Probing the Nanoscopic Thermodynamic Fingerprint of Paramagnetic Ligands Interacting with Amphiphilic Macromolecules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. EPR Spectroscopy
2.2.2. Thermodynamic Analysis of EPR Data
2.2.3. DSC Measurements
3. Results
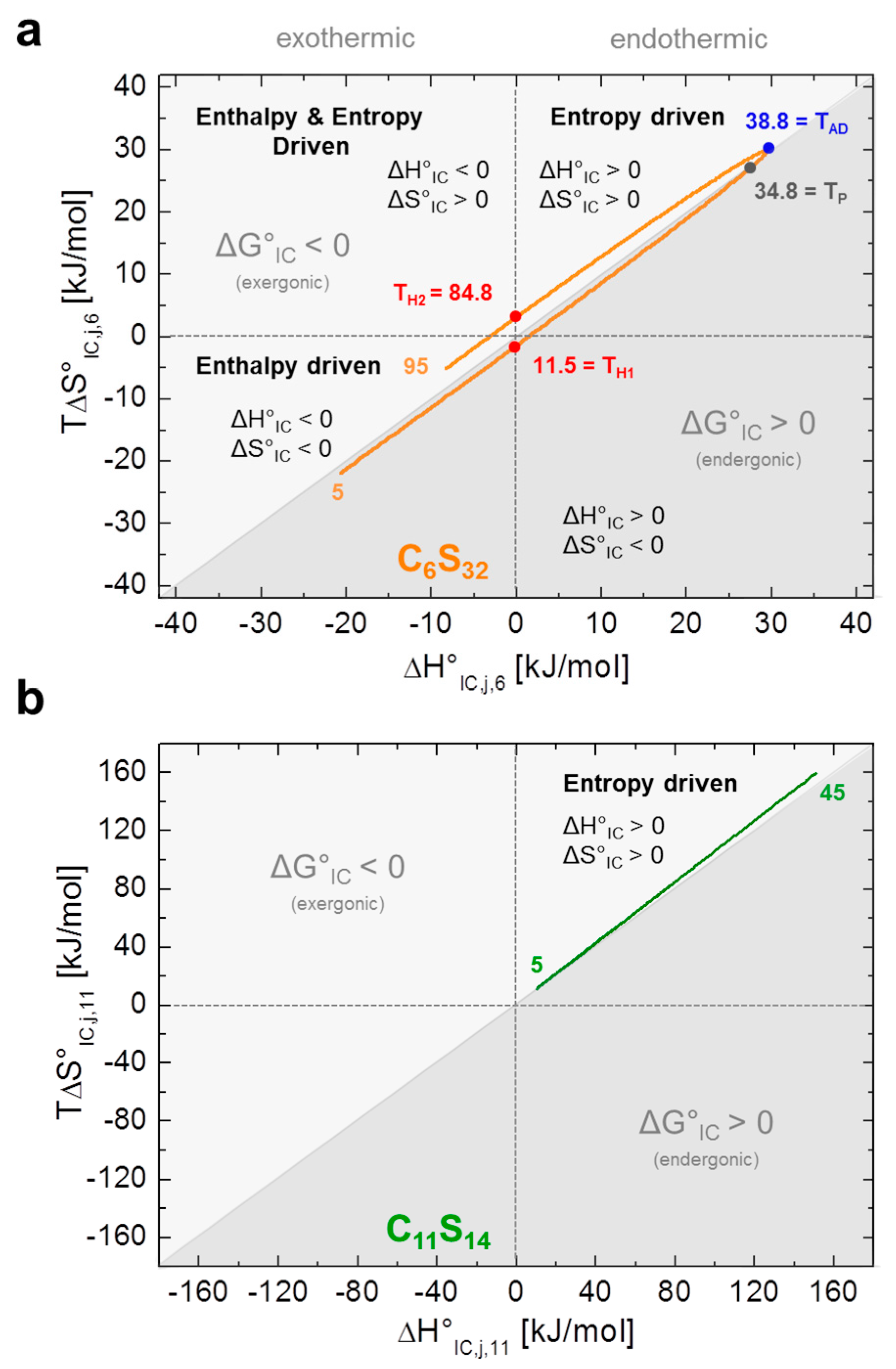
3.1. Thermodynamic Considerations for 16-DSA Ligand Binding to CnSm Core-Shell Polymers
3.2. Differential Scanning Calorimetry (DSC)—A Consistency Check
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Privalov, P.L.; Ptitsyn, O.B.; Birshtein, T.M. Determination of stability of the DNA double helix in an aqueous medium. Biopolymers 1969, 8, 559–571. [Google Scholar] [CrossRef]
- Sturtevant, J.M. Heat capacity and entropy changes in processes involving proteins. Proc. Natl. Acad. Sci. USA 1977, 74, 2236–2240. [Google Scholar] [CrossRef] [PubMed]
- Vuignier, K.; Schappler, J.; Veuthey, J.L.; Carrupt, P.A.; Martel, S. Drug-protein binding: A critical review of analytical tools. Anal. Bioanal. Chem. 2010, 398, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Boysen, R.I.; Jong, A.J.O.; Wilce, J.A.; King, G.F.; Hearn, M.T.W. Role of interfacial hydrophobic residues in the stabilization of the leucine zipper structures of the transcription factors c–Fos and c–Jun. J. Biol. Chem. 2002, 277, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Brandts, J.F. The thermodynamics of protein denaturation. I. The denaturation of chymotrypsinogen. J. Am. Chem. Soc. 1964, 86, 4291–4301. [Google Scholar] [CrossRef]
- Bode, W.; Blume, A. Thermal transitions of proteus mirabilis flagellin as studied by circular dichroism and adiabatic differential calorimetry. FEBS Lett. 1973, 36, 318–322. [Google Scholar] [CrossRef]
- Grant, D.J.W.; Mehdizadeh, M.; Chow, A.H.L.; Fairbrother, J.E. Non-linear van´t Hoff solubility–temperature plots and their pharmaceutical interpretation. Int. J. Pharm. 1984, 18, 25–38. [Google Scholar] [CrossRef]
- Prabhu, N.V.; Sharp, K.A. Heat capacity in proteins. Annu. Rev. Phys. Chem. 2005, 56, 521–548. [Google Scholar] [CrossRef] [PubMed]
- Vailaya, A.; Horvath, C. Retention thermodynamics in hydrophobic interaction chromatography. Ind. Eng. Chem. Res. 1996, 35, 2964–2981. [Google Scholar] [CrossRef]
- Galaon, T.; David, V. Deviation from van’t Hoff dependence in RP-LC induced by tautomeric interconversion observed for four compounds. J. Sep. Sci. 2011, 34, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- Majhi, P.R.; Blume, A. Thermodynamic characterization of temperature-induced micellation and demicellation of detergents studied by differential scanning calorimetry. Langmuir 2001, 17, 3844–3851. [Google Scholar] [CrossRef]
- Boysen, R.I.; Jong, A.J.O.; Hearn, M.T.W. Binding behavior and conformational properties of globular proteins in the presence of immobilized non-polar ligands used in reversed-phase liquid chromatography. J. Chromatogr. A 2005, 1079, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.D.; Basso, L.G.M.; Costa-Filho, A.J. Non-linear van’t Hoff behavior in pulmonary surfactant model membranes. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Kao, T.Y.; Tsai, C.J.; Lan, Y.J.; Chiang, Y.W. The role of conformational heterogeneity on regulating the apoptotic activity of BAX protein. Phys. Chem. Chem. Phys. 2017, 19, 9584–9591. [Google Scholar] [CrossRef] [PubMed]
- Makhatadze, G.I.; Privalov, P.L. Heat capacity of proteins: I. Partial molar heat capacity of individual amino acid residues in aqueous solution: Hydration effect. J. Mol. Biol. 1990, 213, 375–384. [Google Scholar] [CrossRef]
- Privalov, P.L.; Makhatadze, G.I. Heat capacity of proteins: II. Partial molar heat capacity of the unfolded polypeptide chain of proteins: Protein unfolding effects. J. Mol. Biol. 1990, 213, 385–391. [Google Scholar] [CrossRef]
- Paula, S.; Sues, W.; Tuchtenhagen, J.; Blume, A. Thermodynamics of micelle formation as a function of temperature: A high sensitivity titration calorimetry study. J. Phys. Chem. 1995, 99, 11742–11751. [Google Scholar] [CrossRef]
- Kozlov, A.G.; Lohman, T.M. Adenine base unstacking dominates the observed enthalpy and heat capacity changes for the Escherichia coli SSB tetramer binding to single-stranded oligoadenylates. Biochemistry 1999, 38, 7388–7397. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Huang, H.M.; Lin, C.C.; Lin, F.Y.; Chan, Y.C. Effect of temperature on hydrophobic interaction between proteins and hydrophobic adsorbents: Studies by isothermal titration calorimetry and the van’t Hoff equation. Langmuir 2003, 19, 9395–9403. [Google Scholar] [CrossRef]
- Tunon, I.; Silla, E.; Pascual-Ahuir, J.L. Molecular surface area and hydrophobic effect. Protein Eng. 1992, 5, 715–716. [Google Scholar] [CrossRef] [PubMed]
- Pace, C.N. Conformational stability of globular proteins. Trends Biochem. Sci. 1990, 15, 14–17. [Google Scholar] [CrossRef]
- Ramli, R.A.; Laftah, W.A.; Hashim, S. Core-shell polymers: A review. RSC Adv. 2013, 3, 15543–15565. [Google Scholar] [CrossRef]
- Reichenwallner, J.; Thomas, A.; Nuhn, L.; Johann, T.; Meister, A.; Frey, H.; Hinderberger, D. Tunable dynamic hydrophobic attachment of guest molecules in amphiphilic core-shell polymers. Polym. Chem. 2016, 7, 5783–5798. [Google Scholar] [CrossRef]
- Goldman, S.A.; Bruno, G.V.; Polnaszek, C.F.; Freed, J.H. An ESR study of anisotropic rotational reorientation and slow tumbling in liquid and frozen media. J. Chem. Phys. 1972, 56, 716–735. [Google Scholar] [CrossRef]
- Freed, J.H. Theory of slow tumbling ESR spectra for nitroxides. In Spin Labeling: Theory and Applications 1; Berliner, L.J., Ed.; Academic Press: New York, NY, USA, 1976; pp. 53–132. [Google Scholar]
- Thomas, A.; Niederer, K.; Wurm, F.; Frey, H. Combining oxyanionic polymerization and click-chemistry: A general strategy for the synthesis of polyether polyol macromonomers. Polym. Chem. 2014, 5, 899–909. [Google Scholar] [CrossRef]
- Chandler, D. Interfaces and the driving force of hydrophobic assembly. Nature 2005, 437, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Flewelling, R.F.; Hubbell, W.L. Hydrophobic ion interactions with membranes. Thermodynamic analysis of tetraphenylphosphonium binding to vesicles. Biophys. J. 1986, 49, 531–540. [Google Scholar] [CrossRef]
- Earle, K.A.; Budil, D.E.; Freed, J.H. 250-GHz EPR of nitroxides in the slow-motional regime: Models of rotational diffusion. J. Phys. Chem. 1993, 97, 13289–13297. [Google Scholar] [CrossRef]
- Meirovitch, E.; Nayeem, A.; Freed, J.H. Analysis of protein-lipid interactions based on model simulations of electron spin resonance spectra. J. Phys. Chem. 1984, 88, 3454–3465. [Google Scholar] [CrossRef]
- Gurachevsky, A.; Shimanovitch, E.; Gurachevskaya, T.; Muravsky, V. Intra-albumin migration of bound fatty acid probed by spin label ESR. Biochem. Biophys. Res. Commun. 2007, 360, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Anjos, J.L.V.; Santiago, P.S.; Tabak, M.; Alonso, A. On the interaction of bovine serum albumin with ionic surfactants: Temperature induced EPR changes of a maleimide nitroxide reflect local protein dynamics and probe solvent accessibility. Colloids Surf. B 2011, 88, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.K.; Freed, J.H. Thermodynamics of phospatidylcholine-cholesterol mixed model membranes in the liquid crystalline state studied by the orientational order parameter. Biophys. J. 1989, 56, 1093–1100. [Google Scholar] [CrossRef]
- Shin, Y.K.; Budil, D.; Freed, J.H. Thermodynamics and dynamics of phosphatidylcholine-cholesterol mixed model membranes in the liquid crystalline state: Effects of water. Biophys. J. 1993, 65, 1283–1294. [Google Scholar] [CrossRef]
- Copeland, R.A. Enzymes: A Practical Introduction to Structure, Mechanism and Data, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- Yamabe, S.; Furumiya, Y.; Hiraoka, K.; Morise, K. Theoretical van´t Hoff plots of gas-phase ion equilibria of chloride ion in water, methanol and acetonitrile. Chem. Phys. Lett. 1986, 131, 261–266. [Google Scholar] [CrossRef]
- Rehfeld, S.J.; Eatough, D.J.; Plachy, W.Z. The binding isotherms for the interaction of 5-doxyl stearic acid with bovine and human albumin. J. Lipid Res. 1978, 19, 841–849. [Google Scholar] [PubMed]
- Sprague, E.D.; Larrabee, C.E.; Halsall, H.B. Statistical evaluation of alternative models: Application to ligand-protein binding. Anal. Biochem. 1980, 101, 175–181. [Google Scholar] [CrossRef]
- Kirley, T.L.; Sprague, E.D.; Halsall, H.B. The binding of spin-labeled propranolol and spin-labeled progesterone by orosomucoid. Biophys. Chem. 1982, 15, 209–216. [Google Scholar] [CrossRef]
- Gantchev, T.G.; Shopova, M.B. Characterization of spin-labelled fatty acids and hematoporphyrin binding sites interactions in serum albumin. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1990, 1037, 422–434. [Google Scholar] [CrossRef]
- Hauenschild, T.; Reichenwallner, J.; Enkelmann, V.; Hinderberger, D. Characterizing active pharmaceutical ingredient binding to human serum albumin by spin-labeling and EPR spectroscopy. Chem. Eur. J. 2016, 22, 12825–12838. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sturtevant, J.M. Significant discrepancies between van’t Hoff and calorimetric enthalpies. III. Biophys. Chem. 1997, 64, 121–126. [Google Scholar] [CrossRef]
- Atkins, P.W. Physikalische Chemie; Wiley VCH: Weinheim, Germany, 2001. [Google Scholar]
- Finkelstein, A.V.; Ptitsyn, O.B. Protein Physics: A Course of Lectures; Academic Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Becktel, W.J.; Schellman, J.A. Protein stability curves. Biopolymers 1987, 26, 1859–1877. [Google Scholar] [CrossRef] [PubMed]
- Dunitz, J.D. Win some, lose some: Enthalpy-entropy compensation in weak intermolecular interactions. Chem. Biol. 1995, 2, 709–712. [Google Scholar] [CrossRef]
- Leung, D.H.; Bergman, R.G.; Raymond, K.N. Enthalpy-entropy compensation reveals solvent reorganization as a driving force for supramolecular encapsulation in water. J. Am. Chem. Soc. 2008, 130, 2798–2805. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.D.; Lyon, L.A. Shell-restricted swelling and core compression in Poly(N-isopropylacrylamide) core-shell microgels. Macromolecules 2003, 36, 1988–1993. [Google Scholar] [CrossRef]
- Woodward, N.C.; Chowdhry, B.Z.; Snowden, M.J.; Leharne, S.A.; Griffiths, P.C.; Winnington, A.L. Calorimetric Investigation of the influence of cross-linker concentration on the volume phase transition of Poly(N-isopropylacrylamide) colloidal microgels. Langmuir 2003, 19, 3202–3211. [Google Scholar] [CrossRef]
- Keleti, T. Errors in the evaluation of Arrhenius and van´t Hoff plots. Biochem. J. 1983, 209, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Naghibi, H.; Tamura, A.; Sturtevant, J.M. Significant discrepancies between van’t Hoff and calorimetric enthalpies. Proc. Natl. Acad. Sci. USA 1995, 92, 5597–5599. [Google Scholar] [CrossRef] [PubMed]
- Weber, G. van´t Hoff revisited: Enthalpy of association of protein subunits. J. Phys. Chem. 1995, 99, 1052–1059. [Google Scholar] [CrossRef]
- Tellinghuisen, J. Van’t Hoff analysis of K° (T): How good… or bad? Biophys. Chem. 2006, 120, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A. Heat capacity effects in protein folding and ligand binding: A re-evaluation of the role of water in biomolecular thermodynamics. Biophys. Chem. 2005, 115, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Lum, K.; Chandler, D.; Weeks, J.D. Hydrophobicity at small and large length scales. J. Phys. Chem. B 1999, 103, 4570–4577. [Google Scholar] [CrossRef]
- Choudhury, N.; Pettitt, B.M. On the mechanism of hydrophobic association of nanoscopic solutes. J. Am. Chem. Soc. 2005, 127, 3556–3567. [Google Scholar] [CrossRef] [PubMed]
- Ben-Naim, A. On the driving forces for protein-protein association. J. Chem. Phys. 2006, 125, 024901. [Google Scholar] [CrossRef] [PubMed]
- Pace, C.N.; Shirley, B.A.; McNutt, M.; Gajiwala, K. Forces contributing to the conformational stability of proteins. FASEB J. 1996, 10, 75–83. [Google Scholar] [PubMed]
- Pauling, L. The shared-electron chemical bond. Proc. Natl. Acad. Sci. USA 1928, 14, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Pauling, L. The nature of the chemical bond. IV. The energy of single bonds and the relative electronegativity of atoms. J. Am. Chem. Soc. 1932, 54, 3570–3582. [Google Scholar] [CrossRef]
- Parajuli, R. Does the recent IUPAC definition on hydrogen bonding lead to new intermolecular interactions? Curr. Sci. 2016, 110, 495–498. [Google Scholar]
- Dinarvand, R.; D´Emanuele, A. The use of thermoresponsive hydrogels for on-off release of molecules. J. Control. Release 1995, 36, 221–227. [Google Scholar] [CrossRef]
- Haag, R. Supramolecular drug-delivery systems based on polymeric core-shell architectures. Angew. Chem. Int. Ed. 2004, 43, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Haag, R.; Kratz, F. Polymer therapeutics: Concepts and applications. Angew. Chem. Int. Ed. 2006, 45, 1198–1215. [Google Scholar] [CrossRef] [PubMed]
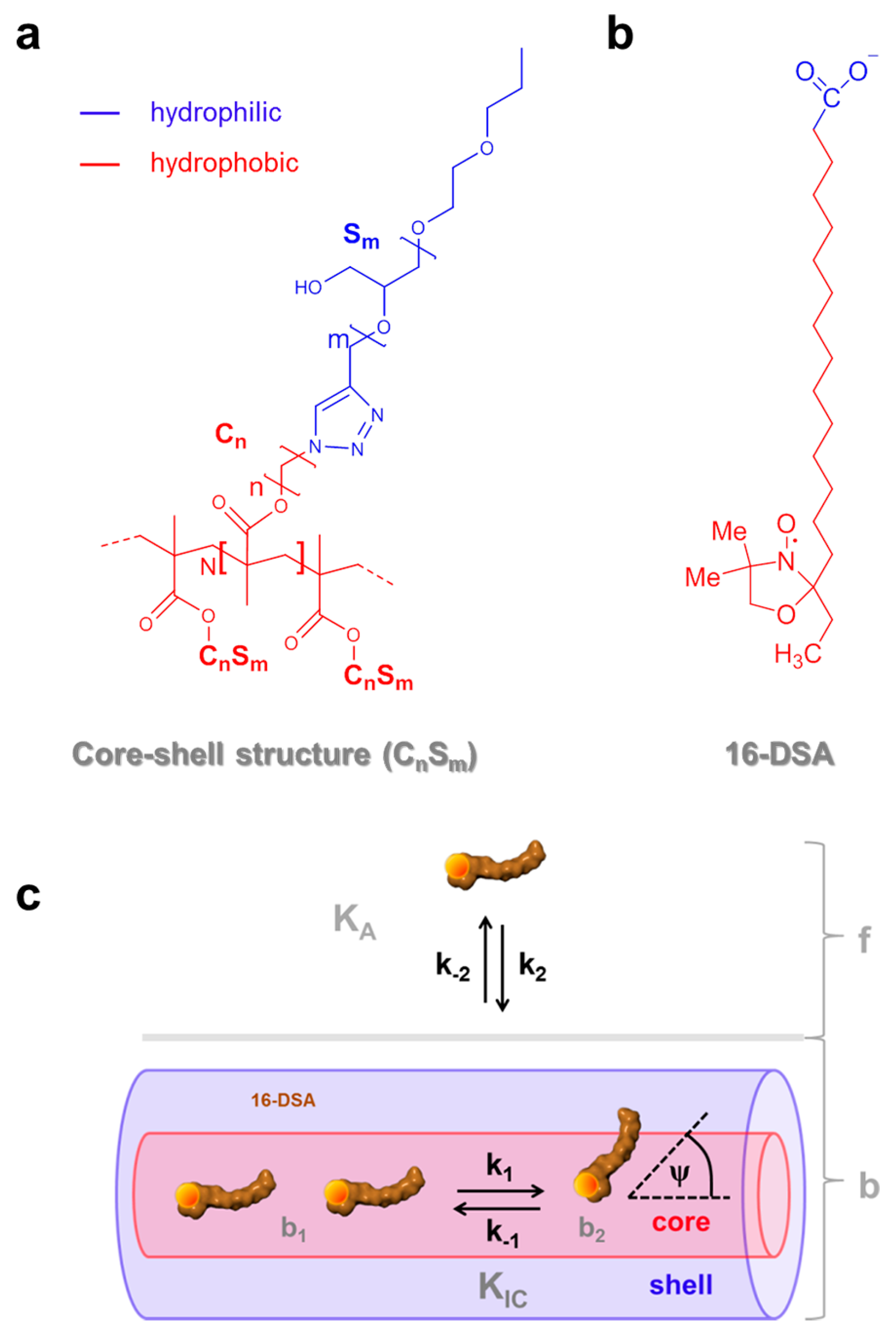
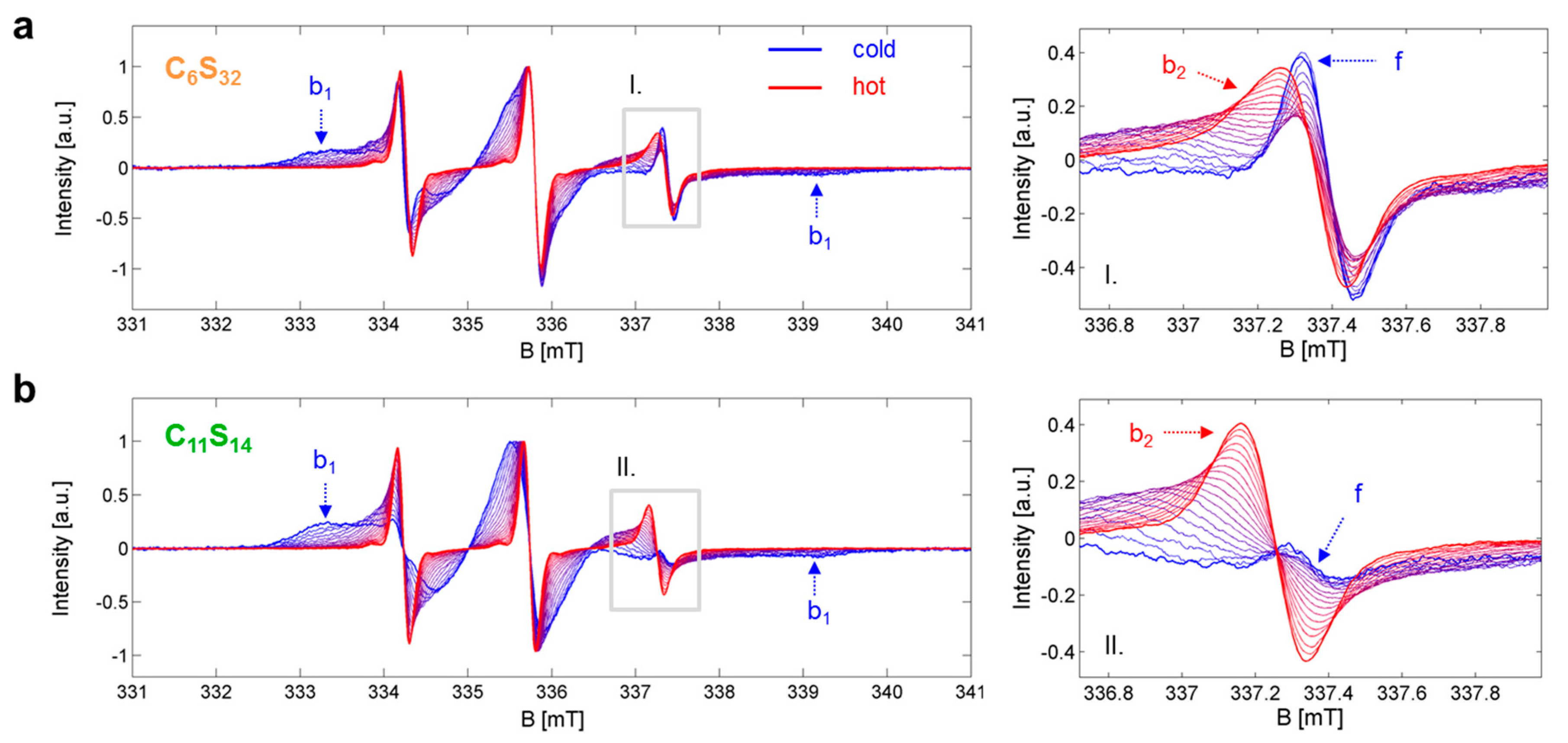
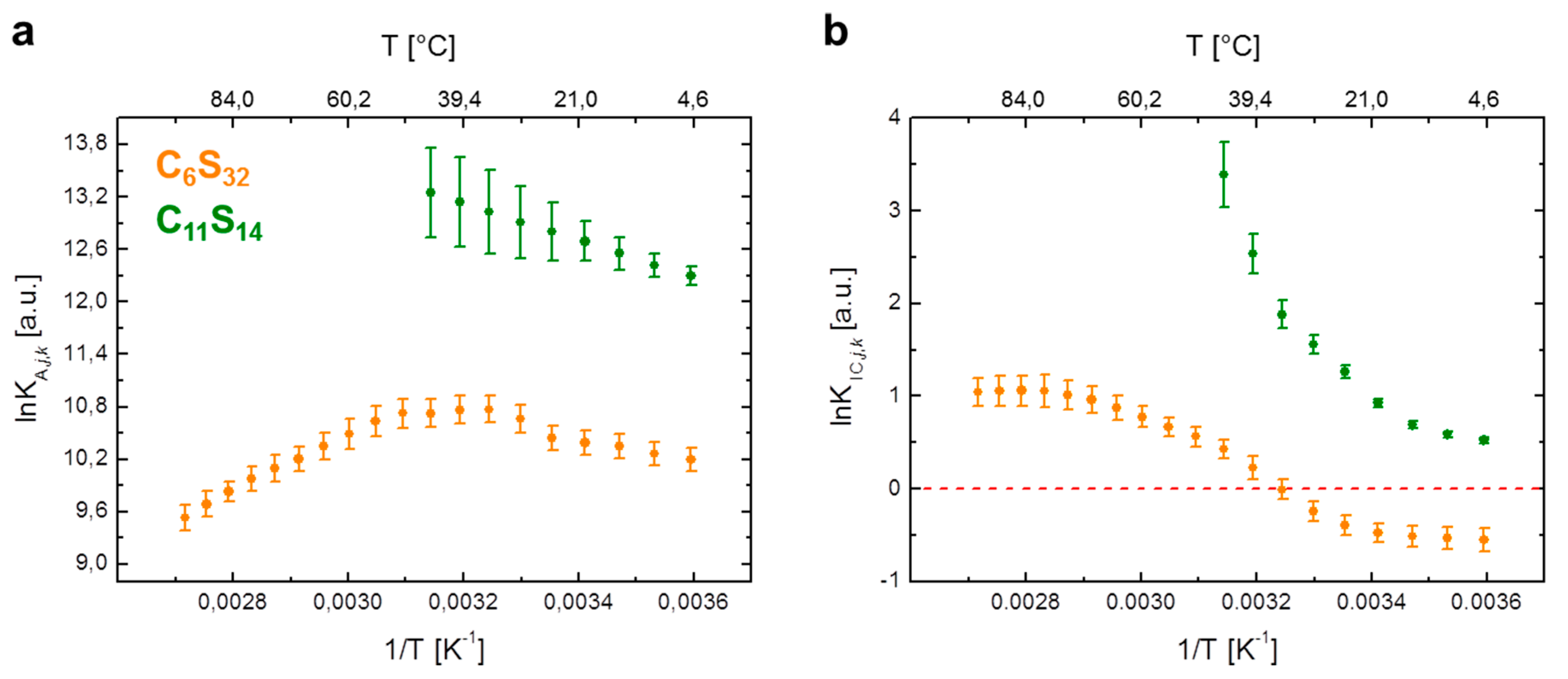
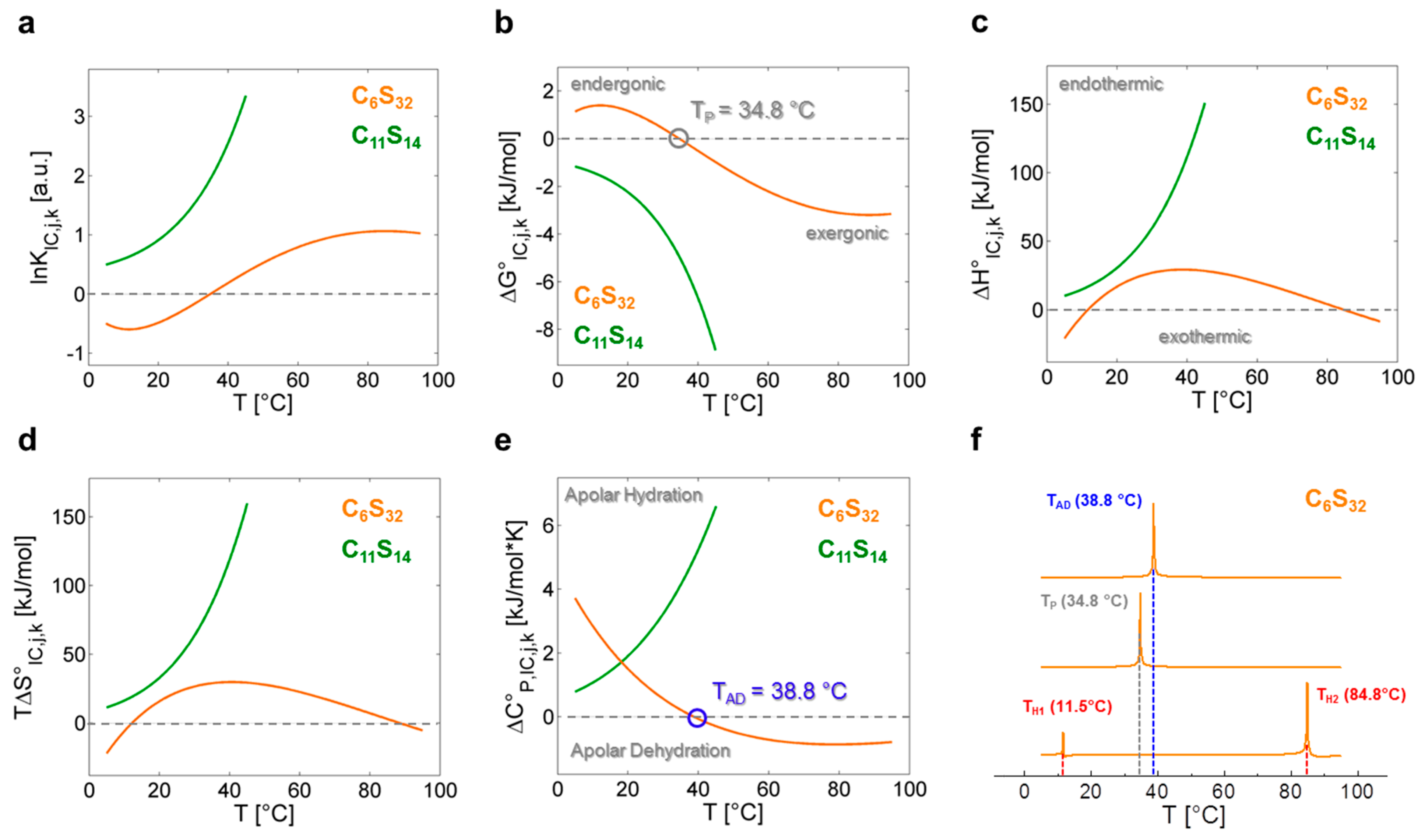
| Method | Parameter | C6S32 | C11S14 |
|---|---|---|---|
| MWMM a (kDa) | 2.72 | 1.46 | |
| SEC MALLS b,c | MW (kDa) | 470.0 | 64.3 |
| N d | 172.8 | 44.0 | |
| PDIMM e | 1.11 | 1.25 | |
| DLS c | HCT f (°C) | 30–40 | – |
| DHAT g (°C) | >35 | <35 | |
| EPR c | DRO h | f, b1, b2 | f, b1, b2 |
| KD,25,k i (µM) | 28.82 ± 2.57 | 2.42 ± 0.35 | |
| KA,25,k i (M−1) | (3.47 ± 0.31) × 104 | (4.13 ± 0.60) × 105 | |
| NL,k j | 11.82 ± 1.37 | 1.96 ± 0.35 | |
| NL,k·cP,k (mM) | 1.005 | 1.219 | |
| aiso,b k (G) | 15.27 | 15.14 | |
| aiso,f l (G) | 15.79 | 15.78 |
| Quantity | lnKA,j,k a | ||
|---|---|---|---|
| j = 25 | C6S32 | C11S14 | |
| (j < 30) | (j > 50) | (j < 45) | |
| ∆G°A,25,k (kJ·mol−1) | −25.9 ± 1.1 | −28.9 ± 1.2 | −31.7 ± 0.4 |
| ∆H°A,25,k (kJ·mol−1) | 8.6 ± 0.5 | −27.1 ± 0.7 | 17.5 ± 0.2 |
| ∆S°A,25,k (J·mol−1·K−1) | 115.7 ± 1.9 | 5.8 ± 1.9 | 165.2 ± 0.7 |
| C6S32 | C11S14 |
|---|---|
| (12) | (16) |
| (13) | (17) |
| (14) | (18) |
| (15) | (19) |
| lnKIC,j,k | ||
|---|---|---|
| j = 25 | C6S32 | C11S14 |
| ∆G°IC,25,k (kJ/mol) | 0.86 | −2.87 |
| ∆H°IC,25,k (kJ/mol) | 23.12 | 42.79 |
| ∆S°IC,25,k (J/mol·K) | 74.71 | 153.24 |
| ∆C°P,IC,25,k (kJ/mol·K) | 1.01 | 2.86 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reichenwallner, J.; Schwieger, C.; Hinderberger, D. Probing the Nanoscopic Thermodynamic Fingerprint of Paramagnetic Ligands Interacting with Amphiphilic Macromolecules. Polymers 2017, 9, 324. https://doi.org/10.3390/polym9080324
Reichenwallner J, Schwieger C, Hinderberger D. Probing the Nanoscopic Thermodynamic Fingerprint of Paramagnetic Ligands Interacting with Amphiphilic Macromolecules. Polymers. 2017; 9(8):324. https://doi.org/10.3390/polym9080324
Chicago/Turabian StyleReichenwallner, Jörg, Christian Schwieger, and Dariush Hinderberger. 2017. "Probing the Nanoscopic Thermodynamic Fingerprint of Paramagnetic Ligands Interacting with Amphiphilic Macromolecules" Polymers 9, no. 8: 324. https://doi.org/10.3390/polym9080324





