Effects of Amino-Functionalized Carbon Nanotubes on the Crystal Structure and Thermal Properties of Polyacrylonitrile Homopolymer Microspheres
Abstract
:1. Introduction
2. Experimental and Methods
2.1. Materials and Amino-CNTs
2.2. Fabrication of Amino-CNT/PAN Microspheres
2.3. Characterization
3. Results and Discussion
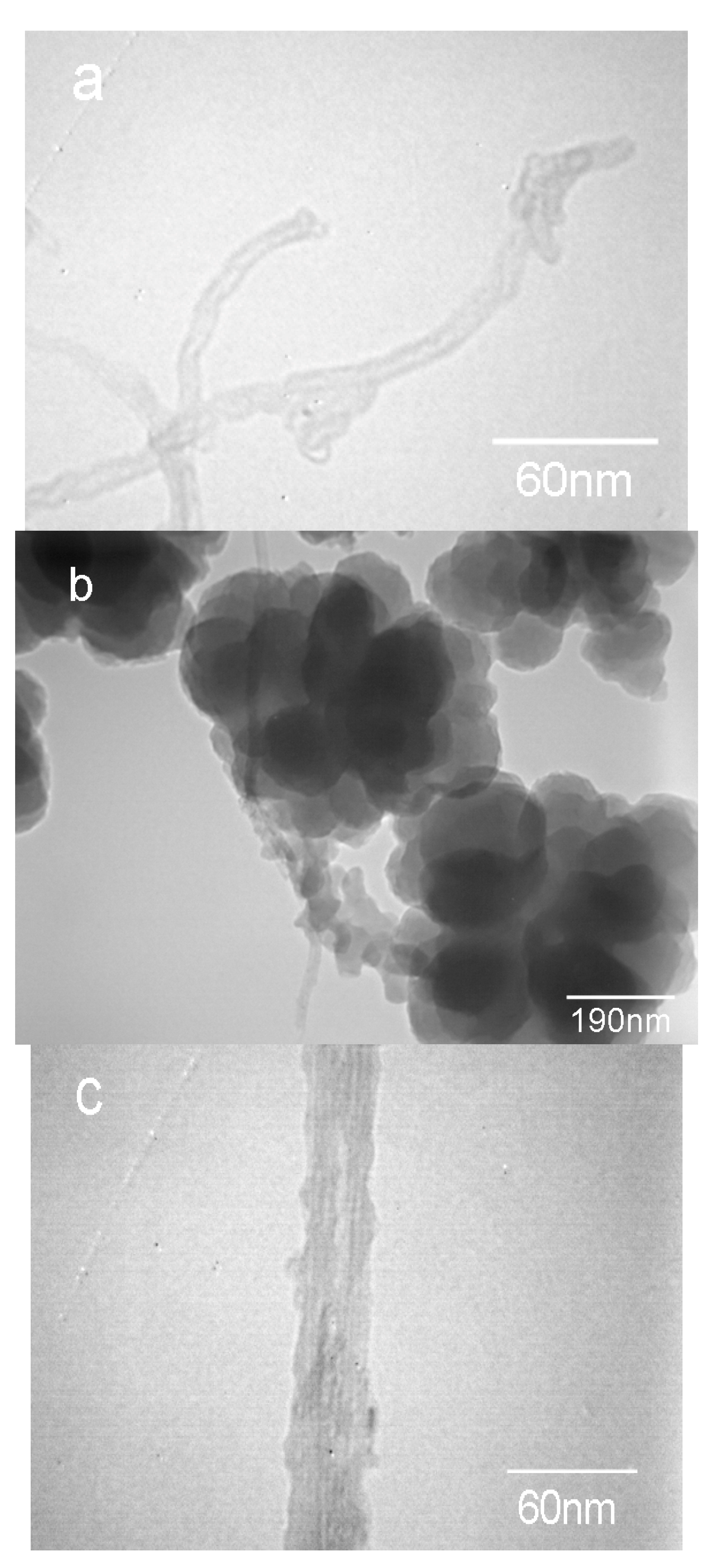
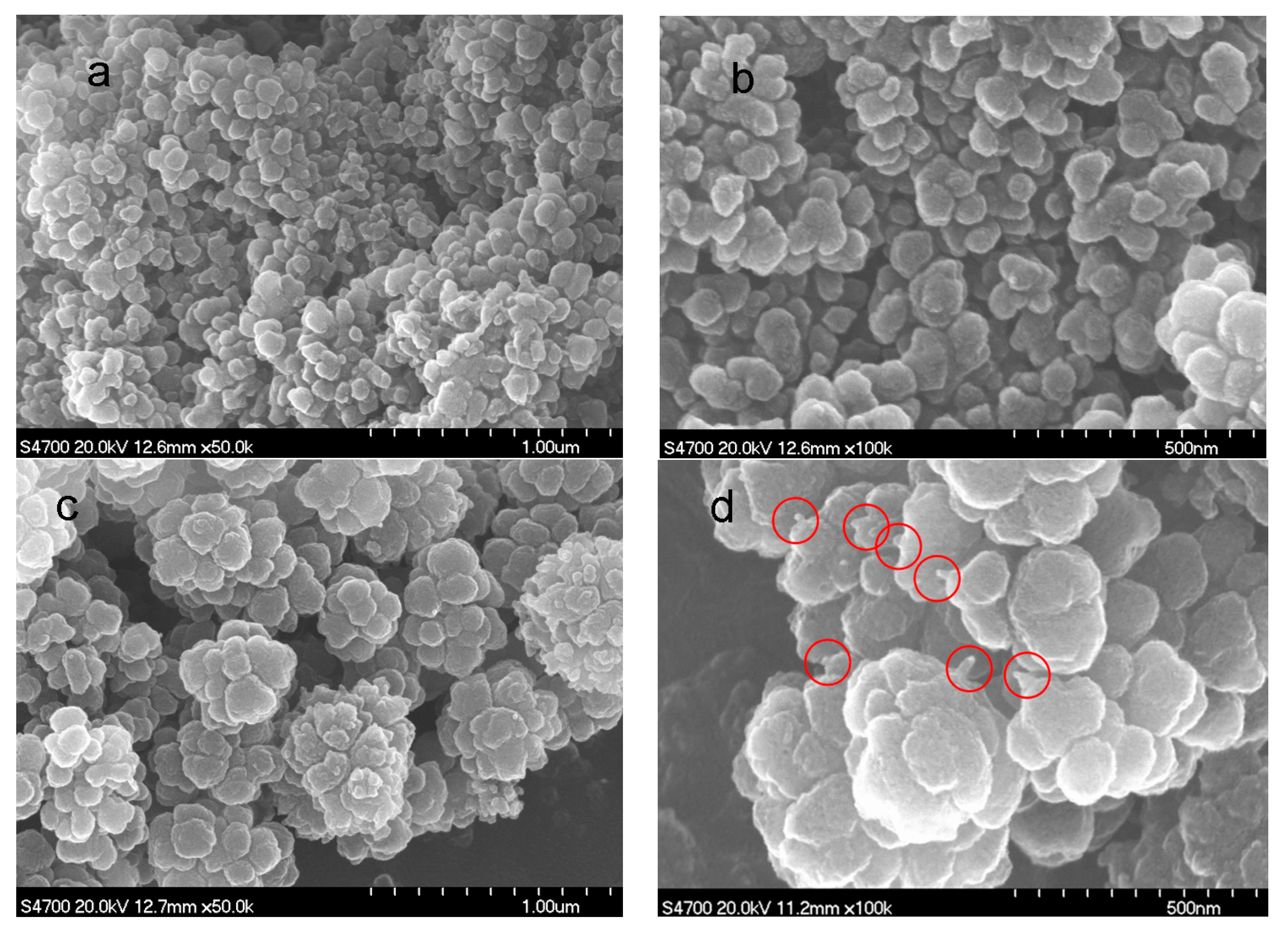
3.1. Morphology of the Amino-CNT/PAN Microspheres
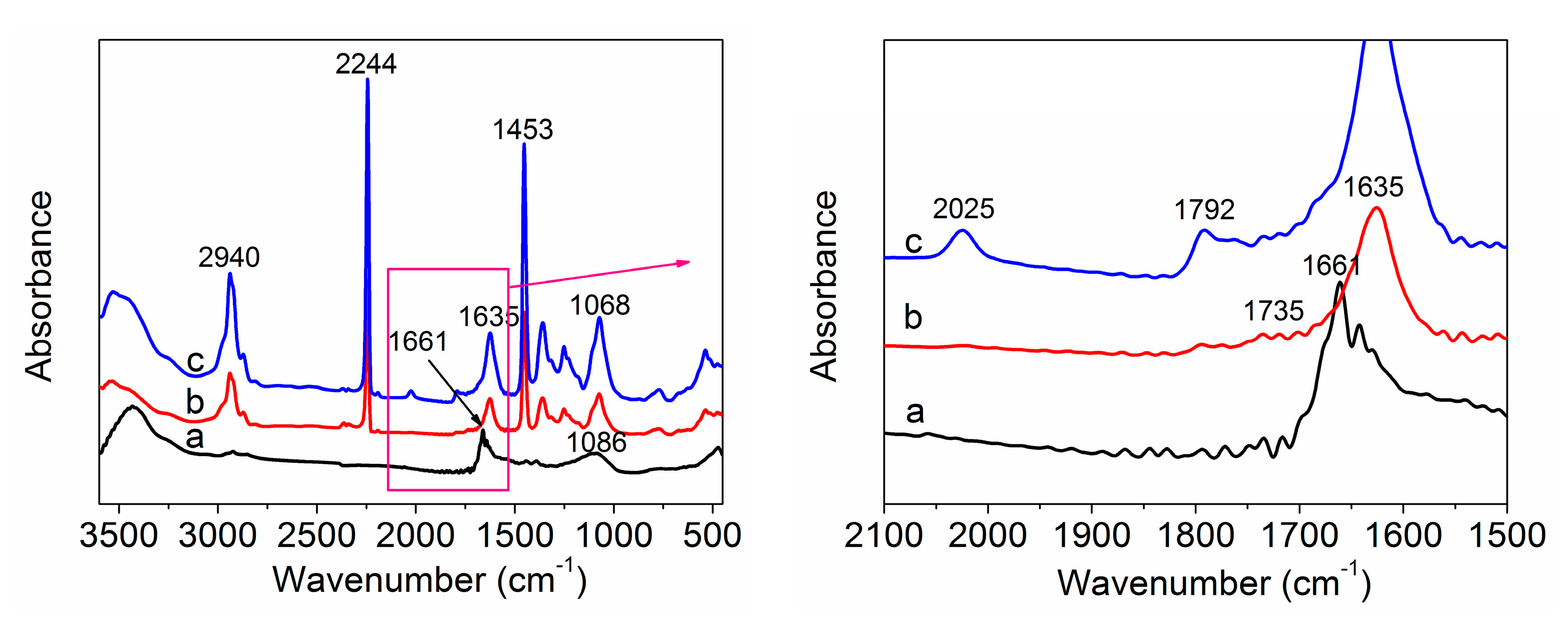
3.2. FTIR Spectra Analysis
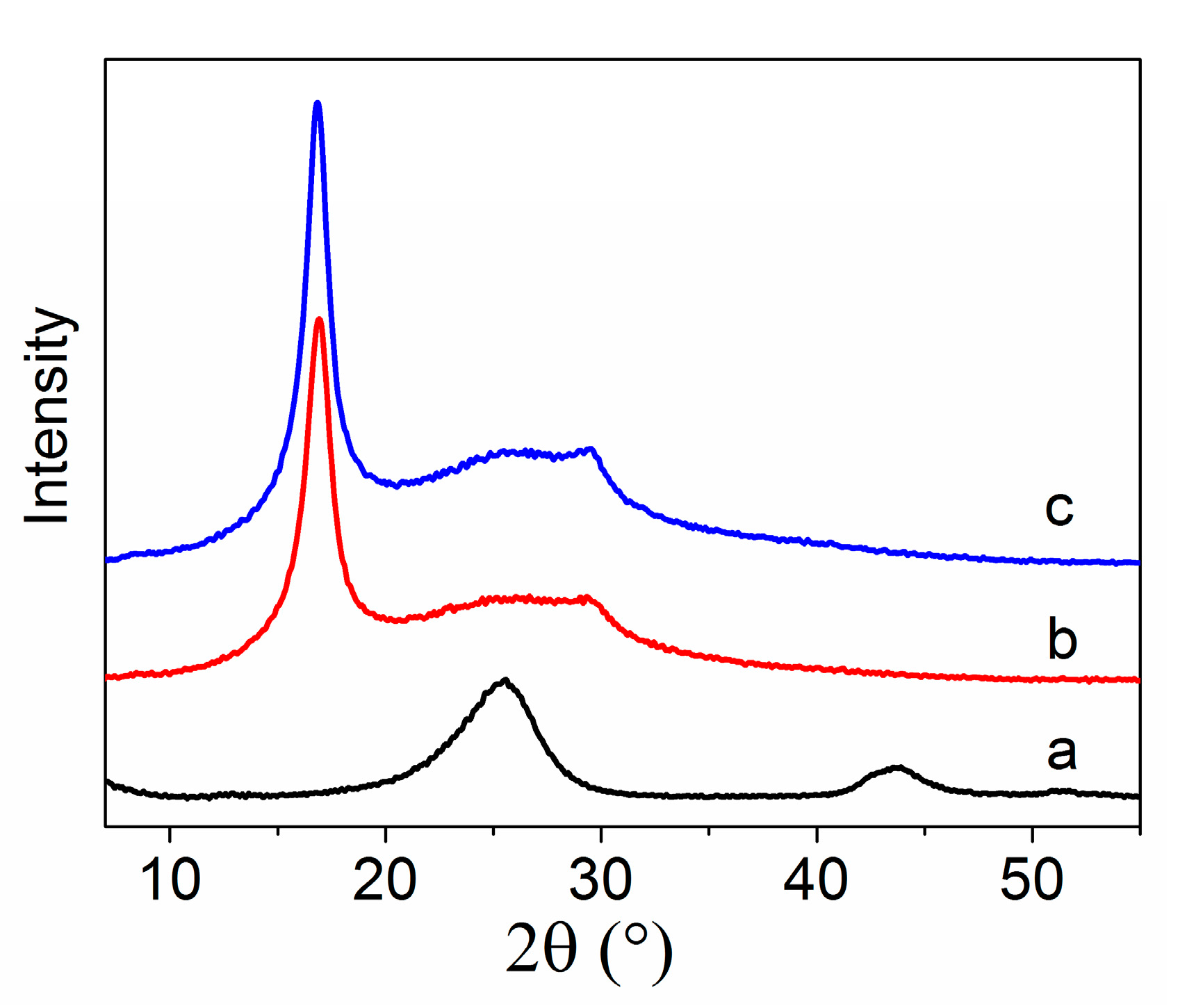
3.3. XRD Curves
3.4. Thermal Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ma, P.C.; Siddiqui, N.A.; Marom, G.; Kim, J.K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Islam, M.F.; Rojas, E.; Bergey, D.M.; Johnson, A.T.; Yodh, A.G. High weight fraction surfactant solubilization of single-wall carbon nanotubes in water. Nano Lett. 2003, 3, 269–273. [Google Scholar] [CrossRef]
- Ma, P.C.; Mo, S.Y.; Tang, B.Z.; Kim, J.K. Dispersion, interfacial interaction and re-agglomeration of functionalized carbon nanotubes in epoxy composites. Carbon 2010, 48, 1824–1834. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Rana, S.; Cho, J.W.; Li, L.; Chan, S.H. Polymer nanocomposites based on functionalized carbon nanotubes. Prog. Polym. Sci. 2010, 35, 837–867. [Google Scholar] [CrossRef]
- Xue, T.J.; Mckinney, M.A.; Wilkie, C.A. The thermal degradation of polyacrylonitrile. Polym. Degrad. Stab. 1997, 58, 193–202. [Google Scholar] [CrossRef]
- Ogawa, H.; Saito, K. Oxidation behavior of polyacrylonitrile fibers evaluated by new stabilization index. Carbon 1995, 33, 783–788. [Google Scholar] [CrossRef]
- Ouyang, Q.; Cheng, L.; Wang, H.J.; Li, K.X. Mechanism and kinetics of the stabilization reactions of itaconic acid-modified polyacrylonitrile. Polym. Degrad. Stab. 2008, 93, 1415–1421. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Wang, C.G.; Bai, Y.J.; Chen, G.W.; Jing, M.; Zhu, B. Property changes of powdery polyacrylonitrile synthesized by aqueous suspension polymerization during heat-treatment process under air atmosphere. J. Colloid Interface Sci. 2009, 329, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Xu, L.H.; Yang, F.Y.; Geng, L. The synthesis of polyacrylonitrile/carbon nanotube microspheres by aqueous deposition polymerization under ultrasonication. Carbon 2010, 48, 688–695. [Google Scholar] [CrossRef]
- Quan, L.; Zhang, H.L.; Xu, L. The non-isothermal cyclization kinetics of amino-functionalized carbon nanotubes/polyacrylonitrile composites by in situ polymerization. J. Therm. Anal. Calorim. 2015, 119, 1081–1089. [Google Scholar] [CrossRef]
- Sreekumar, T.V.; Chandra, L.; Srivastava, A.; Kumar, S. Oxidative stabilization of polyacrylonitrile in the presence of functionalized carbon nanotubes. Carbon 2007, 45, 1114–1116. [Google Scholar] [CrossRef]
- Chae, H.G.; Sreekumar, T.V.; Uchida, T.; Kumar, S. A comparison of reinforcement efficiency of various types of carbon nanotubes in polyacrylonitrile fiber. Polymer 2005, 46, 10925–10935. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, P.; An, F.; Liu, Y.; Lu, C. Dynamic self-stiffening and structural evolutions of polyacrylonitrile/carbon nanotube nanocomposites. ACS Appl. Mater. Interfaces 2017, 9, 5653–5659. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Bao, H.; Lee, B.; Kim, J.W.; Bae, M.K.; Park, Y.M.; Choi, J.K.; Yoon, S.J.; Kim, T.G. Thermal conductivity and electrical resistivity of electrospun polyacrylonitrile-multi wall carbon nanotubes composite carbon nanofibers. J. Nanosci. Nanotechnol. 2016, 16, 12042–12046. [Google Scholar] [CrossRef]
- Akbari, A.; Jazani, O.M.; Saeb, M.R.; Pourabdollah, K.; Soltanolkottabi, F. Towards well-aligned electrospun PAN/MWCNTs composite nanofibers: Design, fabrication, and development. Fibers Polym. 2014, 15, 1230–1235. [Google Scholar] [CrossRef]
- Maitra, T.; Sharma, S.; Srivastava, A.; Cho, T.K.; Madou, M.; Sharma, A. Improved graphitization and electrical conductivity of suspended carbon nanofibers derived from carbon nanotube/polyacrylonitrile composites by directed electrospinng. Carbon 2012, 50, 1753–1761. [Google Scholar] [CrossRef]
- Jin, X.; Ni, Q.Q.; Fu, Y.; Zhang, L.; Natsuki, T. Electrospun nanocomposite polyacrylonitrile fibers containing carbon nanotubes and conbalt ferrite. Polym. Compos. 2012, 33, 317–323. [Google Scholar] [CrossRef]
- Prusty, G.; Das, R.; Swain, S.K. Influence of functionalized single-walled carbon nanotubes on morphology, conducting and oxygen barrier properties of poly(acrylonitrile-co-starch). Compos. Part B Eng. 2014, 62, 236–241. [Google Scholar] [CrossRef]
- Park, C.; Ounaies, Z.; Waston, K.A.; Crooks, R.E.; Smith, J.; Lowther, S.E.; Connell, J.W.; Siochi, E.J.; Harrison, J.S.; St Clair, T.L. Dispersion of single wall carbon nanotubes by in situ polymerization under sonication. Chem. Phys. Lett. 2002, 363, 303–308. [Google Scholar] [CrossRef]
- Deng, H.; Bilotti, E.; Zhang, R.; Wang, K.; Zhang, Q.; Peijs, T.; Fu, Q.A. Improving tensile strength and toughness of melt processed polyamide 6/multiwalled carbon nanotube composites by in situ polymerization and filler surface functionalization. J. Appl. Polym. Sci. 2011, 120, 133–140. [Google Scholar] [CrossRef]
- Huang, Y.C.; Lin, J.H.; Tseng, I.H.; Lo, A.Y.; Lo, T.Y.; Yu, H.P.; Tsai, M.H.; Whang, W.T.; Hsu, K.Y. An in situ fabrication process for highly electrical conductive polyimide/MWCNT composite films using 2,6-diaminoanthraquinone. Compos. Sci. Technol. 2013, 87, 174–181. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Dabiri, M.; Ashrafi, M. Chemical and electrochemical synthesis of conducting graft copolymer of acrylonitrile with aniline. Polym. Int. 2006, 55, 1081–1089. [Google Scholar] [CrossRef]
- Bashir, Z. Thermoreversible gelation and plasticization of polyacrylonitrile. Polymer 1992, 33, 4304–4313. [Google Scholar] [CrossRef]
- Bell, J.P.; Dumbleton, J.H. Changes in the structure of wet-spun acrylic fibers during processing. Text. Res. J. 1971, 41, 196–203. [Google Scholar] [CrossRef]
- Lolla, D.; Gorse, J.; Kisielowski, C.; Miao, J.; Taylor, P.L.; Chase, G.G.; Reneker, D.H. Polyvinylidene fluoride molecules in nanofibers, imaged at atomic scale by aberration corrected electron microscopy. Nanoscale 2016, 8, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.J.; Zhang, D.; Li, Q.; Hou, H.Q.; Graham, M.J.; Dai, L.M.; Harris, F.W.; Cheng, S.Z.D. Multiwalled carbon nanotubes with chemically grafted poletherimides. J. Am. Chem. Soc. 2005, 127, 9984–9985. [Google Scholar] [CrossRef] [PubMed]
- Vuković, G.; Marinković, A.; Obradović, M.; Radmilović, V.; Čolić, M.; Aleksić, R.; Uskoković, P.S. Synthesis, characterization and cytotoxicity of surface amino-functionalized water-dispersible multi-walled carbon nanotubes. Appl. Surf. Sci. 2009, 255, 8067–8075. [Google Scholar] [CrossRef]
- Valentini, L.; Macan, J.; Armentano, I.; Mengoni, F.; Kenny, J.M. Modification of fluorinated single-walled carbon nanotubes with aminosilane molecules. Carbon 2006, 44, 2196–2201. [Google Scholar] [CrossRef]
- Cividanes, L.S.; Brunelli, D.D.; Antunes, E.F.; Corat, E.J.; Sakane, K.K.; Thim, G.P. Cure study of epoxy resin reinforced with multiwalled carbon nanotubes by Raman and luminescence spectroscopy. J. Appl. Polym. Sci. 2013, 127, 544–553. [Google Scholar] [CrossRef]
- Bajaj, P.; Paliwal, D.K.; Gupta, A.K. Influence of metal ions on structure and properties of acrylic fibers. J. Appl. Polym. Sci. 1998, 67, 1647–1659. [Google Scholar] [CrossRef]
- Wu, T.M.; Lin, Y.W.; Liao, C.S. Preparation and characterization of polyaniline/multi-walled carbon nanotube composites. Carbon 2005, 43, 734–740. [Google Scholar] [CrossRef]
- Badawy, S.M.; Dessouki, A.M. Cross-Linked Polyacrylonitrile Prepared by radiation-induced polymerization technique. J. Phys. Chem. B 2003, 107, 11273–11279. [Google Scholar] [CrossRef]
- Pirlot, C.; Willems, I.; Fonseca, A.; Bagy, J.B.; Delhalle, J. Preparation and characterization of carbon nanotube/polyacrylonitrile composites. Adv. Eng. Mater. 2002, 4, 109–114. [Google Scholar] [CrossRef]
- Liu, X.D.; Ruland, W. X-ray studies on the structure of polyacrylonitrile fibers. Macromolecules 1993, 26, 3030–3036. [Google Scholar] [CrossRef]
- Devasia, R.; Nair, C.P.R.; Sivadasan, P.; Ninan, K.N. High char-yielding polyacrylonitrile-co-(itaconic acid)-co-(methyl acrylate): Synthesis and properties. Polym. Int. 2005, 54, 1110–1118. [Google Scholar] [CrossRef]
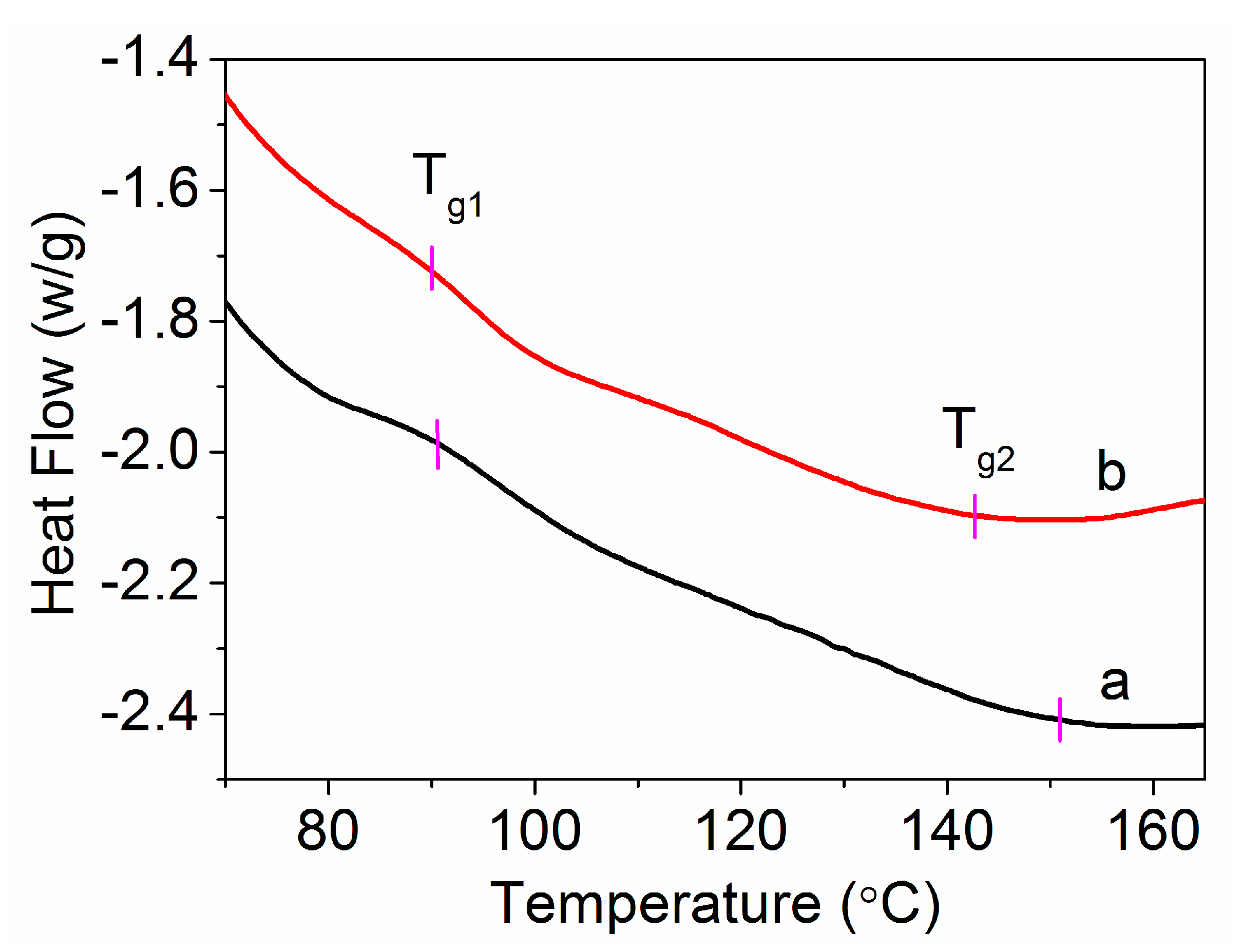
- Bashir, Z. Polyacrylonitrile, an unusual linear homopolymer with two glass transitions. Indian J. Fibre Text. 1999, 24, 1–9. [Google Scholar]
- Zhang, Z.; Jiang, W.; Wang, Y.J.; Wu, Y.C.; Hou, X. Synthesis of monodispersed polyacrylonitrile microspheres by dispersion polymerization. J. Appl. Polym. Sci. 2012, 125, 4142–4148. [Google Scholar] [CrossRef]
- Shin, H.U.; Lolla, D.; Nikolov, Z.; Chase, G.G. Pd-Au nanoparticles supported by TiO2 fibers for catalytic NO decomposition by CO. J. Ind. Eng. Chem. 2016, 33, 91–98. [Google Scholar] [CrossRef]
- Song, Y.H.; Sun, Z.Y.; Xu, L.; Shao, Z.B. Preparation and characterization of highly aligned carbon nanotubes/polyacrylonitrile composite nanofibers. Polymers 2017, 9, 1–13. [Google Scholar] [CrossRef]
- Bashir, Z.; Rastogi, S. The explanation of the increase in slope at the Tg in the plot of d-spacing versus temperature in polyacrylonitrile. J. Macromol. Sci. B 2005, 44, 55–78. [Google Scholar] [CrossRef]
- Kong, Y.; Hay, J.N. The enthalpy of fusion and degree of crystallinity of polymers as measured by DSC. Eur. Polym. J. 2003, 39, 1721–1727. [Google Scholar] [CrossRef]
- Kong, Y.; Hay, J.N. The measurement of the crystallinity of polymers by DSC. Polymer 2002, 43, 3873–3878. [Google Scholar] [CrossRef]
| Sample | Crystallinity (%) | Crystal Size (Å) |
|---|---|---|
| PAN | 35.05 | 53.40 |
| Amino-CNT/PAN | 31.41 | 57.17 |
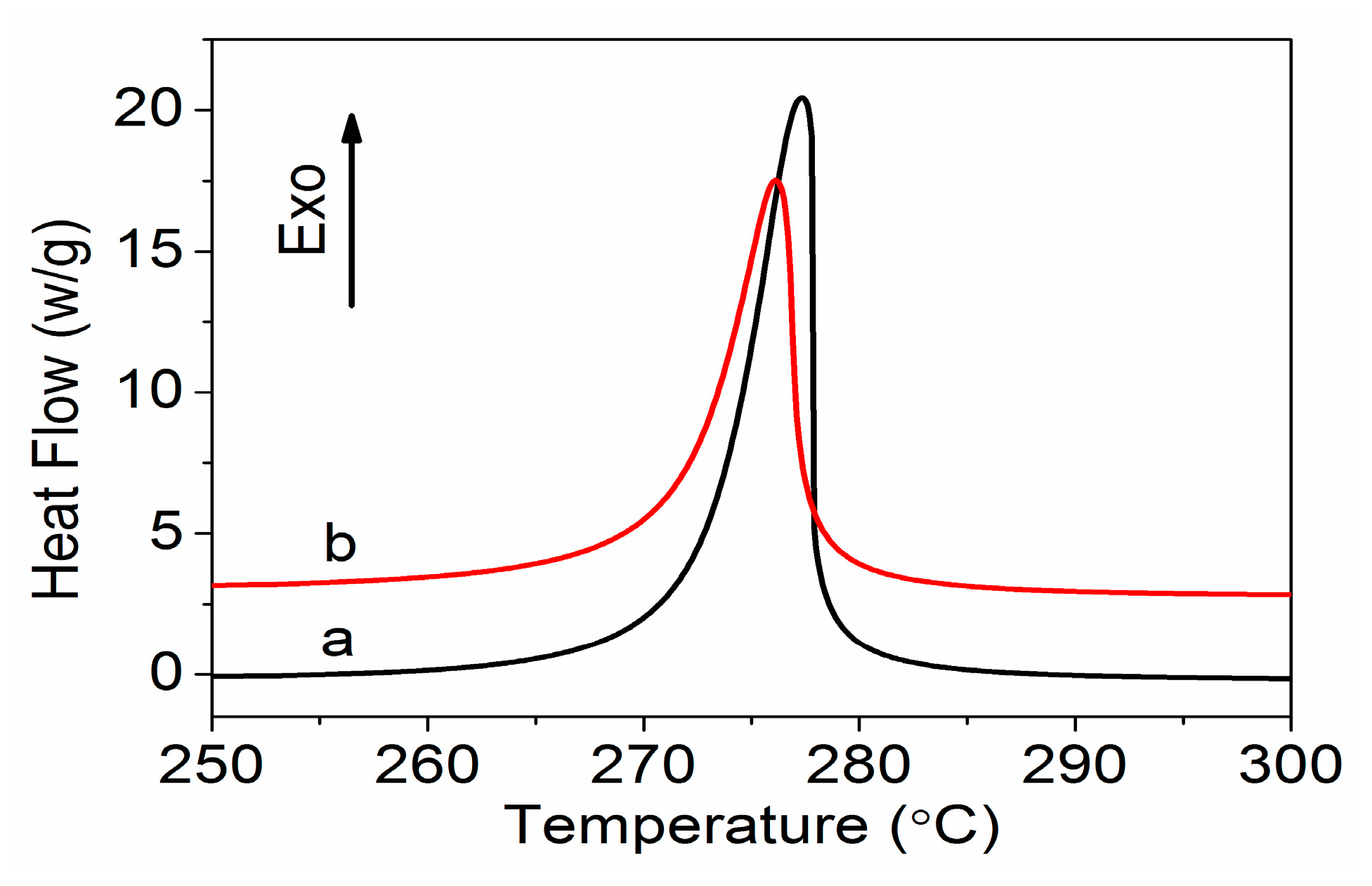
| Sample | Ti (°C) | Tp (°C) | Tf (°C) | ΔH (J g−1) | ΔT (°C) | ΔH/ΔT (J g−1 °C−1) |
|---|---|---|---|---|---|---|
| PAN | 267.0 | 277.3 | 281.5 | 567.1 | 14.5 | 39.1 |
| Amino-CNT/PAN | 263.9 | 276.2 | 281.4 | 565.7 | 17.5 | 32.3 |
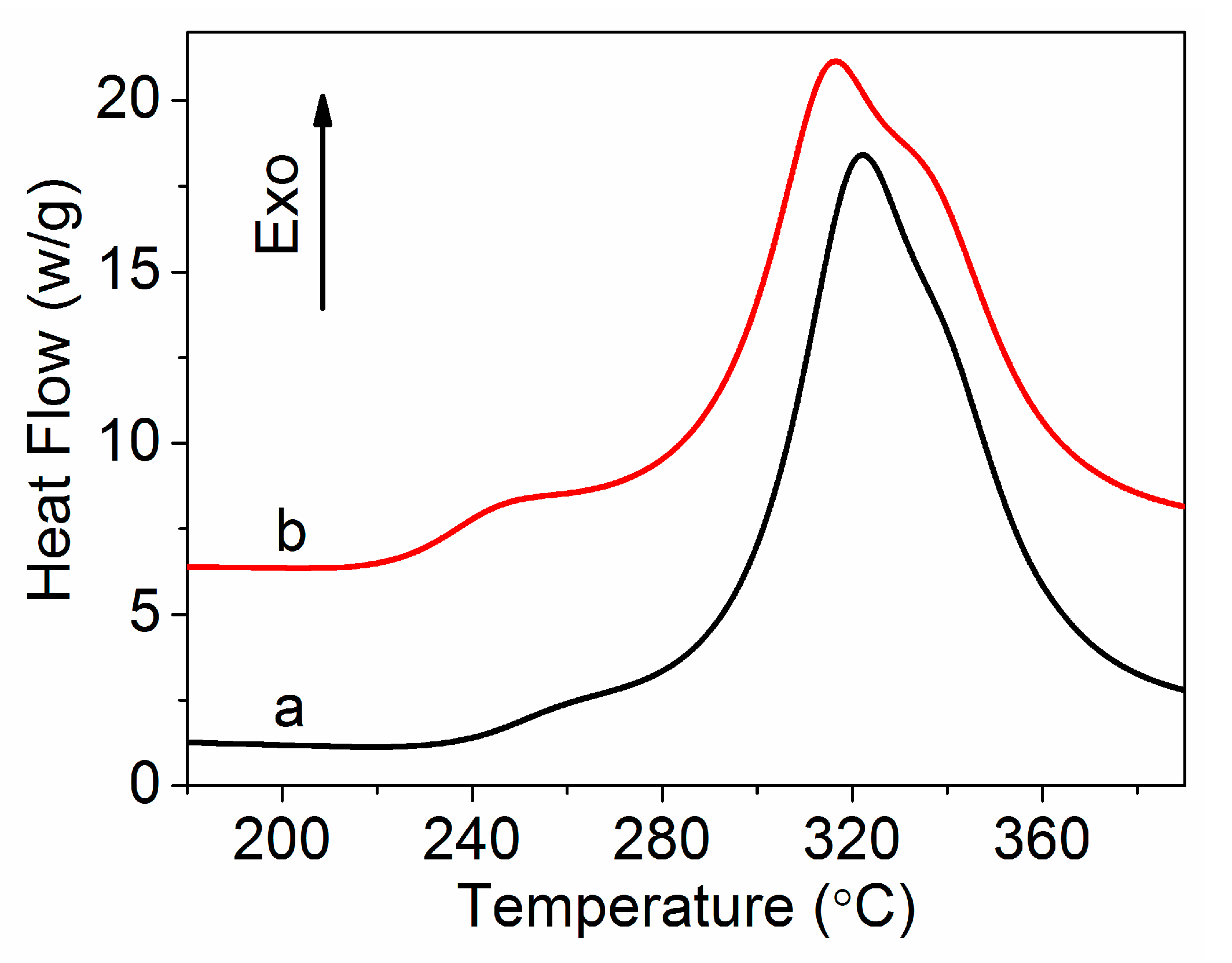
| Sample | Ti (°C) | Tp (°C) | Tf (°C) | ΔH (J g−1) | ΔT (°C) | ΔH/ΔT (J g−1 °C−1) |
|---|---|---|---|---|---|---|
| PAN | 238.1 | 322.1 | 375.2 | 4738 | 137.1 | 34.6 |
| Amino-CNT/PAN | 224.2 | 316.7 | 375.3 | 4758 | 151.1 | 31.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Quan, L.; Xu, L. Effects of Amino-Functionalized Carbon Nanotubes on the Crystal Structure and Thermal Properties of Polyacrylonitrile Homopolymer Microspheres. Polymers 2017, 9, 332. https://doi.org/10.3390/polym9080332
Zhang H, Quan L, Xu L. Effects of Amino-Functionalized Carbon Nanotubes on the Crystal Structure and Thermal Properties of Polyacrylonitrile Homopolymer Microspheres. Polymers. 2017; 9(8):332. https://doi.org/10.3390/polym9080332
Chicago/Turabian StyleZhang, Hailong, Ling Quan, and Lianghua Xu. 2017. "Effects of Amino-Functionalized Carbon Nanotubes on the Crystal Structure and Thermal Properties of Polyacrylonitrile Homopolymer Microspheres" Polymers 9, no. 8: 332. https://doi.org/10.3390/polym9080332






