked soy flour nanocomposites reinforced with cellulose whiskers and nanoclay. Ind. Eng. Chem. Res. 2013, 52, 6969–6983. [Google Scholar] [CrossRef]
- Guo, B.; Tang, Z.; Zhang, L. Transport performance in novel elastomer nanocomposites: Mechanism, design and control. Prog. Polym. Sci. 2016, 61, 29–66. [Google Scholar] [CrossRef]
- Xiong, R.; Hu, K.; Zhang, S.; Lu, C.; Tsukruk, V.V. Ultrastrong freestanding graphene oxide nanomembranes with surface-enhanced Raman scattering functionality by solvent-assisted single-component layer-by-layer assembly. ACS Nano 2016, 10, 6702–6715. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gong, S.; Zhang, Q.; Ming, P.; Wan, S.; Peng, J.; Jiang, L.; Cheng, Q. Graphene-based artificial nacre nanocomposites. Chem. Soc. Rev. 2016, 45, 2378–2395. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Colombo, L.; Ruoff, R.S. Synthesis of graphene films on copper foils by chemical vapor deposition. Adv. Mater. 2016, 28, 6247–6252. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Gao, Q.; Li, J.; Lin, J.M. Graphene materials-based chemiluminescence for sensing. J. Photochem. Photobiol. C 2016, 27, 54–71. [Google Scholar] [CrossRef]
- Kholmanov, I.N.; Magnuson, C.W.; Piner, R.; Kim, J.Y.; Aliev, A.E.; Tan, C.; Kim, T.Y.; Zakhidov, A.A.; Sberveglieri, G.; Baughman, R.H.; et al. Optical, electrical, and electromechanical properties of hybrid graphene/carbon nanotube films. Adv. Mater. 2015, 27, 3053–3059. [Google Scholar] [CrossRef] [PubMed]
- Bondeson, D.; Mathew, A.; Oksman, K. Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose 2006, 13, 171–180. [Google Scholar] [CrossRef]
- Pal, N.; Dubey, P.; Gopinath, P.; Pal, K. Combined effect of cellulose nanocrystal and reduced graphene oxide into poly-lactic acid matrix nanocomposite as a scaffold and its anti-bacterial activity. Int. J. Biol. Macromol. 2017, 95, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Hu, K.; Grant, A.M.; Ma, R.; Xu, W.; Lu, C.; Zhang, X.; Tsukruk, V.V. Ultrarobust transparent cellulose nanocrystal-graphene membranes with high electrical conductivity. Adv. Mater. 2016, 28, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, H.; Li, Y.; Li, J.; He, J. Endogenous cu and zn nanocluster-regulated soy protein isolate films: Excellent hydrophobicity and flexibility. RSC Adv. 2015, 5, 66543–66548. [Google Scholar] [CrossRef]
- Lee, J.A.; Yoon, M.J.; Lee, E.S.; Lim, D.Y.; Kim, K.Y. Preparation and characterization of cellulose nanofibers (cnfs) from microcrystalline cellulose (MCC) and CNF/polyamide 6 composites. Macromol. Res. 2014, 22, 738–745. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Zhu, Q.; Chen, F.; Zhao, X.; Ao, Q. Determination of the domain structure of the 7s and 11s globulins from soy proteins by xrd and ftir. J. Sci. Food Agric. 2013, 93, 1687–1691. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cai, X.; Luo, B.; Yan, M.; Jiang, J.; Shi, W. Mno 2 decorated on carbon sphere intercalated graphene film for high-performance supercapacitor electrodes. Carbon 2016, 107, 426–432. [Google Scholar] [CrossRef]
- Liu, X.; Song, R.; Zhang, W.; Qi, C.; Zhang, S.; Li, J. Development of eco-friendly soy protein isolate films with high mechanical properties through hnts, pva, and ptge synergism effect. Sci. Rep. 2017, 7, 44289. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Yu, X.; Pilla, S. Mechanical and moisture sensitivity of fully bio-based dialdehydecarboxymethyl cellulose cross-linked soy protein isolate films. Carbohyd. Polym. 2017, 157, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Alipoormazandarani, N.; Ghazihoseini, S.; MohammadiNafchi, A. Preparation and characterization of novel bionanocomposite based on soluble soybean polysaccharide and halloysite nanoclay. Carbohydr. Polym. 2015, 134, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Incani, V.; Danumah, C.; Boluk, Y. Nanocomposites of nanocrystalline cellulose for enzyme immobilization. Cellulose 2013, 20, 191–200. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Guo, B.; Liu, M.; Liao, R.; Rabie, A.B.; Jia, D. Poly(vinyl alcohol)/halloysite nanotubes bionanocomposite films: Properties and in vitro osteoblasts and fibroblasts response. J. Biomed. Mater. Res. A. 2010, 93, 1574–1587. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, H.; Ahn, Y.; Lee, Y. High-performance flexible transparent conductive film based on graphene/agnw/graphene sandwich structure. Carbon 2015, 8, 439–446. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Li, B. Nature-inspired one-step green procedure for enhancing the antibacterial and antioxidant behavior of a chitin film: Controlled interfacial assembly of tannic acid onto a chitin film. J. Agric. Food Chem. 2016, 64, 5736–5741. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Z.; Sun, H.; Gao, C. Superstructured assembly of nanocarbons: Fullerenes, nanotubes, and graphene. Chem. Rev. 2015, 115, 7046–7117. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Xiong, R.; Tsukruk, V.V. Probing flexural properties of cellulose nanocrystal-graphene nanomembranes with force spectroscopy and bulging test. Langmuir 2016, 32, 5383–5393. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yang, H.J.; Lee, K.Y.; Song, K.B. Physical properties and application of a red pepper seed meal protein composite film containing oregano oil. Food Hydrocoll. 2016, 55, 136–143. [Google Scholar] [CrossRef]
- Montes, S.; Carrasco, P.M.; Ruiz, V.; Cabanero, G.; Grande, H.J.; Labidi, J.; Odriozola, I. Synergistic reinforcement of poly(vinyl alcohol) nanocomposites with cellulose nanocrystal-stabilized graphene. Compos. Sci. Technol. 2015, 117, 26–31. [Google Scholar] [CrossRef]
- Xie, D.Y.; Song, F.; Zhang, M.; Wang, X.L.; Wang, Y.Z. Roles of soft segment length in structure and property of soy protein isolate/waterborne polyurethane blend films. Ind. Eng. Chem. Res. 2016, 55, 1229–1235. [Google Scholar] [CrossRef]
- Hocker, S.; Hudson-Smith, N.; Schniepp, H.C.; Kranbuehl, D.E. Enhancing polyimide's water barrier properties through addition of functionalized graphene oxide. Polymer 2016, 93, 23–29. [Google Scholar] [CrossRef]
- Xu, S.; Yu, W.; Yao, X.; Zhang, Q.; Fu, Q. Nanocellulose-assisted dispersion of graphene to fabricate poly(vinyl alcohol)/graphene nanocomposite for humidity sensing. Compos. Sci. Technol. 2016, 131, 67–76. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. Effects of carbohydrate/protein ratio on the microstructure and the barrier and sorption properties of wheat starch-whey protein blend edible films. J. Sci. Food Agric. 2017, 97, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Anandjiwala, R.D.; Kumar, A. Thermal and mechanical properties of mandelic acid-incorporated soy protein films. J. Therm. Anal. Calorim. 2015, 123, 1273–1279. [Google Scholar] [CrossRef]

| Samples | Zeta Potential (mV) |
|---|---|
| CNC | −27.6 (0.21) |
| PCNC | 40.4 (0.45) |
| graphene | −30.2 (0.24) |
| Films | Thickness (mm) | TS (MPa) | E (MPa) | EB (%) |
|---|---|---|---|---|
| SPI | 0.256 (0.014) | 3.75 (0.34) | 77.44 (3.39) | 173.69 (0.14) |
| SPI/graphene | 0.293 (0.010) | 5.89 (0.47) | 94.30 (4.23) | 74.92 (0.17) |
| SPI/graphene/CNC | 0.289 (0.011) | 6.26 (0.29) | 82.04 (3.85) | 105.83 (0.13) |
| SPI/graphene/PCNC | 0.263 (0.010) | 7.49 (0.30) | 97.62 (5.43) | 87.14 (0.06) |
| Samples | WVP (10−1·g m−1·h−1·Pa−1) | MC (%) | TSM (%) | WU (%) |
|---|---|---|---|---|
| SPI | 11.04 (0.37) | 17.89 (0.8) | 34.16 (1.9) | 244.90 (7.4) |
| SPI/graphene | 9.29 (0.24) | 11.84 (1.9) | 33.59 (0.9) | 167.01 (4.8) |
| SPI/graphene/CNC | 10.30 (0.13) | 14.07 (1.7) | 33.79 (1.0) | 184.11 (6.1) |
| SPI/graphene/PCNC | 9.55 (0.35) | 13.50 (0.5) | 33.06 (1.8) | 129.35 (5.4) |
| Samples | Ti1 (°C) | Tmax1 (°C) | Ti2 (°C) | Tmax2 (°C) |
|---|---|---|---|---|
| SPI | 143.48 | 232.91 | 291.93 | 307.29 |
| SPI/graphene | 148.41 | 234.19 | 294.80 | 308. 89 |
| SPI/graphene/CNC | 154.16 | 240.88 | 294.79 | 310.67 |
| SPI/graphene/PCNC | 155.06 | 246.05 | 295.57 | 312.83 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Jin, S.; Han, Y.; Li, J.; Chen, H. Improvement in Functional Properties of Soy Protein Isolate-Based Film by Cellulose Nanocrystal–Graphene Artificial Nacre Nanocomposite. Polymers 2017, 9, 321. https://doi.org/10.3390/polym9080321
Li K, Jin S, Han Y, Li J, Chen H. Improvement in Functional Properties of Soy Protein Isolate-Based Film by Cellulose Nanocrystal–Graphene Artificial Nacre Nanocomposite. Polymers. 2017; 9(8):321. https://doi.org/10.3390/polym9080321
Chicago/Turabian StyleLi, Kuang, Shicun Jin, Yufei Han, Jianzhang Li, and Hui Chen. 2017. "Improvement in Functional Properties of Soy Protein Isolate-Based Film by Cellulose Nanocrystal–Graphene Artificial Nacre Nanocomposite" Polymers 9, no. 8: 321. https://doi.org/10.3390/polym9080321