Use of Vanadium Complexes Bearing Naphthalene-Bridged Nitrogen-Sulfonate Ligands as Catalysts for Copolymerization of Ethylene and Propylene
Abstract
:1. Introduction
2. Experimental Section
2.1. General Considerations and Materials
2.2. Synthesis of Complexes
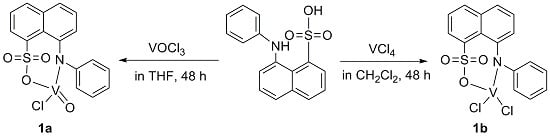
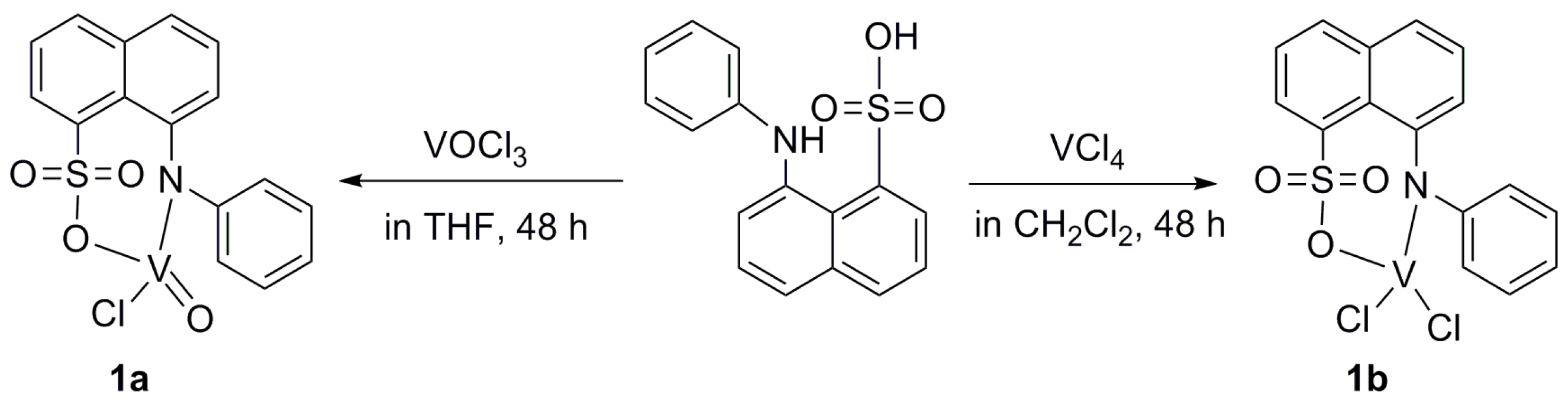
2.2.1. Synthesis of Complex 1a
2.2.2. Synthesis of Complex 1b
2.3. General Procedure for Copolymerization of Ethylene and Propylene
3. Results and Discussion
3.1. Synthesis of Vanadium Complexes
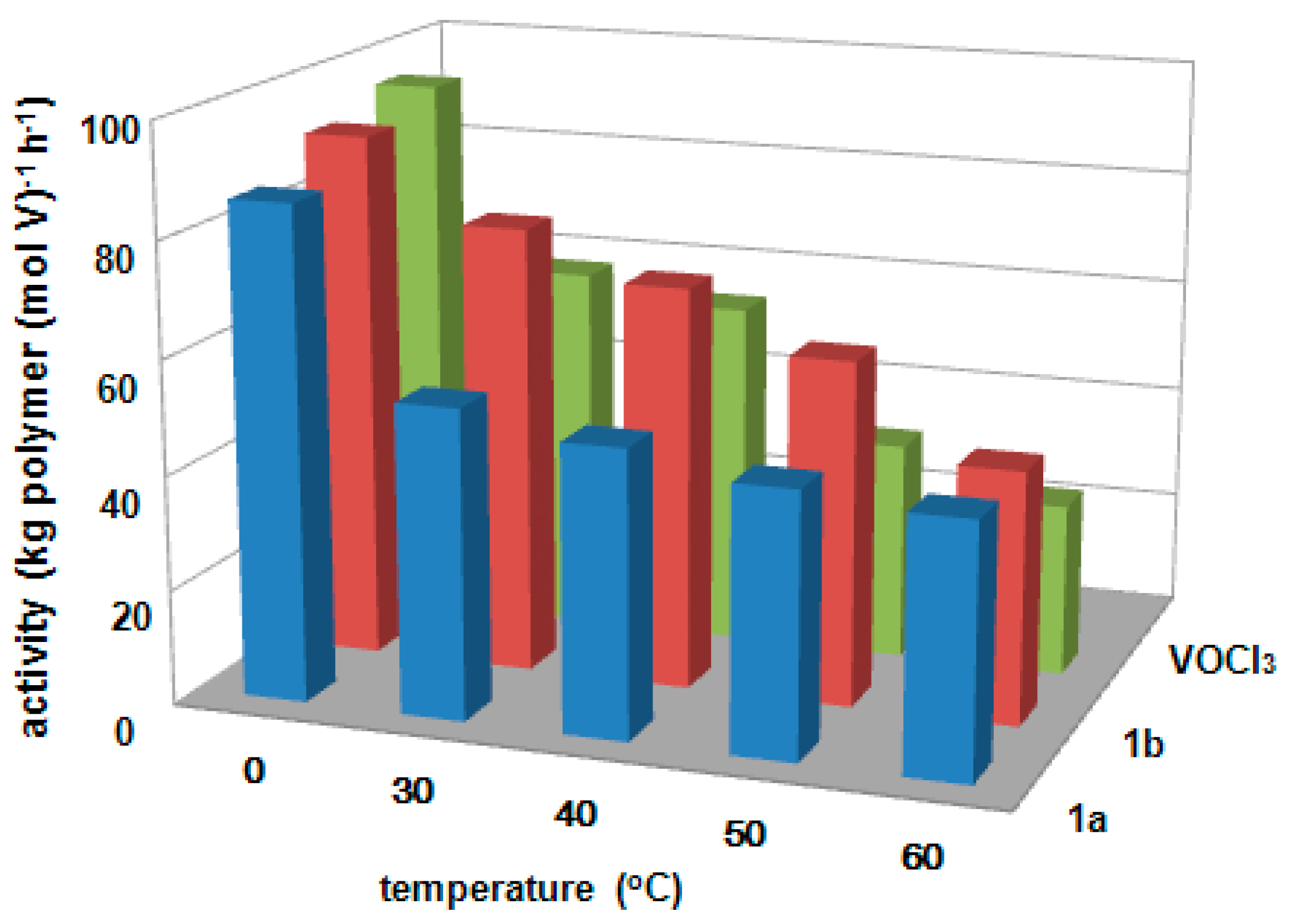
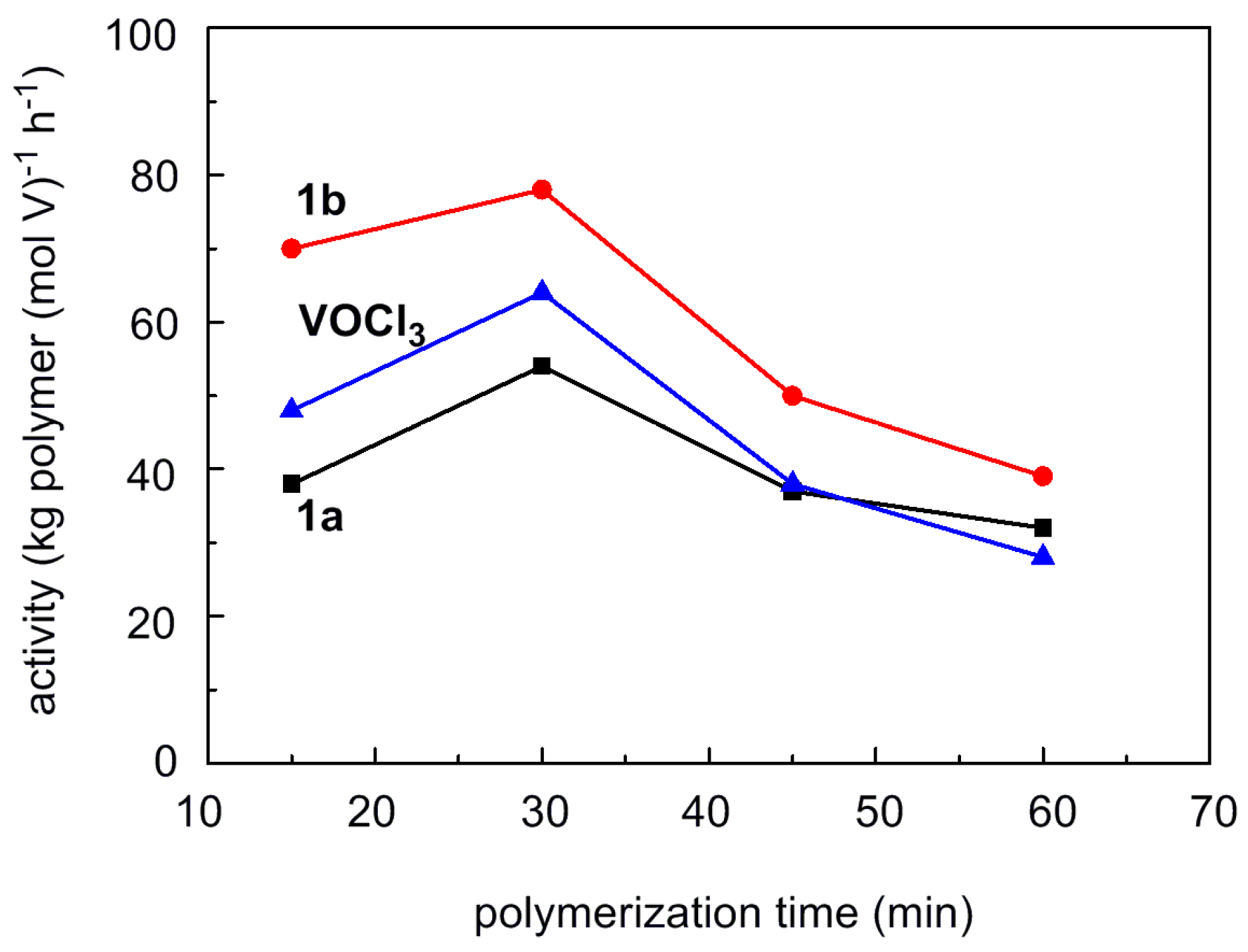
3.2. Ethylene/Propylene Copolymerization
3.3. Polymer Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ziegler, K.; Holzkamp, E.; Breil, H.; Martin, H. Das mülheimer normaldruck-polyäthylen-verfahren. Angew. Chem. 1955, 67, 541–547. [Google Scholar] [CrossRef]
- Natta, G. Stereospezifische katalysen und isotaktische polymere. Angew. Chem. 1956, 68, 393–403. [Google Scholar] [CrossRef]
- Coates, G.W. Precise control of polyolefin stereochemistry using single-site metal catalysts. Chem. Rev. 2000, 100, 1223–1252. [Google Scholar] [CrossRef] [PubMed]
- Ittel, S.D.; Johnson, L.K.; Brookhart, M. Late-metal catalysts for ethylene homo- and copolymerization. Chem. Rev. 2000, 100, 1169–1204. [Google Scholar] [CrossRef] [PubMed]
- Gibson, V.C.; Spitzmesser, S.K. Advances in non-metallocene olefin polymerization catalysis. Chem. Rev. 2003, 103, 283–316. [Google Scholar] [CrossRef] [PubMed]
- Makio, H.; Terao, H.; Iwashita, A.; Fujita, T. FI catalysts for olefin polymerization—A comprehensive treatment. Chem. Rev. 2011, 111, 2363–2449. [Google Scholar] [CrossRef] [PubMed]
- Baier, M.C.; Zuideveld, M.A.; Mecking, S. Post-metallocenes in the industrial production of polyolefins. Angew. Chem. Int. Ed. 2014, 53, 9722–9744. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Zhang, S. Design of vanadium complex catalysts for precise olefin polymerization. Chem. Rev. 2011, 111, 2342–2362. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Q.; Li, Y.S. Well-defined vanadium complexes as the catalysts for olefin polymerization. Coord. Chem. Rev. 2011, 255, 2303–2314. [Google Scholar] [CrossRef]
- Redshaw, C. Vanadium procatalysts bearing chelating aryloxides: Structure–activity trends in ethylene polymerisation. Dalton Trans. 2010, 39, 5595–5604. [Google Scholar] [CrossRef] [PubMed]
- Matsugi, T.; Fujita, T. High-performance olefin polymerization catalysts discovered on the basis of a new catalyst design concept. Chem. Soc. Rev. 2008, 37, 1264–1277. [Google Scholar] [CrossRef] [PubMed]
- Gambarotta, S. Vanadium-based Ziegler-Natta: Challenges, promises, problems. Coord. Chem. Rev. 2003, 237, 229–243. [Google Scholar] [CrossRef]
- Hagen, H.; Boersma, J.; van Koten, G. Homogeneous vanadium-based catalysts for the Ziegler-Natta polymerization of α-olefins. Chem. Soc. Rev. 2002, 31, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Gumboldt, A.; Helberg, J.; Schleitzer, G. Über die reaktivierung der bei der äuthylen/propylen-copolymerisation verwendeten vanadium-katalysatoren. Makromol. Chem. 1967, 101, 229–245. [Google Scholar] [CrossRef]
- Christman, D.L. Preparation of polyethylene in solution. J. Polym. Sci. Part A 1972, 10, 471–487. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, W.C.; Shang, D.D.; Zhang, Z.Q.; Wu, Y.X. Ethylene/propylene copolymerization catalyzed by vanadium complexes containing N-heterocyclic carbenes. Dalton Trans. 2015, 44, 15264–15270. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Igarashi, A.; Katao, S.; Zhang, W.J.; Sun, W.H. Synthesis and structural analysis of (imido)vanadium(V) complexes containing chelate (anilido)methyl-imine ligands: Ligand effect in ethylene dimerization. Inorg. Chem. 2013, 52, 2607–2614. [Google Scholar] [CrossRef] [PubMed]
- Bialek, M.; Czaja, K.; Pietruszka, A. Ethylene/1-olefin copolymerization behaviour of vanadium and titanium complexes bearing salen-type ligand. Polym. Bull. 2013, 70, 1499–1517. [Google Scholar] [CrossRef]
- Igarashi, A.; Zhang, S.; Nomura, K. Ethylene dimerization/polymerization catalyzed by (adamantylimido)vanadium(V) complexes containing (2-anilidomethyl)pyridine ligands: Factors affecting the ethylene reactivity. Organometallics 2012, 31, 3575–3581. [Google Scholar] [CrossRef]
- Zhang, S.; Katao, S.; Sun, W.H.; Nomura, K. Synthesis of (arylimido)vanadium(V) complexes containing (2-anilidomethyl)pyridine ligands and their Use as the catalyst precursors for olefin polymerization. Organometallics 2012, 28, 5925–5933. [Google Scholar] [CrossRef]
- Onishi, Y.; Katao, S.; Fujiki, M.; Nomura, K. Synthesis and structural analysis of (arylimido)vanadium(V) complexes containing phenoxyimine ligands: New, efficient catalyst precursors for ethylene polymerization. Organometallics 2008, 27, 2590–2596. [Google Scholar] [CrossRef]
- Smit, T.M.; Tomov, A.K.; Britovsek, G.J.P.; Gibson, V.C.; White, A.J.P.; Williams, D.J. The effect of imine-carbon substituents in bis(imino)pyridine-based ethylene polymerization catalysts across the transition series. Catal. Sci. Technol. 2012, 2, 643–655. [Google Scholar] [CrossRef]
- Redshaw, C.; Clowes, L.; Hughes, D.L.; Elsegood, M.R.J.; Yamato, T. Ethylene polymerization catalysis by vanadium-based systems bearing sulfur-bridged calixarenes. Organometallics 2011, 30, 5620–5624. [Google Scholar] [CrossRef]
- Clowes, L.; Redshaw, C.; Hughes, D.L. Vanadium-based pro-catalysts bearing depleted 1,3-calix[4]arenes for ethylene or ε-caprolactone polymerization. Inorg. Chem. 2011, 50, 7838–7845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.W.; Lu, L.P.; Long, Y.Y.; Li, Y.S. Synthesis, structural characterization, and ethylene polymerization behavior of (arylimido)vanadium(V) complexes bearing tridentate Schiff base ligands. J. Polym. Sci. Part A 2014, 52, 2633–2642. [Google Scholar]
- Lu, L.P.; Wang, J.B.; Liu, J.Y.; Li, Y.S. Ethylene polymerization and ethylene/hexene copolymerization by vanadium(III) complexes bearing bidentate phenoxy-phosphine oxide ligands. J. Polym. Sci. Part A 2013, 51, 5298–5306. [Google Scholar]
- Zhang, S.W.; Zhang, G.B.; Lu, L.P.; Li, Y.S. Novel vanadium(III) complexes with tridentate phenoxy-phosphine [O,P(O),O] ligands: Synthesis, characterization, and catalytic behavior of ethylene polymerization and copolymerization with 10-undecen-1-ol. J. Polym. Sci. Part A 2013, 51, 844–854. [Google Scholar] [CrossRef]
- Abernethy, C.D.; Codd, G.M.; Spicer, M.D.; Taylor, M.K. A highly stable N-heterocyclic carbene complex of trichloro-oxo-vanadium(V) displaying novel Cl−Ccarbene bonding interactions. J. Am. Chem. Soc. 2003, 125, 1128–1129. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, A.; Kolychev, E.L.; Tamm, M.; Nomura, K. Synthesis of (imido)vanadium(V) dichloride Complexes Containing Anionic N-Heterocyclic Carbenes That Contain a Weakly Coordinating Borate moiety: new MAO-free ethylene polymerization catalysts. Organometallics 2016, 35, 1778–1784. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Endo, K. Vanadium alkoxide catalyzed polymerization of vinyl chloride. J. Polym. Sci. Part A 2011, 49, 1006–1012. [Google Scholar] [CrossRef]
- Wu, J.Q.; Pan, L.; Li, Y.G.; Liu, S.R.; Li, Y.S. Synthesis, structural characterization, and olefin polymerization behavior of vanadium(III) complexes bearing tridentate schiff base ligands. Organometallics 2009, 28, 1817–1825. [Google Scholar] [CrossRef]
- Tomov, A.K.; Gibson, V.C.; Zaher, D.; Elsegood, M.; Dale, S.H. Bis(benzimidazole)amine vanadium catalysts forolefin polymerization and co-polymerisation: thermally robust, single-site catalysts activated by simple alkylaluminium reagents. Chem. Commun. 2004, 17, 1956–1957. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Mitsudome, T.; Lgarashi, A. Synthesis of (adamantylimido)vanadium(V) dimethyl complex containing (2-anilidomethyl)pyridine ligand and selected reactions: exploring the oxidation state of the catalytically active species in ethylene dimerization. Organometallics 2017, 36, 530–542. [Google Scholar] [CrossRef]
- Nomura, K.; Hou, X. Synthesis of vanadium-alkylidene complexes and their use as catalysts for ring opening metathesis polymerization. Dalton Trans. 2017, 46, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Diteepeng, N.; Tang, X.; Hou, X. Ethylene polymerisation and ethylene/norbornene copolymerisation by using aryloxo-modified vanadium(V) complexes containing 2,6-difluoro-, dichloro-phenylimido complexes. Dalton Trans. 2015, 44, 12273–12281. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Ito, S.; Nozaki, K. Coordination−insertion copolymerization of fundamental polar monomers. Chem. Rev. 2009, 109, 5215–5244. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Y.X. Coordination polymerization of polar vinyl monomers by single-site metal catalysts. Chem. Rev. 2009, 109, 5157–5214. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Anselment, T.M.J.; Claverie, J.; Goodall, B.; Jordan, R.F.; Mecking, S.; Rieger, B.; Sen, A.; van Leeuwen, P.W.N.M.; Nozaki, K. Ortho-phosphinobenzenesulfonate: A superb ligand for palladium-catalyzed coordination–insertion copolymerization of polar vinyl monomers. Acc. Chem. Res. 2013, 46, 1438–1449. [Google Scholar] [CrossRef] [PubMed]
- Carrow, B.P.; Nozaki, K. Transition-metal-catalyzed functional polyolefin synthesis: Effecting control through chelating ancillary ligand design and mechanistic insights. Macromolecules 2014, 47, 2541–2555. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, M.; Chen, C. Ethylene polymerization and copolymerization by palladium and nickel catalysts containing naphthalene-bridged phosphine–sulfonate ligands. Organometallics 2016, 35, 1472–1479. [Google Scholar] [CrossRef]
- Bashir, O.; Piche, L.; Claverie, J.P. 18-electron ruthenium phosphine sulfonate catalysts for olefin metathesis. Organometallics 2014, 33, 3695–3701. [Google Scholar] [CrossRef]
- Ma, Y.; Reardon, D.; Gambarotta, S.; Yap, G.; Zahalka, H.; Lemay, C. Vanadium-catalyzed ethylene-propylene copolymerization: the question of the metal oxidation state in Ziegler-Natta polymerization promoted by (β-diketonate)3V. Organometallics 1999, 18, 2773–2781. [Google Scholar] [CrossRef]
- Adisson, E.; Deffieux, A.; Fontanille, M.; Bujadoux, K. Polymerization of ethylene at high temperature by vanadium-based heterogeneous Ziegler—Natta catalysts. II. Study of the activation by halocarbons. J. Polym. Sci. Part A 1994, 32, 1033–1041. [Google Scholar] [CrossRef]
- Janas, Z.; Wisniewska, D.; Jerzykiewicz, L.B.; Sobota, P.; Drabent, K.; Szczegot, K. Synthesis, structural studies and reactivity of vanadium complexes with tridentate (OSO) ligand. Dalton Trans. 2007, 20, 2065–2069. [Google Scholar] [CrossRef] [PubMed]
- Carman, C.J.; Harrington, R.A.; Wilkes, C.E. Microstructure and physical properties of hydrochlorinated 1,4-polyisoprene prepared by butyllithium in nonpolar solvent. Macromolecules 1977, 10, 149–153. [Google Scholar]
- Randall, J.C. Methylene sequence distributions and number average sequence lengths in ethylene-propylene copolymers. Macromolecules 1978, 11, 33–36. [Google Scholar] [CrossRef]
- Cheng, H.N. Carbon-13 NMR analysis of ethylene-propylene rubbers. Macromolecules 1984, 17, 1950–1955. [Google Scholar] [CrossRef]
- Smith, W.V. Sequence distribution in ethylene-propylene copolymers. I. Relations between multads and between multads and the 13C NMR spectrum. J. Polym. Sci. B Polym. Phys. 1980, 18, 1573–1585. [Google Scholar] [CrossRef]
- Randall, J.C. Sequence distributions versus catalyst site behavior of in situ blends of polypropylene and poly(ethylene-co-propylene). J. Polym. Sci. Part A 1998, 36, 1527–1542. [Google Scholar] [CrossRef]

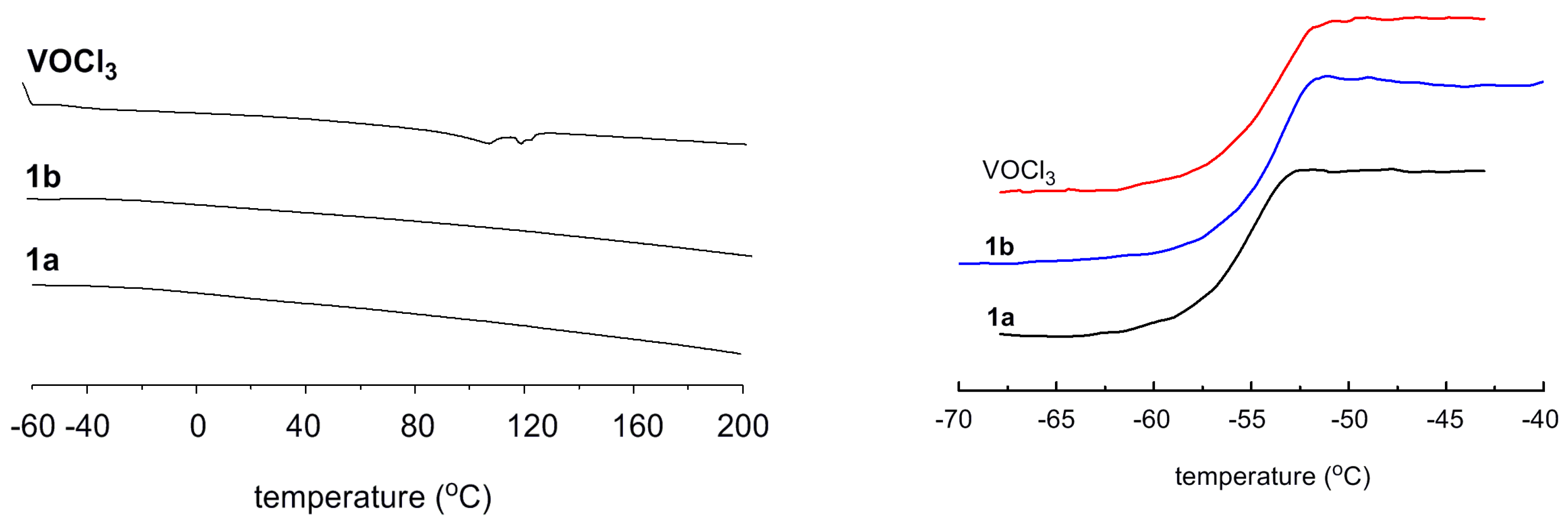
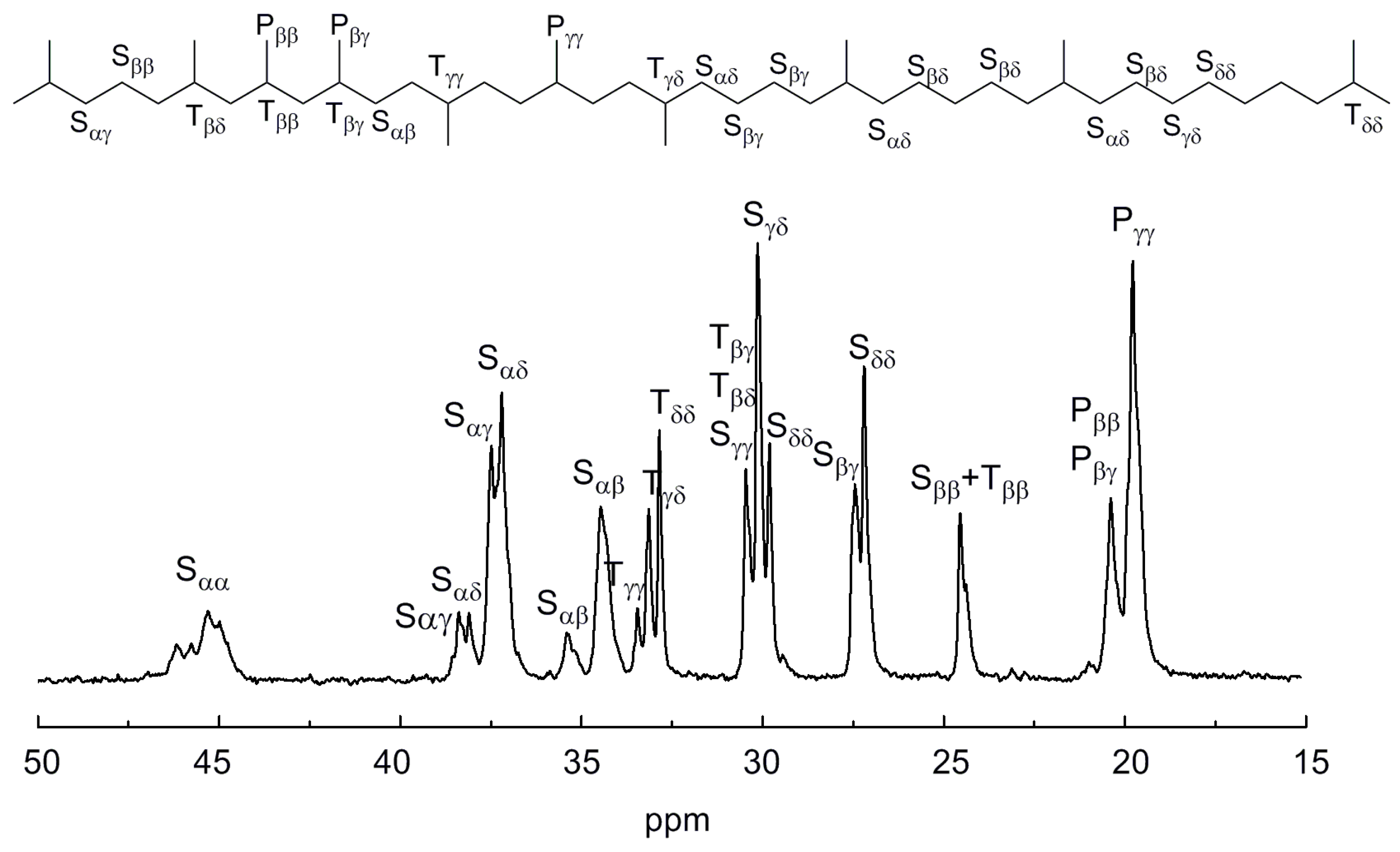
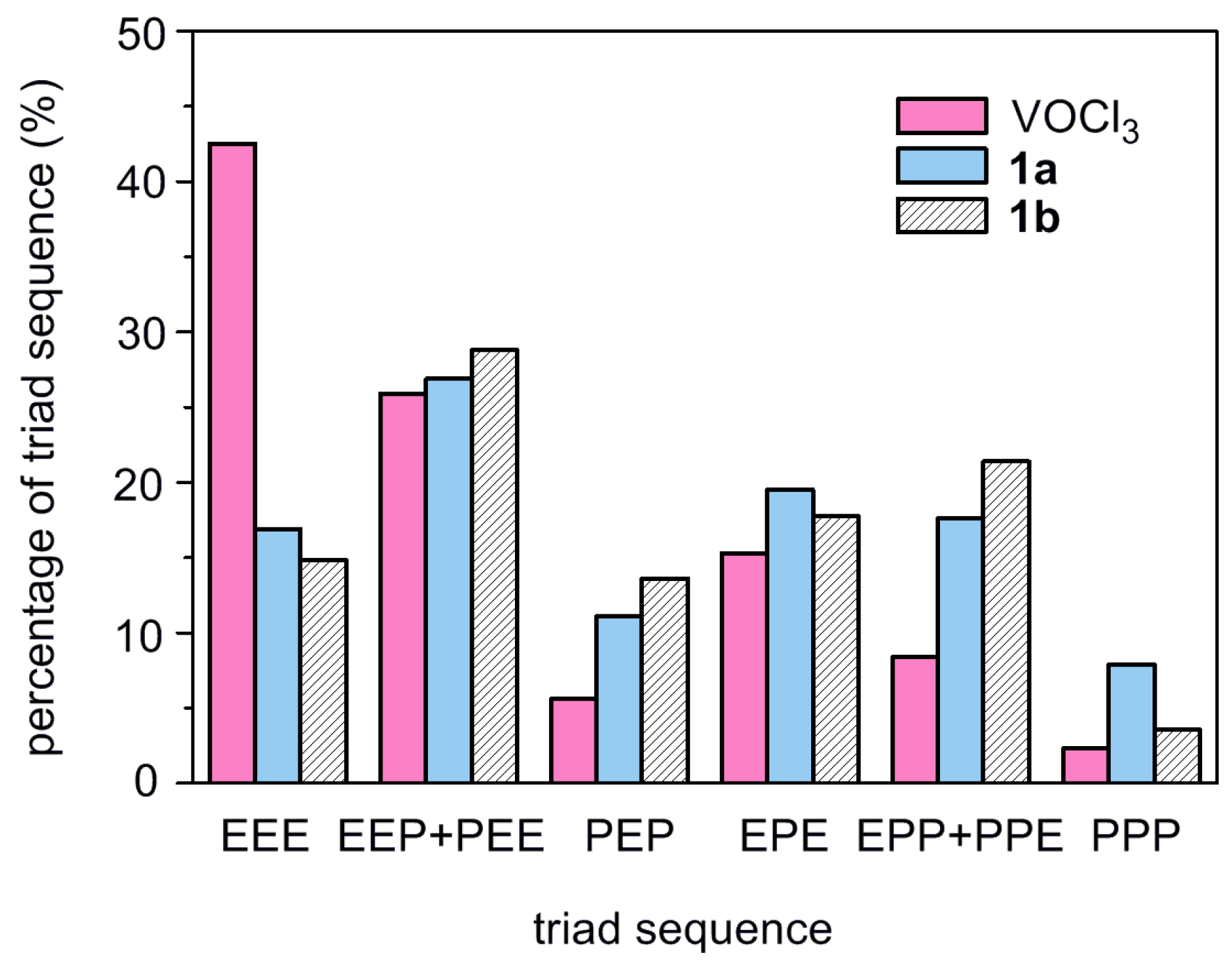
| Run | Cat. | ETCA/V | Yield (g) | Activity b | Mw × 10−4 c | Mw/Mn c | C3 Incorp. (mol%) d |
|---|---|---|---|---|---|---|---|
| 1 | 1a | 0 | 1.03 | 41.2 | 23.1 | 3.16 | 36.0 |
| 2 | 1a | 5 | 1.30 | 52.0 | 12.7 | 2.09 | 39.0 |
| 3 | 1a | 10 | 1.32 | 52.8 | 8.3 | 3.17 | 40.6 |
| 4 | 1a | 20 | 1.22 | 48.8 | 4.0 | 2.66 | 48.2 |
| 5 | 1b | 0 | 0.50 | 20.0 | 28.1 | 3.01 | 33.1 |
| 6 | 1b | 10 | 1.96 | 78.4 | 19.6 | 2.63 | 37.3 |
| 7 | 1b | 20 | 1.55 | 62.0 | 6.1 | 2.77 | 49.4 |
| 8 | VOCl3 | 0 | 0.93 | 18.6 | 19.5 | 5.69 | 25.1 |
| 9 | VOCl3 | 5 | 0.96 | 38.4 | 10.3 | 7.25 | 28.9 |
| 10 | VOCl3 | 10 | 1.60 | 64.0 | 6.4 | 7.69 | 33.0 |
| 11 | VOCl3 | 20 | 1.53 | 61.2 | 2.2 | 5.46 | 40.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, X.; Zhang, C.; Li, L.; Zhang, H.; Hu, Y.; Hao, D.; Zhang, X. Use of Vanadium Complexes Bearing Naphthalene-Bridged Nitrogen-Sulfonate Ligands as Catalysts for Copolymerization of Ethylene and Propylene. Polymers 2017, 9, 325. https://doi.org/10.3390/polym9080325
Hao X, Zhang C, Li L, Zhang H, Hu Y, Hao D, Zhang X. Use of Vanadium Complexes Bearing Naphthalene-Bridged Nitrogen-Sulfonate Ligands as Catalysts for Copolymerization of Ethylene and Propylene. Polymers. 2017; 9(8):325. https://doi.org/10.3390/polym9080325
Chicago/Turabian StyleHao, Xiufeng, Chundi Zhang, Lin Li, Hexin Zhang, Yanming Hu, Daifeng Hao, and Xuequan Zhang. 2017. "Use of Vanadium Complexes Bearing Naphthalene-Bridged Nitrogen-Sulfonate Ligands as Catalysts for Copolymerization of Ethylene and Propylene" Polymers 9, no. 8: 325. https://doi.org/10.3390/polym9080325